Abstract
Objective:
To investigate the effectiveness of exercise and pharmacotherapy interventions in reducing visceral adipose tissue (VAT).
Patients and Methods:
A systematic search of OVID, MEDLINE, SCOPUS, Web of Science, Cochrane Library, ClinicalTrials.gov, New York Academy of Science Grey Literature Report, and Open Grey was combined with hand searches of existing literature. 2,515 titles and abstracts were reviewed. Only randomized controlled trials (RCTs) evaluating the efficacy of monitored exercise or pharmacological interventions for VAT reduction using CT or MRI imaging during a sustained intervention period (≥6 months) were included. Data were independently extracted by reviewers according to PRISMA guidelines and assessed using for quality and risk of bias. Separate analyses for each intervention were performed using random-effect models with pooled estimates of the change in VAT area (cm2) from baseline to follow-up reported as standardized mean difference (SMD, 95% CI).
Results:
3,602 participants from 17 RCTs were included in the final analysis. Both exercise and pharmacological interventions were associated with significant reductions in VAT: pharmacological with a small reduction [SMD: −0.27 (−0.47, −0.07)]; in contrast with more substantial reductions with exercise [SMD −0.54 (−0.63, −0.46)]. Absolute VAT reduction was greater among pharmacologic trials versus exercise. Meta-regression showed a correlation between VAT and weight loss (R2=0.52, exercise and R2=0.88, pharmacologic), but VAT reduction relative to weight loss differed by intervention type.
Conclusion:
Exercise interventions showed greater reduction in VAT relative to weight loss compared with pharmacologic interventions. Preferential lowering of VAT may be clinically meaningful when monitoring success of interventions since weight loss alone may underestimate benefits.
Introduction:
The adverse cardiometabolic effects of obesity are well described, with a growing recognition that visceral adipose tissue (VAT) is a key contributor to the pathogenesis of the metabolic syndrome1. Accumulation of VAT is also associated with increased risk for cardiovascular disease2, type 2 diabetes3, and cancer4.
Interventions aimed at achieving weight loss include lifestyle modification (diet and exercise), pharmacological therapies, and bariatric surgery. Reductions in body weight in general, and in VAT in particular, have the potential to substantially reduce the risk of cardiometabolic disease. For example, exercise has been suggested to produce selective reduction of VAT, even in the absence of overall body weight loss5, 6; however, studies are of modest size and significant heterogeneity and therefore have limited generalizability across interventions. Furthermore, there are no currently published guidelines on recommended therapeutic approaches to reduce VAT since large-scale, sustained duration randomized controlled intervention trials are lacking.
In this study we conducted a systematic review and meta-analysis of randomized controlled trials to assess the relative efficacy of sustained (≥6 months) exercise and pharmacologic interventions on VAT reduction in adults. We hypothesized that monitored exercise interventions would result in a larger and more consistent reduction in VAT relative to overall weight loss when compared with pharmacological therapies, given prior reports that short term aerobic exercise7 and high intensity interval training8 reduce VAT even in the absence of a hypocaloric diet or BMI change.
Patients and Methods:
Data Sources and Search Strategy
A comprehensive computerized search of OVID, MEDLINE, SCOPUS, Web of Science, the Cochrane Library, ClinicalTrials.gov, the New York Academy of Science Grey Literature Report, and Open Grey was conducted for human studies on adults over 18 years of age published in English from date of inception to September 2015 with the expertise of a medical librarian. This was supplemented by hand searching additional relevant articles identified through March 2016 and review of reference lists of selected articles. The online searches contained one or more subject headings or keywords for visceral adiposity (e.g. visceral fat) and desired interventions (e.g. exercise). The initial search included surgical and dietary interventions for weight loss, though these were later excluded from analysis due to lack of sufficient trial data (surgery) or excessive trial heterogeneity (diet). Efforts were made to contact relevant authors to acquire missing information. The search strategy, study selection and analysis were carried out in accord with the PRISMA statement for systematic reviews9. The systematic review protocol and search strategy (registration no. 91187) is publicly available at https://s3-us-west-2.amazonaws.com/utsw-patientcare-web-production/documents/Systematic_Review_Protocol_-_PROSPERO-sm.pdf.
Study Selection
Studies included in this analysis were required to have: (1) a randomized, placebo controlled trial (RCT) design, (2) visceral adipose tissue (VAT) area (cm2) as an outcome, directly measured by computed tomography (CT) or magnetic resonance imaging (MRI), (3) sustained intervention for at least 6 months (since shorter-term interventions, especially ≤3 months, may not reflect routine clinical practice), (4) monitored exercise interventions (for exercise studies), and (5) current U.S. Food and Drug Administration (FDA) approved or previously considered weight loss agents, or agents commonly used for the treatment of weight loss or components of the metabolic syndrome including those used in the treatment of diabetes and cardiovascular disease (for pharmacologic studies). Studies of specific comorbid conditions associated with weight gain, such as polycystic ovarian syndrome and growth-hormone deficiency were excluded as these results were not believed to be generalizable to the general population. Studies with an active control arm (instead of placebo-controlled) and studies that measured VAT in units other than area (e.g. volume) were excluded in order to maintain homogeneity and interpretability between studies. Titles and abstracts were independently screened by two authors (S.R. and B.P.) for potential inclusion.
