Abstract
Background:
There is no accurate, non-invasive measurement to estimate the degree of pulmonary edema in ARDS. We developed the Radiographic Assessment of Lung Edema (RALE) score to evaluate the extent and density of alveolar opacities on chest radiographs. After first comparing the RALE score to gravimetric assessment of pulmonary edema in organ donors, we then evaluated the RALE score in ARDS patients for its relationship to oxygenation and clinical outcomes.
Methods:
We compared radiographs with excised lung weights from 72 organ donors (derivation cohort) and radiographs with clinical data from 174 ARDS patients in the ARDSNet Fluid and Catheter Treatment Trial (validation cohort). To calculate RALE, each radiographic quadrant was scored for extent of consolidation (0–4) and density of opacification (1–3). The product of the consolidation and density scores for each of the four quadrants was summed (maximum score=48).
Results:
Agreement between two independent reviewers for RALE score was excellent (intra-class correlation coefficient=0.93, 95% CI:0.91–0.95). In donors, pre-procurement RALE score correlated with height-adjusted total lung weight (ρ=0.59, p<0.001). In ARDS patients, higher RALE scores were independently associated with lower PaO2/FiO2 and worse survival. Conservative fluid management significantly decreased RALE score over 3 days compared to liberal fluid management.
Conclusions:
The RALE score can be used to assess both the extent of pulmonary edema and the severity of ARDS, by utilizing information that is already obtained routinely, safely, and inexpensively in every ARDS patient. This novel non-invasive measure should be useful for assessing ARDS severity and monitoring response to therapy.
Keywords: Pulmonary edema, Acute Respiratory Distress Syndrome (ARDS), chest radiograph, prognosis, survival, critical care
INTRODUCTION
Pulmonary edema is a key feature of the pathogenesis and prognosis of ARDS1 but the severity of pulmonary edema is only indirectly assessed in current definitions of ARDS by the degree of hypoxemia2. Current methods to quantify the severity of pulmonary edema are either invasive (e.g. PICCO catheter) or require patient transport out of the ICU (e.g. computed tomographic imaging), raising safety concerns. We hypothesized that the chest radiograph, already used routinely to detect the presence of ARDS, can be systematically scored to quantify the severity of pulmonary edema. We further hypothesized that the severity of pulmonary edema as assessed on the routine chest radiograph is associated with the severity of ARDS and clinical outcomes.
To test these two hypotheses, we developed a novel Radiographic Assessment of Lung Edema (RALE) score that assesses both the extent and density of alveolar opacities on the chest radiograph. After establishing that the RALE score correlates with the degree of pulmonary edema by comparing pre-procurement chest radiographs in deceased organ donors to gravimetric measurement of pulmonary edema in excised lungs, we then applied the RALE score to radiographs from patients with ARDS enrolled in the NHLBI Fluid and Catheter Treatment Trial (FACTT).3
MATERIALS AND METHODS
Patient populations.
The initial evaluation of the RALE score was done using chest radiographs and gravimetric measurements of lung edema from a derivation cohort of donors enrolled in the Beta Agonist for Oxygenation in Lung Donors (BOLD) study, a randomized clinical trial of nebulized albuterol in deceased organ donors.4 Following organ resection without perfusion, lungs from enrolled donors declined for transplantation were weighed. Total lung weights were adjusted for patient height and used as a quantitative index of pulmonary edema.5 Included in the study were 72 of 506 donors in BOLD who had matched pre-procurement radiographs and lung weights.
