Abstract
Tricuspid regurgitation natural history and treatment remains poorly understood. Right ventricular function is a key factor in determining prognosis, timing for intervention and longer-term outcome. The right ventricle is a thin walled chamber with a predominance of longitudinal fibres and a shared ventricular septum. In health, the low-pressure pulmonary circulation results in a highly compliant RV well equipped to respond to changes in preload but sensitive to even small alterations in afterload. In Part 1 of this article, discussion focuses on key principles of ventricular function assessment and the importance of right ventricular chamber size, volumes and ejection fraction, particularly in risk stratification in tricuspid regurgitation. Part 2 of this article provides an understanding of the causes of tricuspid regurgitation in the contemporary era, with emphasis on key patient groups and their management.
Keywords: right ventricle, tricuspid regurgitation, pulmonary hypertension
Part 1: Tricuspid regurgitation and the right ventricle in risk stratification and timing of intervention
Introduction
Tricuspid valve regurgitation (TR) presents challenges to modern day clinical practice. Its natural history is not well understood. Previously, uncertainty existed as to whether TR is an independent factor of outcome or rather a surrogate marker of right ventricular disease and other co-morbid conditions including pulmonary hypertension (1). However, more recently studies has shown increasing TR severity is associated with worse survival regardless of left ventricular (LV) function and pulmonary hypertension (2). Both late residual TR seen post mitral valve surgery (3) and isolated severe TR (4) have excess mortality and morbidity. Management guidelines remain ill defined since prospective outcome data are limited (5). Further, surgical TV intervention studies have suggested variable outcomes and this conflicting data have led to confusion as to optimal management in this cohort. This heterogeneous patient group includes right ventricular (RV) dysfunction, pulmonary hypertension and multiple associated comorbid conditions. Recent publications have assisted in defining patient cohorts who may benefit from surgical intervention (6). In Part 1 of this article, discussion focuses on key principles of ventricular function assessment and the importance of right ventricular chamber size, volumes and ejection fraction, particularly in risk stratification in tricuspid regurgitation. Part 2 of this article provides an understanding of the causes of tricuspid regurgitation in the modern era, with emphasis on key patient groups and their management.
Right ventricular physiology with alterations in loading conditions
Anatomy and basic concepts
For a long time, right ventricular (RV) function was considered, based on its morphology (low myocardial mass, thin walls) as simply a transferring chamber; where the cardiac output would remain unaffected by its exclusion. In the last 30 years, publications have highlighted RV dysfunction as an independent prognostic factor in left heart disease. Heart failure morbidity and mortality is worse in those with similar degrees of LV impairment but concomitant RV dysfunction (7, 8, 9, 10). Much of this understanding has evolved through advancements in cardiac imaging.
Fundamental anatomical differences between the right and left ventricles reflect their distinct embryological origin and haemodynamic physiology. The heart is derived from cardiac mesoderm that forms a cardiac crescent (11). Using murine models, this mesoderm has been shown to be partitioned into two cell populations, named the primary and secondary heart fields (12). These fields have distinct transcriptional identities. Although these fields merge to form a single heart tube, which later loops and ultimately forms a four-chambered structure, lineage tracing experiments reveal the primary heart field gives rise to the left and right atria and left ventricle, whereas the secondary heart field gives rise to the right ventricle and outflow tract during development. Taken together with the fact that the right ventricle is exposed to a unique flow and pressure environment compared to the left ventricle as the heart continues to develop after birth, it is plausible that these differences could lead to distinct functionalisations and behaviours between the right and left ventricle that may contribute to differences in disease manifestation.
RV ejection performance and stroke volume are determined by several factors namely preload, afterload, myocardial wall thickness and contractility as well as constraint from the intact pericardium. The RV mass is approximately one-sixth that of the LV, its wall thickness no more than 3–4 mm. The RV is adapted to eject blood against a lower pulmonary vascular resistance. Low afterload results in reduced wall tension and characterises RV physiology as a low pressure highly compliant pumping chamber. An appreciation of myocardial fibre orientation (inner longitudinal fibres extend from base to apex, outer circumferential fibres run parallel to the tricuspid valve (TV) annular plane) and their continuity with the shared ventricular septum assists in our understanding of RV pump action and highlights two key characteristics. Firstly, that of ventricular interdependence determined by the higher LV pressures in normal circumstances; the ventricular septum moves towards the centre of the LV cavity during systole and outwards in diastole (circumferential fibre contraction). Hence, RV contraction has been compared to a bellows-like action where the lateral free wall contracts against a relatively rigid ventricular septum. Secondly, the RV base contracts towards the apex, resulting in longitudinal RV shortening. Thus, cardiac imaging modalities utilise circumferential and longitudinal contraction when assessing RV function.
Right ventricular response in disease states
RV response to disease can result from volume or pressure overload and myocardial disease. In reality a combination of these factors coexists, where understanding the primary aetiology leading to the observed findings is fundamental to risk stratification and further disease management.
The RV is able to accommodate changes in preload far better than afterload. Thus, where the principal lesion is volume overload (i.e. increase in preload) as in the case of primary tricuspid regurgitation or left-to-right shunting, the RV tolerates this high-volume state; remaining well adapted to maintain RV contractility and cardiac output for prolonged periods before progressive RV dilatation, dysfunction and failure ensue. This may occur with or without the development of pulmonary vasculopathy (from chronic high flow). With chronic volume overload, the crescent-shaped RV becomes increasingly spherical and since pericardial capacity is limited, ventricular interdependence shifts the ventricular septum leftward. LV filling is impaired further compounding a fall in cardiac output.
Pressure overload states typically describe increases in RV afterload from pulmonary arterial hypertension (PAH) since pulmonary stenosis is rare. Adaptive mechanisms designed to reduce RV wall stress and maintain normal cardiac output result in ventricular hypertrophy and dilatation. However, progressive functional (secondary) tricuspid regurgitation (consequent to TV annular dilatation as the RV dilates) and paradoxical ventricular septal motion (through increasing RV filling pressure) increase load to the failing RV worsening cardiac output. As with volume overload ventricular interdependence impairs LV filling contributing to the further decline in cardiac output and hypotension.
