Abstract
Hepatitis C virus (HCV) infection is not only an important cause of chronic liver disease, but extra-hepatic manifestations are common and include chronic kidney disease (CKD). HCV is classically associated with cryoglobulinemic glomerulonephritis in the context of mixed cryoglobulinemia syndrome, but other glomerular diseases also occur and may be significantly under-recognized. HCV may cause glomerular disease by immune complex deposition; however, other potential mechanisms by which HCV promotes CKD include a direct cytopathic effect of the virus on renal tissue, and by its association with accelerated atherosclerosis, insulin resistance, and chronic inflammation. Epidemiologic studies show HCV infection confers an increased risk of incident CKD and accelerates progression of CKD to end-stage renal disease (ESRD) in the general population, as well as subpopulations including diabetic patients, those co-infected with human immunodeficiency virus (HIV), and kidney transplant recipients. Patients with CKD and HCV infection experience inferior clinical outcomes, including poorer quality of life and an increased risk of mortality. Treatment with interferon-based regimens is associated with decreased risk of incident CKD and ESRD, though prior studies are limited by the small number of patients with HCV and CKD who underwent treatment. With the advent of new, well-tolerated direct-acting antiviral combinations that are not cleared by the kidneys, it is possible to treat all genotypes of HCV infection in patients with CKD and ESRD. More data on the effect of direct-acting antivirals on CKD incidence and progression are necessary. However there is every expectation that with improved access to HCV treatment, the burden of CKD in patients with HCV could significantly decline.
Keywords: hepatitis C virus, chronic kidney disease, cryoglobulinemia, glomerulonephritis, direct-acting antivirals, interferon
Introduction
The World Health Organization estimates that chronic hepatitis C virus (HCV) infection affects approximately 170 million people worldwide.1 HCV infection causes significant morbidity and mortality, mainly due to the hepatic complications of cirrhosis and hepatocellular carcinoma. However, as many as 74% of patients with HCV experience extra-hepatic manifestations, including mixed cryoglobulinemia, chronic kidney disease (CKD), type 2 diabetes, porphyria cutanea tarda, lichen planus, lymphoproliferative disorders, cardiovascular disease, and depression.2,3 These extra-hepatic manifestations contribute significantly to the burden of the disease, impacting clinical outcomes adversely as well as patient quality of life and health-care costs.3
Soon after its discovery, reports linking HCV infection to kidney disease began to emerge, and accumulating evidence over the last twenty-five years has revealed a complex relationship between these two disease processes. HCV infection is the most common cause of mixed cryoglobulinemia syndrome (MCS), which leads to cryoglobulinemic glomerulonephritis (CGN) Furthermore other immune-complex mediated glomerular diseases have also been identified in patients with HCV.4–20 Beyond glomerular disease, HCV infection has been implicated as a significant risk factor for incident CKD and is associated with an increased risk of progression to end-stage renal disease (ESRD), though the data on this issue have been conflicting.21–54 Furthermore, HCV infection is associated with poorer clinical outcomes and confers an increased risk of mortality in patients with ESRD on hemodialysis (HD).28
Treatment of HCV in patients with CKD and ESRD has been historically very challenging because interferon and ribavirin-based therapies were poorly tolerated and had low rates of sustained virologic response (SVR). Even with the advent of direct-acting antivirals (DAAs), patients with advanced CKD (estimated glomerular filtration rate [eGFR] <30mL/min/1.73m2) were routinely excluded from the pivotal clinical trials conducted in the general population. However, with the approval of DAA-based combinations that were carefully studied in patients with advanced CKD and on dialysis, it is now feasible to treat patients with HCV infection and CKD and improve both hepatic and extra-hepatic outcomes in these individuals. This review will explore the relationship between HCV infection and kidney disease, including its association with glomerular disease, incident CKD, progression to ESRD, and the effect of treatment on these disease processes.
Association of HCV Infection with Glomerular Disease
The earliest reported kidney manifestation in patients with HCV infection was glomerular disease due to what was then referred to as essential MCS.4,5 Though the etiology was previously unknown, HCV infection has since been identified as the cause of MCS in 80–90% of cases.5,55,56 Cryoglobulins are immunoglobulins that precipitate in cooled serum and dissolve on rewarming. In chronic HCV infection, the cryoglobulins consist of viral particles, polyclonal anti-HCV IgG, and monoclonal (type II mixed cryoglobulinemia) or polyclonal (type III) anti-IgG IgM. Persistent stimulation of B cells by chronic infection is thought to be responsible for their production.56 Compared to the non-HCV infected population, HCV infection is associated with a significantly increased risk of cryoglobulinemia (adjusted hazard ratio [aHR] 16.9, 95% confidence interval [CI] 12.0–23.8).41 As many as 50% of HCV-infected patients have circulating cryoglobulins, though fewer than 5% of those develop MCS, a small vessel vasculitis that typically involves the skin, joints, peripheral nervous system, and kidneys.3,56 The clinical manifestations range from mild to life-threatening, and the most common symptoms are purpura, arthralgias, myalgias, weakness, and peripheral neuropathies.56
Renal involvement occurs in approximately 30% of patients with MCS, and it usually develops subsequent to the onset of other manifestations, often by years, although it can also occur concurrently.56,57 In some cases, kidney disease may be the first sign of MCS. CGN typically presents clinically with hypertension, proteinuria, and microscopic hematuria, often accompanied by some degree of renal insufficiency, but it may also manifest as an acute nephritic or even nephrotic syndrome.2,55,58 CGN typically presents as a membranoproliferative pattern of injury (MPGN), with eosinophilic intraluminal thrombi, a double-contoured basement membrane, and mesangial proliferation visible on light microscopy; subendothelial deposits are typically seen on electron microscopy and immunofluorescence confirms that the deposits consist of IgM, IgG, and C3.2,4 Most cases (~80%) show diffuse glomerulonephritis (GN), involving more than 50% of glomeruli, while 10% are focal, affecting less than 50%. The remaining proportion are mesangial proliferative GN, a less severe form without exudative lesions.55
Though HCV is classically associated with CGN, other glomerular diseases in the absence of cryoglobulinemia have also been identified. These are likely due to the deposition of non-cryoglobulin immune complexes in the glomeruli and include non-cryoglobulinemic MPGN, membranous nephropathy, fibrillary or immunotactoid glomerulopathy, and IgA nephropathy.4,10,15–17,59,60 Patients with membranous nephropathy present with nephrotic syndrome without cryoglobulinemia or hypocomplementemia. Multiple small series, including an autopsy study, have reported cases of membranous nephropathy in patients with HCV infection.6,15–17 This association has been the subject of some debate however, as one study found it to be no more common than diabetic nephropathy among a group of HCV-infected patients, and another demonstrated membranous nephropathy in similar rates in a non-HCV control group.18,20
Two less common glomerular diseases, fibrillary and immunotactoid glomerulopathy, have also been reported in patients with HCV. Both are characterized by the deposition of non-amyloid fibrils, thought to be composed of immunoglobulin. These two diseases can be distinguished by the size of the fibrils, which are 16–24nm in diameter in fibrillary and over 30nm in immunotactoid glomerulopathy.8–11,13,14 Both fibrillary and immunotactoid glomerulopathy manifest clinically with hypertension, nephrotic-range proteinuria, hematuria, and renal insufficiency.8–11,13,14 Both glomerulopathies portend a poor outcome, with progression to ESRD in nearly half of the patients within a few years.8–11,13,14
Finally, secondary IgA nephropathy, which may be associated with all forms of advanced liver disease, is seen in patients with HCV infection and cirrhosis.19 Secondary IgA nephropathy in patients with cirrhosis is due to decreased clearance of immune complexes by the diseased liver. While often clinically silent, it can also cause hematuria and varying degrees of renal insufficiency, typically with an indolent progression.7
Other forms of glomerular disease in patients with HCV infection can also be clinically silent and therefore unrecognized. In a study of 30 renal biopsies obtained from patients with HCV at the time of liver transplant, as many as 97% had some form of glomerular abnormality, including 83% with mesangial deposits and 53% with mesangial and subendothelial deposits.12 The most common pattern was MPGN (40%), followed by IgA nephropathy (23%) and mesangial GN (20%). Importantly, many of these patients had no clinical evidence of renal disease: 72% had no urinary abnormalities, and 32% had a serum creatinine of less than 1 mg/dL. An autopsy study in patients with HCV infection similarly found glomerular disease to be prevalent and often subclinical: 55% had glomerular abnormalities (11% MPGN, 18% mesangial proliferative GN, 3% membranous nephropathy, 23% with mesangial thickening), only 18% of which had an abnormal urinalysis in the prior year.6 Together, these studies suggest that the prevalence of glomerular disease in patients with HCV infection may be significantly underestimated.
