Abstract
Background
Electronic cigarette (EC) users may exhale large clouds of aerosol that can settle on indoor surfaces forming ECEAR (EC exhaled aerosol residue). Little is known about the chemical composition or buildup of this residue.
Objective
Our objective was to identify and quantify ECEAR chemicals in two field sites: an EC user’s living room and a multi-user EC vape shop.
Methods
We examined the buildup of ECEAR in commonly used materials (cotton, polyester, or terrycloth towel) placed inside the field sites. Materials were subjected to different lengths of exposure. Nicotine, nicotine alkaloids, and tobacco-specific nitrosamines (TSNAs) were identified and quantified in unexposed controls and field site samples using analytical chemical techniques.
Results
Nicotine and nicotine alkaloids were detected in materials inside the EC user’s living room. Concentrations of ECEAR chemicals remained relatively constant over the first 5 months, suggesting some removal of the chemicals by air flow in the room approximating a steady state. ECEAR chemicals were detected in materials inside the vape shop after 6 hours of exposure and levels continually increased over a month. By 1 month, the nicotine in the vape shop was 60 times higher than in the EC user’s living room. ECEAR chemical concentrations varied in different locations in the vape shop. Control fabrics had either no detectable or very low concentrations of chemicals.
Conclusions
In both field sites, chemicals from exhaled EC aerosols were deposited on indoor surfaces and accumulated over time forming ECEAR. Non-smokers, EC users, and employees of vape shops should be aware of this potential environmental hazard.
Keywords: Electronic Cigarette, Passive Exposure, Vape Shop, Nicotine, Environmental Hazard
1. Introduction
When electronic cigarette (EC) users exhale, the chemicals that are in the aerosol (nicotine, flavorings and solvents) settle on indoor surfaces where they accumulate and form EC exhaled aerosol residue (ECEAR). In a previous study, we showed that nicotine, other alkaloids, and tobacco specific nitrosamines (TSNAs) were transferred from a vape shop where they originated to an adjacent business, in a multiple-tenant retail building, where they formed ECEAR (Khachatoorian et al., 2018). Passive exposure to EC aerosols and ECEAR can occur through inhalation, dermal absorption or ingestion even after EC use has stopped.
The components of inhaled EC aerosols include nicotine, propylene glycol, glycerol, metals, particulate matter, ultra-fine particles, flavor chemicals, and volatile organic chemicals (VOCs) (Goniewicz et al., 2014; Hutzler et al., 2014; Kosmider et al., 2014; Logue et al., 2017; Schripp et al., 2013; Sleiman et al., 2016; Williams et al., 2017; 2013). The process of oxidation and/or dehydration of propylene glycol and glycerol creates several additional chemicals, such as formaldehyde, acetaldehyde and acrolein, which have also been reported in EC aerosols (Flora et al., 2016; Goniewicz et al., 2014; Jensen et al., 2015). TSNAs, some of which are known carcinogens, such as (4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N-nitosnornicotine (NNN)), are present in both some refill fluids (Kim and Shin, 2013) and EC aerosols (Goniewicz et al., 2014; McAuley et al., 2012).
While less is known about the composition of exhaled EC aerosol or secondhand aerosol, several studies have found glycerol, propylene glycol and nicotine, as well as PM2.5 particles and ultrafine particles in exhaled aerosol generated in laboratory settings, vaping conventions, and homes (Fernández et al., 2015; National Academies of Sciences, Engineering, and Medicine et al., 2018). Modeling exposures of participants using ECs inside a vaping bar indicated that ECs contributed to changes in formaldehyde, acetaldehyde, and acrolein levels, which exceeded governmental health hazard reference level limits (Logue et al., 2017). In general, the studies on exhaled EC aerosol have found that indoor air quality deteriorates in situations where vaping occurs.
However, there is very little information on the composition of ECEAR that is deposited on indoor surfaces where ECs are used. In a vape shop where ECEAR was evaluated with surface wipes, nicotine was not detected. In our opinion, this is probably because this particular shop had extremely thorough cleaning procedures, such as cleaning floors, counters, displays, and the bar each night with cleaning agents and bleach, and also had ventilation that included many air supply vents and an exhaust fan that was used during open hours (Zwack et al., 2017). In a laboratory study in which EC users exhaled into a chamber, surface wipes of nicotine were not significantly higher than baseline concentrations (Liu et al., 2017). However, a second laboratory study did find nicotine deposition on surface wipes from an exposure chamber (Goniewicz and Lee, 2015). A pilot study using surface wipes showed no significant differences in the amount of nicotine in EC users’ and non-users’ homes (Bush and Goniewicz, 2015).
The limited and inconsistent data on ECEAR and the lack of data from field sites indicate a clear need for more information on this topic. The purpose of this study was to determine if ECEAR chemicals could be detected and quantified in two field sites, a living room inside an EC user’s home and a typical vape shop with active EC use.
2. Material and methods
2.1. Collection of Fabrics-Living Room Field Site
The field site was a 187.5 ft2 (47.78 m3) living room inside a 4-bedroom family home in Riverside, CA built in 2005. The room was at the west side of the first floor adjacent to a guest bathroom and a bedroom. The room had adequate ventilation including a door into an adjacent bedroom, the front door around the corner, two windows, and a vent providing central cooling and forced air heating. The desk where fabrics were hung was next to a window.