Primary myocardial disease such a RV infarction or primary cardiomyopathy (e.g. arrhythmogenic right ventricular dysplasia) results in RV contractile dysfunction. A fall in RV forward flow reduces LV preload with a reduction in systemic cardiac output. Ventricular interdependence and functional TR (through RV dilatation and wall motion abnormalities disrupting the TV subvalvular complex) with a superimposed volume overload state may further worsen RV dysfunction.
Parameters for right ventricular function assessment
RV function assessment presents challenges to all 2D imaging techniques. The RV lies anteriorly and wraps around the conical-shaped LV. The RV consists of TV inflow, RV body and apex and outflow (infundibulum). Since the RV outflow is orientated in a different plane, integration of multiple image views is necessary for comprehensive assessment. Currently, imaging techniques lack a precise tool to predict RV recovery following intervention. Therefore, the ability to accurately diagnose RV dysfunction remains vital. A checklist of key parameters is summarized in Table 1.
Table 1.
Checklist for right heart chamber assessment in tricuspid regurgitation.
| Parameters | Normal values (cut-off) | |
|---|---|---|
| RV dimensions | Proximal RVOT (and views) | |
| PLAX view AV PSAX view |
≤30 mm ≤35 mm |
|
| Distal RVOT (AV PSAX view) | ≤27 mm | |
| RV dimensions (RV focused A4C view) | ||
| Base Longitudinal Mid cavity |
≤41 mm ≤83 mm ≤35 mm |
|
| RA dimensions | RA indexed volume (RV focused A4C view) | |
| ♂ ♀ |
<25 ± 7 mL/m2 <21 ± 6 mL/m2 |
|
| RV function | TAPSE (focused A4C view) | ≥17 mm |
| Lateral wall S′ TDI (focused A4C view) | ≥9.5 cm/s | |
| Fractional area change (focused A4C view) | ≥35% | |
| 3D echo | ||
| Indexed systolic/diastolic volumes* | ||
| ♂ ♀ RVEF |
27 ± 8.5 mL/m2 / 61 ± 13 mL/m2 22 ± 7 mL/m2 / 53 ± 10.5 mL/m2 58% ± 6.6, where abnormal <45% |
|
| Septal and lateral wall global longitudinal strain | >20–23% (absolute values) | |
| Additional parameters | Eccentricity index | >1.2 |
| Myocardial performance index | Limited use in severe TR | |
| Diastolic function parameters (TV inflow E and A velocities, E/A ratio, Lateral wall E′ TDI) | Limited use in severe TR | |
| Magnetic resonance imaging | ||
| RV dimensions | RV systolic/diastolic indexed volumes* mL/m2 | |
| ♂ ♀ |
39 (±10 NR 19–59)/<91 (±15 NR 61–121) 32 (±10 NR 12–52)/<80 (±48 NR 19–59) |
|
| RV function | RVEF | |
| ♂ ♀ |
62% (±5 NR 52–72) 61% (±6 NR 51–71) |
|
*Where RV volumes and RVEF cannot be calculated with echo or MRI techniques, alternative imaging modalities include CT or radionuclide ventriculography.
3D, three-dimensional; A4C, apical four chamber; AV PSAX, parasternal short axis view at the aortic valve level; PLAX, parasternal long axis; RA, right atrium; RV, right ventricle; RVEF, right ventricular ejection fraction; RVOT, right ventricular outflow tract; TAPSE, tricuspid annular peak systolic excursion; TDI, tissue Doppler imaging; TV, tricuspid valve; ♂, males; ♀, females.
Ventricular interdependence
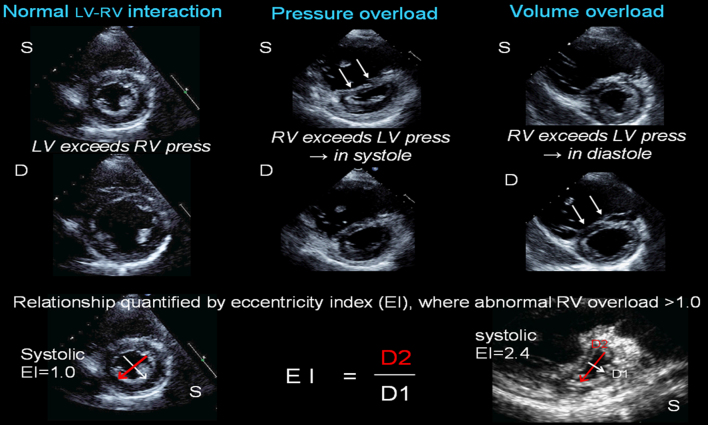
Ventricular interdependence plays an important role in RV dysfunction. RV–LV interaction can be assessed through paradoxical ventricular septum (VS) motion. Since VS configuration is determined by the pressure gradient between the LV and RV throughout the cardiac cycle, its behaviour lends insight into states due to pressure (systolic septum flattening) or volume (diastolic septum flattening) overload. This can be quantified by the eccentricity index (Fig. 1). Indeed VS contraction contributes to RV systolic function by as much as 24% in health to 35% in pathological states, when there is RV-free wall impairment, maintaining RV output (13). Conversely, this mechanical interaction may be the cause of RV dysfunction in the setting of left-sided heart disease, being independent of RV afterload effects, and may predict postoperative RV dysfunction in severe organic mitral regurgitation (14). Figure 1 illustrates the clinical utility of cardiac imaging in differential diagnosis of RV volume and pressure overload states.
Figure 1.