Pathophysiology of Chronic Kidney Disease in Patients with HCV
While glomerular disease due to immune complex deposition is one mechanism of kidney disease in HCV infection, other potential causative factors have also been identified. These include a direct cytopathic effect of the virus on kidney tissue, atherosclerosis, insulin resistance, and chronic inflammation (Figure 1). In addition to depositing as immune complexes, HCV may also more directly provoke kidney injury. HCV-related proteins and RNA have been identified in glomeruli and tubules of kidney biopsies in the absence of antibodies, suggesting a possible direct cytopathic effect of HCV.118–120 In one study, the presence of HCV-related proteins in the mesangium was associated with a higher degree of proteinuria, perhaps indicative of a cytopathic effect in the mesangial cells.51 HCV has the potential for entry into kidney tissue, though this has never been proven.121–123 Even in the absence of cellular entry and replication, HCV could potentially cause cytopathic effects such as endothelial inflammation, mesangial inflammation, and podocyte injury simply by attaching to cell surface receptors.123
Figure 1.
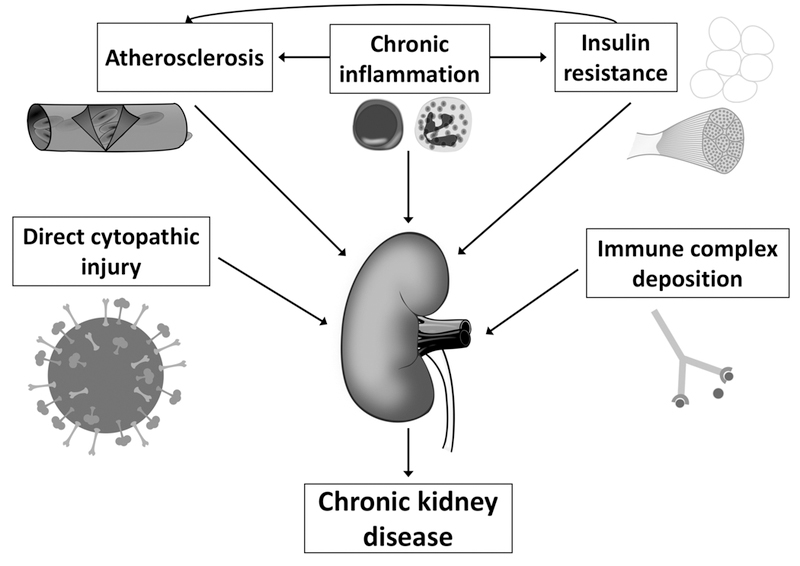
Potential mechanisms of chronic kidney disease in patients with HCV infection.
Legend: Multiple different pathways may contribute to the increased risk of chronic kidney disease and more rapid progression of chronic kidney disease in patients with Hepatitis C virus infection.
Other possible mechanisms for accelerated CKD progression in chronic HCV infection are insulin resistance, atherosclerosis, and chronic inflammation, all of which are likely interrelated. Patients with HCV infection experience insulin resistance and higher levels of fasting insulin. Hyperinsulinemia results in increased production of insulin-like growth factor and transforming-growth-factor-beta which can cause renal cell proliferation and increased expression of angiotensin II in mesangial cells, ultimately enhancing the harmful effects of angiotensin II on the kidney.124 Perhaps related to insulin resistance, accelerated atherosclerotic disease may also promote CKD. HCV has been associated with an increased risk of cardiovascular disease in the general population, and increased atherosclerotic disease in the renal vasculature could result in CKD.125–127 Atherogenesis in HCV infection may be due to direct arterial inflammation or could be related to insulin resistance, hepatic steatosis, and chronic inflammation, among other indirect mechanisms.
Finally, HCV-induced systemic chronic inflammation may also contribute to CKD. In addition to local hepatic inflammation, there is evidence that patients with HCV have a low-grade systemic inflammation, as indicated by elevated inflammatory cytokines and markers of inflammation, including fibrinogen, erythrocyte sedimentation rate, C-reactive protein, and tumor necrosis factor alpha.128–134 This chronic inflammatory state and immune activation could lead to CKD through atherosclerosis or other means.
Association of HCV with Incident CKD
There is mounting epidemiologic evidence that links HCV infection and the development CKD in multiple populations. Soon after its discovery, , HCV was demonstrated to be present in 10–20% of the US dialysis population, a figure significantly more prevalent then that reported in the general population30,39.61–63 . These findings raised the question of the directionality of this relationship: was the disproportionately high prevalence of HCV among patients with ESRD due to nosocomial transmission during HD or was HCV acquired prior to HD and potentially contributed to CKD/ESRD? HCV has unequivocally been shown to be transmissible through HD, and length of time on HD was a risk factor for HCV antibody positivity in multiple studies, though transmission was preventable with the implementation of strict universal precautions.30,64–68 Yet, while nosocomial transmission may have unfortunately resulted in some cases of HCV infection, it cannot fully account for the high proportion of HCV infection in patients new to HD, suggesting a potential causal role for HCV in CKD and its progression to ESRD.22,31
These reports of a high prevalence of HCV infection in patients with ESRD prompted further investigation into the relationship between these disease processes. Many of the early studies were conflicting, with some, but not all, showing an association between HCV infection and prevalent or incident CKD (Table 1). Two cross-sectional analyses of data from the Third National Health and Nutrition Examination Survey found an association with albuminuria but not eGFR, and longitudinal studies of an urban hospital population and a private insurance database did not demonstrate an increased risk of prevalent or incident CKD in HCV-infected patients.21,36,37,46 In addition, the results of multiple analyses of cohorts of US veterans were inconsistent: a longitudinal study of a large number of veterans showed that HCV infection conferred an increased risk of developing incident CKD, and one cross-sectional study reported an increased prevalence of renal insufficiency (defined as a creatinine greater than 1.5 mg/dL) among patients with HCV, while another observed no difference in the prevalence of CKD (eGFR <60 ml/min/1.73m2) in their cohort.23,27,47 A meta-analysis of these early studies did not find a significant association between HCV infection and incident CKD, and there was significant heterogeneity.28
Table 1A.
Chronic kidney disease in patients with hepatitis C virus infection: cross-sectional studies.