Cotton and polyester fabrics (Jo-Anne Fabric and Craft Stores, Riverside, CA, USA) were placed in the field site for different exposure times. The area of 1 gram of cotton and polyester is 43.18 cm2 and 41.91cm2, respectively. Fabrics in the living room field site were collected at the end of each month after 1, 2, 3, 4, 5, and 6 months of exposure. Control fabrics were placed inside a non-smoker’s home for the same amount of time. After exposure, samples were placed in Ziploc® bags and brought to lab on dry ice and either extracted immediately or stored at −80°C in heat sealed, cut to size Mylar bags (ULINE, Pleasant Prairie, Wisconsin, USA). The EC user reported that there was no cigarette smoking inside the house.
2.2. Collection of Fabrics-Vape Shop Field Site
The vape shop was located in San Gabriel, California on a busy street with mostly car traffic. A glass supply store was adjacent to the shop. The vape shop was approximately 600 ft2 (317.9 m3). The store had glass windows facing the street and one entrance. The city of San Gabriel, CA, has prohibited smoking at and within a 25-foot buffer zone of all indoor and outdoor public places, which includes vape shops (http://www.ci.south-pasadena.ca.us/index.aspx?page=375, August 2018). However, vaping is allowed in vape shops. The vape shop we recruited specifically mentioned that they do not support the use of cigarettes and do not allow their use inside the store. Fig. 2A shows the layout of the vape shop. The shop lacks a ventilation system and does not have any system to replenish clean air. The only air circulation was an A/C unit located on the wall of the lounge area (Innova 18,000 BTU split type air conditioner). At its lowest and highest setting, the air conditioner circulated air at 5.7 and 6 cubic feet per hour, respectively. The door to the manager’s office remained closed during business hours. The entrance to the shop was only used when customers entered and exited. The store hours were Mondays to Sundays from 11:00 am to 12:00 am. In the evenings, there were usually 5–15 customers vaping in the shop.
Fig 2: Vape shop field site.
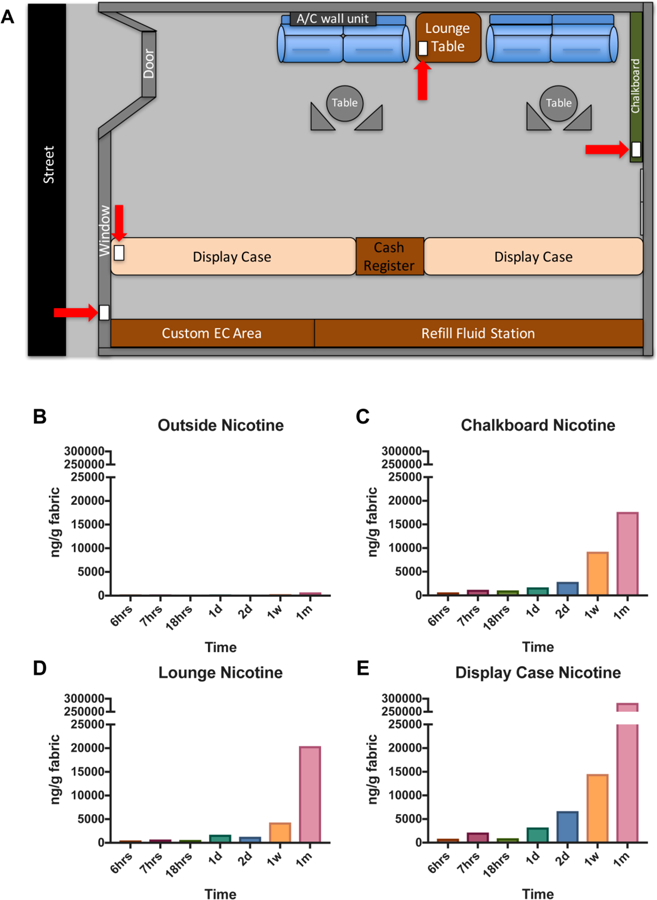
(A) Schematic of the vape shop field site showing placement of terrycloth towels (red arrows). (B-E) Concentrations of nicotine extracted from terrycloth towel that was placed inside the vape shop. The scale is from 0–300,000 ng/gram.
Terrycloth towels (Jo-Anne Fabric and Craft Stores, Riverside, CA, USA) were placed in the field site for different exposure times. The area of 1 gram of terrycloth towel is 26.125 cm2. Fabrics in the vape shop were collected after 6, 7, 18, 24, 48 hours, 1 week and 1 month. Control fabrics were placed outside the vape shop for the same amount of time as the indoor fabrics. After exposure, samples were placed in Ziploc® bags and brought to lab on dry ice and either extracted immediately or stored at −80°C in heat sealed, cut to size Mylar bags (ULINE, Pleasant Prairie, Wisconsin, USA).
2.3. Extraction of Nicotine and its Derivatives in ECEAR Fabrics
ECEAR was extracted from control and exposed samples of cotton, polyester, or terrycloth. Fabrics were cut into small pieces and immersed in cell culture medium consisting of Dulbecco’s Modified Eagle Medium (DMEM) (Invitrogen, Carlsbad, CA) as described in (Bahl et al., 2016; Sarker et al., 2014, Khachatoorian et al., 2018) at a concentration of 0.05 g of fabric/ml of medium. The extracts were aliquoted into 1.5 ml vials for storage at −80 °C and later shipment on dry ice to the Clinical Pharmacology Laboratory at the University of California at San Francisco.
