Abstract
Objective
The performance of Omron HEM-6232T and Omron HEM-6181 for monitoring blood pressure (BP) at the wrist was validated in accordance with the ANSI/AAMI/ISO 81060-2:2013 protocol (ANSI/AAMI/ISO) and the European Society of Hypertension International Protocol revision 2010 (ESH IP2).
Methods
Three trained medical technologists validated the performance of these devices by comparing data obtained from these devices with those obtained using a standard mercury sphygmomanometer.
Results
The mean differences between the devices and mercury readings for SBP and DBP were as follows: HEM-6232T, −0.4±6.7 mmHg and 1.6±5.4 mmHg, respectively; HEM-6181, −0.7±6.2 mmHg and −0.7±5.2 mmHg, respectively, satisfying the ANSI/AAMI/ISO protocol. The mean device–observer measurement difference was −0.9±5.7 mm Hg and 0.2±4.6 mm Hg for SBP and 0.5±4.9 mm Hg and 1.4±3.5 mm Hg for DBP, for HEM-6232T and HEM-6181, respectively, satisfying part 1 of the ESH-IP2. All differences for SBP and DBP in both devices satisfied part 2 of the ESH-IP2. The number of absolute differences in the values obtained using the devices and those measured by the observers fulfilled the requirements of the ANSI/AAMI/ISO and the ESH IP2.
Conclusion
The Omron HEM-6232T and HEM-6181 devices met all the requirements of the ANSI/AAMI/ISO and the ESH IP2.
Keywords: blood pressure measurement, device, guideline, international protocol, validation
Introduction
Home blood pressure (BP) monitoring (HBPM) is now believed to be superior to casual BP measurement for prediction of the future development of cardiovascular diseases.1 Because the recently published clinical guidelines include this superiority of the HBPM rather than the casual clinic BP measurement, HBPM is recommended for diagnostic confirmation.2–4 Home BP measuring devices that enable us to frequently measure BP throughout the activities of daily living are useful for determining more accurate BP levels, because daily variability of BP is known to be a predictive marker for the future development of cardiovascular events.5
Among BP monitors, the wrist device is relatively easy and convenient to use, as the cuff can be easily wrapped around the wrist than around the arm, and undressing is not necessary. The standard location for BP measurements recommended by guidelines for management of treating hypertension is the upper arm,6,7 but wrist devices are being recognized as useful and becoming popular because of their ease of use. There is no substantial difference in the measurement mechanism between devices measuring BP in the upper arm and wrist. Since the wrist devices have the advantage of being smaller and lighter than the upper arm devices, they are more portable when used outside of home.
The one possible disadvantage of the wrist devices is that the wrist must be at heart level when measuring BP. Therefore, recently developed devices are equipped with a warning mechanism when the wrist is in the wrong position.
Another advantage of these devices for HPBM is the secondary use of BP data for titration of antihypertensive medication in hypertensives under treatment.8 Usually, the BP average over a few days is used to evaluate the BP level,24 but this requires the user to memorize data and calculate the mean, which can be difficult. If BP data could be stored on electronic devices, such as personal computers or smart phones, then those data could be easily processed when convenient. Advancements in information communication technology can make this concept a reality, especially since widely-used smartphones can now be used as user-friendly portals for data input.