| Citation | Country | Collection time |
Patients (n) |
HCV+, n (%) |
Outcome | Prevalence: HCV+ vs. HCV− (%) |
aOR (95% CI) |
|---|---|---|---|---|---|---|---|
| Liangpunsakul et al., 200536 |
USA | 1988–1994 | 15,336 | 362 (2.4%) | Proteinuria | 12.4% vs. 7.5%, p=0.001 |
1.99 (1.38–2.85) |
| Tsui et al., 200646 |
USA | 1988–1994 | 15,029 | 366 (2.4%) | Proteinuria | Age ≥40: 46% vs. 24% |
All ages: 1.38 (0.91–2.07) Age 40–59: 1.84 (1.00–3.37) Age ≥60: 2.47 (1.27–4.80) |
| Tsui et al., 200646 |
USA | 1988–1994 | 15,029 | 366 (2.4%) | eGFR <60 | 4.3% vs. 2.0% | 0.89 (0.49–1.62) |
| Huang et al., 200648 |
Taiwan | 2002–2004 | 9,934 | 646 (6.5%) | Proteinuria | 10.2% vs. 7.0%, p=0.004 |
1.648 (1.246– 2.179) |
| Dalrymple et al., 200727 |
USA | 1999–2004 | 25,782 | 1,928 (7.5%) | Creatinine ≥1.5 |
4.8% vs. 6.0%, p=0.04 |
1.40 (1.11–1.76) |
| Tsui et al., 200747 |
USA | 2000–2001 | 474,369 | 52,874 (11.1%) |
eGFR <60 | 9.4% vs. 16.6%, p<0.01 |
0.91 (0.88–0.95) |
| Ishizaka, et al., 200849 |
Japan | 2004–2006 | 12,535 | 72 (0.6%) | Proteinuria | 19% vs. 9%, p=0.007 |
2.00 (1.06–3.76) |
| Ishizaka et al., 200849 |
Japan | 2004–2006 | 12,535 | 72 (0.6%) | eGFR<60 | 31% vs. 15%, p<0.001 |
1.63 (0.95–2.80) |
| Moe et al., 200837 |
USA | 1994–2004 | 13,139 | 3,938 (30%) | eGFR <60 | 6.3% vs. 7.6%, p<0.001 |
0.69 (0.62–0.77) |
| Asrani et al., 201021 |
USA | 2003–2006 | 167,569 | 13,384 (7.9%) |
eGFR <60 | 5.3% vs. 5.1%, p=0.30 |
0.90 (0.36–2.27) |
| Lee et al., 201050 |
Taiwan | 2004 | 54,966 | 5,189 (9.4%) | Proteinuria | 6.4% vs. 5.5% | 1.14 (1.003–1.300) |
| Lee et al., 201050 |
Taiwan | 2004 | 54,966 | 5,189 (9.4%) | eGFR <60 | 19.2% vs. 14.3% | 1.30 (1.20–1.42) |
| Satapathy et al., 201243 |
USA | 2003–2006 | 865 | 552 (63.8%) | eGFR <60 | 3.3% vs. 3.2%, p=0.96 |
NR |
| Li et al., 201452 | Taiwan | 2010–2011 | 24,642 | 1,699 (6.9%) | eGFR <60 | 7.8% vs. 1.5%, p<0.001 |
1.24 (1.05–1.48) |
| Kurbanova et al., 201553 |
USA | 1999–2012 | 33,729 | 659 (1.73%) | Proteinuria | 17.0% vs. 12.6% | 1.50 (1.08–2.08) |
| Lai et al., 201732 |
Taiwan | 1991–1992 | 13,805 | 431 (3.1%) | eGFR <60 | NR | 1.91 (1.27–2.88) |
Abbreviations: aOR, adjusted odds ratio; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HCV, hepatitis C virus; NR, not reported
Recent studies, however, have more consistently demonstrated a link between HCV infection and incident CKD (Table 1).24,25,32,34,38,41,43–45 Two analyses of a nationwide Taiwanese cohort with follow-up of over 5 years observed a significantly increased risk for incident CKD in this population.24,25 One excluded individuals with traditional CKD risk factors (diabetes, hypertension, hyperlipidemia, coronary artery disease) as well as cirrhosis to better isolate the impact of HCV on CKD.25 This study demonstrated a higher risk of CKD (aHR 1.75, 95% CI 1.25–2.43) as well as a lower mean time to diagnosis of CKD in HCV-infected compared to uninfected adults. Another analysis of the same database that did not exclude individuals with the above comorbidities found similar results (aHR 1.28, 95% CI 1.12–1.46).24 In a cohort of over 1 million US veterans, Molnar et al. also demonstrated a significantly increased risk of incident CKD in those with HCV infection (aHR 1.15, 95% CI 1.12–1.17), as did an analysis of over 50,000 patients from a US insurance database (aHR 1.27, 95% CI 1.18–1.37).38,41 These findings have additionally been observed in a nationwide Swedish cohort: Söderholm et al. found a standardized incident ratio of 4.0 for CKD in HCV-infected individuals compared to the general population.44
Furthermore, three recent meta-analyses have all shown an association between HCV infection and incident CKD.29,35,40 One meta-analysis of 9 longitudinal studies and nearly 2 million unique patients demonstrated that positive HCV serology increased the risk of incident CKD (aHR 1.43, 95% CI 1.23–1.63).29 Another pooled dataset from 8 longitudinal studies and found that patients with HCV infection had a 23% increased risk of CKD compared to uninfected individuals (aHR 1.23, 95% CI 1.12–1.32).40 A recent meta-analysis of 12 longitudinal studies similarly showed an increased risk of CKD with HCV (aHR 1.45, 95% CI 1.23–1.71).35 This analysis also observed a graded risk for incident CKD with length of follow-up and duration of HCV infection. In each meta-analysis, the relationship between HCV and CKD remained significant after stratification by country, though the pooled risk was larger in East Asian populations than in US- or European-based cohorts.
Studies performed in other subpopulations, including HCV/HIV-coinfected patients and kidney transplant recipients, have provided further support for an impact of HCV infection on incident CKD. The presence of HCV-coinfection in patients with HIV infection has been identified as a risk factor for CKD in several studies of different cohorts.69–79 A recent meta-analysis incorporating studies that adjusted for potential confounders such as age, race, and history of injection drug use found a pooled aHR of 1.64 (95% CI, 1.28–2.00) for the risk of incident CKD conferred by HCV co-infection.70 Notably, active use of injection drugs has been difficult to account for in many studies, and some evidence suggests that use of injection cocaine may explain part of the relationship between HCV and CKD in HIV co-infected patients.80,81 Studies in patients after kidney transplant have also demonstrated a detrimental role for HCV. HCV infection has been associated with de novo glomerulonephritis and transplant glomerulopathy in these patients, in addition to acute rejection and graft failure.82–88
While some of the data have been conflicting, the discrepancies may indicate potentially significant factors in the development of CKD in patients with HCV infection, including active infection and the degree of viremia, viral genotype, and duration of infection. Most studies used a positive anti-HCV antibody to define HCV infection, which could represent varying degrees of viral activity, including spontaneously cleared or successfully treated infection. A recent study, however, aimed to clarify the association between active HCV infection and CKD by using viral load to define HCV infection.32 In this study, the prevalence of CKD was similar in anti-HCV antibody positive participants with undetectable viral loads and HCV-antibody negative individuals (adjusted odds ratio [aOR] 1.32, 95% CI 0.68–2.56), while patients with HCV viremia (positive HCV RNA) had a significantly increased prevalence of CKD (aOR 1.91, 95% CI 1.27–2.88). Furthermore, among those with a detectable viral load, the degree of viremia was an important risk factor for CKD: there was a dose-dependent trend of HCV RNA level and CKD defined by eGFR, as well as with proteinuria.
These results are consistent with Molnar et al., who found a significant association with incident CKD in HCV-positive viremic patients but not in anti-HCV positive, non-viremic participants in a subgroup analysis, as well as Satapathy et al., who observed that a higher HCV viral load was an important predictor of CKD in their cohort.38,43 In addition, multiple studies in HIV-infected patients have demonstrated an increased risk of CKD with HCV viremia but not seropositivity without HCV viremia.72,74
Yet, these findings contrast with Butt et al., who observed no difference in rates of CKD in viremic vs. non-viremic patients, as well as Lucas et al. who similarly found no significant impact of active HCV viremia in HIV-coinfected patients.23,72 Taken together though, these results, particularly the evidence of a dose-response relationship, do suggest that active HCV infection and the degree of viremia are important factors in the pathophysiology of CKD in HCV-infected patients. Variations in disease activity may underlie some of the conflicting results of previous analyses, and future studies should aim to incorporate HCV viral load in the analyses to further elucidate the importance of active HCV viremia in incident CKD.
Lai et al. examined the impact of HCV genotype and found that genotype 2 infection conferring an increased risk of CKD compared to genotype 1.32 Genotype 1 is the most common HCV genotype in the US and an estimated 15% have genotype 2, whereas as many as 30% of HCV-infected patients in Taiwan have genotype 2.89,90 Notably, nearly all of the studies finding no association between HCV and CKD were performed among presumably genotype 1-predominant US-based cohorts, and meta-analyses have demonstrated an increased risk of incident CKD in East Asian countries.29,35,40 Perhaps a smaller proportion of individuals with genotype 2 in the US cohorts has contributed to some of the inconsistent results.91 There are conflicting results about the role of HCV genotype, however, including an analysis performed by the same group of the same cohort.33 Using the development of ESRD as an endpoint, genotype 1 was more strongly associated than genotype 2, contrasting with the results for CKD. Several other prior studies also explored the role of genotype and found no association.74,92
All of these analyses including genotype data have all been of small cohorts or small subgroups, so it is difficult to draw definitive conclusions. The impact of HCV genotype on the development of CKD and its potential pathophysiologic basis should be explored in future studies.
Beyond viral load and genotype, the duration of HCV infection may also be an important factor in the development of incident CKD. A recent meta-analysis observed a graded risk for incident CKD with duration of HCV infection, and another study with mean follow-up of 6 years after seroconversion demonstrated that recently acquired HCV is not a risk factor for CKD.35,42 A longitudinal study with median follow-up time of 7 years found that the mean time to the development of CKD was 74 months.43 Many analyses finding no association between HCV infection and incident CKD had median follow-up times of only 2 to 3 years, which may not have been sufficient to observe the natural course of the disease.
In addition to the potential roles for degree of viremia, viral genotype, and duration of infection, non-HCV-related risk factors for the development of CKD in patients with HCV infection have also been identified. These include older age23,41,43, male sex29,35, hypertension23,24,41,43, diabetes23,24,35,41,43, hyperlipidemia24, cerebrovascular disease41, peripheral vascular disease41, congestive heart failure41, cirrhosis23,24,41, HIV24, anemia23,41, alcohol or drug abuse23,41, and receipt of angiotensin converting enzyme inhibitors or angiotensin II receptor blockers 23,41, all of which should be taken into account when considering a patient’s risk of CKD.
