Abstract
High affinity natural killer cells (haNKs) are a cell therapy product capable of mediating both direct and antibody-dependent cell-mediated cytotoxicity (ADCC). These cells may be particularly useful in tumors that escape T-cell anti-tumor immunity by harboring antigen processing and presentation defects. Here, we demonstrated that haNKs directly kill both HPV-positive and negative head and neck squamous cell carcinoma cells. Variable tumor cell sensitivity to haNK direct cytotoxicity did not correlated with MHC class I chain-related protein A or B (MICA or MICB) expression. Importantly, haNK killing was significantly enhanced via ADCC mediated by cetuximab or avelumab in cells with higher baseline EGFR or PD-L1 expression, respectively. The ability of IFNγ to induce tumor cell PD-L1 expression correlated with enhanced PD-L1-specific ADCC. IFNγ induced neither tumor cell EGFR expression nor EGFR-specific ADCC. Although a single dose of 8Gy IR did not appear to directly enhance susceptibility to haNK killing alone, enhanced PD-L1- and EGFR-mediated ADCC after IR correlated with increased PD-L1 and EGFR expression in one of four models. This pre-clinical evidence supports the investigation of haNK cellular therapy in combination with ADCC-mediating mAbs, with or without IR, in the clinical trial setting for patients with advanced HNSCCs. Given the MHC-unrestricted nature of this treatment, it may represent an opportunity to treat patients with non-T-cell inflamed tumors.
Keywords: haNK, radiation, ADCC, head and neck cancer, cetuximab, avelumab
Introduction
Through genetic alterations in MHC class I alleles, β−2 microglobulin and antigen processing machinery, many head and neck squamous cell carcinoma (HNSCC) patients harbor subsets of tumor cells that escape T-lymphocyte recognition and destruction[1–3]. Endogenous natural killer (NK) cells recognize and are activated against cells with decreased or absent MHC class I expression and thus play a critical and non-redundant role in cancer surveillance along with T-lymphocytes[4, 5]. While great strides have been made in the development of T-lymphocyte cellular therapies, these sophisticated treatments are not effective against subsets of tumors with low antigenicity or antigen processing or presenting defects[6]. An effective NK-based cell therapy product could be useful in the treatment of solids tumors independent of antigenicity or antigen processing.
Here, we explored the ability of a newly developed NK cell therapy product (high-affinity NK; haNK) engineered to express IL-2 and the high affinity V variant Fc receptor to kill a panel of HPV-positive and negative HNSCC cell lines under different experimental conditions[7]. We assessed whether ionizing radiation (IR) and IgG1 monoclonal antibodies (mAbs) capable of inducing antibody-dependent cell-mediated cytotoxicity (ADCC), alone or in combination, enhance HNSCC tumor cell susceptibility to haNK killing and explore potential biomarkers of response. Taken together, these data provide the foundation for further mechanistic studies involving humanized murine mice and the pre-clinical rationale for the study of haNKs and IgG1 mAbs capable of inducing ADCC in the clinical trial setting.
Methods
Cell culture
haNK cells obtained from NantBioscience (Culver City, CA) through a cooperative research and drug agreement (CRADA) were cultured in phenol-red free XVIVO media (Lonza) supplemented with 5% human AB serum. UM-SCC-1G, −11AG and −47 cells were obtained from Dr. T.E. Carey (University of Michigan). UPCI:SCC-90 cells were obtained from Dr. Robert Ferris (University of Pittsburgh Medical School). Cells were used at low passage number and serially verified to be free of mycoplasma infection. In some experiments, avelumab, obtained from EMD Serono through a CRADA, or cetuximab, obtained from the National Institutes of Health Clinical Center pharmacy, was added to cells at 1 μg/mL. Some cells were exposed to recombinant human interferon-gamma (IFNγ; R&D Systems) at 20 ng/mL for 24 hours.
Irradiation
HNSCC Cells were harvested while in log growth phase and irradiated (8Gy) using a 137Cs source (Gammacell-1000) irradiator at a dose rate of 0.74 Gy/min. Irradiated cells were washed three times before being plated for experiments. haNK cells received 10Gy irradiation 24 hours prior to use in functional assays to mimic the cell therapy product used in clinical trials[7].
xCELLigence real-time impedance assay
HNSCC cell lines were plated onto ACEA 96-well E-plates, and alteration of impedance was acquired using the xCELLigence Real-Time Cell Analysis (RTCA) platform per manufacturer recommendations. Controls for ADCC included IgG1 isotype control antibody and CD16 (FcR) antibody (clone B73.1, Thermo). Percent loss of cell index for a given time point was calculated as: 1-(experimental cell index/control cell index).