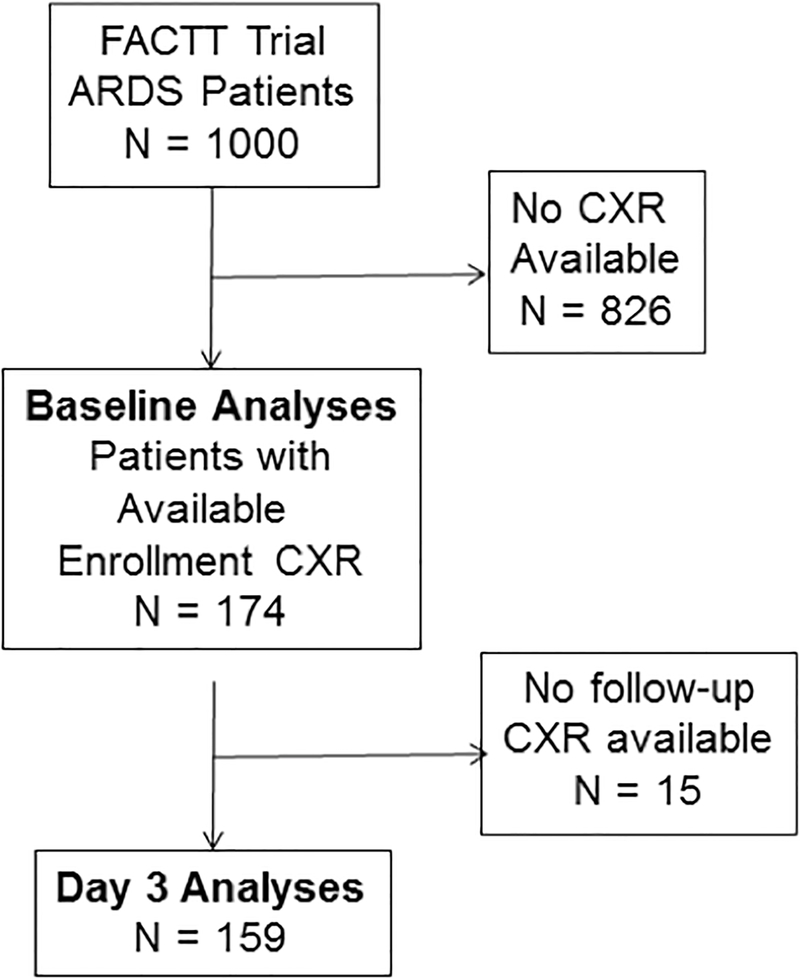
To assess clinical utility of RALE, a validation cohort of patients from the NHLBI ARDS Network Fluid and Catheter Treatment Trial (FACTT) were studied.3 FACTT was a multicenter, randomized trial of conservative versus liberal fluid management in 1000 ARDS patients. Patients (N = 174) were included from five FACTT sites if enrollment chest radiographs were available for scoring; these patients were previously included in a study of vascular pedicle width.6 Patient selection is summarized in Figure 1. Treatment arm, baseline PaO2/FiO2, fluid balance, and clinical outcomes including ventilator-free days (VFDs) and survival were obtained from the trial database.
Figure 1.
Flow diagram of patients included in the baseline (time of enrollment) analyses and the Day 3 analyses of the RALE score. Day 3 analyses were done using chest radiographs from Day 3 after enrollment when available (N = 140). If a chest radiograph was not available from Day 3, a chest radiograph from the next closest day was used with a preference for Day 4 if available (Day 1, N = 1; Day 2, N = 3; Day 4, N = 14; Day 6, N = 1). FACTT, Fluid and Catheter Treatment Trial; ARDS, acute respiratory distress syndrome; CXR, chest radiograph.
Radiograph Scoring.
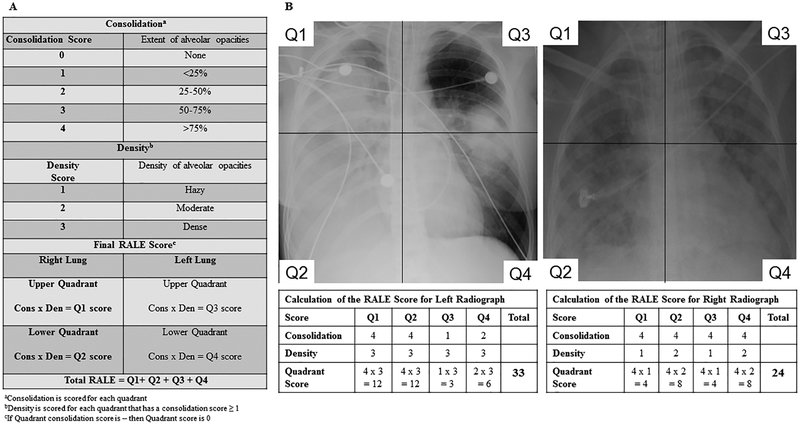
Details of the RALE score are provided in Figure 2. To determine the RALE score, each radiograph was divided into quadrants, defined vertically by the vertebral column and horizontally by the first branch of the left main bronchus. Each quadrant was assigned a consolidation score from 0–4 to quantify the extent of alveolar opacities, based on the percentage of the quadrant with opacification and a density score from 1–3 to quantify the overall density of alveolar opacities, unless the consolidation score for that quadrant was 0. The density score (1=hazy, 2=moderate, 3=dense) allows for more quantitative assessment of the density of opacification by quadrant. To calculate the final RALE score, the product of the consolidation and density score for each quadrant were summed for a final RALE score ranging from 0 (no infiltrates) to 48 (dense consolidation in >75% of each quadrant).