2.4. Chemical Analysis of Nicotine and its Derivatives in ECEAR Fabrics
The quantification of nicotine, nicotine derivatives, and TSNAs was done by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) at the University of California, San Francisco as described previously in detail (Bahl et al., 2014; Hang et al., 2013; Whitehead et al., 2015). The limits of quantification for each chemical was as follows: nicotine = 2 ng/ml, cotinine = 1 ng/ml, n-formylnornicotine = 1 ng/ml, myosmine = 1 ng/ml, NNA (1-(N-methyl-N-nitrosamino)-1-(3-pyridinyl)-4-butanal) = 1 ng/ml.
3. Results
3.1. Living Room Field Site
Fig. 1 shows the six sets of cotton and polyester fabrics hung across the bottom shelf of a bookcase above the computer. The individual started using an Innokin iTaste MVP with an Aspire Nautilus tank in July and changed to a Wotofo ZNA 30 clone by A-mod Technology Co., LTD with an Aspire Nautilus tank in late September. Usage was at 1.9 ohms, 4.0 volts and 8.4 watts. Refill fluids used over the 6 month period are shown in Supplementary Table 1. All refill fluids had a nicotine concentration of 6mg/mL. We asked the user to log the number of puffs when using the EC. The average puff count per day (Fig. 1B) was similar each month; however, the Total Puff count per month (Fig. 1C) was lower during month 6 when the desk was only used for 2 weeks. The user puffed about 3 hours a day and averaged about 15 days of puffing per month.
Fig 1: Living room field site.
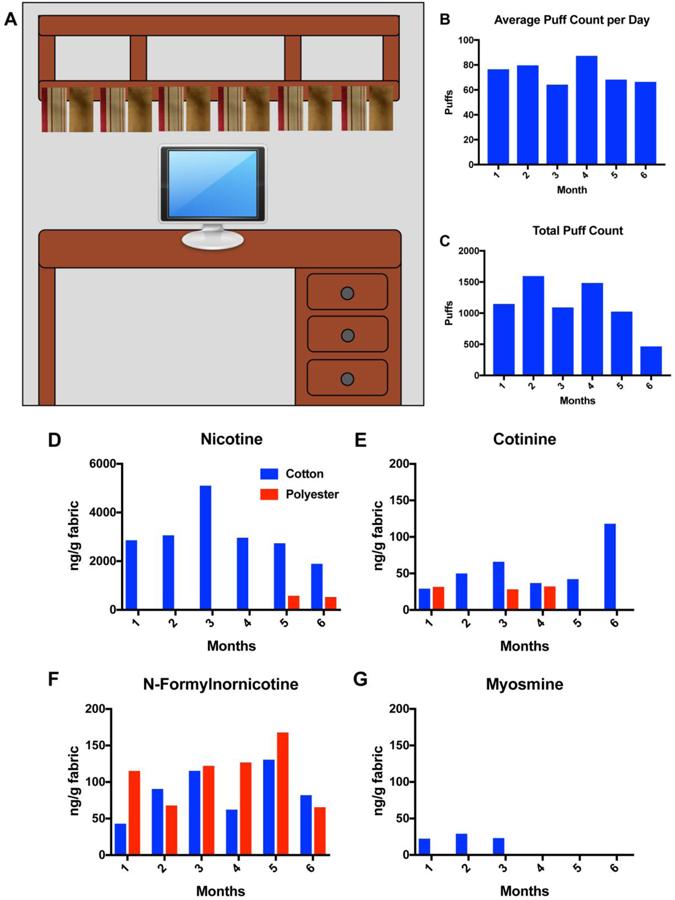
(A) Schematic of six sets of cotton and polyester fabrics hung from a shelf on a desk where EC were used. (B) Average puff count/day for each month over 6 months. The average number of days that the user puffed was 15 days each month. (C) Total puff count for each month over 6 months. Concentrations of nicotine (D), cotinine (E), n-formylnornicotine (F), and myosmine (G) extracted from cotton and polyester fabrics hung inside the living room of an EC user. The scale for Figure D is 0–6000 ng/gram of fabric and the scale for Figure E-G is 0–200 ng/gram of fabric. Cotton – blue. Polyester – red.
3.2. Nicotine and Other Alkaloids Detected in Living Room ECEAR Samples
Nicotine and other minor alkaloids were detected in ECEAR extracts of cotton and polyester from the home of an individual EC user (Fig. 1 D–G). Nicotine was the most abundant marker of ECEAR contamination, and its highest concentration (3 months) was 5,100 ng/gram of cotton fabric (Fig. 1D). During other months, nicotine concentrations varied between 2000 and 3000 ng of nicotine/g of cotton, while nicotine was only detectable in polyester samples after 5 and 6 months of exposure. Cotinine was detected in cotton samples during all months and on polyester during the 1st, 3rd, and 4th month (Fig. 1E). N-Formylnornicotine was detected on both cotton and polyester samples for all exposure times (Fig. 1F). The quantities of N-formyl-nornicotine were similar for both types of fabric, unlike the results with nicotine. Myosmine was only detected in the first 3 months of exposures on cotton fabric (Fig. 1G).
