Abstract
Background:
Abuse of psychostimulants, including methamphetamine (MA), has been linked to heightened impulsivity. While previous research has demonstrated differences in impulsivity between MA users and non-substance users, less is known about variability in impulsivity within MA users and whether the severity of MA use related problems predicts impulsivity within individuals who regularly use MA. This study aims to elucidate the relationship between impulsivity and MA use severity.
Method:
Non-treatment seeking individuals who reported regular MA use (n = 177) completed an impulsivity battery comprising self-report and behavioral measures. A structural equation modeling (SEM) approach was used to test the relationship between the MA use related problem severity and measures of impulsivity.
Results:
The final SEM model of impulsivity and MA use related problems (CFI= 0.897, RMSEA= 0.059, S-B scaled χ2 [260, n = 103] = 406.86) revealed that greater MA use severity was associated with greater self-reported impulsiveness, but no relationship was found between MA use severity and behavioral measures of impulsivity.
Conclusions:
The current findings extend previous research by providing additional evidence that MA use is associated with increased self-reported impulsivity and highlights the importance of evaluating impulsivity as a multidimensional construct.
Keywords: impulsivity, methamphetamine (MA) use
1. Introduction
Methamphetamine (MA) is one of the fastest growing illicit drugs in the world, with approximately 1.2 million past year users (Substance Abuse and Mental Health Services Administration, 2013). MA use also represents a major public health concern (Rawson and Condon, 2007) and has been associated with a host of negative public health outcomes such as decreased quality of life, serious health issues, psychiatric comorbidity, risky sexual behavior, and impairments in daily functioning (Henry et al., 2010; Shoptaw et al., 2003; Walter et al., 2012).
While MA use disorder is a complex diagnostic phenotype, there has been increased interest in the effect of MA use on neurocognitive function. The preponderance of the data appears to support the possibility that MA abuse causes cognitive decline in at least some users of the drug (Dean et al., 2013). The relationship between MA use and impulsivity in particular is of great interest, as impulsivity has been advanced as a critical component of multiple forms of addiction (Goldstein and Volkow, 2002; Jentsch and Taylor, 1999). In fact, the DSM V outlines failure to control one’s impulses for MA despite negative consequences as a diagnostic criterion of MA use disorder, suggesting that MA abusers may have generalized problems with impulse control.
The construct of impulsivity is traditionally defined as acting suddenly and without plan to satisfy an immediate desire (Kreek et al., 2005). It is considered to be multidimensional (De Wit, 2009; Fernie et al., 2010) and can be examined through self-report measures, and by way of several narrower constructs including, but not limited to, response inhibition and delay discounting. Response inhibition refers to an individual’s ability to inhibit his/her thoughts or behaviors, whereas delay discounting involves the individual’s tendency to devalue a reward as time increases before the reward becomes attainable (see review by Jentsch and Taylor, 1999).
In theory, difficulties with response inhibition and failing to value rewards in the future may explain continued drug use despite negative consequences and relapse during periods of abstinence. In a meta-analysis examining delay discounting across the addiction literature, MacKillop and colleagues (2011) found that clinical samples had significantly larger effect sizes (d = .61) than did non-clinical samples (d = .45). These findings suggest that individuals with a substance use disorder have greater delay discounting than non-substance users. Likewise, deficits in response inhibition among individuals who abuse substances compared to non-drug using controls have been found (e.g., Fillmore and Rush, 2002; Lawrence et al., 2009). An individual’s perception of their own level of impulsivity also appears to be related to the initiation and maintenance of substance use (e.g., De Wit, 2009).
Specific to MA use, self-reported impulsivity (i.e., tendency to act without thinking) was associated with greater MA consumption and increased reports of MA “binges” (use of large amounts of MA over an extended period of time) in a large sample of MA users (Semple et al., 2005). Other studies have found that individuals with MA use disorder exhibited greater difficulty with response inhibition (Monterosso et al., 2005) and discounted delayed rewards more steeply (Hoffman et al., 2006) than healthy control individuals. Preclinical studies have also found that, following chronic MA administration (4 mg/kg daily for two weeks), rats valued a delayed reward (water) less as compared to saline administration, suggesting chronic MA use lead to heightened impulsivity in these rodents (Richards et al., 1999).
Although previous clinical studies provide initial evidence of increased impulsivity among stimulant users in comparison to healthy control participants, the current study seeks to extend upon these findings by examining the relationship between impulsivity (i.e., self-report, response inhibition, and delay discounting) and level of MA use disorder severity. It was hypothesized that greater MA use disorder severity will be associated with greater self-reported impulsivity, poorer response inhibition, and increased delay discounting.