Figure 2.
Consolidation and density scoring in the RALE score (Panel A). Calculation of the Radiographic Assessment of Lung Edema (RALE) score (Panel B).
In the organ donors, chest radiographs obtained within 24 hours of lung procurement were scored for each donor, using the radiograph obtained closest to the time of procurement. Radiographs were scored by consensus of two physician reviewers without knowledge of clinical data or lung weight. In FACTT, each radiograph was scored independently by two physician reviewers blinded to treatment arm, in order to examine inter-observer reliability.
Statistical Analysis.
To determine how well the RALE score quantifies pulmonary edema, we evaluated the association between pre-procurement RALE score and total excised lung weight in deceased organ donors using Spearman’s correlations. A subgroup of 36 donors with total lung weight above the median (797 grams) was used to assess the utility of the RALE score in donors with more pulmonary edema.
To assess the reliability of RALE score across independent reviewers, a two-way mixed consistency, average-measures intra-class correlation coefficient (ICC)7 was calculated for baseline and Day 3 RALE scores in the FACTT trial. Bland-Altman plots were used to visualize agreement between independent reviewers.
The clinical utility of the RALE score was assessed using chest radiographs and clinical data from the FACTT trial. To assess the association with ARDS severity, RALE scores were compared to baseline PaO2/FiO2 using multivariable linear regression. Associations between RALE and other clinical outcomes (overall survival, 28-day mortality, 60-day mortality, and VFDs) were also evaluated. For survival, a Cox Proportional Hazard model was fit. Kaplan-Meier estimates were computed for groups defined by baseline RALE score quartiles. For 28-day and 60-day mortality, logistic regression models were used. For VFDs, zero-inflated negative binomial models were used to account for the high frequency of patients with zero VFDs.
To assess the potential value of change in RALE as a study endpoint, we evaluated the association between change in RALE from baseline to Day 3 and FACTT treatment arm (conservative or liberal fluid management) in regression models adjusted for age, gender, BMI, and APACHE III. We also used linear regression with fluid balance as the dependent variable and RALE, age, gender, BMI, and APACHE III as covariates to assess the relationship between RALE and fluid balance.
Continuous variables are summarized with median and interquartile range (IQR). Categorical variables are summarized as frequencies with percentages. Differences between groups were assessed using Wilcoxon rank sum (continuous) and Fisher’s exact test (categorical). For linear regression and Cox proportional hazard models, we report the estimated effect associated with a 5-unit change in RALE score. Statistical significance was considered when P <0.05. Analyses were performed using R (version 3.3.1).8
RESULTS
Validation of the RALE score against gravimetric measurement of lung edema
Characteristics of deceased organ donors are in Supplemental Table 1. The pre-procurement RALE score was significantly correlated with total lung weight (ρ=0.59, p<0.001) (Supplemental Figure 1). Because the extent of pulmonary edema in organ donors is typically lower than in ARDS, we also evaluated the subgroup with more pulmonary edema, as evidenced by lung weight above the median (n=36); the correlation was higher in this subgroup (Supplemental Figure 1) (ρ=0.73, p<0.001).