Flow cytometry
Fresh cultured cells were prepared into single cell suspensions were analyzed. Anti-human HLA-ABC, HLA-E, MICA/B, PD-L1 and EGFR antibodies were purchased from Biolegend. Primary antibodies were applied for 30 minutes at concentrations titrated for each antibody. Dead cells were excluded via 7AAD uptake and a “fluorescence-minus-one” technique was used to validate specific staining in all antibody combinations. Analysis was performed on a BD FACSCanto analyzer running FACSDiva software and interpreted using FlowJo (vX10.0.7r2).
Statistics
Comparison of two sets of data was achieved with student’s t-test. Comparison of multiple sets of data was achieved with one-way analysis of variance (ANOVA). Error bars reflect standard deviation from individual experiments. Analysis was performed using GraphPad Prism v6.
Results
haNKs efficiently kill both HPV-positive and -negative HNSCC cells at low E:T ratios
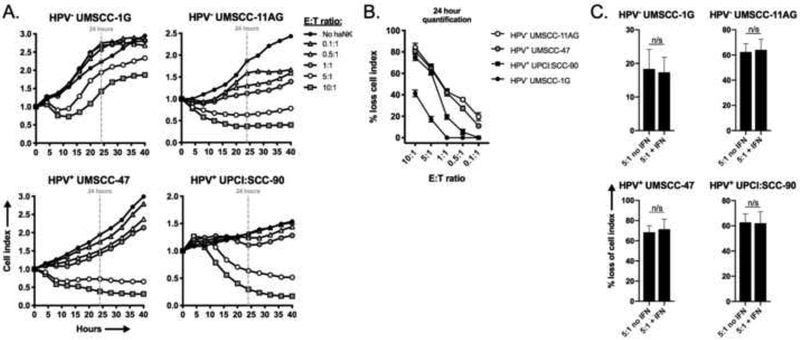
We hypothesized that haNKs, an off-the-shelf NK cell therapy product, could mediate direct cytotoxicity of human HNSCC cells independent of HPV-status. We assessed the ability of haNKs to directly kill two HPV-negative (UM-SCC-1G and UM-SCC-11AG) and 2 HPV-positive (UMSCC-47 and UPCI:SCC-90) cell lines. haNKs induced loss of cell index in a dose dependent fashion as measured by real-time impedance analysis in all cell lines tested (Figure 1A, quantified in 1B). Differential sensitivity to haNK killing was observed between cell lines, with UMSCC-1G and UPCI:SCC-90 demonstrating less sensitivity to direct killing by haNKs compared to UM-SCC-11AG and UM-SCC-47. Tumor cells may be exposed to IFNγ in an inflamed tumor microenvironment, so haNK killing assays were repeated following exposure of tumor cells to IFNγ (Figure 1C). Exposing tumor cells to IFNγ did not enhance susceptibility to direct haNK killing.
Figure 1 – haNKs variably induce cytolysis of a panel of HNSCC cell lines.
HPV-negative cell lines UM-SCC-1G (1×104 cells/well) and −11AG (1×104 cells/well) and HPV-positive cell lines UM-SCC-47 (0.75×104 cells/well) and UPCI:SCC-9 (2×104 cells/well) were plated and allowed to gain impedance overnight prior to the addition of haNKs at the indicated E:T ratios. Cell index plots normalized to the addition of haNKs at time 0. Representative impedance plots shown in (A). Percent loss of cell index relative to control (no haNKs) 24 hours after the addition of haNKs quantified in (B). Cells were pretreated with IFNγ (20 ng/mL for 24 hours) and NK cytolysis was assessed via impedance analysis at select E:T ratios. n/s, non-significant; student’s t-test.