Ventricular interaction. Left panel depicts normal ventricular morphology in systole (S) and diastole (D), where LV pressures exceed RV pressures throughout the cardiac cycle, and the ventricular septum (VS) assumes an outward curve towards the RV, such that the LV cavity is circular. Central panel shows RV pressure overload with significant VS flattening in systole. Right panel shows RV volume overload with significant VS flattening in diastole. Bottom panel shows how eccentricity index is measured, done in both systole and diastole and where EI >1.0, there is increasing volume (seen primarily in diastole) or pressure (seen primarily in systole) overload. Right example shows severe pressure overload with EI 2.4 in systole.
Quantitative assessment of RV function in severe tricuspid regurgitation
Right ventricular size
RV function is the major determinant for morbidity and mortality in many diseases including pulmonary hypertension, left ventricular dysfunction and heart failure and heart valve disease. As described earlier, the RV is sensitive to changes in loading conditions. Typically, early signs in RV disease are manifested through alteration in RV size. RV dilatation can be described by dimension, area and volume measurements. International guidelines for echocardiographic assessment of the right heart define a systematic approach to 2D measurements taken from the RV focused apical four-chamber view and parasternal (PS) windows in end diastole (15) (Table 1). Similarly, RV area is assessed in the RV focused apical four-chamber view. RV volumes can be assessed with three-dimensional echo (3DE) and cardiac magnetic resonance imaging – further details of which are described in the sections below.
RV contractile function
During RV contraction, long axis shortening from base to apex together with lateral wall contraction towards the ventricular septum form the basis for RV function assessment. The LV is conical shaped, where geometric assumptions are more readily applied in volumes and ejection fraction calculations. However, a similar approach to the right ventricle is not feasible given its shape and inability to integrate all three regions within a single imaging plane. Thus, quantitative assessment of RV function by 2D echo modalities relies on surrogate markers and includes long axis function (M-mode and tissue Doppler imaging), fractional area change, RV index of myocardial performance, RV global longitudinal strain and 3D RV volumes and ejection fraction. No single measurement has been validated in different conditions and hence a combined approach integrating several measurements is advocated (15). A large number of potential measurements are described; however, for the purpose of this review, key parameters needed in risk stratification, in routine clinical practice will be discussed (Fig. 2 and Table 1). For a comprehensive overview, the reader is referred to international guidelines (15, 16).
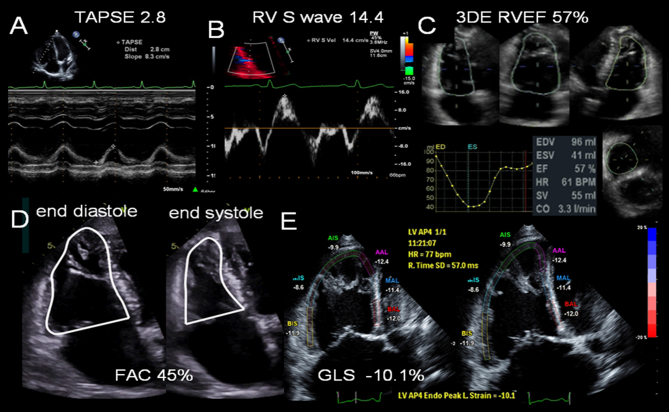
Figure 2.
Right ventricular function parameters measured by echocardiography. (A) Tricuspid annular plane systolic excursion, TAPSE. (B) Tissue Doppler imaging at the RV base demonstrating regional longitudinal shortening, S wave. (C) 3D echo assessment of RV volumes and ejection fraction (RVEF). (D) Fractional area change calculation from the RV focused four-chamber view. The endocardium is traced (beneath the trabeculations) in end-systole and end-diastole. (E) Lateral and septal wall strain (six-segment) imaging to calculate global longitudinal strain, GLS, of the RV. RV strain is reduced −10.1%.
Long axis function
Long axis function, peak systolic motion of the RV base towards the apex, represents longitudinal myocardial fibre shortening. This can be easily documented using M-mode (tricuspid annular systolic plane excursion, TAPSE) and tissue Doppler imaging (TDI S wave) placed over the lateral wall at the TV annular plane. To avoid underestimation, optimal alignment to the annular plane motion in the apical four-chamber view is recommended. Both methods correlate strongly with radionuclide angiography (17), biplane Simpson RV ejection fraction and RV fractional area change (18). It is the most utilised parameter in RV function assessment due to its ease of acquisition and reproducibility (19, 20). A TAPSE <17 mm or TDI S wave <9.5 cm/s are considered abnormal (15). An important limitation is the assumption that lateral wall motion (local basal function in the case of TDI) reflects global function. Therefore, assessment should be in the context of wall motion and integrated with all other RV function parameters. In severe TR with preserved systolic function TAPSE and TDI have supra-normal values (due to a hyperdynamic volume loaded RV); thus, measurements falling into the normal range indicate RV dysfunction.
RV index of myocardial performance
RV index of myocardial performance (MPI) is a global estimate of both systolic and diastolic ventricular function since it includes the isovolumic periods in its calculations. It describes the relationship between ejection and non-ejection work of the RV and has been shown to be a prognostic marker in pulmonary hypertension (25). In the normal RV, isovolumic periods are near absent; however, as RV ejection falls, these intervals gradually extend. The MPI is particularly useful in serial measurements correlating well with clinical status (26). However, there are important limitations to its routine clinical use including load dependency and inaccuracy with irregular heart rates (27). In the setting of severe TR, high right atrial pressures shorten isovolumic periods making this measure unreliable.