Association of RALE scoring with clinical outcomes in ARDS
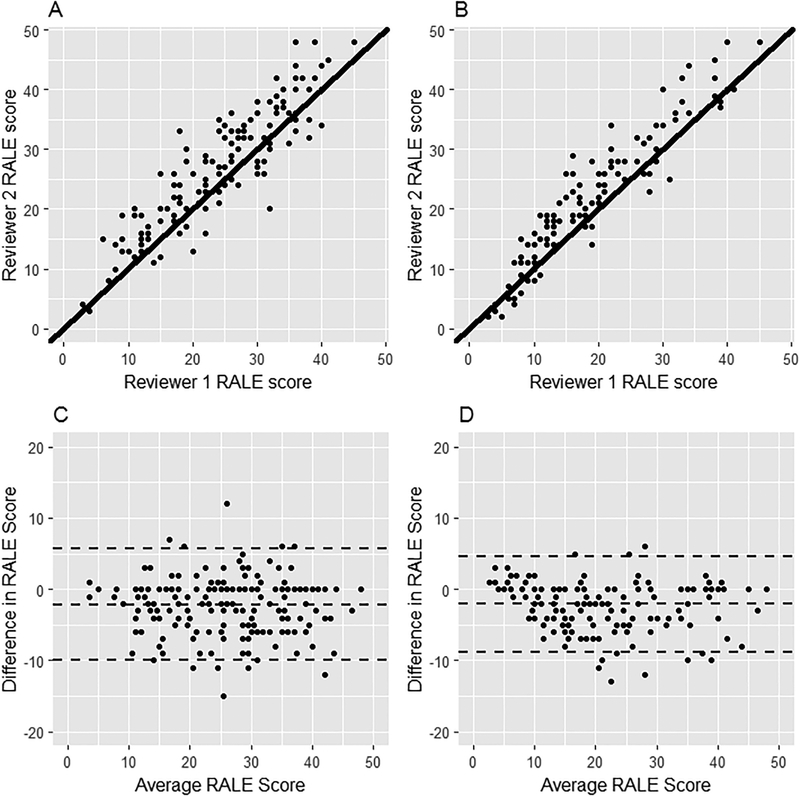
Characteristics of 174 patients from FACTT are in Table 1. Baseline RALE score was substantially higher than in BOLD (median 27, IQR 18–35 vs. 7, IQR 5–15), reflecting the presence of ARDS in FACTT patients. The scores of the two reviewers are compared in Figure 3. ICCs were excellent both at baseline (ICC=0.93, 95%CI:0.91–0.95; Fig 3A) and at Day 3 (ICC=0.96, 95%CI:0.94–0.97; Fig 3B), indicating a high degree of agreement (14). Bland-Altman plots (Figure 3C, D) also showed strong agreement across the range of RALE scores.
Table 1.
Clinical characteristics of patients from FACTT (n=174) who were included in the current study by treatment arm, compared to those who were not included in the current study
| Clinical Characteristic | Included Conservative Fluid Arm (n=82) | Included Liberal Fluid Arm (n=92) | P-value1 | Not included (n=826) |
|---|---|---|---|---|
| Age | 48 (39, 59) | 44 (35, 60) | 0.70 | 49 (39, 61) |
| Male | 44 (54%) | 42 (46%) | 0.29 | 448 (54%) |
| Caucasian | 64 (79%) | 62 (68%) | 0.05 | 513 (62%) |
| BMI (kg/m2) | 28 (25, 34) | 28 (23, 34) | 0.59 | 27 (23, 32) |
| APACHE III | 89 (74, 105) | 95 (81, 121) | 0.07 | 91 (69, 117) |
| 60-Day Mortality | 18 (22%) | 26 (28%) | 0.34 | 225 (27%) |
| RALE Baseline | 28 (17, 35) | 26 (20, 34) | 0.64 | NA |
| RALE Day 3 | 16 (10, 23) | 25 (16, 35) | <0.001 | NA |
| Change in RALE2 | −8 (−1, −16) | −1 (−8, 5) | <0.001 | NA |
Data as median and interquartile range. BMI, body mass index; APACHE III, acute physiology and chronic health evaluation III; RALE, radiographic assessment of lung edema; NA, not available
P-value is for comparison between patients in the conservative and liberal fluid management arms who were included in the current study
Change in RALE for baseline radiograph to Day 3 radiograph. Negative number indicates that the score decreased from baseline to Day 3
Figure 3.
Scatter plots (Panels A and B) and Bland-Altmann plots (Panels C and D) showing agreement between two independent reviewers for RALE scores at Baseline (N = 174, Panel A and C) and at Day 3 after enrollment (N = 159, Panel B and D) for patients in the FACTT trial. To assess agreement between the two reviewers, intra-class correlation coefficients (ICCs) were calculated. ICCs were excellent both at baseline (ICC=0.93, 95%CI:0.91–0.95) and at Day 3 (ICC=0.96, 95%CI:0.94–0.97).