However, the most important factor is to ensure that the BP measuring device is functioning accurately, and to make sure the performance of these devices, the protocols that validate the use of these devices are governed by guidelines.9 The present study aimed to evaluate the accuracy of two self-measuring devices (Omron HEM-6232T and Omron HEM-6181), which are equipped with multiple useful functions for the evaluation of HBPM, according to the ANSI/AAMI/ISO 81060-2:2013 protocol (ANSI/AAMI/ISO).10 and the European Society of Hypertension International Protocol revision 2010 (ESH IP2).11
Materials and methods
Devices
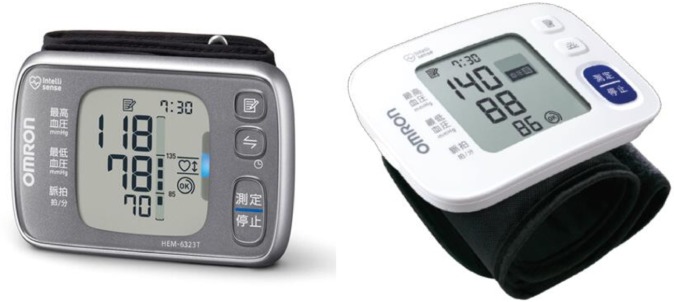
Omron HEM-6232T (Figure 1, left panel, Omron Healthcare Co, Ltd., Kyoto, Japan) is an automatic oscillometric BP measuring device at the wrist with a sensor for the angle of the forearm, and has a pressure range of 0–299 mmHg and a heart rate range of 40–180 beats/minute. SBP, DBP, and pulse rate are displayed on a liquid crystal digital monitor. The entire weight of the device is ~101 g (without batteries), and the dimensions of the device are approximately 91×63.4×13.4 mm (width × height × depth). The cuff can be used for wrist circumferences in the range of 13.5–21.5 cm. It can store 100 BP measurements for two users. It can also calculate an average value based on the three measurements taken within 10 minutes. It detects and displays an irregular pulse, the cuff wrapping status, and body movement during BP measurements. It can quickly and easily synchronize the data of BP and pulse rate recorded on the monitor with a smartphone equipped with Bluetooth.
Figure 1.
Omron HEM-6232T (left) and Omron HEM-6181 (right).
Omron HEM-6181 (Figure 1, right panel, Omron Healthcare Co, Ltd.) is also an automatic oscillometric device for BP measurements at the wrist with a preformed cuff, but without a sensor for the angle of the forearm. The dimensions of the device are approximately 93×62×20 mm (width × height × depth). It can store 60 BP measurements. Otherwise, it functions in a similar manner to Omron HEM-6232T.
Blood pressure measurements
The manufacturer provided standard production device models for each experiment. The validation team for each device consisted of three medical technologists who were experienced in measuring BP. They were also trained by the British and Irish Hypertension Society’s online program (http://www.bihsoc.org). The third observer served as a supervisor who checked both the BP readings by the two observers and device readings. BP measurements were collected by alternating between the Korotkoff method with a mercury sphygmomanometer and the tested device. Simultaneous auscultations were performed by two observers using the double stethoscope (Y tube) when measured with the Korotkoff method. The two observers were blinded to each other’s readings, and the third observer served as a supervisor who checked the BP readings by the two observers.
Subject selection
This study was approved by the institutional review board of the Biwako Central Hospital and the Omron Healthcare Co., Ltd., with written informed consent obtained from each volunteer. The study was performed in a measurement room in the OMRON healthcare Co. or in the Biwako Central Hospital, respectively by the same staff employed by Omron Healthcare Co., Ltd. in accordance with the Declaration of Helsinki. The devices were tested on 85 subjects according to the ANSI/AAMI/ISO, and subsequently, they were tested on 33 subjects according to the ESH IP2 in a separate study. In accordance with the ANSI/AAMI/ISO or the ESH IP2, subjects were screened to ensure that sex, age, wrist circumference, and BP readings fulfilled the participation requirements described in those protocols. Subjects aged <20 years old, having wrist circumference outside of the designated range (13.5–21.5 cm), having arrhythmias, who moved their arms or bodies during the BP measurements, and had unclear Korotkoff sounds were excluded from this study.
Procedure
The study was performed in an isolated room with comfortable room temperature and the subject in the sitting position. Talking during the study was prohibited. After resting for >10 minutes, the BP measurements were started. The subjects were sitting on a chair with a supportive rest for the back, elbow, and forearm, and with their legs uncrossed and feet flat on the floor. First, arm and wrist circumference of the subject was measured, and the appropriate cuff size was adapted. BP was measured on the subject’s left arm at heart level. These devices were validated according to the same arm, sequential method of the ANSI/AAMI/ISO and the ESH IP2.