3.3. Vape Shop Field Site
Terrycloth towels were placed on the display case towards the front of the shop, on the lounge table between the two couches, and hung on the chalkboard towards the back of the store (red arrows in Fig. 2A). Control samples were hung up outside on the window of the vape shop facing the street. Supplementary Table 2 lists the day of the week, length of exposure, and placement of each sample inside the vape shop. The display area was composed of three separate display cases extending from the front to the back of the store. Display cases contained different types of ECs, batteries, tanks, and mouth pieces. Refill fluids were stored on the wall near the display cases. The chalkboard was located towards the back of the store near the manager’s office. There were usually two employees working at any given time of the day, and the manager was usually in his office.
3.4. Nicotine Detected in Vape Shop Field Site ECEAR Samples
Outside control samples had very low levels of nicotine after exposure for 6 hrs, 7 hrs, 1 day, 1 week and 1 month. The latter had the highest nicotine at 719 ng/gram of fabric (Fig. 2B). Samples placed towards the back of the vape shop on the chalkboard had increasing amounts of nicotine as exposure times increased. The 6 hr. exposure had 649 ng of nicotine/gram of terrycloth towel and reached 17,655 ng of nicotine/gram of terrycloth towel at 1 month of exposure (Fig. 2C). The fabrics exposed in the lounge area had a similar result starting at 516 ng of nicotine/gram of terrycloth towel at 6 hrs. of exposure and reaching 20,447 ng of nicotine/gram of terrycloth towel at 1 month of exposure (Fig. 2D). The terrycloth towel placed at the display case toward the front of the vape shop had the highest nicotine concentration starting at 839 ng of nicotine/gram of terrycloth towel at 6 hrs. of exposure and increasing to 283,775 ng of nicotine/gram of terrycloth towel at 1 month of exposure (Fig. 2E).
3.5. Cotinine, N-Formylnornicotine and NNA Detected in Vape Shop Field Site ECEAR Samples
Cotinine was not detected in outside control samples (Fig. 3A), while the chalkboard and lounge areas had detectable levels at 1 month (175 and 209 ng of cotinine/gram of terrycloth towel, respectively) (Fig. 3B-D). N-Formylnornicotine was detected at very low levels in the 1 month outside control fabric (Fig. 3E) but was detected in the 1-day lounge sample (40 ng/gram of fabric). Its concentration increased in later samples, reaching a high of 1,912 ng/gram of fabric for the display case sample (Fig. 3F-H). 1-(N-methyl-N-nitrosamino)-1-(3-pyridinyl)-4-butanal (NNA) was not detected in outside control fabrics (Fig. 3I) nor in the lounge fabrics (Fig. 3K) but was present in the 1-month chalkboard sample at 22 ng/gram of terrycloth and the display case sample at 67 ng/gram of terrycloth (Fig. 3J, L).
Fig 3: Concentrations of cotinine, n-formylnornicotine, and NNA in terrycloth towels from the vape shop field site.
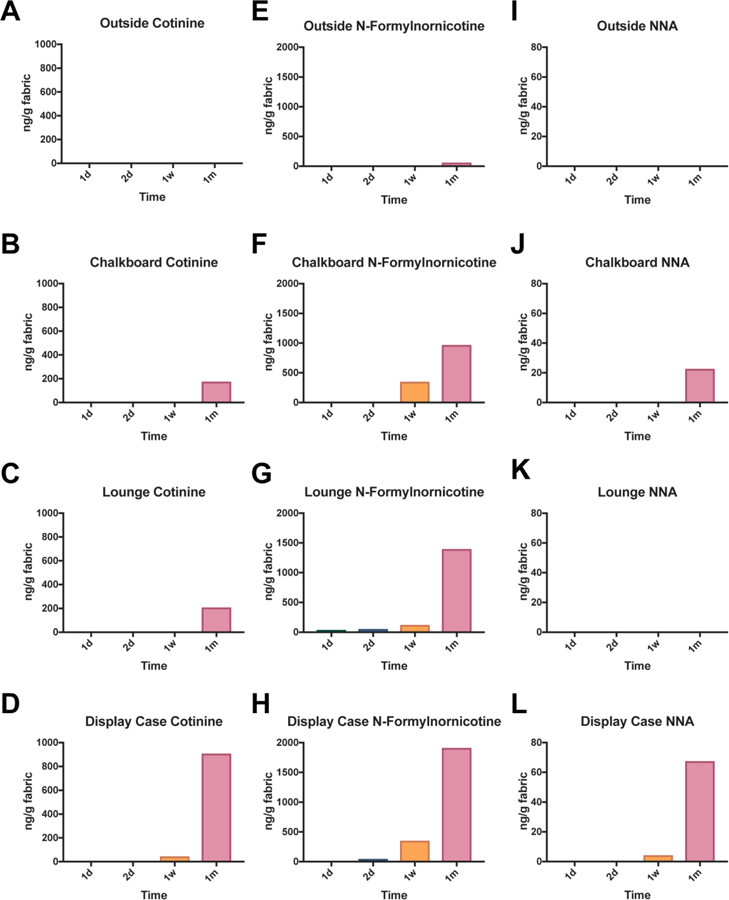
Terrycloth placed outside (A, E, I), on the chalkboard (B, F, J), in the lounge (C, G, K), and on the display case (D, H, L) inside the vape shop had detectable and quantifiable levels of nicotine related alkaloids and a TSNA, NNA. The scale for Figure A-D is 0–1000 ng/gram, E-H is 0–2000 ng/gram, and I-L is 0–80 ng/gram.