Association of HCV with CKD Progression to ESRD
Beyond increasing the risk for incident CKD, HCV infection may also accelerate the progression of CKD and the development of ESRD. This was first demonstrated by Tsui et al. in a large cohort of US veterans; those with HCV infection (defined by antibody positivity) had a more than two-fold risk of developing ESRD.47 In a prospective cohort of Taiwanese adults with CKD, Lee et al. observed that the prevalence of HCV increased with each stage of CKD, and HCV-seropositivity was associated with an increased risk of progression to ESRD.34 Su et al. (Taiwan), Söderholm et al. (Sweden), and Molnar et al. (US) further demonstrated that HCV infection affects CKD progression at the population level.38,44,45
Molnar and colleagues found that HCV conferred not only an increased risk of incident CKD, but also a higher risk of rapid deterioration of kidney function (defined as > 5mL/min/year average eGFR decline), and a two-fold risk of developing ESRD in a large cohort of US veterans.38 In a subgroup analysis, active infection, with a positive HCV viral load, was an important factor in a rapid decline in renal function and progression to ESRD: seropositivity in the absence of viremia was not associated with these outcomes, while viremia was. Lai et al. also observed an increased risk of ESRD among patients with chronic HCV, and this risk grew with a higher viral load, again supporting the role of active infection in the pathogenesis of CKD in HCV.33
In addition, an increased risk of progression of CKD with HCV infection has also been demonstrated in patients with other renal diseases, including diabetic nephropathy, HIV, and chronic GN.26,75,93,94 Among a Japanese cohort of patients with biopsy-proven diabetic nephropathy, those with HCV infection experienced faster decline in renal function, as determined by the slope of the reciprocal creatinine.93 A study of a smaller cohort of diabetic patients, with or without biopsy-proven diabetic nephropathy, similarly observed an increased rate of progression of CKD, as well as an increased risk of ESRD.26 HCV infection has also been identified as a risk factor for accelerated progression of CKD and ESRD in patients co-infected with HIV as well as in individuals with chronic GN of other etiologies, including focal segmental glomerulosclerosis and IgA nephropathy.75,94–97
Importantly, this relationship between HCV and CKD impacts clinical outcomes. In an analysis of National Health and Nutrition Examination Survey data, patients with both HCV infection and CKD experienced an increased risk of mortality (aHR 4.39, 95% CI 2.80–6.88) compared to HCV-infected patients without CKD (aHR 2.41, 95% CI 1.67–3.47) or CKD in the absence of HCV infection (aHR 1.87, 95% CI 1.73–2.00).98 In addition, a meta-analysis of 14 studies demonstrated that HCV seropositive status conferred a 35% increased risk of all-cause mortality in individuals with ESRD on HD.28 HCV infection was associated with an increased risk of liver-related (aHR 3.82, 95% CI 1.92–7.61) as well as cardiovascular death (aHR 1.26, 95% CI 1.10–1.45).28 Furthermore, a recent study of 76,689 patients on HD demonstrated that HCV infection increases the risk of hospitalization and complications of anemia and is associated with a poorer quality of life.99 HCV also affects outcomes in kidney transplant recipients. HCV-infected transplant recipients experience an increased risk of mortality (aHR 1.85, 95% CI 1.49–2.31) and graft loss (aHR 1.76, 95% CI 1.46–2.11).100,101
Role of HCV Treatment in Slowing CKD Progression
Further supporting a causal role for HCV infection in the development of incident and progressive CKD is that antiviral treatment improves kidney function. Treatment of HCV-associated GN, both CGN and non-CGN, has been shown to reduce proteinuria, and it recurs if treatment is stopped or if MCS relapses.4,102–108 In addition, patients treated for HCV may have a decreased risk of CKD and progression to ESRD, although the vast majority of data only exists in patients treated with interferon-based regimens. Satapathy et al. found in their US-based cohort that receiving interferon therapy was a significant negative predictor of incident CKD.43 A study in Japanese patients further demonstrated that among a group of patients with HCV- associated cirrhosis treated with interferon, those who achieved SVR were significantly less likely to have CKD.6
Similar results have been observed at the population level. In two studies of Taiwanese cohorts, treatment with interferon-based therapy resulted in a 58% reduction in incident CKD and an 84% decreased risk of ESRD.109,110 Park et al. utilized pharmacy claims data to further characterize the role of the type and length of therapy on the incidence of CKD in a large US cohort.41 Patients who received the minimally effective HCV treatment, defined as 16 weeks of dual therapy (interferon/ribavirin), 8–12 weeks of triple therapy (interferon/ribavirin plus a protease inhibitor), or 8 weeks of an all-oral DAA therapy, were 30% less likely to develop CKD. Those who received some treatment but did not complete the full course of therapy did not have a decreased risk of CKD after adjustment for other factors.
Improvement in kidney function with antiviral treatment has also been observed in other subpopulations with HCV infection. Hsu et al. found similar results in a nationwide cohort of diabetic patients in Taiwan.110 At 8 years, the cumulative incidence of CKD in those who received treatment was 1.1% compared to 9.3% in the untreated population. In addition, in a study of HCV-infected liver transplant recipients, antiviral treatment and SVR improved kidney function in those with mild CKD.111 Yet, data in patients with HCV/HIV-coinfection have been mixed. Berenguer et al. demonstrated that SVR had a protective effect on CKD in a cohort of co-infected patients in Spain.112 Leone et al., Kovari et al., and Rossi et al., however, did not observe the same impact of SVR in their cohorts of HIV co-infected patients.78,81,113 The impact of HCV treatment on kidney function in HCV/HIV-coinfected patients warrants further study.
Beyond reducing the progression of CKD and improving kidney function in MCS, treatment of HCV infection in patients with kidney disease improves clinical outcomes. Söderholm et al. found a significant survival benefit in treating HCV patients on HD, with antiviral treatment improving survival with an aOR of 3.6 (95% CI 1.6–7.8).44 The primary benefit to treating these patients was a lower risk of CKD- and ESRD-related mortalities, and the survival advantage also extended to the post-transplant period. The current treatment strategy at many transplant centers in the US is to consent the patient to receive a kidney from a HCV-infected deceased organ donor and treat HCV infection after transplantation, resulting in a significantly shortened wait time. While the demonstration of a decreased mortality accompanying successful treatment of HCV while on dialysis is important, these findings must be integrated into the decision tree for each patient on an individual basis depending on variables such as local waiting time, presence of a living donor and patient preference.
Outcome studies in the HCV-infected CKD and HD population have largely been in cohorts receiving interferon-based regimens, which, until the last few years, were the backbone of HCV treatment. These regimens were poorly tolerated, had low rates of SVR, and treatment of patients with CKD was difficult, as interferon and ribavirin are cleared by the kidneys. Use of ribavirin is challenging in patients with kidney disease due to high rates of hemolytic anemia. The advent of DAAs has dramatically altered the treatment landscape in HCV, though cure of patients with CKD remained challenging until very recently. Some regimens still required ribavirin, and other ribavirin-free regimens included sofosbuvir, which is cleared by the kidneys and is not approved for use in patients with a eGFR < 30ml/min/1.73m2. Currently, several DAA regimens have been demonstrated to have high efficacy and safety for patients with advanced CKD and ESRD, including elbasvir/grazoprevir for genotypes 1 and 4 infection, ombitsavir/dasabuvir/paritaprevir/ritonavir for genotype 1 infection, and glecaprevir/pibrentasvir, which effectively treats all genotypes of HCV.114–116
If the mechanism of improvement in proteinuria and incident CKD and ESRD with interferon-based treatment is through viral suppression or cure, then similar results would be expected from DAA regimens. Data on the impact of DAA regimens on kidney function in patients with HCV, however, are limited. A retrospective analysis of 98 patients with HCV and early-stage CKD (1–3) treated with sofosbuvir-based DAA therapies found that in the subgroup with eGFR < 60ml/min/1.73m2 there was a significant improvement eGFR associated with SVR in multivariable models.117 As more data accumulates on HCV-infected patients treated with DAAs, the effect of treatment on kidney function should be explored further.
Evidence for use of DAAs in patients with MCS is also scarce, though the limited data show high rates of SVR and improvement in renal function in many patients. Management of HCV-related MCS involves treatment of the underlying viral infection with DAAs as well as immunosuppression in severe cases. In the VASCUVALDIC study, 24 patients with HCV-related MCS received 24 weeks of sofosbuvir and ribavirin, and 7 patients also received some form of immunosuppression (rituximab, corticosteroids, plasmapheresis).107 A SVR was achieved in 74%, adverse events were rare, and kidney function improved in 4 out of 5 with associated MPGN. A case series of 12 patients with HCV-associated MCS, 7 of whom had active glomerulonephritis, similarly found DAA regimens of sofosbuvir/simeprevir (n=8) or sofosbuvir/ribavirin (n=4) to be effective and to improve kidney function.118 A SVR was achieved in 83% of patients, and GFR and proteinuria both improved. Six out of 7 patients with glomerulonephritis had received immunosuppression, although only one received it concurrent with DAA therapy. Two other case reports also demonstrated improvement in proteinuria and GFR with DAAs, one with daclatasvir and asunaprevir and the other with sofosbuvir, lepidasvir, and ribavirin.119,120 Additional studies will be necessary to further characterize the use of DAAs and their effect on kidney dysfunction in MCS.