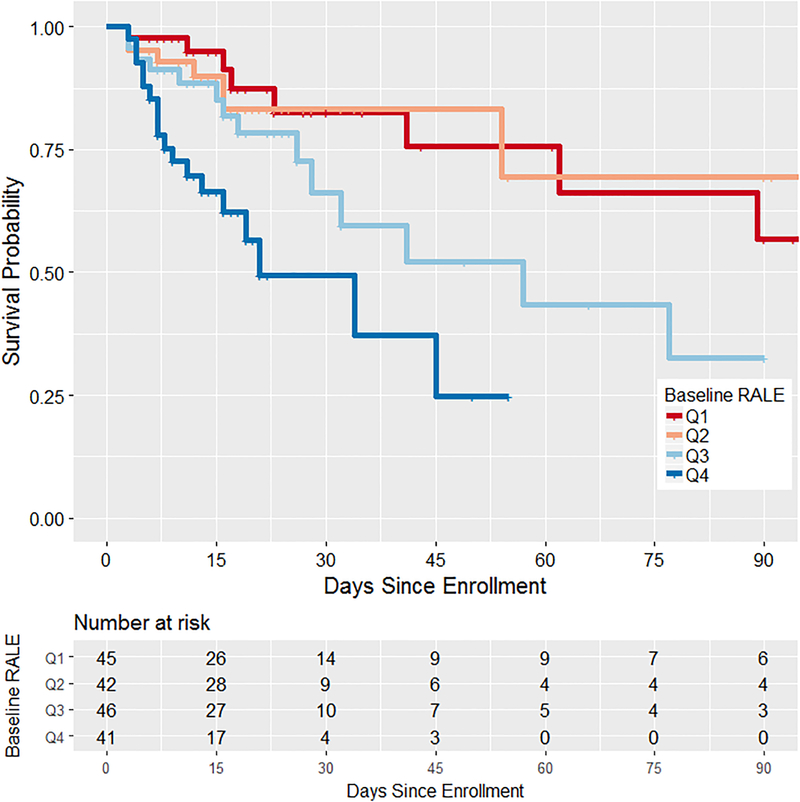
Lower baseline RALE score (reflecting less pulmonary edema) was independently associated with better oxygenation as reflected by higher PaO2/FiO2--for every 5-point decrease in RALE, the baseline PaO2/FiO2 increased by 8.4 mmHg (95% CI:3.2–13.5, p=0.002). Adjusting for age, gender and BMI did not attenuate the association (β=8.4, 95%CI:3.0–13.7, p=0.002, Supplemental Table 2). Lower baseline RALE score was also independently associated with better survival, in both unadjusted analysis (p< 0.001) and analysis controlling for age, gender, BMI, and APACHE III--with every 5-point decrease in RALE score, the adjusted hazard of death decreased by 16% (HR=0.84, 95%CI:0.72–0.99, p=0.032, Supplemental Table 3). Survival curves (Figure 4) show that compared with patients with a high baseline RALE score (in 3rd or 4th quartiles), those with lower baseline RALE score had better survival (p<0.001). Lower baseline RALE score was also associated with better 28-day (OR=0.76, 95%CI:0.62–0.92, p=0.006), and 60-day mortality (OR=0.74, 95%CI:0.61–0.89, p=0.002), in models controlling for age, gender, and BMI. These associations were no longer statistically significant after adjusting for APACHE III (OR=0.86, 95%CI:0.69–1.06, p=0.16 for 28-day, and OR=0.84, 95%CI:0.69–1.03, p=0.097 for 60-day mortality). For 28-day mortality, the area under the curve for mortality association with baseline RALE was 0.82 (95%CI: 0.74–0.90, p<0.001). Baseline RALE score was not associated with VFDs (p=0.59).
Figure 4.
Kaplan Meier estimates of ARDS survival stratified by the baseline RALE score for patients in FACTT. ARDS survival was significantly lower in patients with baseline RALE score above the median (3rd and 4th quartiles, p < 0.001). Quartile 1, Q1; Quartile 2; Q2, Quartile 3, Q3; Quartile 4, Q4.