4. Discussion
This is the first study to compare ECEAR inside a vape shop and an EC user’s living room. Nicotine and related chemicals were detected in both sites; however, chemical concentrations were higher in the vape shop where they increased over time. Our sampling method allowed us to evaluate the buildup of ECEAR. The kinetics of chemical deposition were different in the two sites, with the ECEAR chemicals showing steady state concentrations in the living room, while the vape shop chemicals continually increased in concentration. This difference may be related to the shorter length of time over which vape shop samples were collected or due to better ventilation in the living room field site.
Although it has been reported that EC users retain 94% of the inhaled nicotine (St Helen et al., 2016), we were able to detect a buildup of nicotine in the living room field site where there was a single EC user. During months 1–5, nicotine concentration in ECEAR did not increase with increased EC use, probably because air circulation in this site removed much of the exhaled vapor before it settled, creating a steady state concentration. In the 6th month, the user puffed only 7 days, instead of the usual 15, and the concentration of nicotine was half the amount detected in the prior months. While most chemicals in the living room did not increase in concentration over time, cotinine had a higher concentration at 6 months when there was less puffing. This may have occurred because of oxidation of nicotine in the ECEAR during aging (Goniewicz et al., 2014; Sleiman et al., 2010b). Although a higher ventilation rate results in faster nicotine reduction in indoor air (Rostami et al., 2018), our data show that in a modern home with central air conditioning, nicotine in exhaled EC aerosol accumulates on surfaces where it is likely converted into other alkaloids during aging.
In the living room, nicotine was detected in every cotton sample in contrast to polyester which had no detectable nicotine until the last 2 months of sampling. Others have shown that nicotine is readily removed from cotton but not from polyester (Bahl et al., 2014; Destaillats et al., 2006). This preferential removal from cotton was not observed for all chemicals. For example, n-formylnornicotine was extracted equally efficiently from both cotton and polyester. This may be due to the higher polarity and solubility of N-formylnornicotine, which allows for it to readily extract from a non-polar, hydrophobic material such as polyester. Nicotine has significant lipophilicity and may stay adsorbed on the polyester and not be readily extracted by the aqueous media. These data suggest that re-emission of nicotine into the air can occur more readily from cotton than from polyester, but this does not necessarily hold for all ECEAR chemicals.
In contrast to the living room, the concentration of ECEAR chemicals increased over time inside the vape shop. This could be due to the large amount of aerosol produced by multiple users and the limited circulation of air in the shop. Maximum nicotine levels in the vape shop were about 60 times higher than those in the living room field site (~300,000 ng/gram of fabric in the vape shop versus ~ 5,000 ng/gram of fabric in the living room). The continual buildup of ECEAR chemicals in the vape shop would likely have continued if fabrics were left for periods longer than 1 month. If accumulation of nicotine continued at the same rate over 1 year, its concentration in ECEAR would be approximately 3.6 mg/gram of fabric.
The concentration of ECEAR chemicals varied in the three sampling locations in the vape shop. Highest concentrations were found in the display case samples, which were located in the area of highest vaping by both the customers and employees. Concentrations in the other two sites were about 15 times lower than near the display case. These data show that ECEAR is not evenly distributed within the vape shop and that even in a relatively small field site with poor ventilation, concentrations of nicotine varied significantly. The nicotine accumulation in the vape shop was due to vaping since the outside (control) fabrics contained either no or very low concentrations of nicotine and other ECEAR chemicals.
NNA, a TSNA, was detected in ECEAR and is of interest because it can form from nicotine in the environment (Jacob et al., 2017). NNA is rarely found in tobacco or tobacco smoke, probably because it is too unstable to survive the relatively harsh conditions of tobacco curing and combustion. But under milder conditions, nicotine on surfaces can react with nitrous acid in the environment to form NNA, as well as NNK and NNN (Sleiman et al., 2010a). Our data suggest that chemical reactions occur in ECEAR leading to the formation of TSNAs, such as NNA.
ECEAR concentrations can be affected by ventilation and cleanliness. The vape shop in our study did not have a dedicated exhaust and good ventilation which most likely lead to the continual buildup of ECEAR. In contrast, surface wipes from a vape shop with daily cleaning and air circulation contained no nicotine, although the limit of quantification was not given in the study (Zwack et al., 2017). This particular vape shop shows that ECEAR levels can be reduced by ventilation and cleaning procedures. To mitigate the buildup of ECEAR, regulatory agencies could require specific air exchanges in vape shops to clear exhaled aerosol and reduce ECEAR levels. Currently, the American Society of Heating, Refrigerating, and Air-Conditioning Engineers (ASHRAE) has no standard or guideline for air exchange inside of vape shops (American Society of Heating, 2016), although there are standards for retail stores and cocktail bars. Owners could also establish daily cleaning protocols to further limit the accumulation of ECEAR.