Data Extraction and Quality Assessment
For each study, data were extracted for baseline characteristics of the study population including mean age, sex, weight (kg), BMI (kg/m2), race/ethnicity, waist circumference (cm), and the prevalence of comorbid diabetes. Study methodology including duration and modality of intervention, with associated measures of variance was also extracted. For studies not reporting outcomes as a mean difference between baseline and endpoint measurements, outcomes were calculated using reported baseline and endpoint data. Quality of the included studies were evaluated for risk of bias quantitatively using the Jadad scale10 and qualitatively using the Cochrane risk of bias assessment tool11-13. Studies were given positive indicators in the Cochrane tool for randomized controlled study design, and for providing clear descriptions of blinding processes and allocation concealment. Studies were awarded positive indicators for reporting of loss to follow-up and for providing available data on those not included in endpoint analysis. The Jadad score rates studies on the presence of five characteristics: 1) randomization, 2) appropriateness of randomization scheme, 3) double-blind design, 4) appropriateness of blinding scheme, and 5) description of dropouts and withdrawals.
Outcomes
The primary end point was change in VAT area (in cm2), measured as the standard mean difference change between intervention and control groups from baseline to follow-up. Secondary end points included change in weight, change in BMI, and change in subcutaneous adipose tissue (SAT) area (in cm2). Outcomes were based on the longest follow-up period available for each study.
Data Synthesis and Statistical Analysis
Individual patient-level data were not available for the studies in this analysis; thus, tabular data were used. Quantitative meta-analysis of the outcomes of VAT change from baseline to follow-up were summarized as standardized mean difference (SMD) with 95% confidence interval (CI) at last follow-up between intervention and control groups. SMD was used instead of weighted mean difference given the inclusion of both CT and MRI imaging methods to account for potential variation in scale between these two modalities. Groups were compared using random-effects models, given considerable heterogeneity in study populations and execution of interventions among the included studies. The pooled standard deviations for the net change in all outcomes were obtained or imputed (when not available) assuming a correlation coefficient of 0.90 between baseline and final measurements. For studies comparing different exercise protocols or multiple weight loss agents, each intervention was assessed independently against the control.
Analyses of each intervention were also stratified by exercise regimen and sex. Sensitivity analyses were performed with each study sequentially removed based on the study’s performance on qualitative and quantitative quality assessment and sample size. Heterogeneity was assessed among studies using the I2 statistic within each study group and within subgroups. I2 values of <25%and ≥50% were considered to be minimal and substantial, respectively. Funnel plots were developed and examined to identify publication bias and the Egger test was performed to assess relationships between effect size and sample size14.
All P-values were 2-sided with statistical significance specified at p<0.05. Meta-analysis of the outcomes were conducted using Metan and Metareg functions available for Stata version 12.1 statistical software (Stata Corporation, College Station, TX)15. Risk of bias analysis was performed using Cochrane collaboration’s assessment tool in RevMan (version 5.2 software)11. This meta-analysis has been reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta Analyses (PRISMA) guidelines16, 17.
Role of the Funding Source
This study was funded by the National Institutes of Health (Grant #K23 DK106520). The funder had no role in the study’s design, conduct, or reporting.
Results:
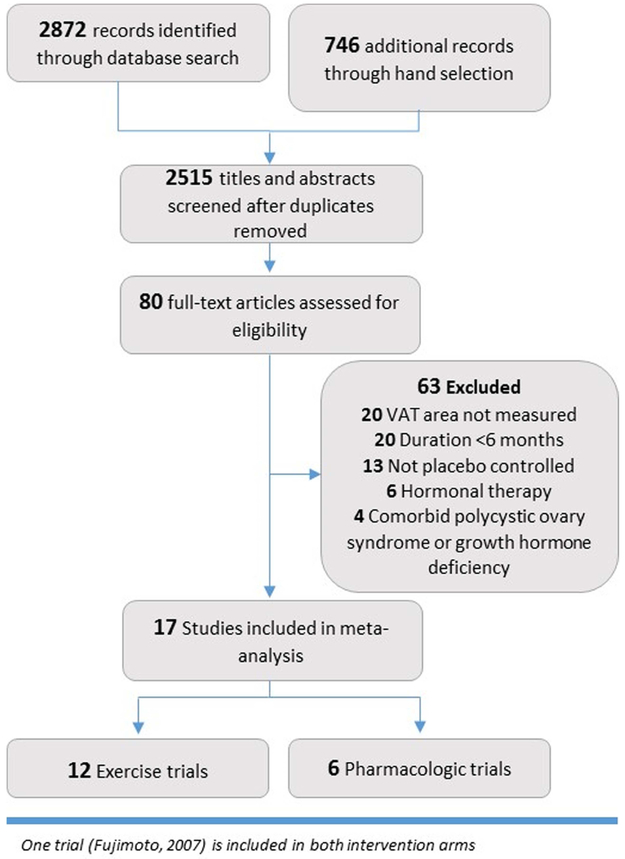
From the 2,515 titles screened for inclusion, 80 were assessed with full text review and 17 were included in the final meta-analysis18-36 (Figure 1). In addition, two pharmacologic studies that met all inclusion criteria except for borderline follow-up time (~5 months) were included in sensitivity analyses only. The study group consisted of twelve exercise trials contributing 2,094 individuals and 6 pharmacologic trials contributing 1,508 individuals (Table 1). Mean (SD) follow-up was 9 (2.9) months among exercise interventions and 8 (2.1) months among pharmacologic interventions. The majority of exercise trials were performed in the United States and Canada, while pharmacologic trials included 3 from the US or Canada, 4 multinational cohorts, 1 Swedish trial and 1 Japanese trial.
Figure 1:
PRISMA flow diagram describing process of study identification and selection.