RV volumes and RV ejection fraction
RV volume changes reflect the degree of volume overload and RV dysfunction. Therefore, quantifying RV volumes is important in diagnosis and risk stratification of patients with tricuspid regurgitation. As described earlier, both inflow and outflow lie in different planes to the body and apex of the RV. Hence, geometric assumptions applied to the LV cannot be reliably used to calculate volumes using the standard 2D echocardiographic views. 3D techniques overcome such limitations. The current gold standard for calculating RV volumes and RV ejection fraction (RVEF) is cardiac magnetic resonance imaging (CMR). Recent pooled data (28) defined normal values for RV end systolic and diastolic volumes indexed to body surface area and RV ejection fraction (Table 1). However, CMR is relatively expensive and not widely available. 3DE techniques are improving and although not available in the majority of Echo Labs as yet show considerable promise. Current guidelines (15) cut-off values are given in Table 1. Despite a systematic underestimation of volumes (although not ejection fraction) of approximately 20% by 3DE (likely reflecting limitations in endocardial border detection and incomplete capture of the entire RV within the 3DE window), studies comparing 3DE with CMR have shown good correlation (29, 30). The semi-automated border detection method is recommended (15).
RV end diastolic volume index (RVEDVI) has been shown to be a predictor of post-surgery RV dysfunction in a number of patient groups in volume overload states. This includes congenital heart disease patients undergoing pulmonary valve replacement in Tetralogy of Fallot’s for pulmonary regurgitation, where a RVEDVI >150–170 mL/m2 suggested reverse remodelling was unlikely to occur. In another study, preoperative RVEDVI of 164 mL/m2 effectively discriminated patients with normal from those with reduced RVEF post surgery, with a sensitivity of 77% and a specificity of 72% (P = 0.01) (31). Therefore, significant RV dilatation can be considered when indexed values reach 150 mL/m2 and beyond.
RV global longitudinal strain
RV global longitudinal strain (GLS) is calculated as the percentage of systolic shortening of the RV, from base to apex. While this parameter is load dependant, it is less confounded by heart rates (as compared to strain rate). RV GLS provides important insights into RV performance, offering useful indicators of prognosis and functional capacity in a number of disease states (32, 33, 34). It has been shown to correlate with myocardial fibrosis, a marker of poor prognosis (35). Two methods for strain analysis exist and each has limitations in image acquisition. Speckle tracking echo relies on image quality, while colour tissue Doppler echo is angle dependant. The RV focused four-chamber view is acquired with care taken to ensure the endocardium and mid wall of all segments are optimised and seen throughout the cardiac cycle, with correct alignment in the case of TDI. Previously, differing cut-off values have been published using a variety of vendors, with a range of different image acquisition and software analysis methodologies, making its clinical use challenging. Data can be analysed from the lateral wall alone or in combination with the septal wall, three or six segment methods respectively. The former method typically yields higher averaged values (absolute value, normal >23%) than the latter (normal >20%) (36). Current published data are limited and guideline recommendations consider normal GLS of the RV lateral (free) wall to be <−20% (absolute value of >20%) (15), although specific vendors may have a slightly higher absolute cut-off value (as in the case of GE healthcare >23% (36)). Recent taskforce recommendations have set out a standardised method for data acquisition and analysis (37) with the aim of improving agreement between vendors in the future. RV GLS in the setting of severe TR would be expected to be ≥20% absolute valve, if the RV contractility remains normal.
RV diastolic dysfunction
RV diastolic dysfunction, similar to the LV, can give important insights into early disease when abnormalities in contractility, through other parameters, may not be evident. Increased wall thickness in response to pressure overload is seen later in disease progression. In addition, diastolic dysfunction is a marker of poor prognosis in many RV pathologies and usefully reflecting response to pharmacological interventions (38, 39). Typically, a combination of trans-tricuspid inflow Doppler pattern, TDI of the basal RV lateral wall and right atrial (RA) size help to describe the severity of diastolic dysfunction. In severe TR, as in other conditions where RA pressure is raised, diastolic function can be challenging and difficult to interpret, particularly in the presence of atrial fibrillation.
Right atrial dilatation
Right atrial dilatation is a marker of poor prognosis and is seen is RV systolic and diastolic dysfunction, pulmonary hypertension with raised RV filling pressures and severe TR. Unlike LA measurements performed using two orthogonal views, the RA size is assessed in the RV focused four-chamber view. The frame where the RA appears largest (usually ventricular end systole) is traced. From this single-plane view, the RA volume is calculated using the area-length or summation of discs method. Absolute RA values tend to be slightly larger in men (25 ± 7 mL/m2) than in women (21 ± 6 mL/m2) and are given as indexed values for body surface area (15). RA dilatation is a common finding in chronic severe TR.
Assessment of RV function during exercise: contractile reserve concept
Dynamic evaluation of cardiac structure and function under physiological or pharmacological stress can unmask underlying abnormalities including the development of symptoms, exercise limitation, valve and ventricular dysfunction and pulmonary hypertension (16). RV function at rest has been shown to be a useful predictor of those with an abnormal cardiopulmonary exercise response in heart failure, where a reduced exercise capacity is known to be a poor prognostic marker (8). However, additional information on RV function and its response to altered loading conditions during exercise has merit. RV contractile reserve has been assessed non-invasively by a number of markers including long axis parameters, FAC and global longitudinal strain (40, 41, 42). In a normal population TAPSE, TDI S wave, pulmonary artery systolic pressure (PASP) and TAPSE/PASP ratio were shown to correlate with maximal exercise capacity (43). RV response during exercise appears to be a surrogate marker of ventricular–arterial coupling in chronic RV pressure overload states (44) and may unmask underlying RV dysfunction in a number of conditions. Further, RV contractile reserve offers incremental prognostic value, in addition to LV contractile reserve, in dilated cardiomyopathy (41).