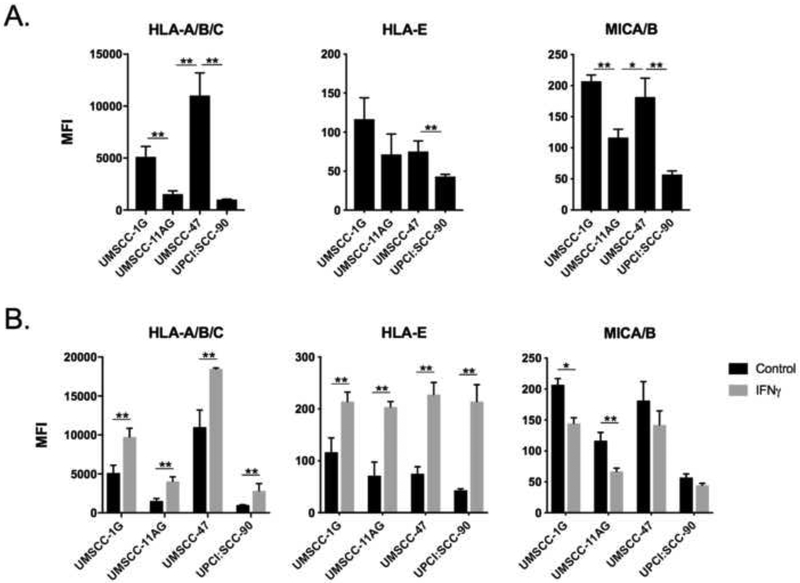
haNKs lack expression of inhibitory killer-cell immunoglobulin-like receptors (KIRs) that are activated by target-cell MHC class I molecules[8]. Accordingly, expression of HLA-A/B/C or –E expression on HNSCC target cells should not correlate with sensitivity to haNK killing. To test this hypothesis, we measured baseline (Figure 2A) and IFNγ-inducible (Figure 2B) expression of cell surface HLA molecules. No clear correlation between surface HLA expression and sensitivity to haNK killing was present. haNK cells do express the activating receptor natural killer group 2D (NKG2D)[7]. Despite expression of NKG2D on haNK cells, HNSCC target cell expression of NKG2D ligands MHC class I chain-related protein A or B (MICA and MICB) did not predict sensitivity to haNK killing, suggesting the presence of other determinants of haNK sensitivity. Cumulatively, these data demonstrated that haNKs are capable of killing both HPV-positive and -negative HNSCC cells, but neither activating or inhibitory NK receptors, nor their corresponding ligands, consistently predicted sensitivity under these experimental conditions.
Figure 2 – HPV-positive and negative cells variably express inhibitory and activating ligands for NK cell killing.
HNSCC cell lines were analyzed for baseline (A) and IFNγ-inducible (B) expression of inhibitory and activating ligands for NK cell function by flow cytometry. IFNγ (20 ng/mL) exposure was for 24 hours. Representative results from one of at least two independent assays shown, each performed in at least technical triplicate. *, p<0.05; **, p<0.01; student’s t-test or ANOVA.
A single 8Gy dose of IR variably sensitizes HNSCC cells to haNK killing
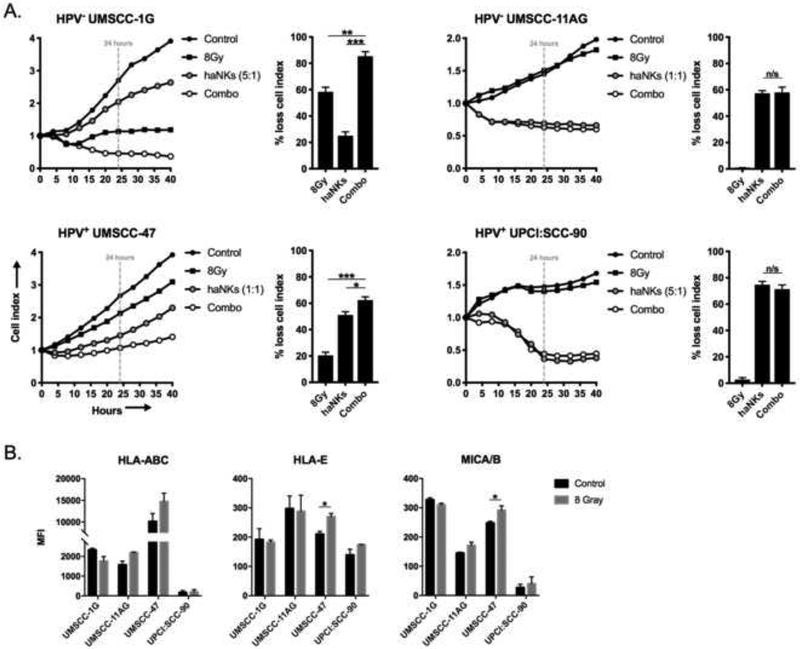
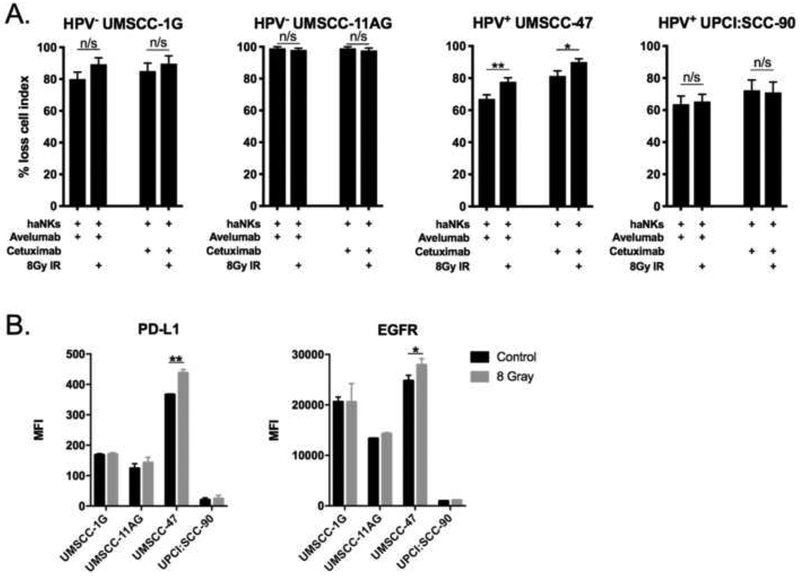
IR sensitizes tumor cells to killing by antigen-specific T-cells through a number of mechanisms[9]. While standard IR treatment for HNSCC utilizes low dose (2Gy) daily fractionation, higher doses of IR (8Gy) sensitize tumor cells to T-cell killing to a greater degree[10]. We hypothesized that IR could similarly enhance HNSCC tumor cell sensitivity to haNK killing. UM-SCC-1G and −47 cells demonstrated sensitivity to IR and loss of cell index following