2. Methods
2.1. Participants
Non-treatment seeking individuals who regularly use MA were recruited from the Greater Los Angeles area. Inclusion criteria consisted of the following: (1) English fluency; (2) aged 18 to 50; and (3) ability to produce a MA positive urine prior to study entry. Exclusion criteria included the following: (1) in treatment for MA dependence, a history of treatment in the 30 days before enrollment, or treatment seeking; (2) current (last 12 months) DSM-IV diagnosis of drug dependence other than MA; (3) lifetime DSM-IV diagnosis of schizophrenia, bipolar disorder, or any psychotic disorder; (4) current major depressive disorder with suicidal ideation; and (5) current use of psychoactive drug, other than marijuana and MA, determined by toxicology screen.
2.2. Procedures
Participants were recruited from the community through radio, Internet, and newspaper advertisements. Interested individuals called into the laboratory and completed a brief phone screen to assess for eligibility. Following the phone screen, eligible individuals were invited to the lab for assessment. During the in-person assessment, participants provided a urine sample for verification of recent MA use and completed questionnaires and interviews to assess for individual differences, MA and other substance use, and impulsivity. Participants received $50 for participating in the assessment visit.
2.3. Measures
2.3.1. Individual differences measures.
(1) A Demographics Questionnaire was used to collect information on age, sex, marital status, socioeconomic status, occupation, income, education, and ethnicity; (2) Current smoking was assessed by the Fagerstrőm Test for Nicotine Dependence (FTND; Heatherton et al., 1991); and (3) The Beck Anxiety (BAI; Beck et al., 1988) and Beck Depression (BDI; Beck et al., 1996) Inventories were used to assess for physical and cognitive symptoms of anxiety and depression, respectively.
2.3.2. Methamphetamine use severity.
MA use disorder and other exclusionary psychiatric diagnoses were assessed using the Structured Clinical Interview for DSM-IV (SCID; First et al., 1995). A total symptom count (indicator variable “SX_Count” in the model) was derived from the SCID, as well as age of first MA use (“Onset”), and total years using MA (“Yrs_Use”) (see Figure 1). The indicator variable, “MAWQ,” was calculated from the MA Withdrawal Questionnaire (MAWQ), a self-report questionnaire of physical, emotional, and functional symptoms of MA withdrawal (Srisurapanont et al., 1999a, b) and the “MAUQ” variable was derived from the MA Urge Questionnaire (MAUQ), a self-report measure of craving. Frequency of MA use over the past 30 days (“Frequency”) was calculated from the 30-day Timeline Followback (TLFB) interview, which obtained baseline of quantity and frequency of MA use (Sobell et al., 1996; Sobell and Sobell, 1980). Thus, the indicator variables that make up the MA use severity latent construct included: (i) SX_Count, (ii) Onset, (iii) Yrs_Use, (iv) MAWQ, (v) MAUQ, and (vi) Frequency.
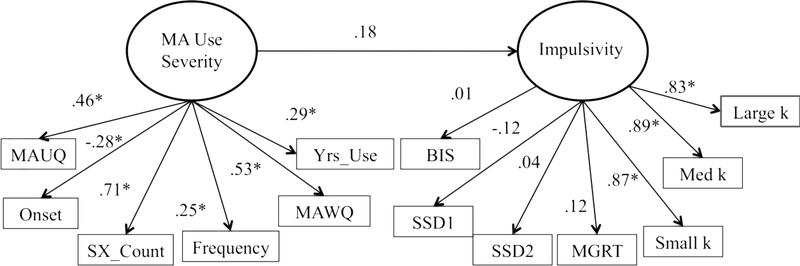
Figure 1.
Results for the initial structural equation model for the relationship between MA use severity and impulsivity. Note: MA Urge Questionnaire (MAUQ); age of onset of MA use (Onset); number of diagnostic criteria met for MA use disorder (SX_Count); number of days using MA out of the 30 days (Frequency); MA Withdrawal Questionnaire (MAWQ); total years using MA (Yrs_Use); Barratt Impulsivity Scale (BIS); stop signal delay ladder 1 (SSD1); stop signal delay ladder 2 (SSD2); and mean go reaction time (MGRT).