Nicotine was the dominant chemical in our field site ECEAR samples. This is of concern because nicotine is water and lipid soluble, and readily permeates into skin (Maina et al., 2017; 2016), where it can accumulate and be released into the blood after exposure (Bekö et al., 2017). Nicotine can cause dermatitis, irritation, and skin sensitization in humans (Berlin et al., 2014; Berner et al., 1990; Bircher et al., 1991; Gourlay et al., 1999; Greenland et al., 1998). Patients using transdermal nicotine therapy have reported erythema, rash, pruritis, irritation and edema (Gourlay et al., 1999), and in several studies of nicotine patches, significant increases in dizziness, nausea and sleep disorders were reported (Greenland et al., 1998). It is interesting that the main symptoms associated with patch users are all symptoms frequently reported by EC users in online websites (Hua and Talbot, 2016). Although ECEAR uptake through the skin and other routes has not yet been analyzed, the data with patches and EC user reported health effects are consistent with the idea that ECEAR could cause skin and systemic responses. It will be important in future studies to determine how much exposure and uptake of EC chemicals occur in both EC users and non-users in vape shops or other indoor environments where EC are used. Such uptake could be due to both the aerosol and ECEAR.
Indoor settings where ECs are used could cause involuntary inhalation, ingestion, or dermal uptake of EC aerosols and ECEAR chemicals. This is especially true for employees and customers inside a vape shop and family members inside a home where ECs are used. Most worrisome is the exposure that toddlers and small children would receive in a home with ECEAR due to their frequent mouthing of fabrics and touching surfaces and floors. The FDA currently prohibits the sale of EC products to minors under the age of 18, but there are no stringent regulations concerning the age limit of patrons inside vape shops (Food and Drug Administration, HHS, 2016). California business and professions code #22962C prohibits anyone under the age of 18 from entering a vape shop unless accompanied by a parent or guardian, but there is no “enforcement” agency for this law (http://leginfo.legislature.ca.gov, September 2018). Federal and state governmental agencies may consider tightening and restricting access to vape shops to adults 21 and over only.
In the vape shop, we detected the equivalent of approximately 108 mg of nicotine/m2 after 1 month of accumulation. This is considerably higher than the nicotine concentration found in surface wipes from a smokers living room (73 µg/m2) (Matt et al., 2004). Even in our living room field site, the concentration of nicotine (1,181 µg/m2) in the 3 month cotton sample was considerably higher than in the smoker’s home. Our data may be higher since we left fabric in the field sites, while wipes were taken in the smoker’s home.
Our study was based on one home and one vape shop. Other field sites may produce different concentrations of chemicals in ECEAR depending on the amount of use, the type of EC, its nicotine content, topography of the users, cleaning protocols, and ventilation (Zwack et al., 2017; Maloney et al., 2016). In addition, our samples were collected over a relatively short period of time. Most indoor facilities and homes accumulate ECEAR over much longer times, suggesting that concentrations of nicotine and TSNAs could be higher than reported in our study.
Conclusion
In summary, this study shows that nicotine and other chemicals in exhaled EC aerosol can build up on indoor surfaces where they could result in exposure of EC users and non-smokers through dermal contact, inhalation, or ingestion. Further research on ECEAR deposition is needed to provide a broader perspective on ECEAR levels in indoor environments. Employees, whether they are EC users or not, who work long hours inside vape shops are exposed to ECEAR and EC aerosols, which may be an occupational health hazard. Given the accumulation of nicotine and related chemicals in ECEAR, it is important for government agencies to look closely into age restrictions inside vape shops and the health effects of ECEAR on exposed populations.
Supplementary Material
Highlights.
ECEAR was detected and quantified in an EC user’s living room and vape shop
ECEAR contained mainly nicotine, plus other alkaloids and TSNAs
In the well-ventilated living room, nicotine levels were constant over 5 months
In the vape shop ECEAR was detected after 6 hours and continually built up over time
Unwanted exposure to ECEAR chemicals could occur in environments where ECs are used
Acknowledgments
We thank Olivia Yturralde and Polly Cheung for their help in analyzing samples.
Funding
This research was supported by the Tobacco-Related Disease Research Program of California (TRDRP) [26IR-0018, 2017]; the National Institute on Drug Abuse [P30 DA012393]; the National Center for Research Resources [S10 RR026437]. The content is solely the responsibility of the authors and does not necessarily represent the official views of TRDRP, NIH, or other granting agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests
All authors declare that they have no actual or potential competing financial interest.
Patient consent
The project did not involve humans.
Data sharing statement
All of the relevant data are included in the manuscript.