IR alone (Figure 3A). Combination IR and haNK treatment enhanced killing of UMSCC-1G and −47 cells, but not UM-SCC-11AG or UPCI:SCC-90 cells, over either treatment alone. Loss of cell impedance in UM-SCC-1G and −47 cells with combination treatment appeared to be additive and more dependent upon baseline sensitivity of the tumor cells to IR. Despite prior evidence that IR enhances MICA/B expression on tumor cells to sensitize them to NK killing[11], IR modestly enhanced MICA/B expression on UM-SCC-47 cells only (Figure 3B). Thus, a single dose of 8Gy IR did not sensitize HNSCC cells to haNK killing, as combination IR and haNK treatment produced additive and not synergistic loss of tumor cell index.
Figure 3 – Ionizing radiation variably enhances direct haNK cytolysis of HNSCC cell lines.
(A) HNSCC cell lines were plated with and without prior exposure to 8Gy IR were plated and allowed to gain impedance overnight prior to the addition of haNKs at the indicated E:T ratios. Cell index plots normalized to the addition of haNKs at time 0. Percent loss of cell index relative to control (no haNKs) 24 hours after the addition of haNKs quantified to the right of each cell index plot. (B) HNSCC cells were assessed for changes in inhibitory and activating ligands for NK function 24 hours after a single dose of 8Gy IR by flow cytometry. Representative results from one of at least two independent assays shown, each performed in at least technical triplicate. *, p<0.05; **, p<0.01; ***, p<0.001; student’s t-test or ANOVA.
Avelumab and cetuximab enhance haNK killing of HNSCC cells via ADCC
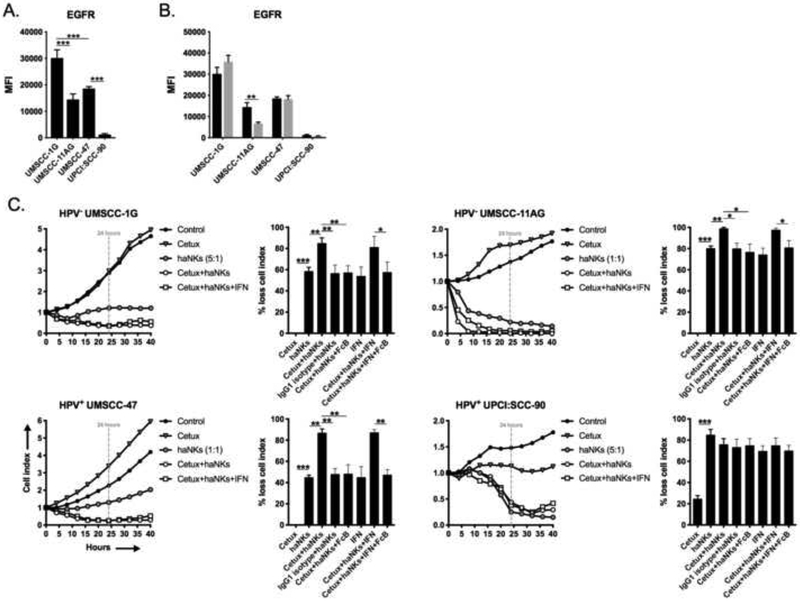
Through expression of the high-affinity V variant of CD16 (Fc receptor), haNKs efficiently mediate ADCC when combined with IgG1 isotype antibodies[12, 13]. The IgG1 EGFR-targeting mAb cetuximab carries multiple FDA-approved indications for the treatment of locoregionally advanced or recurrent/metastatic HNSCC[14]. The IgG1 PD-L1-targeting mAb avelumab is currently being investigated in combination with chemoradiation in the upfront treatment of advanced HNSCC in the phase III trial setting (NCT02952586). We hypothesized that both avelumab and cetuximab mAbs could enhance haNK killing of HNSCC cells.