Conclusion
In summary, HCV and kidney disease are intricately linked. HCV infection increases the risk of CKD and its progression to ESRD, likely due to multiple mechanisms, and increases adverse outcomes in patients with CKD. Further investigation into these mechanisms and the role of HCV viremia, genotype, and duration of infection, and other risk factors will be required to fully understand this relationship. Historically, interferon-based antiviral treatment was associated with improved outcomes, and studies suggested that achieving a cure of HCV might slow the progression of CKD. Interpretation of these studies was limited by the small numbers of patients with HCV and CKD who received treatment. With new, well-tolerated DAA combinations that are not renally-eliminated, it is now possible to treat patients with all genotypes of HCV infection in the setting of CKD and ESRD. Additional data on the effect of DAAs on kidney function, proteinuria, and CKD incidence and progression are still necessary. However, there is every expectation that with improved access to HCV treatment, the burden of kidney disease in patients with HCV infection could significantly decline.
Table 1B.
Chronic kidney disease in patients with hepatitis C virus infection: longitudinal studies.
| Author (year) |
Country | Collection time |
Patients (n) |
HCV+, n (%) |
Follow- up, years |
Outcome | Cumulative incidence: HCV+ vs. HCV− |
Incidence rate: HCV+ vs. HCV− |
aHR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Tsui et al., 200747 |
USA | 2000–2004 | 474,369 | 52,874 (11.1%) |
3.4 | ESRD | NR | 4.26 vs. 3.05 per 1000 person-years |
2.80 (2.43– 3.23) |
| Moe et al., 200837 |
USA | 1994–2004 | 7,038 | 2,243 (31.8%) |
3.5 | eGFR<60 | NR | NR | 0.896 (0.790– 1.015) |
| Asrani et al., 201021 |
USA | 2003–2006 | 88,822 | 8,063 (9.1%) |
2.1 | eGFR<60 | 5.3% vs. 5.1%, p=0.10 |
NR | 0.92 (0.79– 1.08) |
| Hofmann et al., 201154 |
Sweden | 1990–2006 | 223,536 | 25,412 (11.4%) |
9.3 | CKD stages 1–5 |
NR | NR | Male: 3.9 (3.2–4.8) Female: 5.8 (4.2–7.9) |
| Butt et al., 201123 |
USA | 2001–2006 | 43,139 | 18,002 (41.7%) |
3.15 | eGFR<60 | 17.4% vs. 14.9%, p<0.001 |
NR | 1.30 (1.23– 1.37) |
| Su et al., 201245 |
Taiwan | 2000–2005 | 37,746 | 6,291 (16.6%) |
5.58 | ESRD | NR | NR | 1.53 (1.17– 2.01) |
| Satapathy et al., 201243 |
USA | 2003–2006 | 865 | 552 (63.8%) |
7 | eGFR<60 | 8.3% vs. 4.5%, p=0.03 |
NR | NR |
| Chen et al., 201325 |
Taiwan | 1998–2004 | 15,190 | 3,182 (20.9%) |
5.88 | CKD stages 1–5 |
2.0% vs. 1.5%, p=0.03 |
3.42 vs. 2.48 per 1,000 person-years, p=0.02 |
1.75 (1.25– 2.43) |
| Chen et al., 201424 |
Taiwan | 1996–2010 | 47,150 | 9,430 (20.0%) |
7.1 | CKD stages 1–5 |
3.8% vs. 2.5% |
5.46 vs. 3.43 per 1,000 person-years, p<0.001 |
1.28 (1.12– 1.46) |
| Lee et al., 201434 |
Taiwan | 2002–2009 | 4,185 | 317 (7.6%) |
2.2 | ESRD | 5-year cumulative incidence: 52.6% vs. 38.4% |
13.2 vs. 4.9 per 100 person-years |
1.32 (1.07– 1.62) |
| Molnar et al., 201538 |
USA | 2004–2006 | 1,021,049 | 100,518 | 8 | eGFR <60 |
11.2% vs. 10.4% |
16.7 vs. 14.9 per 1,000 person-years |
1.15 (1.12– 1.17) |
| Molnar et al., 201538 |
USA | 2004–2006 | 1,021,049 | 100,518 | 8 | ESRD | 0.9% vs. 0.3% |
1.2 vs. 0.36 per 1,000 person- years |
1.98 (1.81– 2.16) |
| Rogal et al., 201642 |
USA | 2001–2014 | 71,528 | 2,589 (3.6%) |
5.99 | eGFR <60 |
35.6% vs. 30.8%, p<0.001 |
NR | 0.86 (0.79– 0.92) |
| Lai et al., 201733 |
Taiwan | 1991–2008 | 19,984 | 591 (3.0%) |
16.8 | ESRD | 3.4% vs. 1.1% |
194.3 vs. 60.2 per 100,000 person-years |
2.33 (1.40– 3.89) |
| Park et al., 201741 |
USA | 2008–2015 | 225,792 | 56,448 (25.0%) |
NR | CKD stages 3–5 |
NR | 10.36 vs. 5.72 per 1,000 person-years |
1.27 (1.18– 1.37) |
| Söderholm et al., 201844 |
Sweden | 1997–2013 | 248,216 | 45,522 (18.3%) |
6.22 | CKD stages 1–5 |
2.5% | 3.84 vs. 0.97 per 1,000 person-years |
SIR 4.0 (3.7– 4.2) |
Abbreviations: aHR, adjusted hazard ratio. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HCV, hepatitis C virus; NR, not reported; SIR, standard incidence ratio.
Acknowledgments
MES has received grant support from Gilead Sciences, Abbvie, Merck & Co. and has participated in scientific advisory board meetings for Abbvie and Merck & Co and is a Scientific Consultant to Abbvie. MES was supported by NIH K23 DK117014.
Abbreviations:
- aHR
adjusted hazard ratio
- aOR
adjusted odds ratio
- CGN
cryoglobulinemic glomerulonephritis
- CKD
chronic kidney disease
- DAA
direct-acting antiviral
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- HCV
hepatitis C virus
- HD
hemodialysis
- HIV
human immunodeficiency virus
- MCS
mixed cryoglobulinemic syndrome
- MPGN
membranoproliferative glomerulonephritis
- SVR
sustained virologic response
Footnotes
Disclosures:
JBH has no conflicts to declare.