Table 1:
Characteristics of Interventions and Populations at Baseline in Included RCTsa
| Study characteristics | Intervention arm baseline data | Quality assessment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | Treatment | Control | N | Setting | Follow- up, mo |
Men, n (%) | Mean age, y | Baseline BMI | Diabetes | Jadad score |
Dropoutb | Quality range |
|
| Barone 2009 | Exercise | Placebo | 104 | US | 6 | 51 (49.0) | 64.6 (5.7) | 29.4 (3.8) | Excluded | −1 | 10% | Low | |
| Brochu 2009 | Exercise with caloric restriction | Caloric restriction | 107 | CA | 6 | 0 (0) | 57.2 (5) | 32.6 (4.9) | Excluded | 4 | 22% | High | |
| Dobrosielski, 2012 | Exercise | Placebo | 140 | Baltimore, US | 6 | 81 (58.0) | 57 (6) | 33.0 (0.6) | Included (100%) | 4 | 19% | High | |
| Donnelly 2003 | Exercise | Placebo | 74 | Nebraska and Kansas, US | 16 | 31 (41.3) | W: 24 (5) M: 22 (4) |
W: 28.7 (3.2) M: 29.7 (2.9) |
Excluded | 4 | 44% | High | |
| Friedenreich 2011 | Exercise | Placebo | 320 | 2 centers in Alberta, CA | 12 | 0 (0) | 61.2 (5.4) | 29.1 (4.5) | Excluded | 4 | 2.8% | High | |
| Fujimoto 2007 | Exercise | Placebo | 497 | Diabetes Prevention Program: 27 centers, US |
12 | 163 (32.8) | W:51.2 (10.4) M: 57.3 (10.9) |
W: 33.2 (5.3) M: 31.8 (4.7) |
Excluded | 1 | 2.4% | Low | |
| Hunter 2010 | Aerobic Exercise Resistance Exercise |
Placebo | 69c | Alabama, US | 12 | 0 (0) | 34.7 (8.4) 34.1 (7.2) |
23.5 (1) 23.9 (1) |
Excluded | 1 | Not reported | Low | |
| Mctiernan 2007 | Exercise | Placebo | 202 | Gastroenterology practices, US | 12 | 102 (69.4) | W: 54.4 (7.1) M: 56.6 (7.6) |
W: 28.5 (4.8) M: 30.1 (4.8) |
Excluded | 3 | 7%d | High | |
| Poehlman 2000 | Endurance Exercise Resistance Exercise |
Placebo | 51 | Vermont, US | 6 | 0 (0) | 29 (5) 28 (3) |
22 (2) 22 (2) |
Excluded | 4 | 36% | High | |
| Sigal 2007 | Combined Exercise Aerobic Exercise Resistance Exercise |
Placebo | 251 | 8 community based facilities in Ottawa, CA | 6 | 160 (63.7) | 53.5 (7.3) 53.9 (6.6) 54.7 (7.5) |
35.0 (9.6) 35.6 (10.1) 34.1 (9.6) |
Included (100%) | 4 | 12% | High | |
| Slentz 2005 | Low/moderate Exercise Low/vigorous Exercise High/vigorous Exercise |
Placebo | 175 | North Carolina, US | 8 | 91 (52.0) | 54 (5.4) 53 (7) 51.5 (5.3) |
29.8 (3.2) 29.7 (3.1) 29.1 (2.4) |
Excluded | 3 | 32% | High | |
| Stewart 2005 | Exercise | Placebo | 104 | Baltimore, US | 6 | 51 (49.0) | W: 64.3 (5.8) M: 61.7 (4.5) |
W: 29.1 (4.4) M: 29.7 (3) |
Excluded | 2 | 10% | Low | |
| Astrup 2012e, f | Liraglutide 1.2 mg Liraglutide 1.8 mg Liraglutide 2.4 mg Liraglutide 3.0 mg Orlistat |
Placebo | 84 | 19 research sites in 8 European countries | 5 | 156 (28.0) | 47.2 (9.7) 45.5 (10.9) 45.0 (11.1) 45.9 (10.7) 45.9 (9.1) |
34.8 (2.6) 35.0 (2.6) 35.0 (2.8) 34.8 (2.8) 34.1 (2.6) |
Excluded | 4 | 30% | High | |
| Despres 2009 | Rimonabant | Placebo | 799 | 53 centers in 14 countries | 12 | 370 (46.3) | 49.9 (12.3) | 36.3 (6.4) | Included | 3 | 20% | High | |
| Dumont 2001 | Gemfibrozil | Placebo | 64 | Quebec, CA | 6 | 64 (100.0) | 46 (6) | 31.6 (2.7) | Excluded | −1 | Not reported | Low | |
| Fujimoto 2007 | Metformin | Placebo | 474 | DPP: 27 centers, US | 12 | 176 (34.9) | W: 51.3 (9.2) M: 52.6 (11.0) |
W: 32.9 (5.6) M: 31.7 (4.4) |
Excluded | 0 | 2% | Low | |
| Jansson 2011g | Rosuvastatin | Placebo | 54 | Gothenburg, SE | 6 | 54 (100) | 54 (5.2) | Not reported | Excluded | 3 | 7% | Low | |
| Kelley 2004 | Orlistat | Placebo | 39 | Pittsburgh, US | 6 | 13 (33.3) | 50.3 (1.9) | 34.0 (1.0) | Included (100%) | 3 | 25% | High | |
| Ridderstrale 2014f | Empaglifozin and metformin | Glimepiride and metformin | 91 | 173 sites in 23 countries | 26 | 40 (44.0) | 57.6 (8.6) | 31.5 (4.6) | Included (100%) | 4 | 16%h | High | |
| Takase 2012 | Ezetimibe | Placebo | 78 | Hamamatsu, JP | 6 | 50 (64.1) | 63.8 (11.4) | 27.8 (2.3) | Included | 2 | 0% | Low | |
BMI = body mass index, M = male, mo = months, W = women
Reported for full study population
Not full sample: excludes non-adherers
For intervention group only; dropouts among controls not reported
All data apart from VAT and SAT are for full pool of participants for which n=95, 90, 93, 93, 95, 98, respectively. VAT and SAT measured in subset of patients for whom n is presented in this table
Included in sensitivity analysis only
Unpublished data, available through clinicaltrials.gov
Dropout rate for full study, not reported for VAT sub-study
Participants enrolled in exercise cohorts were predominantly female (65.1%) with a mean (SD) age of 54 (7.3) years and mean BMI (SD) at enrollment of 31 (5.4) kg/m2. Diabetics were excluded from all but two exercise trials20, 27 which included diabetic patients only. Mean dropout rate amongst exercise trials was 17.9%. Pharmacologic trials included studies of rimonabant, gemfibrozil, metformin, rosuvastatin, orlistat and ezetimibe. Additional studies of liraglutide and empagliflozin were included in sensitivity analysis. Participants in pharmacologic trials were also predominantly female (52.7%) with a mean (SD) age of 51 (11.0) years and mean (SD) BMI at enrollment of 34 (5.6) kg/m2. Dropout rates were lower at 12%. Similar to exercise trials, diabetic patients were excluded from the majority of trials, but were included in trials of orlistat33 and rimonabant31.