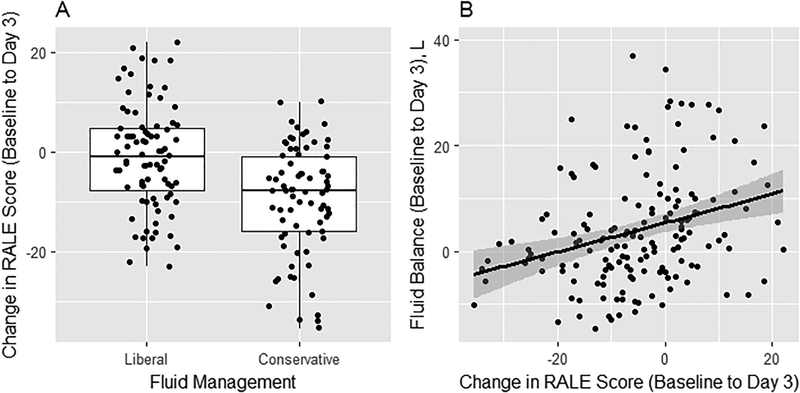
RALE scores at baseline were not significantly different between liberal and conservative fluid arms (Table 1). However, Day 3 RALE score was significantly lower in the conservative arm compared to the liberal arm (Table 1; p<0.001). From baseline to Day 3, the RALE score declined significantly more in the conservative fluid arm than the liberal arm (Table 1 and Figure 5A). This treatment effect remained significant in multivariable analysis (β=6.98, 95%CI:3.73–10.22, p<0.001). Furthermore, a decrease in RALE score from baseline to Day 3 was associated with lower cumulative fluid balance. Specifically, for every 5-point decrease in RALE score, cumulative fluid balance decreased by 0.85L (95%CI:0.18–1.53, p=0.01) (Figure 5B).
Figure 5.
The change in RALE score differed by treatment arm in FACTT, with a significant drop in score from enrollment to Day 3 in the conservative fluid management arm compared to the liberal fluid management arm (Panel A). This treatment effect remained significant in multivariable analysis (β=6.98, 95%CI:3.73–10.22, p<0.001). Independent of the treatment arm, the change in RALE score from enrollment to Day 3 was significantly associated with fluid balance (Panel B). Specifically, for every 5-point decrease in RALE score, cumulative fluid balance decreased by 0.85L (95%CI:0.18–1.53, p=0.01).
Discussion
The primary objectives of this study were to determine whether the chest radiograph can be systematically scored to quantify the severity of pulmonary edema and to determine whether this radiographic scoring is associated with the severity of ARDS and clinical outcomes. To this end, we developed the RALE score and tested it in deceased organ donors and in patients with ARDS.
In deceased organ donors, pre-procurement RALE scores correlated with total excised lung weights, evidence that the RALE score provides a quantitative index of lung edema. In patients with ARDS, there was a significant, independent correlation between higher baseline RALE score and lower PaO2/FiO2. The RALE score was also strongly and independently associated with ARDS outcomes including overall survival and 28-day and 90-day mortality. Calculation of the score is simple, and was robustly reproducible when independent readings were compared. Taken together, these findings indicate that the RALE score provides a clinically significant assessment of the extent of pulmonary edema in patients with ARDS that is reflected both by the severity of hypoxemia and by adverse clinical outcomes. Furthermore, the RALE score declined in response to treatment targeted at reducing pulmonary edema (the conservative fluid arm of FACTT), suggesting potential value both for assessment of therapeutic response and as a radiographic clinical outcome.
There are multiple potential applications for the RALE score in patients with ARDS both for patient care and for clinical research. RALE scores could be used for clinical identification of patients at highest risk of mortality, leading to earlier detection, intervention, and treatment of high-risk patients with ARDS. A similar approach could be used for risk stratification for enrollment of ARDS patients into therapeutic clinical trials. Assessment of ARDS severity has been recently used with some success to enrich clinical trials for the more severely ill,9, 10 but the only severity assessment used to date has been the PaO2/FiO2 ratio. Since chest radiographs are routinely obtained in patients with ARDS, use of RALE for risk stratification would not require additional testing or cost and could be done without delay in all patients, providing additional information about ARDS severity beyond that provided by the PaO2/FiO2 ratio. Serial assessment of RALE scores could be used to assess response to treatment clinically, or as an endpoint in clinical trials.