Analysis
Data were analyzed according to the ANSI/AAMI/ISO and the ESH IP2 requirements. Data were expressed as the mean ± SD, and the minimum and maximum values were also described. The mean of each pair of observer measurements was calculated as a reference value. The difference between the mean observer value and the test values were calculated according the protocols and were displayed as Bland–Altman plots against the mean between the two values.
Results
Omron HEM-6232T
According to the ANSI/AAMI/ISO, a total of 94 subjects were screened for the present experiment. Nine subjects were excluded from the study according to the criteria specified in Table 1, a total of 85 subjects, 38 men (45%) and 47 women (55%), fulfilled the inclusion criteria. Other baseline characteristics of the subjects according to the ANSI/AAMI/ISO are listed in Table 2.
Table 1.
Screening and recruitment details according to the ANSI/AAMI/ISO 81060-2:2013 protocol
| Omron HEM-6232T | Omron HEM-6181 | |
|---|---|---|
| Total screened | 94 | 92 |
| Total excluded | 9 | 7 |
| Arrhythmias | 0 | 0 |
| Poor quality sounds | 0 | 0 |
| Cuff size unavailable | 0 | 0 |
| Movement during BP measurement | 0 | 0 |
| BP variation | 9 | 7 |
| Total recruited | 85 | 85 |
Abbreviation: BP, blood pressure.
Table 2.
Baseline characteristics of the subjects according to the ANSI/AAMI/ISO 81060-2:2013 protocol
| Omron HEM-6232T | Omron HEM-6181 | |
|---|---|---|
| Women (%) | 55 | 61 |
| Age (years), mean ± SD (min to max) | 57±12.7 (23 to 80) | 55±12.2 (23 to 84) |
| Wrist circumference (cm), mean ± SD (min to max) | 17.0±2.2 (13.5 to 21.4) | 17.1±2.2 (13.5 to 21.4) |
| 13.5–17.4 cm (%) | 59 | 56 |
| 17.5–21.5 cm (%) | 41 | 44 |
| 13.5–15.4 cm (%) | 32 | 31 |
| 19.5–21.5 cm (%) | 20 | 20 |
The mean value of the 255 measurements by using the standard mercury sphygmomanometer, were 123±21.8 (range, 83–197) for SBP and 80±14.2 (range, 52–112) mmHg for DBP.
BP distribution for the ANSI/AAMI/ISO (N=255) is listed in Table 3.
Table 3.
Blood pressure distribution according to the ANSI/AAMI/ISO 81060-2:2013 protocol (n=255)
| Omron HEM-6232T | Omron HEM-6181 | |
|---|---|---|
| SBP (mmHg), mean ± SD (min to max) | 123±21.8 (83 to 197) | 124±25.9 (85 to 195) |
| ≥160 mmHg (%) | 5 | 11 |
| ≥140 mmHg (%) | 20 | 28 |
| ≤100 mmHg (%) | 12 | 18 |
| DBP (mmHg), mean ± SD (min to max) | 80±14.2 (52 to 112) | 81±16.2 (50 to 118) |
| ≥100 mmHg (%) | 9 | 15 |
| ≥85 mmHg (%) | 26 | 35 |
| ≤60 mmHg (%) | 8 | 9 |
The differences between the two observers were 0±1.5 mmHg and 0±1.5 mmHg for SBP and DBP, respectively. The mean differences between the two observers and the Omron HEM-6232T were −0.4±6.7 mmHg (range, −33 to 19 mmHg) for SBP and 1.6±5.4 mmHg (range, −12 to 18 mmHg) for DBP according to criterion 1 (Table 4). These data fulfilled the ANSI/AAMI/ISO requirements of ≤5±≤8 mmHg. The mean differences between the two observers and the Omron HEM-6232T were −0.4±5.6 mmHg (range, −24 to 10 mmHg) for SBP and 1.6±4.8 mmHg (range, −10 to 14 mmHg) for DBP according to criterion 2 (Table 4). Thereby, the SD for SBP is calculated to be <6.9 mmHg and for DBP, 6.7 mmHg by criterion l (Table 4). These results are in accordance with the ANSI/AAMI/ISO requirements for criteria 1 and 2.