2.3.3. Impulsivity battery.
The following measures were collected during the behavioral visit in order to capture aspects of impulsivity: (1) The Barratt Impulsivity Scale −11 (BIS; Patton et al., 1995), a brief, self-report, 30-item measure that assessed participants for trait impulsivity. (2) The Stop Signal Task (SST; Logan et al., 1984), a computer task which captures both inhibitory and activational responding and requires participants to respond to go signals and attempt to inhibit their response when occasional stop signals are presented. The SST consists of 64 trials in which participants are instructed to quickly press the key that corresponds to the direction the arrow is pointing on the screen; however, on 25% of the trials a tone is sounded which signals the participant to attempt to inhibit their response (‘Stop trials’). Of note, the stop-signal delay (SSD) or the time between the go and stop signal varied across trials such that if the participant was successful in inhibiting his/her response, the delay would increase by 50 ms and would decrease by 50 ms if they failed. The SSD began at 250 ms for ladder one and 350 ms for ladder two of the task. Thus, an average SSD was computed for each ladder of the task (SSD 1 and SSD 2; (Logan, 1994), (3) The Delay Discounting Task (DDT; Kirby et al., 1999), a questionnaire-based computerized task which captures participants’ ability to delay rewards as it requires participants to choose between an immediate, smaller monetary gain and a larger, delayed gain. The DDT task utilized in this study was developed on E-Prime 2.0. During the task, participants were presented with two options in which they had to choose from hypothetical monetary amounts across 27 trials. Across each trial, the monetary amounts and the hypothetical duration of the delay period in which they would “receive” this monetary reward varied. The order of the trials remained the same across participants and the values were identical to that from Kirby et al. (1999). The dependent variable derived from the task is a k score, which represents the degree to which a participant values larger delayed rewards over smaller immediate rewards. Three k scores corresponding to three levels of different magnitudes of reward were extracted for each participant as discount rates typically decrease as the magnitude of rewards increase (Kirby et al., 1999): “Small k” mean = $25, “Medium k” mean = $55, and “Large k” mean = $85. The indicator variables constituting the impulsivity latent construct included: (i) total score from the BIS (“BIS”), (ii) the stop signal delay ladder 1 and ladder 2 extracted from the SST task (“SSD1” and “SSD2”), (iii) the mean go reaction time extracted from the SST task (“MGRT”), and (iv) the preference for smaller, immediate over larger delayed rewards as indexed by three k values (“Small k”, “Medium k”, and “Large k”) calculated from the DDT task.
2.4. Data analysis
A multivariate structural equation modeling (SEM) approach was used in order to simultaneously capture associations between the various measures of impulsivity and MA use disorder severity. These latent constructs included observed variables as described in the measures section. Modeling analyses were conducted using the EQS version 6.1 for Windows SEM program (Bentler, 1995). Robust statistical estimates were used due to the non-normal distribution of the MA indicator variables. Statistical model fit was assessed with the Satorra-Bentler scaled chi-squared fit index (Satorra and Bentler, 2001). A relative estimate (ratio of chi-square to degrees of freedom) was also calculated, as the use of the chi-squared likelihood ratio to assess the model fit has been deemed unsatisfactory for numerous reasons (Tanaka, 1993). Values less than 2 on the relative chi-square indicate adequate model fit (Byrne, 1989). Descriptive model fit was assessed with the robust versions of the comparative fit index (CFI; Bentler, 1990) and the root mean square error of approximation (RMSEA; Browne and Cudeck, 1993). Both the CFI and the RMSEA are sensitive to model misspecifications and are minimally affected by sample size (Hu and Bentler, 1995). The CFI ranges from 0 to 1, with values above 0.90 indicating acceptable fit (Bentler, 1990). The RMSEA ranges from 0 to 8, where fit values less than 0.05 indicate close fit and values less than 0.10 indicate reasonable fit (Steiger, 1990). A standard significance threshold of p < .05 was employed for all analyses.
A two-step approach using the Bentler-Weeks model was taken (Bentler and Weeks, 1980). First, an a priori measurement model was specified for each of the constructs as described below. This enabled examination of the fit of the indicator variables to their respective constructs via critical ratios, which are distributed as z-values. Practical fit of the standardized indicator variable loadings was also examined and variables were trimmed accordingly to produce the most parsimonious latent constructs. Secondly, a path predicting the MA Use Severity construct from the Impulsivity construct was defined and estimated.
3. Results
A total of 203 participants completed the in-person assessment battery. Twenty-six participants were removed from the analyses for providing unreliable data (twelve tested positive for other drugs, nine endorsed symptoms of psychosis, and five provided invalid/missing data on measures), leaving a total of 177 participants for the analysis (127 men, 50 women). Of these 177 participants, 65% met DSM-IV criteria for both MA dependence and abuse, 19.8% met criteria for MA dependence only, 9.6% met criteria for MA abuse only, 4.5% were diagnostic orphans (i.e., endorsed less than three symptoms of MA dependence), and 1.1% did not endorse any symptoms of either MA abuse or dependence. Demographic, MA use, and impulsivity variables are presented in Table 1. Correlations between MA use variables and impulsivity variables are presented in Table 2.
Table 1.