To our knowledge, apart from a prior study of a radiographic consolidation score that was used as a clinical outcome in the BOLD clinical trial in deceased organ donors,11 the current study is the only human study that compares a chest radiographic score to the quantitative assessment of pulmonary edema by direct measurement of lung weight. Several other studies comparing a simpler radiographic score to extravascular lung water (EVLW) measured by single- or double-indicator methods were limited in the ability to show reliable correlation, perhaps due to limitations of prior radiographic scoring methods.12–14 Both computerized axial tomography and ultrasound have been used to assess the presence and distribution of lung edema,15 although neither would be as practical as RALE scoring for daily assessment. However, it would be potentially valuable in the future to compare ultrasound assessments with RALE score since like chest radiography, lung ultrasound can be done non-invasively at the bedside. The only other semi-quantitative score that has been used to assess the severity of pulmonary edema on the chest radiograph in ARDS patients is the radiographic component of the Murray Lung Injury Score (LIS).16 However, the radiographic component of the LIS provides very little discrimination, only scoring each quadrant for the presence or absence of opacities. In a study of the prognostic value of the LIS in ARDS, the radiographic component of the LIS provided virtually no discrimination of outcomes compared to the other components of the LIS.17
This study has several strengths. First, in contrast to prior studies that estimated the extent of pulmonary edema by EVLW, we directly quantified the degree of pulmonary edema using total lung weight in donor lungs. Second, in FACTT, chest radiographs were independently scored by two clinician investigators to assess inter-observer reliability. The high level of agreement supports the potential applicability of the RALE score for clinicians at the bedside. Third, patients from FACTT originated from multiple different study sites and had a variety of underlying causes of ARDS, supporting broad applicability of the findings.
There are also some limitations. We were only able to study a subset of patients from the FACTT trial due to limited availability of chest radiographs. This limited our power to explore some aspects of the association between RALE score and ARDS severity and outcomes such as non-linear associations. Despite this, we found clinically meaningful associations between RALE score and oxygenation, clinical outcomes, and fluid management. Because the FACTT patients were derived from a clinical trial with highly selective inclusion and exclusion criteria, future studies of the RALE score in ARDS should include a broader population of patients to enhance generalizability. Trauma patients were underrepresented in the FACTT cohort, so the applicability of the current findings to trauma-associated ARDS is uncertain. Also, application of the RALE score may be challenging in patients who have extensive atelectasis, pleural effusions or morbid obesity. Furthermore, the level of positive end-expiratory pressure could affect the RALE score.18 Although PEEP levels were protocolized in FACTT, this is an important area for further study. Finally, it will be important to validate the RALE score in other clinical setting and data sets, and to further investigate the utility of the scoring system in comparison to lung ultrasonography and computed tomography imaging in the assessment of the extent of pulmonary edema.
CONCLUSIONS
Radiographic assessment of the extent of pulmonary edema using the RALE score correlates well with direct gravimetric assessment of pulmonary edema. In patients with ARDS, the RALE score was independently association with both ARDS severity, response to conservative fluid management, and clinical outcomes. Taken together, these findings suggest that the RALE score provides an innovative new method to leverage information that is already collected routinely in patients with ARDS to non-invasively assess both the extent of pulmonary edema and the severity of ARDS.
Supplementary Material
Key Messages:
What is the key question?
We asked whether the chest radiograph can be systematically scored to quantify the severity of pulmonary edema in ARDS and whether this radiographic score is associated with the severity of ARDS and clinical outcomes.
What is the bottom line?
The Radiographic Assessment of Lung Edema (RALE) score was independently associated with severity of ARDS as assessed by oxygenation and clinical outcomes including mortality.
Why read on?
The RALE score is a simple non-invasive measure that can be used to assess the severity of ARDS utilizing information that is already obtained routinely, safely, and inexpensively in every ARDS patient.
Acknowledgements:
We would like to acknowledge the BOLD study investigators and the NHLBI ARDSNet FACTT trial investigators for collection of the primary clinical data that was analyzed in this study.
Funding: Funding for this study was provided by NIH HL103836, R37 HL51856, HL131621, HL126671, Courtney’s Race for the ARDS Cure and the Courtney Charneco Family.