Table 4.
Validation results according to the ANSI/AAMI/ISO 81060-2:2013 protocol
| Omron HEM-6232T, (mean ± SD [min – max]) | Omron HEM-6181, mean ± SD (min – max) | |
|---|---|---|
| Criterion 1 | ||
| SBP (mmHg) | −0.4±6.7 (−33 to 19) | −0.7±6.2 (−19 to 16) |
| DBP (mmHg) | 1.6±5.4 (−12 to 18) | −0.7±5.2 (−19 to 12) |
| Criterion 2 | ||
| SBP (mmHg) | −0.4±5.6 (−24 to 10) | −0.7±5.2 (−13 to 9) |
| DBP (mmHg) | 1.6±4.8 (−10 to 14) | −0.7±4.7 (−17 to 9) |
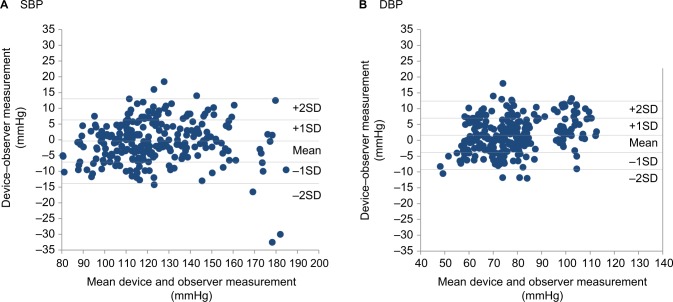
Figure 2A, B shows the differences in the SBP and DBP readings in relation to the mean differences between the HEM-6232T and mercury sphygmomanometer.
Figure 2.
Bland–Altman plots of the differences between the Omron HEM-6232T readings and the observer measurements for: (A) SBP and (B) DBP according to the ANSI/AAMI/ISO 81060-2:2013 protocol.
A total of 46 subjects were screened for this experiment according to the ESH IP2 (Table 5). Thirteen subjects were excluded from the study according to the criteria, and 17 men and 16 women were included fulfilling the requirements. Other baseline characteristics of the subjects according to the ESH IP2 are listed in Table 6. Observer measurements in each recruitment range according to the ESH IP2 are listed in Table 7. The differences between the two observers were 0±1.5 mmHg and 0±1.8 mmHg for SBP and DBP, respectively. The mean differences between the two observers and the Omron HEM-6232T were −0.9±5.7 mmHg (range, −17 to 18 mmHg) for SBP and 0.5±4.9 mmHg (range, −12 to 18 mmHg) for DBP. The number of measurements that differed from the mercury standard by 5, 10, and 15 mmHg or less is shown in Table 8 fulfilling the requirements of the ESH IP2.
Table 5.
Screening and recruitment details according to the ESH IP2
| Omron HEM-6232T | Omron HEM-6181 | |
|---|---|---|
| Total screened | 46 | 37 |
| Total excluded | 13 | 4 |
| Range complete | 0 | 0 |
| Range adjustment | 9 | 3 |
| Arrhythmias | 1 | 0 |
| Device failure | 0 | 0 |
| Poor quality sounds | 0 | 0 |
| Cuff size unavailable | 0 | 0 |
| Observer disagreement | 1 | 0 |
| Other reasons | 2 | 1 |
| Total recruited | 33 | 33 |
Abbreviation: ESH IP2, European Society of Hypertension International Protocol revision 2010.
Table 6.