Quality assessment
In all, 8 of the exercise trials and 4 of the pharmacologic trials received a “high” quality Jadad score, corresponding to a Jadad score of ≥ 3. Quality assessment using the Cochrane tool is shown in Supplemental Figure 1. Low scores corresponded to studies that failed to describe attrition bias or provide information on the effect of loss to follow-up on subsequent analysis. Publication bias was assessed visually by a funnel plot and using Egger’s test for bias (Supplemental Figure 2). The summary estimate of included studies is represented by the solid vertical line, with smaller studies represented by open circles gathered at the base of the plot and larger studies at the peak. Symmetry of the funnel plot along with a non-significant p-value in Egger’s test suggest together that there was no significant publication bias (P=.32).
Primary Outcome: Visceral Adipose Tissue Reduction
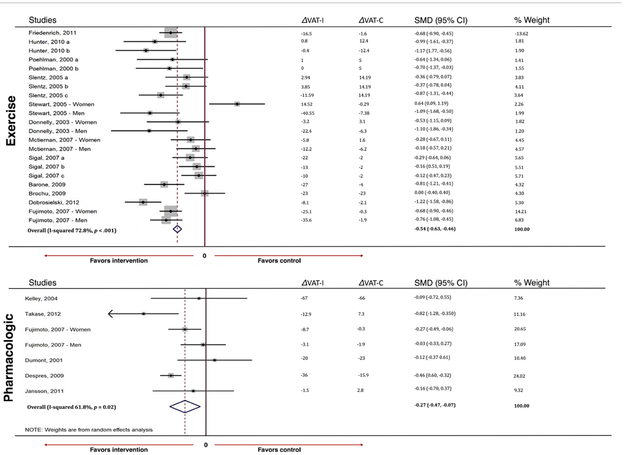
In pooled analyses, exercise intervention was associated with a medium reduction in VAT (standardized mean difference [SMD] −0.54, 95% confidence interval [CI] −0.63, −0.46) compared to a small reduction seen with pharmacologic interventions (SMD −0.27, 95% CI −0.47, −0.07) (Figure 2a). Both results reached statistical significance. Although exercise interventions more effectively reduced VAT when compared to controls, mean absolute VAT reduction was more pronounced among pharmacologic trials which produced a VAT reduction of 23.9 cm2 (SD = 37.8) compared to a reduction of 15.3 cm2 (SD = 40.4) with exercise. This discrepancy can be attributed to large VAT reductions seen among control groups in pharmacologic trials. Among exercise trials, aerobic regimens reduced VAT the most, producing an absolute reduction of 16.4 cm2 (SD = 37.8), followed by combined aerobic/resistance regimens (14.0 cm2, SD = 23.6) and resistance-only regimens (12.2 cm2, SD = 46.5) (Supplemental Table 1). Among pharmacological trials, the greatest reduction in VAT was seen in the cohort given three times daily orlistat 120 mg, with a mean reduction in absolute VAT of 67 cm2, followed by rimonabant and gemfibrozil. Consistent reductions in VAT were demonstrated both with liraglutide and combination of empagliflozin with metformin (Supplemental Table 2). We found substantial heterogeneity among studies for both exercise (I2=73%) and pharmacological (I2=62%) interventions. Given that loss of VAT in response to diet, exercise, or pharmacotherapy is correlated with baseline VAT (more likely to have greater VAT loss with higher baseline VAT) and that baseline VAT is related to sex (higher in males vs. females), we evaluated the effects of the interventions stratified by sex and found similar effects for exercise and pharmacological interventions on VAT loss in both sexes. Given the small number of patients with diabetes included in the studies, we were unable to evaluate for any differential effects on VAT for exercise or medications between those with and without diabetes.