REFERENCES
- American Society of Heating, Refrigerating and Air-Conditioning Engineers: ANSI/ASHRAE Standard 62.1–2016 – Ventilation for Acceptable Indoor Air Quality Atlanta, Ga.: ASHRAE, 2016, ISSN: 1041–2336. [Google Scholar]
- Bahl V, Jacob P, Havel C, Schick SF, Talbot P, 2014. Thirdhand Cigarette Smoke: Factors Affecting Exposure and Remediation. PLoS ONE 9, e108258–9. 10.1371/journal.pone.0108258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl V, Weng NJH, Schick SF, Sleiman M, Whitehead J, Ibarra A, Talbot P, 2016. Cytotoxicity of Thirdhand Smoke and Identification of Acrolein as a Volatile Thirdhand Smoke Chemical That Inhibits Cell Proliferation. Toxicol. Sci 150, 234–246. 10.1093/toxsci/kfv327 [DOI] [PubMed] [Google Scholar]
- Bekö G, Morrison G, Weschler CJ, Koch HM, Pälmke C, Salthammer T, Schripp T, Eftekhari A, Toftum J, Clausen G, 2017. Dermal uptake of nicotine from air and clothing: Experimental verification. Indoor Air 28, 247–257. 10.1111/ina.12437 [DOI] [PubMed] [Google Scholar]
- Berlin I, Grange G, Jacob N, Tanguy ML, 2014. Nicotine patches in pregnant smokers: randomised, placebo controlled, multicentre trial of efficacy. BMJ 348, g1622–g1622. 10.1136/bmj.g1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner B, Wilson DR, Steffens RJ, Mazzenga GC, Hinz R, Guy RH, Maibach HI, 1990. The Relationship between pKa and Skin Irritation for a Series of Basic Penetrants in Man. Fundam. Appl. Toxicol 760–766. [DOI] [PubMed]
- Bircher AJ, Howald H, Rufli T, 1991. Adverse skin reactions to nicotine in a transdermal therapeutic system. Contact Derm 25, 230–236. [DOI] [PubMed] [Google Scholar]
- Bush D, Goniewicz ML, 2015. A pilot study on nicotine residues in houses of electronic cigarette users, tobacco smokers, and non-users of nicotine-containing products. Int. J. Drug Policy 26, 609–611. 10.1016/j.drugpo.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaillats H, Singer BC, Lee SK, Gundel LA, 2006. Effect of ozone on nicotine desorption from model surfaces: evidence for heterogeneous chemistry. Environ. Sci. Technol 40, 1799–1805. [DOI] [PubMed] [Google Scholar]
- Fernández E, Ballbè M, Sureda X, Fu M, Saltó E, Martínez-Sánchez JM, 2015. Particulate Matter from Electronic Cigarettes and Conventional Cigarettes: a Systematic Review and Observational Study. Curr Envir Health Rpt 2, 423–429. 10.1007/s40572-015-0072-x [DOI] [PubMed] [Google Scholar]
- Flora JW, Meruva N, Huang CB, Wilkinson CT, Ballentine R, Smith DC, Werley MS, McKinney WJ, 2016. Characterization of potential impurities and degradation products in electronic cigarette formulations and aerosols. Regulatory Toxicology and Pharmacology 74, 1–11. 10.1016/j.yrtph.2015.11.009 [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration, HHS, 2016. Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products. Final rule. Fed Regist 81, 28973–29106. [PubMed] [Google Scholar]
- Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P, Benowitz N, 2014. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 23, 133–139. 10.1136/tobaccocontrol-2012-050859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Lee L, 2015. Electronic cigarettes are a source of thirdhand exposure to nicotine. Nicotine Tob Res 17, 256–258. 10.1093/ntr/ntu152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlay SG, Forbes A, Marriner T, McNeil JJ, 1999. Predictors and Timing of Adverse Experiences During Transdermal Nicotine Therapy. Drug Saf 20, 545–555. 10.2165/00002018-199920060-00007 [DOI] [PubMed] [Google Scholar]
- Greenland S, Satterfield MH, Lanes SF, 1998. A meta-analysis to assess the incidence of adverse effects associated with the transdermal nicotine patch. Drug Saf 18, 297–308. 10.2165/00002018-199818040-00005 [DOI] [PubMed] [Google Scholar]
- Hang B, Sarker AH, Havel C, Saha S, Hazra TK, Schick S, Jacob P, Rehan VK, Chenna A, Sharan D, Sleiman M, Destaillats H, Gundel LA, 2013. Thirdhand smoke causes DNA damage in human cells. Mutagenesis 28, 381–391. 10.1093/mutage/get013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua M, Talbot P, 2016. Potential health effects of electronic cigarettes: A systematic review of case reports. Prev Med Rep 4, 169–178. 10.1016/j.pmedr.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutzler C, Paschke M, Kruschinski S, Henkler F, Hahn J, Luch A, 2014. Chemical hazards present in liquids and vapors of electronic cigarettes. Arch Toxicol 88, 1295–1308. 10.1007/s00204-014-1294-7 [DOI] [PubMed] [Google Scholar]
- Jacob P III, Benowitz NL, Destaillats H, Gundel L, Hang B, Martins-Green M, Matt GE, Quintana PJE, Samet JM, Schick SF, Talbot P, Aquilina NJ, Hovell MF, Mao J-H, Whitehead TP, 2017. Thirdhand Smoke: New Evidence, Challenges, and Future Directions. Chem. Res. Toxicol 30, 270–294. 10.1021/acs.chemrestox.6b00343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH, 2015. Hidden Formaldehyde in E-Cigarette Aerosols. N Engl J Med 372, 389–392. 10.1056/nejmc1413069 [DOI] [PubMed] [Google Scholar]
- Khachatoorian C, Jacob P III, Benowitz NL, Talbot P, 2018. Electronic cigarette chemicals transfer from a vape shop to a nearby business in a multiple-tenant retail building. Tob Control 10.1136/tobaccocontrol-2018-054316 [DOI] [PMC free article] [PubMed]