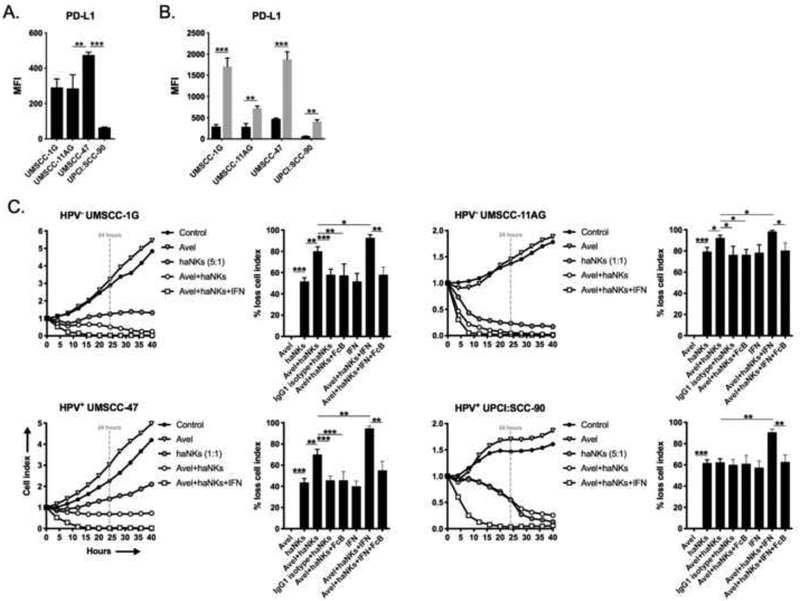
Baseline expression of PD-L1, the target of avelumab, was variable between the cell lines (Figure 4A) but significantly increased upon exposure to IFNγ in all lines (Figure 4B). The addition of avelumab in the absence of tumor cell exposure to IFNγ significantly increased haNK killing in all cell lines except UPCI:SCC-90 (Figure 4C). Avelumab alone did not alter tumor cell impedance. UPCI:SCC-90 cells express the least PD-L1 at baseline compared to the other three cell lines. Avelumab enhanced haNK killing was PD-L1-specific and mediated through ADCC as it was abrogated in the presence of IgG1 isotype control and FcR blocking antibodies, respectively. Tumor cell pre-exposure to IFNγ significantly enhanced ADCC killing beyond that observed without IFNγ in all cell lines that was reversed in the presence of FcR blocking antibodies. These data suggested that baseline avelumab-induced ADCC can be enhanced through increased expression of its ligand, PD-L1, upon exposure to IFNγ.
Figure 4 – Avelumab variably enhances haNK cytolysis of HNSCC cell lines via ADCC.
Baseline (A) and IFNγ-inducible (B) expression of cell surface PD-L1 in HNSCC cells assessed by flow cytometry. (C) HNSCC lines were plated in the presence or absence of avelumab (1 μg/mL), with or without IFNγ, and allowed to gain impedance overnight prior to the addition of haNKs at the indicated E:T ratios. Cell index plots normalized to the addition of haNKs at time 0. For each model, representative impedance plots for select conditions shown on the left, and percent loss of cell index relative to control (no haNKs) 24 hours after the addition of haNKs quantified on the right. IgG1 isotype control antibody and CD16 (FcR) blocking antibodies used as controls. Representative results from one of at least two independent assays shown, each performed in at least technical triplicate. *, p<0.05; **, p<0.01; ***, p<0.001; student’s t-test or ANOVA.