Demographic and methamphetamine use variables for the study sample (N = 177)
| Variable | Mean (SD) | Frequency (%) |
|---|---|---|
| Age | 35.44 (8.8) | |
| Sex (Male) | 127 (71.5%) | |
| Ethnicity | ||
| Caucasian | 54 (30.5%) | |
| African American | 39 (22.0%) | |
| Asian | 5 (2.8%) | |
| Latino | 57 (32.2%) | |
| Native American | 1 (0.6%) | |
| Multiple Ethnicities | 20 (11.3%) | |
| Education (years) | 12.64 (3.0) | |
| Age of First Use | 22.79 (7.9) | |
| Years of MA Use | 12.53 (8.6) | |
| Met Criteria for Abuse Only | 17 (9.6%) | |
| Met Criteria for Dependence Only | 35 (19.8%) | |
| Met Criteria for Abuse & Dependence | 115 (65.0%) | |
| Met No Diagnosis | 2 (1.1%) | |
| Diagnostic Orphan | 8 (4.5%) | |
| Average Symptom Count | 5.90 (2.4) | |
| Symptom Count | ||
| 0 | 2 (1.1%) | |
| 1 | 4 (2.3%) | |
| 2 | 7 (4.0%) | |
| 3 | 23 (13.0%) | |
| 4 | 16 (9.0%) | |
| 5 | 25 (14.1%) | |
| 6 | 24 (13.6%) | |
| 7 | 27 (15.3%) | |
| 8 | 22 (12.4%) | |
| 9 | 16 (9.0%) | |
| 10 | 7 (4.0%) | |
| 11 | 4 (2.3%) | |
| Days of MA Use in Past 30 Days | 19.0 (8.8) | |
| MA Use Questionnaire | 17.36 (11.5) | |
| MA Withdrawal Questionnaire | 15.27 (11.5) | |
| Beck Depression Inventory | 13.50 (10.2) | |
| Beck Anxiety Inventory | 9.84 (10.2) | |
| Primary Route of Administration | ||
| Smoke | 110 (74.8%) | |
| Inject | 10 (6.8%) | |
| Snort | 23 (15.6%) | |
| Ingest | 4 (2.7%) | |
| Fagerstrom Test of Nicotine Dependence | 5.54 (2.5) | |
Table 2.
Pearson bivariate correlations between demographic, MA use variables, and impulsivity indicators
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | 1 | ||||||||||||||
| 2. Education (years) | − 0.11 | 1 | |||||||||||||
| 3. Years of MA Use | 0.58* | − 0.17* | 1 | ||||||||||||
| 4. Age of First Use | 0.47* | 0.08 | − 0.44* | 1 | |||||||||||
| 5. Frequency of MA Use | − 0.06 | 0.02 | 0.11 | − 0.21* | 1 | ||||||||||
| 6. MAUQ | − 0.06 | − 0.02 | 0.04 | − 0.12 | 0.11 | 1 | |||||||||
| 7. MAWQ | − 0.06 | − 0.11 | − 0.02 | − 0.08 | 0.08 | 0.30* | 1 | ||||||||
| 8. Average Symptom Count | 0.09 | − 0.13 | 0.22* | − 0.14 | 0.17* | 0.31* | 0.40* | 1 | |||||||
| 9. Barratt Impulsivity Scale | − 0.03 | − 0.05 | 0.22* | − 0.29* | 0.09 | 0.29* | 0.49* | 0.45* | 1 | ||||||
| 10. Small k | 0.26* | − 0.18* | 0.22* | 0.089 | 0.039 | 0.09 | 0.09 | 0.12 | − 0.02 | 1 | |||||
| 11. Medium k | 0.21* | − 0.12 | 0.22* | 0.02 | 0.04 | 0.04 | 0.10 | 0.07 | − 0.05 | 0.78* | 1 | ||||
| 12. Large k | 0.13 | − 0.04 | 0.12 | 0.04 | 0.12 | 0.08 | 0.12 | 0.09 | −0.009 | 0.73* | 0.75* | 1 | |||
| 13. Stop Signal Delay Ladder 1 (SSD1) | 0.03 | 0.08 | 0.05 | − 0.06 | 0.01 | 0.07 | 0.10 | − 0.03 | 0.14 | − 0.05 | − 0.04 | − 0.08 | 1 | ||
| 14. Stop Signal Delay Ladder 2 (SSD2) | 0.05 | 0.09 | 0.003 | 0.03 | − 0.04 | 0.05 | 0.06 | − 0.04 | 0.05 | − 0.02 | − 0.001 | − 0.005 | 0.80* | 1 | |
| 15. Mean Go Reaction Time (MGRT) | 0.20* | 0.001 | 0.09 | 0.09 | − 0.04 | 0.02 | 0.04 | − 0.01 | 0.02 | 0.03 | 0.05 | 0.08 | 0.57* | 0.69* | 1 |
p < .05
3.1. SEM model testing
The initial model that was tested was not found to fit well descriptively (CFI= 0.694, RMSEA= 0.146) or statistically (S-B scaled χ2 [63, n = 103] = 291.33; relative χ2 = 4.62; Figure 1). Importantly, the MA use severity indicator variables were all found to load significantly onto the MA Use Severity latent construct. The DDT k values indicator variables loaded significantly onto the Impulsivity factor; however, the other indicator variables, BIS, SSD1, SSD2, and MGRT did not load significantly onto the Impulsivity factor. Additionally, the path from MA Use Severity to Impulsivity was not found to be statistically significant. Given the poor descriptive and statistical fit of the initially proposed model, additional iterations of the model were run in order to improve fit.
A second model was run, which attempted to improve fit on the side of the outcomes. This was successfully achieved by splitting the singular Impulsivity factor into four separate constructs: BIS: Cognitive Impulsivity, BIS: Behavioral Impulsivity (Reise et al., 2013), Stop Signal Task, and Delay Discounting Task. The second model was found to fit relatively well descriptively (CFI= 0.875, RMSEA= 0.064) and well statistically (S-B scaled χ2 [265, n = 103] = 443.14; relative χ2 = 1.67).