Figure 2:
Pooled changes in visceral adiposity (a) and changes in weight (b) by intervention type. VAT change designated in cm2, weight change designated in kg. % weight refers to the individual contribution of each study to the overall pooled estimate. VAT= visceral adipose tissue
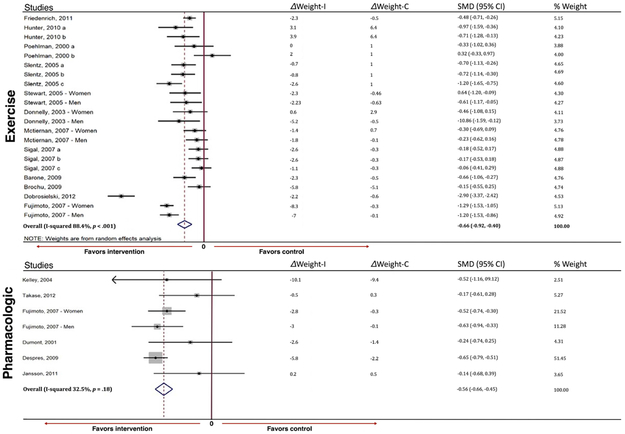
Secondary Outcomes: Weight, Body Mass Index, and Subcutaneous Adipose Tissue Reduction
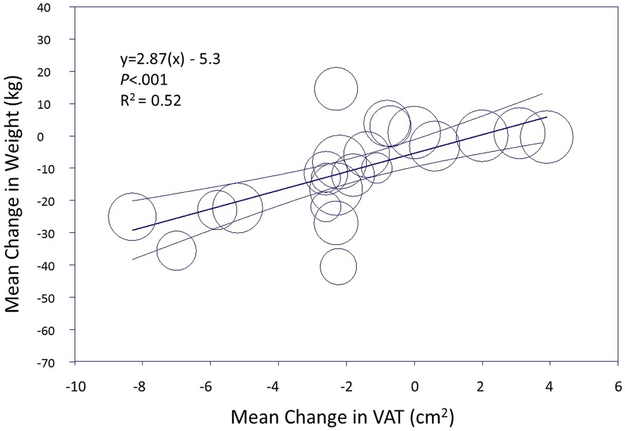
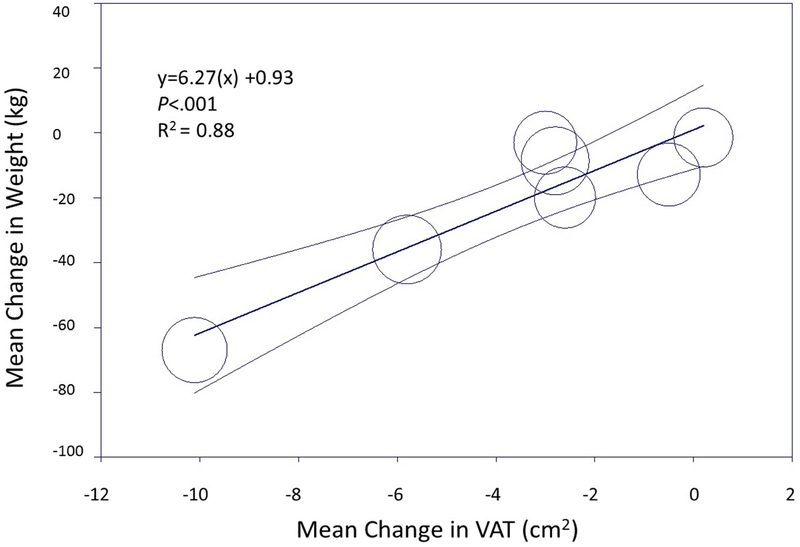
Both exercise and pharmacologic interventions resulted in a medium and statistically significant reduction in weight (SMD −0.66, 95% CI −0.92, −0.40 for exercise interventions and SMD −0.56, 95% CI −0.66, −0.45 for pharmacologic interventions) (Figure 2b). Meta-regression modeling demonstrated a linear correlation between change in weight and change in VAT among both exercise and pharmacologic interventions (R2=0.52 for exercise and R2=0.88 for pharmacologic interventions). However, the reduction of VAT relative to weight loss for each intervention type differed (based on the slope of the best-fit regression line), with greater VAT loss relative to weight at smaller achieved weight reductions with pharmacologic interventions in contrast to greater VAT loss relative to weight at larger achieved weight reductions for exercise (Figure 3a). For example, using meta-regression, for a ~7 kg reduction in weight with exercise, the expected VAT reduction would 0.5 cm2, compared with the same VAT reduction achieved with only ~2 kg of weight loss with pharmacologic therapy (Figure 3b). In contrast, to achieve −3 cm2 reduction in VAT with medication, ~18 kg of weight loss would be required compared with only ~14 kg of weight loss with exercise. BMI and SAT showed modest reductions for exercise interventions (SMD −0.61, 95% CI −0.70, −0.53 and SMD −0.61, 95% CI −0.69, −0.52, respectively) and small effects for pharmacologic studies in pooled analysis (SMD −0.34, 95% CI −0.44, −0.24 and SMD - 0.34, 95% CI −0.54, −0.14, respectively) (Supplemental Figure 3), and were correlated with reductions in VAT.
Figure 3:
Relationship between weight loss and visceral adiposity reduction among exercise trials (a) and pharmacologic trials (b). Data represent the mean change (95% confidence interval) in weight by visceral adipose tissue reduction in a random effects model.
Discussion:
Accumulation of visceral fat has been linked to the development of the metabolic syndrome and has been hypothesized to be the driver of an unfavorable metabolic profile in obesity 37-40. Both lifestyle and pharmacological interventions have the potential to reduce VAT to improve cardiometabolic outcomes. We demonstrate that among overweight and obese adults, both long-term, sustained monitored exercise and pharmacologic interventions reduce VAT, as well as SAT, weight, and BMI. While neither intervention preferentially targeted VAT over SAT, exercise interventions produced a modest and sustained reduction and appeared to reduce VAT more than pharmacological regimens relative to control. Moreover, the degree of VAT reduction relative to weight loss differed by intervention type, suggesting that monitoring success in exercise and pharmacologic interventions using weight loss alone may underestimate benefits. Indeed, emerging evidence supports the notion that a lifestyle-modification program characterized by an increase in physical activity and a balanced diet can reduce the risk of obesity-related comorbid conditions despite minimal or no weight loss. The benefits of such an approach may include reductions in visceral fat and cardiometabolic risk factors, and increases in both skeletal muscle mass and cardiorespiratory fitness5, 41. Differences in VAT loss relative to changes in weight between intervention types may reflect concomitant loss of lean mass in pharmacologic trials not present in exercise interventions that can maintain or increase lean mass. Overall, these findings suggest that both exercise and pharmacologic therapies effectively impact VAT reduction compared with placebo, while also resulting in modest reductions in both SAT and weight.