Baseline characteristics of the subjects according to the ESH IP2
| Omron HEM-6232T | Omron HEM-6181 | |
|---|---|---|
| Women (%) | 48 | 48 |
| Age (years), mean ± SD (min–max) | 54±9.6 (42–76) | 54±12.1 (25–74) |
| Wrist circumference (cm), mean ± SD (min–max) | 17.7±1.6 (15.3–21.2) | 17.3±2.0 (13.9–20.8) |
| SBP (mmHg), mean ± SD (min–max) | 142±26.4 (92–210) | 141±26.7 (89–178) |
| Low (n) (<90 mmHg) | 0 | 1 |
| (90–129 mmHg) | 12 | 10 |
| Medium (n) (130–160 mmHg) | 10 | 12 |
| High (n) (161–180 mmHg) | 10 | 10 |
| (>180 mmHg) | 1 | 0 |
| DBP (mmHg), mean ± SD (min–max) | 89±17.8 (46–122) | 87±19.6 (56–118) |
| Low (n) (<40 mmHg) | 0 | 0 |
| (40–79 mmHg) | 11 | 12 |
| Medium (n) (80–100 mmHg) | 11 | 11 |
| High (n) (101–130 mmHg) | 11 | 10 |
| (>130 mmHg) | 0 | 0 |
Abbreviation: ESH IP2, European Society of Hypertension International Protocol revision 2010.
Table 7.
Observer measurements in each recruitment range according to the ESH IP2
| Omron HEM-6232T | Omron HEM-6181 | |
|---|---|---|
| SBP (mmHg) | ||
| Overall range (low : high) | 87:205 | 90:188 |
| Low (<130 mmHg) | 33 | 36 |
| Medium (130–160 mmHg) | 41 | 38 |
| High (>160 mmHg) | 25 | 25 |
| Maximum difference | 16 | 13 |
| DBP (mmHg) | ||
| Overall range (low : high) | 50:122 | 49:120 |
| Low (<80 mmHg) | 33 | 33 |
| Medium (80–100 mmHg) | 37 | 38 |
| High (>100 mmHg) | 29 | 28 |
| Maximum difference | 8 | 10 |
Abbreviation: ESH IP2, European Society of Hypertension International Protocol revision 2010.
Table 8.
Validation results according to the ESH IP2 for the Omron HEM-6232T
| Part 1 | ≤5 mmHg | ≤10 mmHg | ≤15 mmHg | Grade 1 | Mean (mmHg) | SD (mmHg) |
|---|---|---|---|---|---|---|
| Required | ||||||
| Two of | 73 | 87 | 96 | |||
| All of | 65 | 81 | 93 | |||
| Achieved | ||||||
| SBP | 70 | 91 | 97 | Pass | −0.9 | 5.7 |
| DBP | 73 | 96 | 98 | Pass | 0.5 | 4.9 |
| Part 2 | 2/3≤5 mmHg | 0/3≤5 mmHg | Grade 2 | Grade 3 | ||
| Required | ≥24 | ≤3 | ||||
| Achieved | ||||||
| SBP | 24 | 1 | Pass | Pass | ||
| DBP | 25 | 0 | Pass | Pass | ||
Abbreviation: ESH IP2, European Society of Hypertension International Protocol revision 2010.
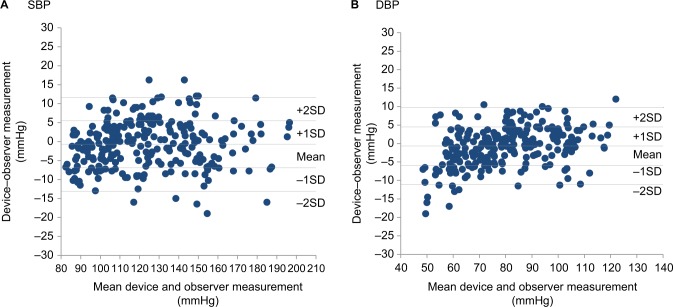
Figure 3A, B shows the differences in the SBP and DBP readings in relation to the mean differences between the HEM-6232T and mercury sphygmomanometer.
Figure 3.
Bland–Altman plots of the differences between the Omron HEM-6232T readings and the observer measurements for: (A) SBP and (B) DBP according to the ESH IP2.
Abbreviation: ESH IP2, European Society of Hypertension International Protocol revision 2010.