A third and final model was undertaken in order to attempt to further improve model fit on the side of the predictor. In order to do so, a principal component analysis was conducted to derive factor scores from the MA use severity indicator variables. The principal factor method followed by promax (oblique) rotation revealed the six indicator variables split into two meaningful factors (Eigenvalue = 1.95 and 1.32). Sx_Count, MAUQ, and MAWQ, comprised the first factor while Yrs_Use, Onset, and Frequency made up the second factor, with each index loading on to its respective factor at 0.40 or greater. The third factor fell below the 1.0 cutoff; thus, only two factors were maintained.
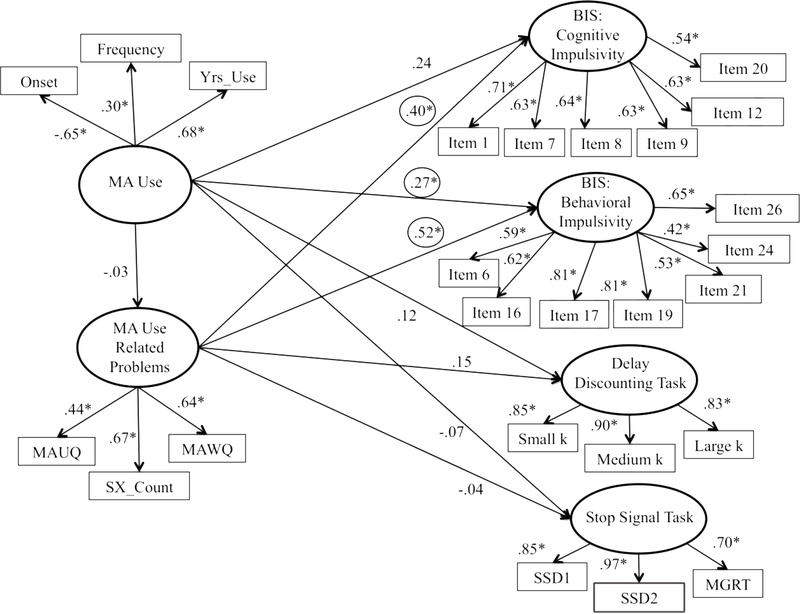
The final SEM model split the MA Use Severity factor into two factors consistent with the principal component analysis. This model was found to fit relatively well descriptively (CFI= 0.897, RMSEA= 0.059) and well statistically (S-B scaled χ2 [260, n = 103] = 406.86; relative χ2 = 1.56). The final model is presented in Figure 2. The indicator variables, MAUQ (β = .44), MAWQ (β = .64) and SX_Count (β = .67) loaded significantly onto the MA Use Related Problems factor as did the indicator variables for the MA Use factor, Onset (β = −.65), Frequency (β = .30), and Yrs_Use (β = .68). Additionally, all of the indicator variables continued to load significantly onto their respective Stop Signal Task, Delay Discounting Task, BIS: Behavioral Impulsivity, and BIS: Cognitive Impulsivity factors (all βs > .40).
Figure 2.
The final structural equation model for the relationship between MA use severity and impulsivity. Model revised to improve fit. Note: MA Urge Questionnaire (MAUQ); age of onset of MA use (Onset); number of diagnostic criteria met for MA use disorder (SX_Count); number of days using MA out of the 30 days (Frequency); MA Withdrawal Questionnaire (MAWQ); total years using MA (Yrs_Use); Barratt Impulsivity Scale (BIS); stop signal delay ladder 1 (SSD1); stop signal delay ladder 2 (SSD2); and mean go reaction time (MGRT).
The path from MA Use to BIS: Behavioral Impulsivity (β= 0.27) was significant, such that increased MA use was associated with increased self-reported behavioral impulsivity. The paths from both BIS factors (Cognitive Impulsivity and Behavioral Impulsivity) to MA Use Related Problems were found to be statistically significant (β= 0.40 and β= 0.52, respectively), such that greater MA use related problems were associated with increased self-reported behavioral and cognitive impulsivity (see Figure 2).
4. Discussion
Individuals with MA use disorder have been shown to demonstrate increased impulsivity (Vocci, 2008), decreased response inhibition (Monterosso et al., 2005), and poorer delay discounting (Hoffman et al., 2006) when compared to healthy control samples. Clinical findings are consistent with preclinical studies showing that MA administration increases impulsivity in animal models (Richards et al., 1999). While the mechanisms of impulsivity are not entirely understood, there is evidence to suggest that the impulsivity observed in MA users may be linked to the neuromodulation of the dopaminergic system that occurs following repeated MA use (Lee et al., 2009). Given the lack of research investigating the continuous relationship between MA use/problems and impulsivity across levels of MA Use Severity, the current study used a multivariate approach that simultaneously accounted for clinical variables of MA use, such as age of onset, frequency of use, craving, and diagnostic symptoms, in a large sample of MA users.