References
- 1.Global Hepatitis Report 2017 Geneva: World Health Organization;2017. [Google Scholar]
- 2.Cacoub P, Gragnani L, Comarmond C, Zignego AL. Extrahepatic manifestations of chronic hepatitis C virus infection. Dig Liver Dis 2014;46 Suppl 5:S165–173. [DOI] [PubMed] [Google Scholar]
- 3.Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of Hepatitis C: A Meta-analysis of prevalence, quality of life, and economic burden. Gastroenterology 2016;150(7):1599–1608. [DOI] [PubMed] [Google Scholar]
- 4.Johnson RJ, Gretch DR, Yamabe H, et al. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med 1993;328(7):465–470. [DOI] [PubMed] [Google Scholar]
- 5.Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med 1992;327(21):1490–1495. [DOI] [PubMed] [Google Scholar]
- 6.Arase Y, Ikeda K, Murashima N, et al. Glomerulonephritis in autopsy cases with hepatitis C virus infection. Intern Med 1998;37(10):836–840. [DOI] [PubMed] [Google Scholar]
- 7.Ji F, Li Z, Ge H, Deng H. Successful interferon-alpha treatment in a patient with IgA nephropathy associated with hepatitis C virus infection. Intern Med 2010;49(22):2531–2532. [DOI] [PubMed] [Google Scholar]
- 8.Alpers CE, Kowalewska J. Fibrillary glomerulonephritis and immunotactoid glomerulopathy. J Am Soc Nephrol 2008;19(1):34–37. [DOI] [PubMed] [Google Scholar]
- 9.Javaugue V, Karras A, Glowacki F, et al. Long-term kidney disease outcomes in fibrillary glomerulonephritis: a case series of 27 patients. Am J Kidney Dis 2013;62(4):679–690. [DOI] [PubMed] [Google Scholar]
- 10.Markowitz GS, Cheng J-T, Colvin RB, Trebbin WM, D ‘agati VD. Hepatitis C viral infection is associated with fibrillary glomerulonephritis and immunotactoid glomerulopathy. J Am Soc Nephrol 1998;9:2244–2252. [DOI] [PubMed] [Google Scholar]
- 11.Coroneos E, Truong L, Olivero J. Fibrillary glomerulonephritis associated with hepatitis C viral infection. Am J Kidney Dis 1997;29(1):132–135. [DOI] [PubMed] [Google Scholar]
- 12.McGuire BM, Julian BA, Bynon JS Jr., et al. Brief communication: Glomerulonephritis in patients with hepatitis C cirrhosis undergoing liver transplantation. Annals Int Med 2006;144(10):735–741. [DOI] [PubMed] [Google Scholar]
- 13.Nasr SH, Valeri AM, Cornell LD, et al. Fibrillary glomerulonephritis: a report of 66 cases from a single institution. Clin J Am Soc Nephrol 2011;6(4):775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenstock JL, Markowitz GS, Valeri AM, Sacchi G, Appel GB, D’Agati VD. Fibrillary and immunotactoid glomerulonephritis: Distinct entities with different clinical and pathologic features. Kidney Int 2003;63(4):1450–1461. [DOI] [PubMed] [Google Scholar]
- 15.Stehman-Breen C, Alpers CE, Couser WG, Willson R, Johnson RJ. Hepatitis C virus associated membranous glomerulonephritis. Clin Nephrol 1995;44(3):141–147. [PubMed] [Google Scholar]
- 16.Sumida K, Ubara Y, Hoshino J, et al. Hepatitis C virus-related kidney disease: various histological patterns. Clin Nephrol 2010;74(6):446–456. [PubMed] [Google Scholar]
- 17.Yamabe H, Johnson RJ, Gretch DR, et al. Hepatitis C virus infection and membranoproliferative glomerulonephritis in Japan 1995;6(2):220–223. [DOI] [PubMed] [Google Scholar]
- 18.Cosio FG, Roche Z, Agarwal A, Falkenhain ME, Sedmak DD, Ferguson RM. Prevalence of hepatitis C in patients with idiopathic glomerulopathies in native and transplant kidneys. Am J Kidney Dis 1996;28(5):752–758. [DOI] [PubMed] [Google Scholar]
- 19.Dey AK, Bhattacharya A, Majumdar A. Hepatitis C as a potential cause of IgA nephropathy. Indian J Nephrol 2013;23(2):143–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Serag HB, Hampel H, Yeh C, Rabeneck L. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology 2002;36(6):1439–1445. [DOI] [PubMed] [Google Scholar]
- 21.Asrani SK, Buchanan P, Pinsky B, Rey LR, Schnitzler M, Kanwal F. Lack of association between hepatitis C infection and chronic kidney disease. Clin Gastroenterol Hepatol 2010;8(1):79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergman S, Accortt N, Turner A, Glaze J. Hepatitis C infection is acquired pre-ESRD. Am J Kidney Dis 2005;45(4):684–689. [DOI] [PubMed] [Google Scholar]
- 23.Butt AA, Wang X, Fried LF. HCV infection and the incidence of CKD. Am J Kidney Dis 2011;57(3):396–402. [DOI] [PubMed] [Google Scholar]
- 24.Chen YC, Lin HY, Li CY, Lee MS, Su YC. A nationwide cohort study suggests that hepatitis C virus infection is associated with increased risk of chronic kidney disease. Kidney Int 2014;85(5):1200–1207. [DOI] [PubMed] [Google Scholar]
- 25.Chen YC, Chiou WY, Hung SK, Su YC, Hwang SJ. Hepatitis C virus itself is a causal risk factor for chronic kidney disease beyond traditional risk factors: a 6-year nationwide cohort study across Taiwan. BMC Nephrol 2013;14:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crook ED, Penumalee S, Gavini B, Filippova K. Hepatitis C is a predictor of poorer renal survival in diabetic patients. Diabetes Care 2005;28(9):2187–2191. [DOI] [PubMed] [Google Scholar]
- 27.Dalrymple LS, Koepsell T, Sampson J, et al. Hepatitis C virus infection and the prevalence of renal insufficiency. Clin J Am Soc Nephrol 2007;2(4):715–721. [DOI] [PubMed] [Google Scholar]
- 28.Fabrizi F, Martin P, Dixit V, Messa P. Hepatitis C virus infection and kidney disease: a meta-analysis. Clin J Am Soc Nephrol 2012;7(4):549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fabrizi F, Verdesca S, Messa P, Martin P. Hepatitis C virus infection increases the risk of developing chronic kidney disease: a systematic review and meta-analysis. Dig Dis Sci 2015;60(12):3801–3813. [DOI] [PubMed] [Google Scholar]
- 30.Fissell RB, Bragg-Gresham JL, Woods JD, et al. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney international 2004;65(6):2335–2342. [DOI] [PubMed] [Google Scholar]
- 31.Iwasa Y, Otsubo S, Sugi O, et al. Patterns in the prevalence of hepatitis C virus infection at the start of hemodialysis in Japan. Clin Exp Nephrol 2008;12(1):53–57. [DOI] [PubMed] [Google Scholar]
- 32.Lai TS, Lee MH, Yang HI, et al. High hepatitis C viral load and genotype 2 are strong predictors of chronic kidney disease. Kidney Int 2017;92(3):703–709. [DOI] [PubMed] [Google Scholar]
- 33.Lai TS, Lee MH, Yang HI, et al. Hepatitis C viral load, genotype, and increased risk of developing end-stage renal disease: REVEAL-HCV study. Hepatology 2017;66(3):784–793. [DOI] [PubMed] [Google Scholar]
- 34.Lee JJ, Lin MY, Chang JS, et al. Hepatitis C virus infection increases risk of developing end-stage renal disease using competing risk analysis. PloS one 2014;9(6):e100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li M, Wang P, Yang C, et al. A systematic review and meta-analysis: Does hepatitis C virus infection predispose to the development of chronic kidney disease? Oncotarget 2017;8:10692–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liangpunsakul S, Chalasani N. Relationship between hepatitis C and microalbuminuria: results from the NHANES III. Kidney Int 2005;67(1):285–290. [DOI] [PubMed] [Google Scholar]
- 37.Moe SM, Pampalone AJ, Ofner S, Rosenman M, Teal E, Hui SL. Association of hepatitis C virus infection with prevalence and development of kidney disease. Am J Kidney Dis 2008;51(6):885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molnar MZ, Alhourani HM, Wall BM, et al. Association of hepatitis C viral infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology 2015;61(5):1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niu MT, Coleman PJ, Alter MJ. Multicenter study of hepatitis C virus infection in chronic hemodialysis patients and hemodialysis center staff members. Am J Kidney Dis 1993;22(4):568–573. [DOI] [PubMed] [Google Scholar]
- 40.Park H, Adeyemi A, Henry L, Stepanova M, Younossi Z. A meta-analytic assessment of the risk of chronic kidney disease in patients with chronic hepatitis C virus infection. J Viral Hepat 2015;22(11):897–905. [DOI] [PubMed] [Google Scholar]
- 41.Park H, Chen C, Wang W, Henry L, Cook RL, Nelson DR. Chronic hepatitis C virus (HCV) increases the risk of chronic kidney disease (CKD) while effective HCV treatment decreases the incidence of CKD. Hepatology 2018;67(2): 492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogal SS, Yan P, Rimland D, et al. Incidence and progression of chronic kidney disease after hepatitis C seroconversion: results from ERCHIVES. Dig Dis Sci 2016;61(3):930–936. [DOI] [PubMed] [Google Scholar]
- 43.Satapathy SK, Lingisetty CS, Williams S. Higher prevalence of chronic kidney disease and shorter renal survival in patients with chronic hepatitis C virus infection. Hepatol Int 2012;6(1):369–378. [DOI] [PubMed] [Google Scholar]