Omron HEM-6181
In accordance with the ANSI/AAMI/ISO requirements, a total of 92 subjects were screened for this experiment. Seven subjects were excluded according to the criteria (Table 1), and 33 men (39%) and 52 women (61%) were included. Other baseline characteristics of the subjects according to the ANSI/AAMI/ISO are listed in Table 2. Using the standard mercury sphygmomanometer, the mean values of the 255 measurements were 124±25.9 (range, 85 to 195) for SBP and 81±16.2 (range, 50 to 118) mmHg for DBP.
BP distribution for the ANSI/AAMI/ISO is shown in Table 3 fulfilling the requirements.
The differences between the two observers were 0±1.5 mmHg and 1±1.6 mmHg for SBP and DBP, respectively. The mean differences between the two observers and the Omron HEM-6232T were −0.7±6.2 mmHg (range, −19 to 16 mmHg) for SBP and −0.7±5.2 mmHg (range, −19 to 12 mmHg) for DBP according to criterion 1 (Table 4). These data fulfilled the ANSI/AAMI/ISO requirements of ≤5±≤8 mmHg. The mean differences between the two observers and the Omron HEM-6181 were –0.7±5.2 mmHg (range, −13 to 9 mmHg) for SBP and −0.7±4.7 mmHg (range, −17 to 9 mmHg) for DBP according to criterion 2 (Table 4). Thereby, the SD for SBP is calculated to be <6.9 mmHg and for DBP 6.9 mmHg by criterion l (Table 4). These results are in accordance with the ANSI/AAMI/ISO requirements for criteria 1 and 2.
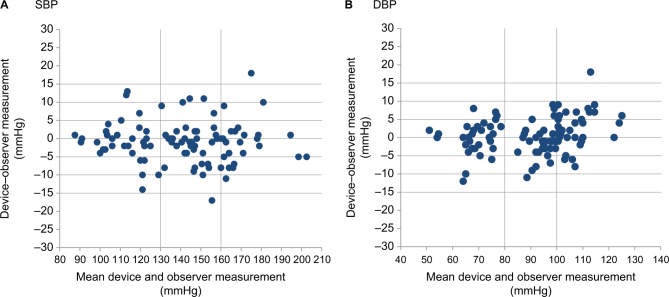
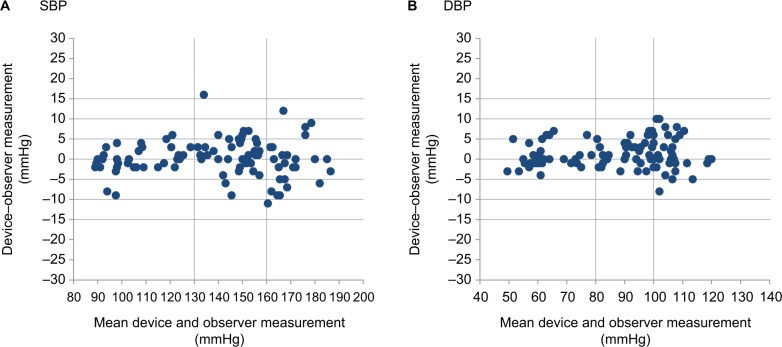
Figure 4A, B shows the differences in the SBP and DBP readings in relation to the mean differences between the HEM-6181 and mercury sphygmomanometer.
Figure 4.
Bland–Altman plots of the differences between the Omron HEM-6181 readings and the observer measurements for: (A) SBP and (B) DBP according to the ANSI/AAMI/ISO 81060-2:2013 protocol.
A total of 37 subjects were screened for this experiment in the ESH IP2 (Table 5). Four subjects were excluded from the study according to the criteria, 17 men and 16 women were included fulfilling the subjects requirements. Baseline characteristics of the subjects according to the ESH IP2 are listed in Table 6 fulfilling the requirements. Observer measurements in each recruitment range according to the ESH IP2 are shown in Table 7 fulfilling the requirements. The differences between the two observers were 0±1.8 mmHg and 0±1.9 mmHg for SBP and DBP, respectively. The mean differences between the two observers and the Omron HEM-6181 were 0.2±4.6 mmHg (range, −11 to 16 mmHg) for SBP and 1.4±3.5 mmHg (range, −8 to 10 mmHg) for DBP. The number of measurements that differed from the mercury standard by 5, 10, and 15 mmHg or less is shown in Table 9 fulfilling the requirements of the ESH IP2.