Contrary to PD-L1, baseline expression of EGFR (Figure 5A) was minimally altered upon exposure to IFNγ (Figure 5B). The addition of cetuximab in the absence of tumor cell exposure to IFNγ significantly increased haNK killing in all cell lines except UPCI:SCC-90 (Figure 5C), which also expressed EGFR to a lesser degree that the other lines. EGFR-specific ADCC was again verified with the use of IgG1 isotype control and FcR blocking antibodies. Pre-treatment of tumor cells with IFNγ did not enhance EGFR-specific ADCC, which correlated with a lack of EGFR induction following exposure to IFNγ. These data suggested that although cetuximab was able to induce ADCC, lack of induction of EGFR may have prevented enhanced ADCC following exposure to IFNγ. This data, along with evidence of enhanced PD-L1-specific ADCC with avelumab after tumor cell pre-treatment with IFNγ, suggested that the ability of IgG1 antibodies to mediate ADCC is strongly linked to target expression.
Figure 5 – Cetuximab variably enhances haNK cytolysis of HNSCC cell lines via ADCC.
Baseline (A) and IFNγ-inducible (B) expression of cell surface EGFR in HNSCC cells assessed by flow cytometry. (C) HNSCC lines were plated in the presence or absence of cetuximab (1 μg/mL), with or without IFNγ, and allowed to gain impedance overnight prior to the addition of haNKs at the indicated E:T ratios. Cell index plots normalized to the addition of haNKs at time 0. For each model, representative impedance plots for select conditions shown on the left, and percent loss of cell index relative to control (no haNKs) 24 hours after the addition of haNKs quantified on the right. IgG1 isotype control antibody and CD16 (FcR) blocking antibodies used as controls. Representative results from one of at least two independent assays shown, each performed in at least technical triplicate. *, p<0.05; **, p<0.01; ***, p<0.001; student’s t-test or ANOVA.
Increased antibody-enhanced haNK killing of HNSCC cells after IR correlates with changes in antibody target expression
We next assessed whether a single dose of 8Gy IR could increase avelumab or cetuximabenhanced haNK killing of HNSCC cells (Figure 6A). haNK killing of UM-SCC-47 cells in the presence of avelumab or cetuximab was enhanced by radiation in UM-SCC-47 only. Adding further support to the concept that antibody target expression predicts enhanced ADCC killing of HNSCC cells by haNKs, cell surface expression of PD-L1 and EGFR was enhanced following IR in UM-SCC-47 cells only (Figure 6B). These data suggested that IR could enhance tumor cell killing when combined with an IgG1 mAb capable of inducing haNK-mediated ADCC through increased antibody target cell surface expression.
Figure 6 – Ionizing radiation variably alters cetuximab or avelumab-enhanced cytolysis of HNSCC cells by haNKs.
(A) Quantification of percent loss of cell index relative to control (no haNKs) 24 hours after the addition of haNKs. HNSCC cells with or without prior exposure to a single dose of 8Gy IR were plated and allowed to gain impedance overnight before the addition of haNKs with or without avelumab or cetuximab (1 μg/mL). (B) HNSCC cells were assessed for changes in PD-L1 and EGFR expression 24 hours after a single dose of 8Gy IR by flow cytometry. Representative results from one of at least two independent assays shown, each performed in at least technical triplicate. *, p<0.05; **, p<0.01; student’s t-test.
Discussion
Expanded tumor infiltrating lymphocytes (TILs) or TCR engineered T-lymphocyte cellular therapies show promise in the treatment of solid tumors such as squamous cell carcinomas[15, 16], but have significant drawbacks. These include the need for lympho-depleting conditioning chemotherapy before infusion, post-infusion IL-2 that can cause capillary leak syndrome and cytokine storm, and HLA-restriction for MHC-restricted T-lymphocyte antigens[17]. These challenges may be overcome with the use of NK cell therapy, which can be administered as a monotherapy without the need for chemotherapy preconditioning and is both antigen- and HLA-unrestricted. haNKs are an off-the-shelf NK cell therapy product derived from the NK-92 cell line and engineered to express IL-2 and the high affinity V variant of the Fc receptor that enhances its ability for ADCC[7]. The use of haNKs in the clinical trial setting to treat many solid tumor types including HNSCC in underway in both the United States and China (NCT03169764, NCT03008330).
Here, we established several pre-clinical findings important for the clinical translation of haNKs to treat HNSCC. First, haNKs harbored the capacity to efficiently kill both HPV-positive and negative HNSCC cells in a dose-dependent fashion. Our use of highly sensitive impedance analysis to measure loss of cell viability in real-time demonstrated that haNKs can kill HNSCC cell targets at very low, physiologic E:T ratios. Other methods of assessing target cell cytolysis rely on the release of radioactive compounds, have high background, and require high E:T ratios. Knowing that haNKs can kill HNSCC cell targets at low E:T ratios is critical as tumor penetrance after adoptive cell transfer is likely to be quite low[18, 19].