The initial theoretical model was not found to fit well. Although all indicator variables loaded significantly onto MA Use Severity, only three impulsivity variables loaded significantly onto the singular Impulsivity factor. Further, the various dimensions of impulsivity were not found to correlate with one another when separated in subsequent models, in line with previous models in alcohol users (Courtney et al., 2012). These findings highlight the multidimensionality of impulsivity (De Wit, 2009; Fernie et al., 2010). Despite previous findings that individual constructs of impulsivity share common frontostriatal mechanisms (see review by Jentsch et al., 2014), the current findings offer further support that each dimension of impulsivity may be distinct and unique in its clinical presentation. Furthermore, there was no significant relationship between MA Use Severity and behavioral measures of impulsivity observed in the estimated models while self-reported impulsivity was found to be associated with MA Use Severity.
Following two iterations described in the results section, the final model found that increased MA use related problems were related to greater self-reported behavioral and cognitive impulsivity. High self-reported impulsivity has previously been associated with increased craving among MA dependent individuals, and severity of withdrawal and self-reported craving following drug use, among MA dependent and cocaine dependent individuals (Tziortzis et al., 2011). The current study expands on these findings as multiple MA use related problems, indexed by increased craving, withdrawal, and DSM-IV symptoms, were significantly associated with greater self-reported impulsivity on the BIS.
Interestingly, the relationship between the MA use severity factors (i.e., MA Use and MA Use Related Problems) and the behavioral measures of impulsivity remained nonsignificant in the final model, suggesting that response inhibition and delay discounting may not be associated with the level of severity of MA use. These findings are surprising, given other clinical and preclinical studies that have shown that individuals with MA use disorders exhibit greater difficulty with response inhibition (Monterosso et al., 2005) and discount delayed rewards more steeply (Hoffman et al., 2006) than healthy controls. The current findings suggest that these behavioral measures of impulsivity may be better at distinguishing between users and non-users than predicting levels of MA use severity. Similarly, Monterosso and colleagues (2005) found an association between worsened performance on the SST and recent amount of MA used (in grams), but no relationship with performance and frequency of use in a small group of MA abusers (n = 11). Hoffman et al. (2006) reported that variables of MA use, such as severity of MA use, years of use, and average amount used daily (in grams), were not related to any neuropsychiatric variables, although it is unclear if the authors included the DDT in that analysis. The current findings suggest that, in a much larger sample (n = 177), response inhibition and delay discounting may not be affected by severity of MA use as captured by both MA use related problems and MA use. However, there are a few potential explanations for these results that warrant discussion. It may be the nature of the tasks precludes the detection of a significant relationship between MA use and behavioral measures of impulsivity. For example, Bickel and colleagues (2011) found that the rate of delay discounting varied depending on the commodity being offered such that stimulant users tended to discount delayed rewards of the drug at higher rates than money. Thus, it is possible that the results found in this study may have differed if the commodity of reward was drug rather than money. Another important point of discussion is the role that acute intoxication may play in performance on behavioral measures of response inhibition and delay discounting as some research has shown that these measures of impulsivity have been impacted by acute stimulant administration (Fillmore and Rush, 2002; Johnson et al., 2017). This consideration is important to note because it may not be the case that MA use severity is not related to these behavioral measures of impulsivity, but rather it may be the null finding is a product of the conditions in which they were captured.
Findings from the current study may have implications for treatment. In a recent multisite treatment study, MA-dependent individuals’ scores on a subscale of the BIS, which has been thought to capture acting without thinking, were related to treatment non-completion rates (d = 0.53; Winhusen et al., 2013). These findings suggest that self-reported impulsiveness on the BIS may be useful in predicting those at risk of treatment dropout. The current study may be useful to clinicians as both the BIS (Winhusen et al., 2013) and the MA Use Related Problems factor could potentially be utilized to identify patients at increased risk for not completing treatment.
The findings should be considered in light of the strengths and limitations of the study. Although the sample size is considered large for the nature of the population, it is not sufficiently powered for moderation analyses of sample characteristics such as the influence of ethnicity and gender within the specified SEM model. Further, this was a cross-sectional examination of regular users of MA, thereby precluding causal inferences about the progression of the disorder within individual participants and about the causal relationships between MA use severity and impulsivity dimensions. Future research might address these limitations by increasing sample size and investigating these relationships in a longitudinal design. Lastly, generalizability of the findings is limited due to the exclusion of treatment-seeking individuals, those who met criteria for a lifetime DSM-IV diagnosis of psychiatric disorders, and current use of psychoactive drugs (other than MA or marijuana). However, as this study was initially part of a larger-scale MA administration study (Ray et al., 2015), the exclusionary criteria were developed with safety and ethical considerations in mind.