Table 9.
Validation results according to the ESH IP2 for the Omron HEM-6181
| Part 1 | ≤5 mmHg | ≤10 mmHg | ≤15 mmHg | Grade 1 | Mean (mmHg) | SD (mmHg) |
|---|---|---|---|---|---|---|
| Required | ||||||
| Two of | 73 | 87 | 96 | |||
| All of | 65 | 81 | 93 | |||
| Achieved | ||||||
| SBP | 78 | 96 | 98 | Pass | 0.2 | 4.6 |
| DBP | 81 | 99 | 99 | Pass | 1.4 | 3.5 |
| Part 2 | 2/3≤5 mmHg | 0/3≤5 mmHg | Grade 2 | Grade 3 | ||
| Required | ≥24 | ≤3 | ||||
| Achieved | ||||||
| SBP | 27 | 0 | Pass | Pass | ||
| DBP | 28 | 1 | Pass | Pass | ||
Abbreviation: ESH IP2, European Society of Hypertension International Protocol revision 2010.
Figure 5A, B shows the differences in the SBP and DBP readings in relation to the mean differences between the HEM-6181 readings and mercury sphygmomanometer measurements.
Figure 5.
Bland–Altman plots of the differences between the Omron HEM-6181 readings and the observer measurements for SBP (A) and DBP (B) for ESH IP2.
Discussion
Both the Omron HEM-6232T and the Omron HEM-6181 were found to pass the validation requirements of the International Protocols in the present validation study. On the performance of additional functions, such as detection of inadequate wrapping of the cuff, body movement, the positioning sensor of wrist, and irregular pulses were recorded during the process of screening subjects, and found to be properly functioning. The two devices used in the present study contribute to secure the compliance of a BP measurement by the foregoing functions.
The dabl Educational Trust web site (http://www.dabledu-cational.org/) and BHS web site (https://bihsoc.org/) provide recommendation lists that contain many BP monitors for home use that have been validated according to either the guidelines of the International Protocol, the BHS, ESH, or the AAMI. There are some important points that clinicians should be aware of when authorizing the use of a BP monitor at home by ordinary people. One is the possibility of incorrectly wrapping the cuff and another is the importance of keeping the devise at the level of the heart during BP measurement. Wrapping the cuff perfectly around the wrist leads to an accurate reading.12,13 If the BP monitor is not used correctly, the measurements will be incorrect, leading users to distrust the monitor. Furthermore, correctly wrapping the cuff requires experience, and some users will not use a BP monitor because they find wrapping the cuff to be difficult. Especially in the wrist device, the position of the device substantially influences the BP reading, and while measuring BP, it is necessary to keep the cuff position at heart level. The Omron HEM-6232T senses the angle of the forearm, and lets us achieve the proper angle with blue light or arrow display. Only when it is blue light, the device starts to measure BP. A 10 cm difference between the heart level and the cuff position breeds 7 mmHg difference by hydrostatic pressure between the heart level and the cuff position.14,15 The upper arm device has no influence by the hydrostatic pressure on the measurement. Thus, the guidelines2–4,16 do not recommend use of wrist device for home BP monitoring. These issues were addressed by the two devices validated in this study, which may make them useful for ensuring compliance in using a home BP monitor. Collectively, these devices are useful not only for healthcare professionals but also for ordinary subjects who are not familiar with BP measurements.
Conclusion
The results of this study show that the Omron HEM-6232T and the Omron HEM-6181 devices met the requirements of the international protocols, ANSI/AAMI/ISO and ESH IP2, and may be useful for BP measurement at home.
Footnotes
Disclosure
Kanako Saito and Yukiko Hishiki are employed by Omron Healthcare Co, Ltd., the manufacturer of Omron HEM-6232T and Omron HEM-6181. The authors report no other conflicts of interest in this work.