Next, we demonstrated enhanced loss of HNSCC viability in two of four HNSCC cell lines with combination IR and haNKs over either treatment alone. However, both cell lines that demonstrated enhanced loss of cell viability with combination treatment were the same cell lines that displayed sensitivity to IR alone, suggesting that the observed effect with combination treatment is additive and not synergistic. This is in contrast to the ability of IR to enhance tumor cell susceptibility to antigen-specific T-lymphocyte killing through mechanisms that include at least enhanced MHC class I and cell surface calreticulin expression[20, 21]. This finding may further be explained by a lack of change in MICA and MICB expression in these cells that has been described in other models following IR[11], suggesting inter-model variability in the ability of IR to enhance tumor cell susceptibility to NK killing.
This work established that haNK killing of HNSCC targets was more significantly enhanced with the addition of cetuximab and avelumab, two clinically relevant IgG1 mAbs capable of inducing ADCC. Cetuximab is routinely used to treat advanced HNSCC, and the role of ADCC mediated by endogenous NK cells in the clinical efficacy of systemic cetuximab in patients with HNSCC has been clearly established in a series of elegant works by Ferris and colleagues[22, 23]. Cetuximab may be an ideal inducer of ADCC given the near universal expression of EGFR on HNSCC tumor cells[24]. However, widespread expression of EGFR outside the tumor may induce on-target but off-tumor toxicity, suggesting this combination will be to be studied carefully in the clinical setting. Alternatively, the use of PD-L1-targeting avelumab could dually activate both T-lymphocyte and NK immunity through PD-L1 blockade and initiation of ADCC. Yet, PD-L1 expression on HNSCC cells in vivo is likely to be less consistent than EGFR given that PD-L1 expression is largely a reflection of underlying tumor inflammation and the presence of cytokines such as interferon[25]. Deciphering which of these antibodies best enhances the anti-tumor effect of haNKs, while minimizing immune-related adverse events, will likely require head-to-head multi-arm clinical trials.
Though highly correlative, findings in this work could inform biomarker hypotheses in larger, confirmatory studies. Baseline EGFR and both baseline and IFNγ-induced PD-L1 expression on the surface of HNSCC correlated with the ability of cetuximab and avelumab, respectively, to enhance haNK killing. Further, IR increased expression of EGFR and PD-L1 on the surface of UM-SCC-47 cells only, and IR enhanced ADCC killing in these cells only. This is in contrast to baseline MICA and MICB expression on HNSCC cells, which did not correlate with baseline susceptibility to NKG2D+ haNK killing. Thus, while IR did not appear to directly enhance HNSCC susceptibility to haNK killing, it may be useful in combination with IgG1 mAb and haNK treatment via increased antibody target expression. Enhancement of tumor cell PDL1 expression appears to be model dependent but was consistently inducible upon exposure of IFNγ in all models tested here[26, 27]. Tumor cell EGFR or PD-L1 expression could serve as predictive biomarkers of response in combination clinical trials testing haNKs in combination with cetixumab or avelumab.
In conclusion, haNKs are an off-the-shelf NK cell therapy product that may be useful in the treatment of HNSCC. We demonstrated that haNKs efficiently kill both HPV-positive and negative HNSCC cells at very low E:T ratios that may be achievable with adoptive cell transfer. The addition of IgG1 mAbs cetuximab and avelumab enhanced haNK killing via ADCC in three of four cell models. Tumor cell pre-treatment with IFNγ enhanced PD-L1 expression and PD-L1 specific ADCC, suggesting that PD-L1-specific ADCC with avelumab could be enhanced in inflamed tumors. Importantly, although IR alone did not appear to directly enhance susceptibility to haNK killing, IR may indirectly promote tumor cell killing through enhanced ADCC antibody target expression. These data strongly support the investigation of haNKs in combination with IgG1 mAbs capable of inducing ADCC, with or without IR, in the clinical trial setting. Given the MHC-unrestricted nature of this treatment, and evidence that a significant number of HNSCCs harbor subsets of cells with antigen processing and presentation defects[1, 3], these treatments may represent a treatment option for non-T-cell inflamed tumors.