Prior studies have assessed the impact of exercise interventions on weight and body fat distribution7, 42-50. However, many prior studies comparing different modalities for weight and VAT reduction have not examined these outcomes with long-term follow-up, randomized control design, or assessment of other adipose depots. Our study addresses many of these limitations in the literature and confirms findings in the meta-analyses by Ismail et al.44 and Vissers et al.7, that exercise alone can produce reductions in VAT in overweight and obese individuals and provides further evidence to support the role of aerobic exercise and combined aerobic and resistance regimens in VAT reduction. Aerobic exercise in particular may improve cardiorespiratory fitness and multiple metabolic biomarkers. Furthermore, although it is evident from our study and others that aerobic exercise compared with resistance training results in greater VAT reduction, alternative exercise variables such as the volume (i.e. amount of exercise per unit time) and intensity (i.e. aerobic level of a given exercise type during training) of an exercise program may also impact VAT51. Our study also goes beyond the findings of those prior studies in demonstrating reductions in SAT as well as VAT and in correlating changes in these adipose depots with overall weight loss. These findings suggest that specific markers of VAT loss are likely important when monitoring the success of weight loss interventions. Initiatives designed to better assess lifestyle and pharmacological interventions for weight loss using direct imaging based assessments of VAT or alternative surrogate markers such as the hypertriglyceridemic waist52, rather than weight or BMI in isolation, are likely to demonstrate that preferential VAT loss beyond BMI is clinically meaningful.
To our knowledge, this study is the first systematic review and meta-analysis of sustained pharmacologic and exercise interventions on VAT and weight. Two prior meta-analyses have aimed to assess different modalities for reduction in VAT42, 45. Our study differs in two key aspects: 1) we limit our inclusion to randomized trials only, and 2) we assess studies with follow-up ≥6 months in order to test our hypothesis for sustained weight loss. More recent analysis by Merlotti et al extends these findings to surgical interventions as well, and support our finding that reductions in VAT are correlated with reductions in SAT regardless of intervention type45. That analysis is also limited by inclusion of non-randomized data as well as studies with relatively short follow-up.
Prior studies have proposed mechanisms for the modulation of visceral adiposity and its effect on cardiovascular risk. Early hypotheses associated excess VAT with cardiovascular risk by means of impaired liver metabolism that in turn contributes to impaired glucose tolerance and hypertriglyceridemia. However, more recent studies suggest an overactive hypothalamic-pituitary-adrenal axis may be the primary driver of an unfavorable cardiometabolic profile resulting in increased VAT and CVD risk53. Accumulation of VAT is believed to result in increased circulating blood volume and systemic pro-atherogenic inflammatory factors and adipokines, which together translate to an increased risk for the development of heart failure and atherosclerotic cardiac disease54.
Our finding that absolute VAT reduction was greater among pharmacologic trials compared with exercise studies may potentially be attributed to larger VAT reductions seen among control groups in the pharmacologic trials. Pharmacologic trials uniformly include caloric restriction protocols/counseling in both the experimental and control arms since medications are considered for approval as adjunctive therapies to diet. The presence of caloric restriction leading to greater VAT reduction in both arms of pharmacologic studies may therefore underlie this finding. The mechanisms of action of the pharmacologic agents included in this study vary substantially and are summarized in Supplemental Table 3. Although rimonabant, a cannabinoid receptor (CB1) blocker, was not approved by the FDA and was suspended worldwide in the late 2000’s due to adverse effects, other agents targeting CB1 remain in the pipeline, suggesting value in continued investigation of this pathway55. While orlistat and GLP-1 analogs including liraglutide remain the mainstays of FDA-approved weight loss therapy in the United States, there has been increased interest in the newer SGLT2 inhibitors given their demonstrable benefits in the treatment of diabetes and cardiovascular disease. Individually, however, only rimonabant, ezetimibe (unproven weight loss mechanism but may be related to reduction in intestinal fat absorption), and empagliflozin/metformin reached statistical significance for VAT reduction or weight loss.
Strengths and Limitations
Strengths of the current study include the inclusion of only randomized trials and a large sample size with a diverse population of overweight and obese adults that allows for generalization to the general population. Furthermore, we evaluated multiple weight loss modalities over long-term follow-up, with potentially greater clinical relevance than studies of short-term interventions. Several limitations merit comment. We were able to access aggregate data only rather than patient-level data, which may influence the effect estimates. Furthermore, many randomized-controlled trials of weight-loss interventions do not include body fat distribution outcomes, so we were unable to assess the impact of other FDA-approved agents for weight loss on VAT reduction. In addition, many trials lacked data on the impact of weight and VAT loss on other metabolic risk factors and biomarkers and thus we cannot draw direct conclusions about improvements in cardiovascular health as a result of these interventions. Finally, as with all meta-analyses, selection bias cannot be completely ruled out because articles were only retrieved from published trials.
Clinical Implications
In pooled analyses, exercise demonstrated a medium improvement in visceral adiposity, subcutaneous adiposity and weight, while pharmacologic interventions for weight loss demonstrated smaller overall effects. Importantly, change in weight was shown to be an overall predictor of VAT change, but may underestimate the effect on VAT reduction in exercise studies. Prior work has demonstrated that the regional distribution of body fat is more important than excess adiposity per se in driving the cardiovascular disease risk associated with excess of body weight53. Since the relationship between reduction in visceral fat and weight is variable, body weight in isolation may be an inadequate clinical marker and prognostic indicator of cardiovascular risk in obesity. Our findings support the use of more specific markers of VAT when monitoring the success of weight loss interventions. Additionally, future studies of weight loss interventions should embed assessments of body fat distribution, such as VAT, in order to determine clinical benefits. Interventions that result in substantial VAT loss with less impact on overall weight may still be clinically meaningful.