Therefore, RV contractile reserve evaluation during exercise may add valuable information. Physiological (treadmill or bike) and pharmacological (dobutamine) stress echo are described in the assessment of RV contractile reserve. Exercise bike stress echocardiography (our preferred method) allows evaluation of symptoms and acquisition of serial data. PASP is known to fall towards resting levels rapidly and peak stress data should be acquired within 1 min of exercise cessation. The key RV parameters assessed should include TAPSE, TDI S wave, FAC, TR velocity and GLS. LV parameters are usually concomitantly assessed along with other valve lesions depending on the clinical scenario (16). Contractile reserve is considered present where RV cavity reduces in size with augmentation of all RV function parameters. Currently data are limited and standardised values are yet to be established. Studies demonstrate an increase in each value by at least a few points (typically TAPSE >3 mm, TDI S >2 cm/s, FAC >5% or GLS >2%) where RV contractile reserve is associated with improved prognosis (45, 46). When compared to baseline, no increase or reduction in values during exercise indicates absence of contractile reserve and underlying RV dysfunction, where early intervention may need to be considered.
Summary
Tricuspid regurgitation natural history and treatment remains poorly understood. Right ventricular function is a key factor in determining prognosis, timing for intervention and longer-term outcome. Central to the assessment of TR is defining more precisely RV function. A practical and systematic approach should include RV dimensions, volumes and surrogate markers of contractility (RV long axis function, FAC) as well as RV EF (either 3DE or MRI). Where further clarification in terms of risk stratification may be required, GLS and functional testing with assessment of contractile reserve should be utilised. Part two of this article will provide an understanding of the causes of tricuspid regurgitation in the current era, with emphasis on key patient groups and their management.
Part 2: Tricuspid regurgitation and the right ventricle in risk stratification and timing of intervention
Assessment of tricuspid regurgitation severity
Traditional severity grading has focused on colour Doppler regurgitant jet imaging and assessed from multiple echo windows. Reliance on jet size and expansion within the right atrium (RA) is limited by a number of technical and haemodynamic factors. Vena contracta width is the recommended parameter in TR severity grading, where ≥7 mm is indicative of severe TR (47). Quantitative proximal isovelocity surface area (PISA) method (also known as flow convergence method) is possible, but less often used, where severe TR is defined as an effective regurgitant orifice (EROA) ≥40 mm2 with a regurgitant volume ≥45 mL (47). Available study data are less well defined than for mitral regurgitation, and discrepancies in parameters may reflect the regurgitant orifice morphology, in particular for functional TR, being more often elliptical rather than circular in shape. Newer techniques such as vena contracta area (48) are likely to offer improved diagnostic accuracy. TR defined by the EROA cut-off ≥40 mm2 has been shown to be associated with excess mortality and morbidity during 10-year follow up in a recent publication (49). Evaluation of TR severity requires a holistic approach with supportive evidence for severe regurgitation (Fig. 3). In the era of transcatheter tricuspid valve therapies, it has become clear that standard 2D methods underestimate the true severity of TR. This is evident through 3D assessment of the regurgitant orifice (50). A new grading system, utilising 3D vena contracta area as an additional parameter, has been proposed with three grades within the ‘severe’ category (severe, massive and torrential) (51, 52).
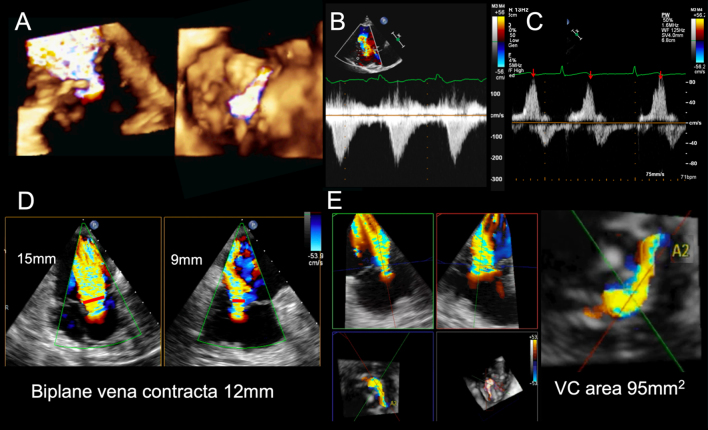
Figure 3.
Selection of tricuspid valve regurgitation parameters. (A) Is a 3D colour flow full-volume acquisition of severe TR and demonstrates the flow convergence, vena contracta and jet expansion upwards into the RA (left image) and the en face view of the flow convergence viewed from the RV aspect (right image). The regurgitant orifice is an irregular elliptical shape. (B) Continuous wave Doppler profile of severe tricuspid regurgitation, demonstrating a triangular low velocity jet. In this setting, estimation of RV systolic pressures will be underestimated due to rapid pressure equalization between RA and RV. (C) Depicts systolic flow reversal, seen in severe tricuspid regurgitation, during hepatic vein flow interrogation. (D) 3D echo format, X-plane, where orthogonal planes in 2D are imaged simultaneously. The vena contracta (red arrows) is longer in the left view and highlights an elliptical regurgitant orifice, confirming the findings in image A. (E) Shows multiplane reconstruction format of the 3D vena contracta and measurement of the vena contracta area and demonstrates massive TR VC area 95 mm2. VC, vena contracta.
Aetiology: modern day causes of tricuspid regurgitation and clinical groups presenting with severe tricuspid regurgitation
The relationship of the RV with TR is complex. Treatment options are governed by the underlying aetiology (Table 2) and hence an understanding of the mechanisms for TR is essential (53) (Fig. 4). While mild tricuspid regurgitation is a common finding, it is usually benign. However, severe tricuspid regurgitation is associated with poor outcomes (4). TR can occur as a result of primary valve disease (20%) or more often be secondary to annular dilatation usually from RV dilatation and dysfunction (80%). However, in the case of functional TR, an increasingly recognised cause is right atrial dilatation in the setting of atrial fibrillation (53). Commonly encountered clinical groups who may be consideration for intervention are discussed below.
Table 2.