References
- 1.Imai Y, Obara T, Asamaya K, Ohkubo T. The reason why home blood pressure measurements are preferred over clinic or ambulatory blood pressure in Japan. Hypertens Res. 2013;36(8):661–672. doi: 10.1038/hr.2013.38. [DOI] [PubMed] [Google Scholar]
- 2.Dasgupta K, Quinn RR, Zarnke KB, et al. The 2014 Canadian hypertension education program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2014;30(5):485–501. doi: 10.1016/j.cjca.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence Hypertension: clinical management of primary hypertension in adults (clinical guideline 127) [Accessed 2018]. Available from: http://guidance.nice.org.uk/CG127.
- 4.Chiang CE, Wang TD, Li YH, et al. 2010 guidelines of the Taiwan Society of Cardiology for the management of hypertension. J Formos Med Assoc. 2010;109(10):740–773. doi: 10.1016/S0929-6646(10)60120-9. [DOI] [PubMed] [Google Scholar]
- 5.Chowdhury EK, Owen A, Krum H, et al. Second Australian National Blood Pressure Study Management Committee Systolic blood pressure variability is an important predictor of cardiovascular outcomes in elderly hypertensive patients. J Hypertens. 2014;32:525–533. doi: 10.1097/HJH.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 6.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;71(6):1269–1324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 7.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31(7):1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 8.Ralston JD, Cook AJ, Anderson ML, et al. Home blood pressure monitoring, secure electronic messaging and medication intensification for improving hypertension control: a mediation analysis. Appl Clin Inform. 2014;5(1):232–248. doi: 10.4338/ACI-2013-10-RA-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parati G, Asmar R, Stergiou GS, et al. ESH Working Group on blood pressure monitoring European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779–785. doi: 10.1038/jhh.2010.54. [DOI] [PubMed] [Google Scholar]
- 10.Association for the Advancement of Medical Instrumentation American National Standard. ANSI/AAMI/ISO 81060-2:2013 Non-invasive Sphygmomanometers - Part 2: Clinical investigation of automated measurement type. [Accessed February 25, 2019]. Available from: http://my.aami.org/aamiresources/previewfiles/8106002_1306_preview.pdf#search=%27American+National+Standard.+ANSI%2FAAMI%2FISO+810602%3A2013+Noninvasive%27.
- 11.O’Brien E, Atkins N, Stergiou G, et al. Working group on blood pressure monitoring of the European Society of Hypertension European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010;15:23–38. doi: 10.1097/MBP.0b013e3283360e98. [DOI] [PubMed] [Google Scholar]
- 12.Anast N, Olejniczak M, Ingrande J, Brock-Utne J. The impact of blood pressure cuff location on the accuracy of noninvasive blood pressure measurements in obese patients: an observational study. Can J Anaesth. 2016;63(3):298–306. doi: 10.1007/s12630-015-0509-6. [DOI] [PubMed] [Google Scholar]
- 13.Banner TE, Gravenstein JS. Comparative effects of cuff size and tightness of fit on accuracy of blood pressure measurements. J Clin Monit. 1991;7(4):281–284. doi: 10.1007/BF01619345. [DOI] [PubMed] [Google Scholar]
- 14.Kikuya M, Chonan K, Imai Y, Gotob E, Ishii M, Research group to assess the validity of automated blood pressure measurement devices in Japan Accuracy and reliability of wrist-cuff devices for self-measurement of blood pressure. J Hypertens. 2002;20:629–638. doi: 10.1097/00004872-200204000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Yarows SA. Comparison of the Omron HEM-637 wrist monitor to the auscultation method with the wrist position sensor on or disabled. Am J Hypertens. 2004;17(1):54–58. doi: 10.1016/j.amjhyper.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Shimamoto K, Ando K, Fujita T. Japanese Society of Hypertension Committee for guidelines for the management of hypertension. Hypertens Res. 2014;37:253–390. doi: 10.1038/hr.2014.20. [DOI] [PubMed] [Google Scholar]