More information is needed regarding the effects of newer agents for cardiometabolic disease, such as SGLT2 inhibitors, in modulating visceral fat, as they are likely to play an increasingly important role in the management of complications of obesity such as type 2 diabetes. While the present findings support the use of exercise over pharmacotherapy in achieving weight loss and VAT reductions, the potential synergistic effects of both therapies combined compared with either alone were not able to be determined in our study and will require further investigation.
Conclusion
Exercise interventions showed greater reduction in VAT relative to weight loss compared with pharmacologic interventions. Preferential lowering of VAT may be clinically meaningful and is important when monitoring success of interventions since weight loss alone may underestimate benefits. The reduction in VAT seen with both pharmacotherapy and exercise, in addition to empirical improvements in VAT with a calorie restricted diet, suggests a role for a multimodality approach to the treatment of overweight/obesity using a combination of strategies to help guide therapy and lower cardiovascular risk.
Supplementary Material
Acknowledgments
Sources of Funding
Dr. Neeland is supported by grant K23 DK106520 from the National Institutes of Health.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- FDA
U.S. Food and Drug Administration
- RCT
randomized controlled trial
- SAT
subcutaneous adipose tissue
- SD
standard deviation
- SMD
standardized mean difference
- VAT
visceral adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Dr. Neeland has received honoraria, consulting/speaking fees, and other research support from Boehringer-Ingelheim (significant), a research grant from Novo Nordisk (significant), and is a member of the scientific advisory board of Advanced MR Analytics (modest). Dr. Després is the scientific director of the International Chair on Cardiometabolic Risk that is supported by the Fondation de l’Université Laval (significant).
References:
- 1.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. [DOI] [PubMed] [Google Scholar]
- 2.Neeland IJ, Turer AT, Ayers CR, et al. Body fat distribution and incident cardiovascular disease in obese adults. J Am Coll Cardiol. 2015;65:2150–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neeland IJ, Turer AT, Ayers CR, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308:1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62:921–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross R, Bradshaw AJ. The future of obesity reduction: beyond weight loss. Nature reviews. Endocrinology. 2009;5:319–325. [DOI] [PubMed] [Google Scholar]
- 6.Despres JP. Obesity and cardiovascular disease: weight loss is not the only target. Can J Cardiol. 2015;31:216–222. [DOI] [PubMed] [Google Scholar]
- 7.Vissers D, Hens W, Taeymans J, Baeyens JP, Poortmans J, Van Gaal L. The effect of exercise on visceral adipose tissue in overweight adults: a systematic review and meta-analysis. PLoS ONE [Electronic Resource]. 2013;8:e56415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maillard F, Rousset S, Pereira B, et al. High-intensity interval training reduces abdominal fat mass in postmenopausal women with type 2 diabetes. Diabetes Metab. 2016;42:433–441. [DOI] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ. 2001;323:42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger VW, Alperson SY. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris R, Bradburn M, Deeks J, Harbord R, Altman D, Sterne J. Metan: fixed- and random-ffects meta-analysis. Stata J. 2008;8:3–28. [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. [DOI] [PubMed] [Google Scholar]
- 18.Barone BB, Wang NY, Bacher AC, Stewart KJ. Decreased exercise blood pressure in older adults after exercise training: contributions of increased fitness and decreased fatness. Br J Sports Med. 2009;43:52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brochu M, Malita MF, Messier V, et al. Resistance training does not contribute to improving the metabolic profile after a 6-month weight loss program in overweight and obese postmenopausal women. J Clin Endocrinol Metab. 2009;94:3226–3233. [DOI] [PubMed] [Google Scholar]
- 20.Dobrosielski DA, Gibbs BB, Ouyang P, et al. Effect of exercise on blood pressure in type 2 diabetes: a randomized controlled trial. J Gen Intern Med. 2012;27:1453–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donnelly JE, Hill JO, Jacobsen DJ, et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the Midwest Exercise Trial. Arch Intern Med. 2003;163:1343–1350. [DOI] [PubMed] [Google Scholar]
- 22.Friedenreich CM, Woolcott CG, McTiernan A, et al. Adiposity changes after a 1-year aerobic exercise intervention among postmenopausal women: a randomized controlled trial. Int J Obes (Lond). 2011;35:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujimoto WY, Jablonski KA, Bray GA, et al. Body size and shape changes and the risk of diabetes in the diabetes prevention program. Diabetes. 2007;56:1680–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunter GR, Brock DW, Byrne NM, Chandler-Laney PC, Del Corral P, Gower BA. Exercise training prevents regain of visceral fat for 1 year following weight loss. Obesity (Silver Spring). 2010;18:690–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McTiernan A, Sorensen B, Irwin ML, et al. Exercise effect on weight and body fat in men and women. Obesity (Silver Spring). 2007;15:1496–1512. [DOI] [PubMed] [Google Scholar]
- 26.Poehlman ET, Dvorak RV, DeNino WF, Brochu M, Ades PA. Effects of resistance training and endurance training on insulin sensitivity in nonobese, young women: a controlled randomized trial. J Clin Endocrinol Metab. 2000;85:2463–2468. [DOI] [PubMed] [Google Scholar]