- Kim H-J, Shin H-S, 2013. Determination of tobacco-specific nitrosamines in replacement liquids of electronic cigarettes by liquid chromatography-tandem mass spectrometry. Journal of Chromatography A 1291, 48–55. 10.1016/j.chroma.2013.03.035 [DOI] [PubMed] [Google Scholar]
- Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, Goniewicz ML, 2014. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res 16, 1319–1326. 10.1093/ntr/ntu078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liang Q, Oldham MJ, Rostami AA, Wagner KA, Gillman IG, Patel P, Savioz R, Sarkar M, 2017. Determination of Selected Chemical Levels in Room Air and on Surfaces after the Use of Cartridge- and Tank-Based E-Vapor Products or Conventional Cigarettes. Int. J. Environ. Res. Publ. Health 14, 969 10.3390/ijerph14090969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue JM, Sleiman M, Montesinos VN, Russell ML, Litter MI, Benowitz NL, Gundel LA, Destaillats H, 2017. Emissions from Electronic Cigarettes: Assessing Vapers’ Intake of Toxic Compounds, Secondhand Exposures, and the Associated Health Impacts. Environ. Sci. Technol 51, 9271–9279. 10.1021/acs.est.7b00710 [DOI] [PubMed] [Google Scholar]
- Maina G, Castagnoli C, Ghione G, Passini V, Adami G, Filon FL, Crosera M, 2017. Skin contamination as pathway for nicotine intoxication in vapers. Toxicology in Vitro 41, 102–105. 10.1016/j.tiv.2017.02.022 [DOI] [PubMed] [Google Scholar]
- Maina G, Castagnoli C, Passini V, Crosera M, Adami G, Mauro M, Filon FL, 2016. Transdermal nicotine absorption handling e-cigarette refill liquids. Regul. Toxicol. Pharmacol 74, 31–33. 10.1016/j.yrtph.2015.11.014 [DOI] [PubMed] [Google Scholar]
- Maloney JC, Thompson MK, Oldham MJ, Stiff CL, Lilly PD, Patskan GP, Shafer KH, Sarkar MA (2016). Insights from two industrial hygiene pilot e-cigarette passive vaping studies, Journal of Occupational & Environmental Hygiene, 13(4):279–87. 10.1080/15459624.2015.1116693 [DOI] [PubMed] [Google Scholar]
- Matt GE, Quintana PJE, Hovell MF, Bernert JT, Song S, Novianti N, Juarez T, Floro J, Gehrman C, Garcia M, Larson S, 2004. Households contaminated by environmental tobacco smoke: sources of infant exposures. Tob Control 13, 29–37. 10.1136/tc.2003.003889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley TR, Hopke PK, Zhao J, Babaian S, 2012. Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhalation Toxicology 24, 850–857. 10.3109/08958378.2012.724728 [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems, Eaton DL, Kwan LY, Stratton K, 2018. Public Health Consequences of E-Cigarettes 10.17226/24952 [DOI]
- Rostami AA, Agyemang S, Pithawalla YB, 2018. A distributed computational model for estimating room air level of constituents due to aerosol emission from e-vapor product use. Food and Chemical Toxicology 116, 114–128. 10.1016/j.fct.2018.04.020 [DOI] [PubMed] [Google Scholar]
- Sarker AH, Chatterjee A, Williams M, Lin S, Havel C, Jacob P, Boldogh I, Hazra TK, Talbot P, Hang B, 2014. NEIL2 protects against oxidative DNA damage induced by sidestream smoke in human cells. PLoS ONE 9, e90261 10.1371/journal.pone.0090261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schripp T, Markewitz D, Uhde E, Salthammer T, 2013. Does e-cigarette consumption cause passive vaping? Indoor Air 23, 25–31. 10.1111/j.1600-0668.2012.00792.x [DOI] [PubMed] [Google Scholar]
- Sleiman M, Destaillats H, Smith JD, Liu C-L, Ahmed M, Wilson KR, Gundel LA, 2010a. Secondary organic aerosol formation from ozone-initiated reactions with nicotine and secondhand tobacco smoke. Atmospheric Environment 44, 4191–4198. 10.1016/j.atmosenv.2010.07.023 [DOI] [Google Scholar]
- Sleiman M, Gundel LA, Pankow JF, Jacob P, Singer BC, Destaillats H, 2010b. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proc. Natl. Acad. Sci. U.S.A 107, 6576–6581. 10.1073/pnas.0912820107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleiman M, Logue JM, Montesinos VN, Russell ML, Litter MI, Gundel LA, Destaillats H, 2016. Emissions from Electronic Cigarettes: Key Parameters Affecting the Release of Harmful Chemicals. Environ. Sci. Technol 50, 9644–9651. 10.1021/acs.est.6b01741 [DOI] [PubMed] [Google Scholar]
- St Helen G, Ross KC, Dempsey DA, Havel CM, Jacob P, Benowitz NL, 2016. Nicotine Delivery and Vaping Behavior During ad Libitum E-cigarette Access. Tob Regul Sci 2, 363–376. 10.18001/TRS.2.4.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead TP, Havel C, Metayer C, Benowitz NL, Jacob P, 2015. Tobacco alkaloids and tobacco-specific nitrosamines in dust from homes of smokeless tobacco users, active smokers, and nontobacco users. Chem. Res. Toxicol 28, 1007–1014. 10.1021/acs.chemrestox.5b00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Bozhilov K, Ghai S, Talbot P, 2017. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS ONE 12, e0175430–24. 10.1371/journal.pone.0175430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P, 2013. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS ONE 8, e57987 10.1371/journal.pone.0057987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwack LM, Stefaniak AB, LeBouf RF, 2017. Evaluation of chemical exposures at a vape shop, Health, Hazard Evaluation Report, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Health Hazard Evaluation Report 2015-0107-3279, http://www.cdc.gov/niosh/hhe/reports/pdfs/2015-0107-3279.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