Causes of tricuspid regurgitation.
| Cause | Underlying aetiology |
|---|---|
| Primary valve disease | |
| Congenital (e.g. Ebstein’s anomaly, atrioventricular septal defect) | |
| Leaflet prolapse | |
| Rheumatic heart disease | |
| Infection | |
| Carcinoid | |
| Radiation | |
| Blunt trauma | |
| Iatrogenic | |
| Pacing or defibrillator lead implantation | |
| Rare causes | |
| Drugs (e.g. methysergide, pergolide) | |
| Autoimmune disorders (e.g. SLE) | |
| Cardiac tumours | |
| Endomyocardial fibrosis | |
| Secondary (associated with RV or RA dilatation) | |
| Pressure overload states | Secondary to raised pulmonary vascular resistance Pre-capillary pulmonary (arterial) hypertension |
| Post-capillary pulmonary (venous) hypertension | |
| Volume overload | Shunts e.g. atrial septal defects |
| Pulmonary regurgitation | |
| Intrinsic myocardial disease | RV ischaemia |
| RV cardiomyopathy (e.g. arrhythmogenic RV cardiomyopathy) | |
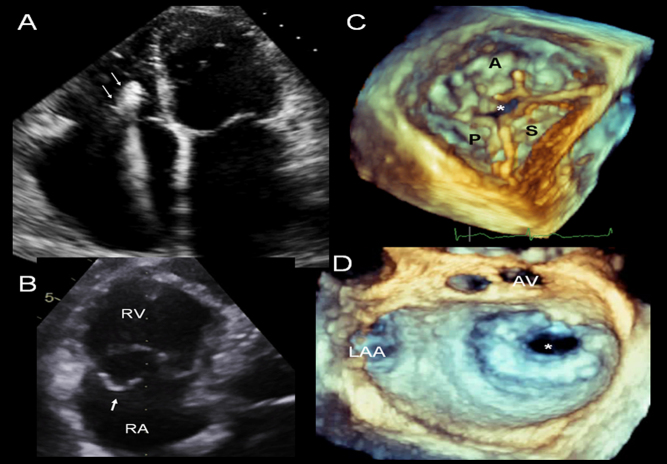
Figure 4.
Examples of modern day aetiologies responsible for tricuspid regurgitation. Four-chamber view showing RV pacing lead with echo bright regions (likely reflecting fibrosis) and tethering of the lead to the septal TV leaflet, which resulted in severe regurgitation. (A) 3D imaging is an important adjunct in providing details of the underlying abnormalities and leaflet involvement; en face views from the RV surface and multiplane reconstruction formats are particularly useful in this regard. (B) TV posterior leaflet prolapse and flail segment resulting from a sheering injury with severe regurgitation. (C) 3D zoom mode echo image of tricuspid valve viewed from the RV surface, demonstrating loss of coaptation in the central portion of the valve (*). This has resulted from TV annular dilatation as a consequence of RV dilatation in the setting of pulmonary hypertension. There is massive tricuspid regurgitation in, S septal, P posterior and A, anterior leaflets. (D) 3D zoom mode surgical view of the mitral valve depicting severe mitral stenosis (* denotes mitral valve orifice), a typical left heart valve lesion that may result in post capillary pulmonary hypertension and functional tricuspid regurgitation. AV aortic valve; LAA, left atrial appendage; RA, right atrium; RV, right ventricle.
Primary tricuspid valve regurgitation
Primary tricuspid valve leaflet disease can occur from a number of aetiologies (Table 2). RV size and function along with right atrial size are usually initially preserved with an absence of pulmonary hypertension (PH). RV volume overload causes progressive RA and RV dilatation and over time RV dysfunction. Clues to the possible aetiology are often evident from the patient’s history (e.g. previous blunt trauma to the chest or radiotherapy treatment) and clinical examination (e.g. autoimmune disorders). A detailed assessment of the TV anatomy is essential and 3D echo imaging plays a crucial role. Identification of abnormalities including leaflet prolapse with chordal rupture or leaflet thickening with retraction and chordal shortening may support the diagnosis. Isolated TV surgery is indicated in symptomatic severe primary TR (Class I) and should be considered in asymptomatic or mildly symptomatic patients with RV enlargement or deteriorating RV function (Class IIa) (54, 55). In asymptomatic severe primary TR, functional testing may be useful to unmask symptoms and assess for the abnormal RV contractile reserve (see Part 1). If serial follow-up demonstrates evidence of gradual RV dilatation and particularly if RV contractile reserve is abnormal or falling, despite remaining asymptomatic, then in our institution, consideration is given to early surgery if TV repair is likely.
Left heart disease and tricuspid regurgitation
The presence of TR in patients undergoing left heart (LH) valve surgery requires careful assessment to decide whether concomitant TV repair is indicated. Severe TR, even if functional in origin, does not predictably improve following correction of the left heart valve lesion despite a fall in RV afterload. Surgical correction for severe TR at the time of LH valve disease is a Class I indication (54, 55, 56). Further, less than severe TR may progress over months to years to severe symptomatic TR following left heart valve surgery with an associated poor survival (3). Therefore, concomitant TV repair should be considered in those undergoing left heart valve surgery, where TR is mild or moderate, and there is evidence of TV annular dilatation (>40 mm or 21 mm/m2 measured on echo RV focused four chamber view) Class IIa indication for surgery.