- 27.Sigal RJ, Kenny GP, Boule NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:357–369. [DOI] [PubMed] [Google Scholar]
- 28.Slentz CA, Aiken LB, Houmard JA, et al. Inactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol (1985). 2005;99:1613–1618. [DOI] [PubMed] [Google Scholar]
- 29.Stewart KJ, Bacher AC, Hees PS, Tayback M, Ouyang P, Jan de Beur S. Exercise effects on bone mineral density relationships to changes in fitness and fatness. Am J Prev Med. 2005;28:453–460. [DOI] [PubMed] [Google Scholar]
- 30.Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond). 2012;36:843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Després JP, Ross R, Boka G, Alméras N, Lemieux I. Effect of rimonabant on the high-triglyceride/low-HDL-cholesterol dyslipidemia, intraabdominal adiposity, and liver fat the ADAGIO-lipids trial. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:416–423. [DOI] [PubMed] [Google Scholar]
- 32.Dumont M, Mauriege P, Bergeron J, Despres JP, Prud'homme D. Effect of a six month gemfibrozil treatment and dietary recommendations on the metabolic risk profile of visceral obese men. Int J Obes Relat Metab Disord. 2001;25:1136–1143. [DOI] [PubMed] [Google Scholar]
- 33.Kelley DE, Kuller LH, McKolanis TM, Harper P, Mancino J, Kalhan S. Effects of moderate weight loss and orlistat on insulin resistance, regional adiposity, and fatty acids in type 2 diabetes. Diabetes Care. 2004;27:33–40. [DOI] [PubMed] [Google Scholar]
- 34.Ridderstrale M, Andersen KR, Zeller C, et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691–700. [DOI] [PubMed] [Google Scholar]
- 35.Takase H, Dohi Y, Okado T, Hashimoto T, Goto Y, Kimura G. Effects of ezetimibe on visceral fat in the metabolic syndrome: a randomised controlled study. Eur J Clin Invest. 2012;42:1287–1294. [DOI] [PubMed] [Google Scholar]
- 36.Jansson J-O, Ohlsson C, Nilsson A, Karason K. Rosuvastatin in Visceral Adiposity (RIVIERA). clinicaltrials.gov: Göteborg University; 2015. [Google Scholar]
- 37.Chaston TB, Dixon JB. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes (Lond). 2008;32:619–628. [DOI] [PubMed] [Google Scholar]
- 38.Leenen R, van der Kooy K, Droop A, et al. Visceral fat loss measured by magnetic resonance imaging in relation to changes in serum lipid levels of obese men and women. Arterioscler Thromb. 1993;13:487–494. [DOI] [PubMed] [Google Scholar]
- 39.Bays HE. Adiposopathy is "sick fat" a cardiovascular disease? J Am Coll Cardiol. 2011;57:2461–2473. [DOI] [PubMed] [Google Scholar]
- 40.Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring). 2006;14:336–341. [DOI] [PubMed] [Google Scholar]
- 41.Janiszewski PM, Ross R. Physical activity in the treatment of obesity: beyond body weight reduction. Appl Physiol Nutr Metab. 2007;32:512–522. [DOI] [PubMed] [Google Scholar]
- 42.Verheggen RJ, Maessen MF, Green DJ, Hermus AR, Hopman MT, Thijssen DH. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: distinct effects on body weight and visceral adipose tissue. Obes Rev. 2016;17:664–690. [DOI] [PubMed] [Google Scholar]
- 43.Irwin ML, Yasui Y, Ulrich CM, et al. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. JAMA. 2003;289:323–330. [DOI] [PubMed] [Google Scholar]
- 44.Ismail I, Keating SE, Baker MK, Johnson NA. A systematic review and meta-analysis of the effect of aerobic vs. resistance exercise training on visceral fat. Obes Rev. 2012;13:68–91. [DOI] [PubMed] [Google Scholar]
- 45.Merlotti C, Ceriani V, Morabito A, Pontiroli AE. Subcutaneous fat loss is greater than visceral fat loss with diet and exercise, weight-loss promoting drugs and bariatric surgery: a critical review and meta-analysis. Int J Obes (Lond). 2017;41:672–682. [DOI] [PubMed] [Google Scholar]
- 46.Okura T, Nakata Y, Tanaka K. Effects of exercise intensity on physical fitness and risk factors for coronary heart disease. Obes Res. 2003;11:1131–1139. [DOI] [PubMed] [Google Scholar]
- 47.Thomas EL, Brynes AE, McCarthy J, et al. Preferential loss of visceral fat following aerobic exercise, measured by magnetic resonance imaging. Lipids. 2000;35:769–776. [DOI] [PubMed] [Google Scholar]
- 48.Vissers D, Hens W, Hansen D, Taeymans J. The Effect of Diet or Exercise on Visceral Adipose Tissue in Overweight Youth. Med Sci Sports Exerc. 2016;48:1415–1424. [DOI] [PubMed] [Google Scholar]
- 49.Gutin B, Barbeau P, Owens S, et al. Effects of exercise intensity on cardiovascular fitness, total body composition, and visceral adiposity of obese adolescents. Am J Clin Nutr. 2002;75:818–826. [DOI] [PubMed] [Google Scholar]
- 50.Irving BA, Davis CK, Brock DW, et al. Effect of exercise training intensity on abdominal visceral fat and body composition. Med Sci Sports Exerc. 2008;40:1863–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao T, Fu YF. Resistance training vs. aerobic training and role of other factors on the exercise effects on visceral fat. Eur Rev Med Pharmacol Sci. 2015;19:1779–1784. [PubMed] [Google Scholar]
- 52.Lemieux I, Pascot A, Couillard C, et al. Hypertriglyceridemic waist: A marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. 2000;102:179–184. [DOI] [PubMed] [Google Scholar]
- 53.Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–1313. [DOI] [PubMed] [Google Scholar]
- 54.Neeland IJ, Winders BR, Ayers CR, et al. Higher natriuretic peptide levels associate with a favorable adipose tissue distribution profile. J Am Coll Cardiol. 2013;62:752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodgers RJ, Tschop MH, Wilding JP. Anti-obesity drugs: past, present and future. Dis Model Mech. 2012;5:621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.