Despite the limitations, there are several strengths of the present study. This study used a multivariate approach that simultaneously accounted for clinical variables of MA use problems (e.g., withdrawal, craving, and DSM-IV symptoms of abuse/dependence) to examine the relationship between MA use severity and measures of impulsivity. This approach is thought to better capture an existing relationship between MA use severity and impulsivity, as it is able to account for multiple variables, associations, and error, concurrently. The current study is unique as it examined impulsivity in the context of a range of MA use related problems. Because impulsivity differences between MA users and control individuals have been established in the literature (e.g., Dean et al., 2012), the current study advances the field by examining the continuous relationship between MA use and these constructs within a group of individuals who regularly use MA. Additionally, the study was able to examine these relationships through the use of multiple variables for each construct, allowing for a more in-depth and reliable evaluation of the associations between MA-use related problems and impulsivity. Lastly, a considerable strength of the study was the development of a clinically relevant MA Use Severity factor comprised of measures of withdrawal, craving, and DSM-IV abuse/dependence symptoms. As the field awaits improvements in evidence-based treatments for stimulant use disorders, having instruments that facilitate the evaluation of MA use severity may enable tailored approaches to improve the efficacy of current treatments.
In conclusion, the current findings extend previous research examining the relationship between MA use and the BIS (e.g., Lee et al., 2009; Tziortzis et al., 2011; Winhusen et al., 2013) by providing additional evidence for increased self-reported impulsivity among MA users. Additionally, the findings further support the multidimensionality of the impulsivity construct and highlight the importance of separately evaluating the distinct dimensions.
Highlights.
Non-treatment seeking methamphetamine (MA) users completed impulsivity assessments.
Analyses examined dimensions of impulsivity in relation to MA use severity.
Higher severity of MA use predicted higher self-reported impulsivity.
Acknowledgments
Role of Funding Source
This research was supported by a grant from the National Institute on Drug Abuse (R21 DA029831) to LAR. Support for this study was also provided by the UCLA Clinical and Translational Science Institute (CTSI), grants UL1RR033176 and UL1TR000124. NRM and KEC were supported by the UCLA Training Program in Translational Neuroscience of Drug Abuse (T32 DA024635). KEC is currently supported by a training grant from the National Institute on Alcohol Abuse and Alcoholism (T32 AA013525). None of the funding sources had any further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Conflict of Interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beck AT, Epstein N, Brown G, Steer RA, 1988. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol 56, 893–897. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Beck Depression Inventory: Second edition manual. The Psychological Corporation, San Antonio. [Google Scholar]
- Bentler PM, 1990. Comparative fit indexes in structural models. Psychol. Bull 107, 238–246. [DOI] [PubMed] [Google Scholar]
- Bentler PM, 1995. EQS: Structural Equations Program Manual Multivariate Software. Encino, CA. [Google Scholar]
- Bentler PM, Weeks DG, 1980. Linear structural equations with latent variables. Psychometrika 45, 289–308. [Google Scholar]
- Bickel WK, Landes RD, Christensen DR, Jackson L, Jones BA, Kurth-Nelson Z, Redish AD, 2011. Single- and cross-commodity discounting among cocaine addicts: The commodity and its temporal location determine discounting rate. Psychopharmacol. 217, 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne MW, Cudeck R, 1993. Alternative ways of assessing model fit In: Bollen KAL, J.S. (Ed.), Testing Structural Models. Sage, New York: pp. 136–162. [Google Scholar]
- Byrne BM, 1989. A Primer of LISREL: Basic Applications and Programming for Confirmatory Factor Analytic Models. Springer-Verlag, New York. [Google Scholar]
- Courtney KE, Arellano R, Barkley-Levenson E, Galvan A, Poldrack RA, Mackillop J, Jentsch JD, Ray LA, 2012. The relationship between measures of impulsivity and alcohol misuse: An integrative structural equation modeling approach. Alcohol. Clin. Exp. Res 36, 923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit H, 2009. Impulsivity as a determinant and consequence of drug use: A review of underlying processes. Addict. Biol 14, 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AC, Groman SM, Morales AM, London ED, 2013. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacol. 38, 259–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie G, Cole JC, Goudie AJ, Field M, 2010. Risk-taking but not response inhibition or delay discounting predict alcohol consumption in social drinkers. Drug Alcohol Depend. 112, 54–61. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, 2002. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 66, 265–273. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2002. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry 159, 1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO, 1991. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict 86, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Perry W, 2010. Effect of methamphetamine dependence on everyday functional ability. Addict. Behav 35, 593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH, 2006. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacol. 188, 162–170. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM, 1995. Evaluating model fit In: Hoyle RH (Ed.), Structural Equation Modeling: Concepts, Issues, and Applications Sage, New York: pp. 76–99. [Google Scholar]