Footnotes
Competing Interests: Dr. Bastarache has received research funding from the National Institutes of Health and Global Blood Therapeutics. Dr. Rice has received consulting fees from Avisa Pharma, LLC and Cumberland Pharmaceuticals, Inc. Dr. Calfee has received research funding from the National Institutes of Health, the Food and Drug Administration, the Department of Defense, Bayer, and Glaxo Smith Kline. She has received consulting fees from GlaxoSmithKline, Boehringer Ingelheim, Bayer, Prometic, CSL Behring, and Roche/Genentech. Dr. Matthay has received research grants from Amgen and GlaxoSmithKline and has a current research grant from Bayer Pharmaceuticals for ARDS studies. He has received consultation fees from CSL Behring, Boehinger Ingelheim, Cerus Therapeutics, Quark Pharmaceuticals, Thesan Pharmaceuticals, and Bayer Pharmaceuticals. He was Chair of a DSMB for Asthma trials for Roche-Genentech (2013–2017). He also receives grant support for research and clinical trials from the NHLBI, FDA, and the Department of Defense. Dr. Ware has received research funding from the National Institutes of Health, Boehringer Ingelheim and Global Blood Therapeutics and consultant fees from CSLBehring. The other authors report no competing interests.
REFERENCES
- 1.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. The Journal of Clinical Investigation 2012;122(8):2731–40. doi: 10.1172/jci60331 [published Online First: 2012/08/02] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307(23):2526–33. doi: 10.1001/jama.2012.5669 [published Online First: 2012/07/17] [DOI] [PubMed] [Google Scholar]
- 3.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354(24):2564–75. [DOI] [PubMed] [Google Scholar]
- 4.Ware LB, Landeck M, Koyama T, et al. A randomized trial of the effects of nebulized albuterol on pulmonary edema in brain-dead organ donors. Am J Transplant 2014;14(3):621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staub NC. Pulmonary edema. Physiol Reviews 1974;54:678–811. [DOI] [PubMed] [Google Scholar]
- 6.Rice TW, Ware LB, Haponik EF, et al. Vascular pedicle width in acute lung injury: correlation with intravascular pressures and ability to discriminate fluid status. Crit Care 2011;15(2):R86. doi: 10.1186/cc10084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psych Methods 1996;1:30–46. [Google Scholar]
- 8.R: a language and environment for statistical computing [program]. Vienna, Austria: R Foundation for Statistical Computing, 2016. [Google Scholar]
- 9.Guerin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368(23):2159–68. doi: 10.1056/NEJMoa1214103 [DOI] [PubMed] [Google Scholar]
- 10.Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363(12):1107–16. doi: 10.1056/NEJMoa1005372 [DOI] [PubMed] [Google Scholar]
- 11.Ware LB, Neyrinck A, O’Neal HR, et al. Comparison of chest radiograph scoring to lung weight as a quantitative index of pulmonary edema in organ donors. Clinical transplantation 2012:65–71. doi: 10.1111/j.1399-0012.2011.01591.x [published Online First: 2012/02/14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sivak ED, Richmond BJ, O’Donavan PB, et al. Value of extravascular lung water measurement vs portable chest x-ray in the management of pulmonary edema. Crit Care Med 1983;11(7):498–501. [published Online First: 1983/07/01] [DOI] [PubMed] [Google Scholar]
- 13.Halperin BD, Feeley TW, Mihm FG, et al. Evaluation of the portable chest roentgenogram for quantitating extravascular lung water in critically ill adults. Chest 1985;88(5):649–52. [published Online First: 1985/11/01] [DOI] [PubMed] [Google Scholar]
- 14.Brown LM, Calfee CS, Howard JP, et al. Comparison of thermodilution measured extravascular lung water with chest radiographic assessment of pulmonary oedema in patients with acute lung injury. Ann Intensive Care 2013;3(1):25. doi: 10.1186/2110-5820-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellani G, Rouby JJ, Constantin JM, et al. Looking closer at acute respiratory distress syndrome: the role of advanced imaging techniques. Curr Opin Crit Care 2017;23(1):30–37. doi: 10.1097/mcc.0000000000000380 [DOI] [PubMed] [Google Scholar]
- 16.Murray JF, Matthay MA, Luce JM, et al. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988;138:720–23. [DOI] [PubMed] [Google Scholar]
- 17.Kangelaris KN, Calfee CS, May AK, et al. Is there still a role for the lung injury score in the era of the Berlin definition ARDS? Ann Intensive Care 2014;4(1):4. doi: 10.1186/2110-5820-4-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallet F, Delannoy B, Haquin A, et al. Evaluation of recruited lung volume at inspiratory plateau pressure with PEEP using bedside digital chest X-ray in patients with acute lung injury/ARDS. Respir Care 2013;58(3):416–23. doi: 10.4187/respcare.01893 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.