- 44.Soderholm J, Millbourn C, Busch K, et al. Higher risk of renal disease in chronic hepatitis C patients: Antiviral therapy survival benefit in patients on hemodialysis. J Hepatol 2018;68(5):904–911. [DOI] [PubMed] [Google Scholar]
- 45.Su FH, Su CT, Chang SN, et al. Association of hepatitis C virus infection with risk of ESRD: a population-based study. Am J Kid Dis 2012;60(4):553–560. [DOI] [PubMed] [Google Scholar]
- 46.Tsui JI, Vittinghoff E, Shlipak MG, O’Hare AM. Relationship between hepatitis C and chronic kidney disease: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol 2006;17(4):1168–1174. [DOI] [PubMed] [Google Scholar]
- 47.Tsui JI, Vittinghoff E, Shlipak MG, et al. Association of hepatitis C seropositivity with increased risk for developing end-stage renal disease. Arch Int Med 2007;167(12):1271–1276. [DOI] [PubMed] [Google Scholar]
- 48.Huang JF, Chuang WL, Dai CY, et al. Viral hepatitis and proteinuria in an area endemic for hepatitis B and C infections: another chain of link? J Intern Med 2006;260(3):255–262. [DOI] [PubMed] [Google Scholar]
- 49.Ishizaka N, Ishizaka Y, Seki G, Nagai R, Yamakado M, Koike K. Association between hepatitis B/C viral infection, chronic kidney disease and insulin resistance in individuals undergoing general health screening. Hepatol Res 2008;38(8):775–783. [DOI] [PubMed] [Google Scholar]
- 50.Lee JJ, Lin MY, Yang YH, Lu SN, Chen HC, Hwang SJ. Association of hepatitis C and B virus infection with CKD in an endemic area in Taiwan: a cross-sectional study. Am J Kidney Dis 2010;56(1):23–31. [DOI] [PubMed] [Google Scholar]
- 51.Yanik EL, Lucas GM, Vlahov D, Kirk GD, Mehta SH. HIV and proteinuria in an injection drug user population. Clin J Am Soc Nephrol 2010;5(10):1836–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li WC, Lee YY, Chen IC, Wang SH, Hsiao CT, Loke SS. Age and gender differences in the relationship between hepatitis C infection and all stages of chronic kidney disease. J Viral Hepat 2014;21(10):706–715. [DOI] [PubMed] [Google Scholar]
- 53.Kurbanova N, Qayyum R. Association of hepatitis C virus infection with proteinuria and glomerular filtration rate. Clin Transl Sci 2015;8(5):421–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hofmann JN, Torner A, Chow WH, Ye W, Purdue MP, Duberg AS. Risk of kidney cancer and chronic kidney disease in relation to hepatitis C virus infection: a nationwide register-based cohort study in Sweden. Eur J Cancer Prev 2011;20(4):326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roccatello D, Fornasieri A, Giachino O, et al. Multicenter study on hepatitis C virus-related cryoglobulinemic glomerulonephritis. Am J Kidney Dis 2007;49(1):69–82. [DOI] [PubMed] [Google Scholar]
- 56.Fabrizi F, Plaisier E, Saadoun D, Martin P, Messa P, Cacoub P. Hepatitis C virus infection, mixed cryoglobulinemia, and kidney disease. Am J Kidney Dis 2013;61(4):623–637. [DOI] [PubMed] [Google Scholar]
- 57.Ferri C, Sebastiani M, Giuggioli D, et al. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum 2004;33(6):355–374. [DOI] [PubMed] [Google Scholar]
- 58.D’Amico G Renal involvement in hepatitis C infection: cryoglobulinemic glomerulonephritis. Kidney Int 1998;54(2):650–671. [DOI] [PubMed] [Google Scholar]
- 59.Sansonno D, Gesualdo L, Manno C, Schena FP, Dammacco F. Hepatitis C virus-related proteins in kidney tissue from hepatitis C virus-infected patients with cryoglobulinemic membranoproliferative glomerulonephritis. Hepatology 1997;25(5):1237–1244. [DOI] [PubMed] [Google Scholar]
- 60.Fabrizi F, Pozzi C, Farina M, et al. Hepatitis C virus infection and acute or chronic glomerulonephritis: an epidemiological and clinical appraisal. Nephrol Dial Transplant 1998;13(8):1991–1997. [DOI] [PubMed] [Google Scholar]
- 61.Esteban J, R E, Viladomiu L, et al. Hepatitis C virus antibodies among risk groups in Spain. Lancet 1989;2(8658):294–297. [DOI] [PubMed] [Google Scholar]
- 62.Zeldis J, Depner T, Kuramoto I, Gish R, Holland P. The prevalence of hepatitis C virus antibodies among hemodialysis patients. Ann Intern Med 1990;112(12):958–960. [DOI] [PubMed] [Google Scholar]
- 63.Jeffers LJ, Perez GO, de Medina MD, et al. Hepatitis C infection in two urban hemodialysis units. Kidney Int 1990;38(2):320–322. [DOI] [PubMed] [Google Scholar]
- 64.Alter MJ, Hadler SC, Judson FN, et al. Risk factors for acute non-A, non-B hepatitis in the United States and association with hepatitis C virus infection. JAMA 1990;264(17):2231–2235. [PubMed] [Google Scholar]
- 65.Jadoul M, Cornu C, van Ypersele de Strihou C. Incidence and risk factors for hepatitis C seroconversion in hemodialysis: a prospective study. The UCL Collaborative Group. Kidney Int 1993;44(6):1322–1326. [DOI] [PubMed] [Google Scholar]
- 66.Corcoran GD, Brink NS, Millar CG, et al. Hepatitis C virus infection in haemodialysis patients: a clinical and virological study. J Infect 1994;28(3):279–285. [DOI] [PubMed] [Google Scholar]
- 67.Allander T, Gruber A, Naghavi M, et al. Frequent patient-to-patient transmission of hepatitis C virus in a haematology ward. Lancet 1995;345(8950):603–607. [DOI] [PubMed] [Google Scholar]
- 68.Jadoul M, Cornu C, van Ypersele de Strihou C. Universal precautions prevent hepatitis C virus transmission: a 54 month follow-up of the Belgian Multicenter Study. The Universitaires Cliniques St-Luc (UCL) Collaborative Group. Kidney Int 1998;53(4):1022–1025. [DOI] [PubMed] [Google Scholar]
- 69.Rossi C, Raboud J, Walmsley S, et al. Hepatitis C co-infection is associated with an increased risk of incident chronic kidney disease in HIV-infected patients initiating combination antiretroviral therapy. BMC Infect Dis 2017;17(1):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fabrizi F, Dixit V, Martin P, Messa P. Hepatitis C virus increases the risk of kidney disease among HIV-positive patients: Systematic review and meta-analysis. J Med Virol 2016;88(3):487–497. [DOI] [PubMed] [Google Scholar]
- 71.Mocroft A, Lundgren JD, Ross M, et al. Development and validation of a risk score for chronic kidney disease in HIV infection using prospective cohort data from the D:A:D study. PLoS Med 2015;12(3):e1001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lucas GM, Jing Y, Sulkowski M, et al. Hepatitis C viremia and the risk of chronic kidney disease in HIV-infected individuals. J Infect Dis 2013;208(8):1240–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abraham AG, Althoff KN, Jing Y, et al. End-stage renal disease among HIV-infected adults in North America. Clin Infect Dis 2015;60(6):941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peters L, Grint D, Lundgren JD, et al. Hepatitis C virus viremia increases the incidence of chronic kidney disease in HIV-infected patients. AIDS 2012;26(15):1917–1926. [DOI] [PubMed] [Google Scholar]
- 75.Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int 2004;66(3):1145–1152. [DOI] [PubMed] [Google Scholar]
- 76.Kalayjian RC, Lau B, Mechekano RN, et al. Risk factors for chronic kidney disease in a large cohort of HIV-1 infected individuals initiating antiretroviral therapy in routine care. AIDS 2012;26(15):1907–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Flandre P, Pugliese P, Cuzin L, et al. Risk factors of chronic kidney disease in HIV-infected patients. Clin J Am Soc Nephrol 2011;6(7):1700–1707. [DOI] [PubMed] [Google Scholar]
- 78.Kovari H, Rauch A, Kouyos R, et al. Hepatitis C infection and the risk of non-liver-related morbidity and mortality in HIV-infected persons in the Swiss HIV Cohort Study. Clin Infect Dis 2017;64(4):490–497. [DOI] [PubMed] [Google Scholar]
- 79.Wyatt CM, Malvestutto C, Coca SG, Klotman PE, Parikh CR. The impact of hepatitis C virus coinfection on HIV-related kidney disease: a systematic review and meta-analysis. AIDS 2008;22(14):1799–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rossi C, Cox J, Cooper C, et al. Frequent injection cocaine use increases the risk of renal impairment among hepatitis C and HIV coinfected patients. AIDS 2016;30(9):1403–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rossi C, Saeed S, Cox J, et al. Hepatitis C virus cure does not impact kidney function decline in HIV co-infected patients. AIDS 2018;32(6):751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scott DR, Wong JK, Spicer TS, et al. Adverse impact of hepatitis C virus infection on renal replacement therapy and renal transplant patients in Australia and New Zealand. Transplantation 2010;90(11):1165–1171. [DOI] [PubMed] [Google Scholar]