Research Highlights.
haNKs are an off-the-shelf cellular therapy that kill head and neck cancer cells
Cetuximab and Avelumab enhance tumor cell killing by haNKs through ADCC
Ionizing radiation may enhance ADCC through increased expression of antibody targets
haNKs plus cetuximab or avelumab warrant evaluation in clinical trials for HNSCC
Acknowledgments
Funding: This work was supported by the Intramural Research Program of the NIH, NIDCD, project number ZIA-DC000087.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
JL is an employee of NantKWest. NantKWest supplied haNK cells. No financial support was involved. All other authors report no conflict of interest.
References
- [1].Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lopez-Albaitero A, Nayak JV, Ogino T, Machandia A, Gooding W, DeLeo AB, et al. Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J Immunol. 2006;176:3402–9. [DOI] [PubMed] [Google Scholar]
- [3].Grandis JR, Falkner DM, Melhem MF, Gooding WE, Drenning SD, Morel PA. Human leukocyte antigen class I allelic and haplotype loss in squamous cell carcinoma of the head and neck: clinical and immunogenetic consequences. Clin Cancer Res. 2000;6:2794–802. [PubMed] [Google Scholar]
- [4].Ljunggren HG, Karre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985;162:1745–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Garrido F, Aptsiauri N, Doorduijn EM, Garcia Lora AM, van Hall T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr Opin Immunol. 2016;39:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jochems C, Hodge JW, Fantini M, Fujii R, Morillon YM 2nd, Greiner JW, et al. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget. 2016;7:86359–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Maki G, Klingemann HG, Martinson JA, Tam YK. Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92. J Hematother Stem Cell Res. 2001;10:369–83. [DOI] [PubMed] [Google Scholar]
- [9].Morisada M, Chamberlin M, Allen C. Exploring the rationale for combining ionizing radiation and immune checkpoint blockade in head and neck cancer. Head & neck. 2018;40:1321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Morisada M, Moore EC, Hodge R, Friedman J, Cash HA, Hodge JW, et al. Dose-dependent enhancement of T-lymphocyte priming and CTL lysis following ionizing radiation in an engineered model of oral cancer. Oral oncology. 2017;71:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim JY, Son YO, Park SW, Bae JH, Chung JS, Kim HH, et al. Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Exp Mol Med. 2006;38:474–84. [DOI] [PubMed] [Google Scholar]
- [12].Fujii R, Schlom J, Hodge JW. A potential therapy for chordoma via antibody-dependent cell-mediated cytotoxicity employing NK or high-affinity NK cells in combination with cetuximab. J Neurosurg. 2018;128:1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jochems C, Hodge JW, Fantini M, Tsang KY, Vandeveer AJ, Gulley JL, et al. ADCC employing an NK cell line (haNK) expressing the high affinity CD16 allele with avelumab, an anti-PD-L1 antibody. Int J Cancer. 2017;141:583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. [DOI] [PubMed] [Google Scholar]
- [15].Hinrichs CS. Cell-based molecularly targeted therapy: targeting oncoproteins with T cell receptor gene therapy. J Clin Invest. 2018;128:1261–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stevanovic S, Pasetto A, Helman SR, Gartner JJ, Prickett TD, Howie B, et al. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science. 2017;356:200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Svane IM, Verdegaal EM. Achievements and challenges of adoptive T cell therapy with tumor-infiltrating or blood-derived lymphocytes for metastatic melanoma: what is needed to achieve standard of care? Cancer Immunol Immunother. 2014;63:1081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fisher B, Packard BS, Read EJ, Carrasquillo JA, Carter CS, Topalian SL, et al. Tumor localization of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J Clin Oncol. 1989;7:250–61. [DOI] [PubMed] [Google Scholar]
- [19].Slaney CY, Kershaw MH, Darcy PK. Trafficking of T cells into tumors. Cancer Res. 2014;74:7168–74. [DOI] [PubMed] [Google Scholar]
- [20].Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:1858–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Trivedi S, Srivastava RM, Concha-Benavente F, Ferrone S, Garcia-Bates TM, Li J, et al. Anti-EGFR Targeted Monoclonal Antibody Isotype Influences Antitumor Cellular Immunity in Head and Neck Cancer Patients. Clin Cancer Res. 2016;22:5229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24:2666–72. [DOI] [PubMed] [Google Scholar]
- [25].Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127:2930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. The Journal of clinical investigation. 2014;124:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Concha-Benavente F, Srivastava RM, Trivedi S, Lei Y, Chandran U, Seethala RR, et al. Identification of the Cell-Intrinsic and -Extrinsic Pathways Downstream of EGFR and IFNgamma That Induce PD-L1 Expression in Head and Neck Cancer. Cancer research. 2016;76:1031–43. [DOI] [PMC free article] [PubMed] [Google Scholar]