Left heart disease (LHD) with progressive TR is associated with pulmonary hypertension (PH) resulting from an increase in pulmonary venous (post-capillary) pressure, through backward passive transmission of filling pressures and is termed passive or isolated post-capillary PH (IpcPH) (57). Pulmonary arterial pressure and hence pulmonary vascular resistance (PVR) are initially not raised. This is typically seen with mitral valve disease (although increasingly aortic stenosis) and LV dysfunction (both systolic and diastolic). Increasing RV afterload results in RV dilatation. Progressive RV contractile dysfunction and remodelling exacerbates functional tricuspid regurgitation through a combination of tricuspid annulus dilatation and papillary muscle displacement from RV remodelling. Hence, a superimposed volume overload state further adds to RV dysfunction triggering a vicious cycle of RV dilatation and progressive functional TR. Prolonged chronic IpcPH can result in a reactive pulmonary arterial vasoconstriction characterised by an increase in PVR with a raised transpulmonary gradient. The increased pulmonary artery vasomotor tone initially is reversible. In some cases, progressive pathological changes with fixed and obstructive remodelling of the pulmonary arteries may also occur. This combined post- and pre-capillary PH (CpcPH) causes further increases in pulmonary arterial pressures and PVR, further increasing RV afterload. TR may further worsen as RV dilation and dysfunction progress. Differentiation between isolated and combined pulmonary hypertension has important implications in risk stratification and treatment options. A useful additional parameter is diastolic pressure gradient (DPG), which is thought to be more reliable in differentiating a true vasculopathy. A DPG ≥7 mmHg in combination with a raised PVR >3 wood units suggests CpcPH (57, 58). This finding is associated with a worse pulmonary arterial compliance, RV ejection fraction, exercise tolerance and life expectancy as well as higher surgical risk. Hence, careful assessment including right heart catheterisation and RV function assessment may be necessary when considering treatment options.
Residual tricuspid regurgitation post MV surgery
Significant TR late after LH valve surgery is found frequently ranging from 8% to as much as 49% (59, 60, 61). Studies highlighting the association of TR severity with heart failure, hospital admissions and death often failed to assess RV function (59, 60, 61, 62). Kammerlander et al. demonstrated RV dysfunction rather than severe TR to be the best predictor of outcome during long-term follow-up in 539 patients after LH valve surgery (1). Following surgery, the TV annulus may continue to dilate despite the improvement and/or absence of pulmonary hypertension. The reasons for this are not fully understood; however, age, presence of AF and left atrial dilatation are predictors of significant late TR (59, 60, 61, 62). Treatment of this patient group is challenging. Redo TV surgery carries high early mortality (63); Guenther et al. demonstrated operative mortality of 19% at 30 days (64). Careful risk stratification before considering corrective TV surgery should include not only RV function status and pulmonary vascular haemodynamic assessment, but also the consequences of chronic systemic venous hypertension resulting in congestion and hypoperfusion. Perioperative mortality usually results from right heart failure and hepatorenal syndrome, progressing to multi-organ failure and death. Patients who demonstrate a raised bilirubin and creatinine with evidence of hypersplensim where haemoglobin and platelet counts are reduced, despite optimisation of right heart failure, typically reflect end-stage disease. Conventional surgical intervention carries high mortality and those who might survive often show little symptomatic improvement.
Pacemaker, cardiac resynchronisation or cardioverter-defibrillator lead-associated tricuspid regurgitation
Two patient groups exist, those with bradyarrhythmia indicated pacemakers who usually have preserved LV and RV function and those with LV impairment qualifying for LV function optimisation and/or prevention of sudden death. The incidence of TR has been reported between 6 and 10% (2, 65, 66). Common to both groups is lead placement across the TV into the RV and its potential to interfere with valve function. Mechanical complications of lead insertion were noticed during post-mortem studies (67, 68) and confirmed during surgical procedures to correct pacing lead-related severe TR (69), falling into three categories: TV chordal apparatus entanglement, septal leaflet impingement and leaflet perforation. A longer-term fibrous reaction has been documented at the site of lead contact with endocardium resulting in lead encapsulation, ensheathment or entrapment further compounding valve dysfunction through tethering the leaflets and subvalvular apparatus. Detail imaging is essential and 3D echo and multiplane reconstruction formats are particularly useful to ascertain the relationship of the RV lead with the TV leaflets and subvalve apparatus.
The aetiology of TR and its treatment should be placed in the context of baseline ventricular function and the existence and severity of TR prior to RV lead implantation. Pre-existing functional TR as a result of LH disease and pulmonary hypertension may worsen following pacing lead implantation, where relatively small increases result in clinically significant TR. Accurate assessment of RV function, pulmonary hypertension severity, LV dysfunction along with the consequences of venous congestion is required to appropriately risk stratify and decide on the merits of treatment. CMR may be contra-indicated in this patient group (although increasingly devices are becoming CMR compatible). RV volumes and RVEF can be obtained using 3DE, but if not available, cardiac computerised tomography may be a useful alternative. Treatment options may include minimising pacing burden (in the case of brady-pacing where the native heart beat allows for a synchronised ventricular contraction), lead repositioning with preferential His-bundle pacing to improve RV dysfunction and subsequently TR severity or surgical lead explanation and TV repair (70, 71). The latter is usually reserved for those with previously preserved biventricular function where TR is a consequence of RV lead interference. The RV is often dilated and function hyperdynamic from volume overload. Patients typically present with progressive breathlessness sometime after pacemaker implantation and if not recognised may progress to right heart failure. At this stage, careful assessment of RV function is needed to ensure a point where significant RV dysfunction has not been reached, where surgically addressing the TR will not confer any benefit but instead present significant perioperative risks. Lastly, TR may also result from trauma during lead extraction or lead-related infection. Assessment of RV function should include RV contractile reserve, RV volumes and RVEF. Pulmonary hypertension is not usually a feature in this patient group unless there is LH disease.
AF-associated tricuspid regurgitation
Recent studies have demonstrated isolated functional TR is associated with RA enlargement in the setting of chronic atrial fibrillation (72). RA remodelling has been shown to result in TV annular dilatation with a loss of annular leaflet coverage reserve without leaflet tethering (73). The TV annulus is more circular in shape, more dilated and planar when compared to functional TR due to LH disease in sinus rhythm. While TV annular area has been shown to be principally associated with RV volumes in LH-associated TR, in the case of functional TR secondary to AF, it strongly correlates with RA volumes. Typically, patients are more often older, female, hypertensive, have a greater degree of RA dilation compared to the left atrium and lower pulmonary artery pressures. However, in terms of treatment, surgical correction is considered only after heart rhythm and rate control, as well as ventricular function and heart failure, has been optimised. Once achieved waiting up to 6 months may be necessary to see the full effects of RA remodelling and TR improvements. If TR remains severe, then the standard work up focused on quantifying any RV dysfunction, pulmonary hypertension, associated co-morbidities and signs of end-organ damage will help determine the appropriateness for surgical intervention.