- 83.Mahmoud IM, Sobh MA, El-Habashi AF, et al. Interferon therapy in hemodialysis patients with chronic hepatitis C: study of tolerance, efficacy and post-transplantation course. Nephron Clin Pract 2005;100(4):c133–139. [DOI] [PubMed] [Google Scholar]
- 84.Cruzado JM, Carrera M, Torras J, Grinyo JM. Hepatitis C virus infection and de novo glomerular lesions in renal allografts. Am J Transplant 2001;1(2):171–178. [PubMed] [Google Scholar]
- 85.Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G. Hepatitis C virus antibody status and survival after renal transplantation: meta-analysis of observational studies. Am J Transplant 2005;5(6):1452–1461. [DOI] [PubMed] [Google Scholar]
- 86.Mathurin P, Mouquet C, Poynard T, et al. Impact of hepatitis B and C virus on kidney transplantation outcome. Hepatology 1999;29(1):257–263. [DOI] [PubMed] [Google Scholar]
- 87.Hanafusa T, Ichikawa Y, Kishikawa H, et al. Retrospective study on the impact of hepatitis C virus infection on kidney transplant patients over 20 years. Transplantation 1998;66(4):471–476. [DOI] [PubMed] [Google Scholar]
- 88.Chute DF, Chung RT, Sise ME. Direct-acting antiviral therapy for hepatitis C virus infection in the kidney transplant recipient. Kidney Int 2018;93(3):560–567. [DOI] [PubMed] [Google Scholar]
- 89.Messina JP, Humphreys I, Flaxman A, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 2015;61(1):77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee CM, Lu SN, Hung CH, et al. Hepatitis C virus genotypes in southern Taiwan: prevalence and clinical implications. Trans R Soc Trop Med Hyg 2006;100(8):767–774. [DOI] [PubMed] [Google Scholar]
- 91.Lucas GM. Association between hepatitis C virus and chronic kidney disease: heterogeneity begets heterogeneity. Kidney Int 2017;92(3):546–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mocroft A, Neuhaus J, Peters L, et al. Hepatitis B and C co-infection are independent predictors of progressive kidney disease in HIV-positive, antiretroviral-treated adults. PLoS One 2012;7(7):e40245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Soma J, Saito T, Taguma Y, et al. High prevalence and adverse effect of hepatitis C virus infection in type II diabetic-related nephropathy. J Am Soc Nephrol 2000;11(4):690–699. [DOI] [PubMed] [Google Scholar]
- 94.Noureddine LA, Usman SA, Yu Z, Moorthi RN, Moe SM. Hepatitis C increases the risk of progression of chronic kidney disease in patients with glomerulonephritis. Am J Nephrol 2010;32(4):311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jotwani V, Li Y, Grunfeld C, Choi AI, Shlipak MG. Risk factors for ESRD in HIV-infected individuals: traditional and HIV-related factors. Am J Kidney Dis 2012;59(5):628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bickel M, Marben W, Betz C, et al. End-stage renal disease and dialysis in HIV-positive patients: observations from a long-term cohort study with a follow-up of 22 years. HIV Med 2013;14(3):127–135. [DOI] [PubMed] [Google Scholar]
- 97.Tsui J, Vittinghoff E, Anastos K, et al. Hepatitis C seropositivity and kidney function decline among women with HIV: data from the Women’s Interagency HIV Study. Am J Kidney Dis 2009;54(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lazo M, Nwankwo C, Daya NR, et al. Confluence of epidemics of hepatitis C, diabetes, obesity, and chronic Kidney disease in the United States population. Clin Gastroenterol Hepatol 2017;15(12):1957–1964 e1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Goodkin DA, Bieber B, Jadoul M, Martin P, Kanda E, Pisoni RL. Mortality, hospitalization, and quality of life among patients with hepatitis C infection on hemodialysis. Clin J Am Soc Nephrol 2017;12(2):287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fabrizi F, Martin P, Dixit V, Messa P. Meta-analysis of observational studies: hepatitis C and survival after renal transplant. J Viral Hepat 2014;21(5):314–324. [DOI] [PubMed] [Google Scholar]
- 101.Heo NY, Mannalithara A, Kim D, Udompap P, Tan JC, Kim WR. Long-term patient and graft survival of kidney transplant recipients with Hepatitis C virus infection in the United States. Transplantation 2018;102(3):454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alric L, Plaisier E, Thebault S, et al. Influence of antiviral therapy in hepatitis C virus-associated cryoglobulinemic MPGN. Am J Kidney Dis 2004;43(4):617–623. [DOI] [PubMed] [Google Scholar]
- 103.Rossi P, Bertani T, Baio P, et al. Hepatitis C virus-related cryoglobulinemic glomerulonephritis: long-term remission after antiviral therapy. Kidney Int 2003;63(6):2236–2241. [DOI] [PubMed] [Google Scholar]
- 104.Sabry AA, Sobh MA, Sheaashaa HA, et al. Effect of combination therapy (ribavirin and interferon) in HCV-related glomerulopathy. Nephrol Dial Transplant 2002;17(11):1924–1930. [DOI] [PubMed] [Google Scholar]
- 105.Cua IH, Kwan V, Henriquez M, Kench J, George J. Long term suppressive therapy with pegylated interferon for chronic hepatitis C associated membranoproliferative glomerulonephritis. Gut 2006;55(10):1521–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Orlent H, Mathot RA, Van Bommel EF, Vulto AG, Schalm SW, Brouwer JT. Peginterferon and dose-titrated ribavirin for hepatitis C-associated nephrotic membranoproliferative glomerulonephritis type 1. Dig Dis Sci 2005;50(10):1804–1806. [DOI] [PubMed] [Google Scholar]
- 107.Saadoun D, Thibault V, Si Ahmed SN, et al. Sofosbuvir plus ribavirin for hepatitis C virus-associated cryoglobulinaemia vasculitis: VASCUVALDIC study. Ann Rheum Dis 2016;75(10):1777–1782. [DOI] [PubMed] [Google Scholar]
- 108.Bruchfeld A, Lindahl K, Stahle L, Soderberg M, Schvarcz R. Interferon and ribavirin treatment in patients with hepatitis C-associated renal disease and renal insufficiency. Nephrol Dial Transplant 2003;18(8):1573–1580. [DOI] [PubMed] [Google Scholar]
- 109.Chen YC, Hwang SJ, Li CY, Wu CP, Lin LC. A Taiwanese nationwide cohort study shows interferon-based therapy for chronic hepatitis C reduces the risk of chronic kidney disease. Medicine (Baltimore) 2015;94(32):e1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hsu YC, Lin JT, Ho HJ, et al. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology 2014;59(4):1293–1302. [DOI] [PubMed] [Google Scholar]
- 111.Ble M, Aguilera V, Rubin A, et al. Improved renal function in liver transplant recipients treated for hepatitis C virus with a sustained virological response and mild chronic kidney disease. Liver Transpl 2014;20(1): 25–34. [DOI] [PubMed] [Google Scholar]
- 112.Berenguer J, Rodriguez-Castellano E, Carrero A, et al. Eradication of hepatitis C virus and non-liver-related non-acquired immune deficiency syndrome-related events in human immunodeficiency virus/hepatitis C virus coinfection. Hepatology 2017;66(2):344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leone S, Prosperi M, Costarelli S, et al. Incidence and predictors of cardiovascular disease, chronic kidney disease, and diabetes in HIV/HCV-coinfected patients who achieved sustained virological response. Eur J Clin Microbiol Infect Dis 2016;35(9):1511–1520. [DOI] [PubMed] [Google Scholar]
- 114.Roth D, Nelson DR, Bruchfeld A, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet 2015;386(10003):1537–1545. [DOI] [PubMed] [Google Scholar]
- 115.Pockros PJ, Reddy KR, Mantry PS, et al. Efficacy of direct-acting antiviral combination for patients with hepatitis C virus genotype 1 infection and severe renal impairment or end-stage renal disease. Gastroenterology 2016;150(7):1590–1598. [DOI] [PubMed] [Google Scholar]
- 116.Gane E, Lawitz E, Pugatch D, et al. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N Engl J Med 2017;377(15):1448–1455. [DOI] [PubMed] [Google Scholar]
- 117.Sise ME, Backman E, Ortiz GA, et al. Effect of sofosbuvir-based Hepatitis C virus therapy on kidney function in patients with CKD. Clin J Am Soc Nephrol 2017;12(10):1615–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sise ME, Bloom AK, Wisocky J, et al. Treatment of hepatitis C virus-associated mixed cryoglobulinemia with direct-acting antiviral agents. Hepatology 2016;63(2):408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shimada M, Nakamura N, Endo T, et al. Daclatasvir/asunaprevir based direct-acting antiviral therapy ameliorate hepatitis C virus-associated cryoglobulinemic membranoproliferative glomerulonephritis: a case report. BMC Nephrol 2017;18(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Flemming JA, Lowe CE. Successful treatment of hepatitis C, genotype 3, with sofosbuvir/ledipasvir in decompensated cirrhosis complicated by mixed cryoglobulinaemia. BMJ Case Rep 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