- Jentsch JD, Ashenhurst JR, Cervantes MC, Groman SM, James AS, Pennington ZT, 2014. Dissecting impulsivity and its relationships to drug addictions. Ann. N. Y. Acad. Sci 1327, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR, 1999. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacol. 146, 373–390. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Herrmann ES, Sweeney MM, LeComte RS, Johnson PS, 2017. Cocaine administration dose-dependently increases sexual desire and decreases condom use likelihood: The role of delay and probability discounting in connecting cocaine with HIV. Psychopharmacol. 234, 599–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK, 1999. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J. Exp. Psychol. Gen 128, 78–87. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS, 2005. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat. Neurosci 8, 1450–1457. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, Clark L, 2009. Impulsivity and response inhibition in alcohol dependence and problem gambling. Psychopharmacol. 207, 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA, 2009. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J. Neurosci 29, 14734–14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, 1994. On the ability to inhibit thought and action: A users’ guide to the stop signal paradigm In: Dagenbach D, Carr TH (Ed.), Inhibitory processes in attention, memory, and language. Academic Press, San Diego: pp. 189–239. [Google Scholar]
- Logan GD, Cowan WB, Davis KA, 1984. On the ability to inhibit simple and choice reaction time responses: A model and a method. J. Exp. Psychol 10, 276–291. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafo MR, 2011. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacol. 216, 305–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED, 2005. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 79, 273–277. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES, 1995. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol 51, 768–774. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Condon TP, 2007. Why do we need an Addiction supplement focused on methamphetamine? Addiction 102 Suppl. 1, 1–4. [DOI] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Courtney KE, Moallem NR, Lunny K, Roche D, Leventhal AM, Shoptaw S, Heinzerling K, London ED, Miotto K, 2015. The effects of naltrexone on subjective response to methamphetamine in a clinical sample: A double-blind, placebo-controlled laboratory study. Neuropsychopharmacol. 40, 2347–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reise SP, Moore TM, Sabb FW, Brown AK, London ED, 2013. The Barratt Impulsiveness Scale-11: Seassessment of its structure in a community sample. Psychol. Assess 25, 631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Sabol KE, de Wit H, 1999. Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacol. 146, 432–439. [DOI] [PubMed] [Google Scholar]
- Satorra A, Bentler P, 2001. A scaled difference chi-square test statistic for moment structure analysis. Psychometrika 66, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Peck J, Reback CJ, Rotheram-Fuller E, 2003. Psychiatric and substance dependence comorbidities, sexually transmitted diseases, and risk behaviors among methamphetamine-dependent gay and bisexual men seeking outpatient drug abuse treatment. J. Psychoactive Drugs 35 Suppl. 1, 161–168. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Brown J, Leo GI, Sobell MB, 1996. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend 42, 49–54. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 1980. Convergent validity: An approach to increasing confidence in treatment outcome conclusions with alcohol and drug abusers, in evaluating alcohol and drug abuse treatment effectiveness: Recent advances. Pergamon Press, Elmsford, NY. [Google Scholar]
- Srisurapanont M, Jarusuraisin N, Jittiwutikan J, 1999a. Amphetamine withdrawal: I. Reliability, validity and factor structure of a measure. Aust. N. Z. J. Psychiatry 33, 89–93. [DOI] [PubMed] [Google Scholar]
- Srisurapanont M, Jarusuraisin N, Jittiwutikan J, 1999b. Amphetamine withdrawal: II. A placebo-controlled, randomised, double-blind study of amineptine treatment. Aust. N. Z. J. Psychiatry 33, 94–98. [DOI] [PubMed] [Google Scholar]
- Steiger JH, 1990. Structural model evaluation and modification: An interval estimation approach. Multivariate Behav. Res 25, 173–180. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA), 2013. Results from the 2012 National Survey on Drug Use and Health: Summary of national findings. SAMHSA, Rockville, MD. [Google Scholar]
- Tanaka JS, 1993. Multifaceted conceptions of fit in structural equation models In: Bollen KAL, J.S. (Ed.), Testing Structural Equation Models. Sage, Newbury Park, CA. [Google Scholar]
- Tziortzis D, Mahoney JJ 3rd, Kalechstein AD, Newton TF, De La Garza R, 2nd, 2011. The relationship between impulsivity and craving in cocaine- and methamphetamine-dependent volunteers. Pharmacol. 98, 196–202. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, 2008. Cognitive remediation in the treatment of stimulant abuse disorders: A research agenda. Exp. Clin. Psychopharmacol 16, 484–497. [DOI] [PubMed] [Google Scholar]
- Walter AW, Bachman SS, Reznik DA, Cabral H, Umez-Eronini A, Nath A, Flournoy MW, Young NS, 2012. Methamphetamine use and dental problems among adults enrolled in a program to increase access to oral health services for people living with HIV/AIDS. Pub. Health Rep 127 Suppl. 2, 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen T, Lewis D, Adinoff B, Brigham G, Kropp F, Donovan DM, Seamans CL, Hodgkins CC, Dicenzo JC, Botero CL, Jones DR, Somoza E, 2013. Impulsivity is associated with treatment non-completion in cocaine- and methamphetamine-dependent patients but differs in nature as a function of stimulant-dependence diagnosis. J. Subst. Abuse Treat 44, 541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]