Tricuspid regurgitation secondary to pulmonary hypertension and RV pressure overload
Functional TR in the setting of progressive pulmonary arterial hypertension (PAH) reflects disease of the RV. RV dilatation and dysfunction are the precursor for secondary TR. Therefore, treatment is focused on diagnosing the cause of PAH and targeting appropriate therapy, which may include pulmonary vasodilators. Prognosis is associated with RV function and targeted therapy aims to reduce RV afterload with a concomitant improvement in RV function. Severity of TR often improves with RV reverse remodelling. Where severe TR persists, it usually reflects persistent RV dysfunction, where residual TR may paradoxically work to off-load the failing RV. Therefore, TV surgical intervention is rarely considered and reserved for carefully selected patients undergoing treatment. This includes pulmonary thromboendarterectomy, where the expected reduction in pulmonary hypertension and RV afterload will preserve RV function and support any concurrent TV intervention.
Transcatheter therapies
Lastly, for patients who are deemed high risk for surgery, emerging transcatheter TV technologies may offer hope. These include leaflet repair for example, MitraClip (Abbott Vascular); annuloplasty repair e.g. TriCinch (4TECH), Trialign (Mitraling), Cardioband (Edwards Lifescience); valve replacement e.g. Caval valve replacement (CAVI) or a combination of these techniques (74). While feasibility has been established, this technology is at an early stage with patient selection and outcomes yet to be clearly defined.
Summary
Tricuspid regurgitation natural history and treatment remain poorly understood. Right ventricular function is a key factor in determining prognosis, timing for intervention and longer-term outcome. The paucity of published data reflects some uncertainty in the management of tricuspid regurgitation. Historic data have suggested surgical treatment of tricuspid regurgitation carries high mortality. Central to the assessment of severe TR is a clear understanding of the underlying aetiology and defining more precisely RV dysfunction. RV function assessment is central to assessment in TR. Early surgery should be considered in primary severe tricuspid regurgitation with RV dilatation even prior to significant symptoms and underlines the importance of functional assessment and exercise contractile reserve. Functional severe TR represents a different cohort of patients who have pulmonary hypertension and/or primary RV dysfunction responsible for tricuspid annular dilatation and severe secondary TR. Those presenting with RV failure reflect a potentially high-risk group with often complex disease, involving RV dysfunction, pulmonary hypertension, hepatic and renal complications, with additional pulmonary and left heart disease, who may have undergone previous cardiac surgery. Optimising medical therapy is essential following which careful assessment and work-up are needed to ensure intervention is performed in a timely and appropriate manner (Table 3).
Table 3.
RV function in risk stratification for surgical intervention in severe TR.
| Summary of key points |
|
Declaration of interest
Petros Nihoyannopoulos is the co-editor-in-chief of Echo Research and Practice. He was not involved in the review or editorial process for this paper, on which he is listed as an author. The other authors have nothing to disclose.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- 1.Kammerlander AA, Marzluf BA, Graf A, Bachmann A, Kocher A, Bonderman D, Mascherbauer J. Right ventricular dysfunction, but not tricuspid regurgitation, is associated with outcome late after left heart valve procedure. Journal of the American College of Cardiology 2014. 64 2633–2642. ( 10.1016/j.jacc.2014.09.062) [DOI] [PubMed] [Google Scholar]
- 2.Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. Journal of the American College of Cardiology 2004. 43 405–409. ( 10.1016/j.jacc.2003.09.036) [DOI] [PubMed] [Google Scholar]
- 3.Ruel M, Rubens FD, Masters RG, Pipe AL, Bédard P, Mesana TG. Late incidence and predictors of persistent or recurrent heart failure in patients with mitral prosthetic valves. Journal of Thoracic and Cardiovascular Surgery 2004. 128 278–283. ( 10.1016/j.jtcvs.2003.11.048) [DOI] [PubMed] [Google Scholar]
- 4.Topilsky Y, Nkomo VT, Vatury O, Michelena HI, Letourneau T, Suri RM, Pislaru S, Park S, Mahoney DW, Biner S, et al Clinical outcome of isolated tricuspid regurgitation. JACC: Cardiovascular Imaging 2014. 7 1185–1194. ( 10.1016/j.jcmg.2014.07.018) [DOI] [PubMed] [Google Scholar]
- 5.Antunes MJ, Rodríguez-Palomares J, Prendergast B, De Bonis M, Rosenhek R, Al-Attar N, Barili F, Casselman F, Folliguet T, Iung B, et al Management of tricuspid valve regurgitation. European Journal of Cardio-Thoracic Surgery 2017. 52 1022–1030. ( 10.1093/ejcts/ezx279) [DOI] [PubMed] [Google Scholar]
- 6.Kim YJ, Kwon DA, Kim HK, Park JS, Hahn S, Kim KH, Kim KB, Sohn DW, Ahn H, Oh BH, et al Determinants of surgical outcome in patients With isolated tricuspid regurgitation. Circulation 2009. 120 1672–1678. ( 10.1161/CIRCULATIONAHA.109.849448) [DOI] [PubMed] [Google Scholar]
- 7.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. Journal of the American College of Cardiology 2001. 37 183–188. ( 10.1016/S0735-1097(00)01102-5) [DOI] [PubMed] [Google Scholar]
- 8.Webb-Peploe KM, Henein MY, Coats AJS, Gibson DG. Echo derived variables predicting exercise tolerance in patients with dilated and poorly functioning left ventricle. Heart 1998. 80 565–569. ( 10.1136/hrt.80.6.565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis JF, Webber JD, Sutton LL, Chesoni S, Curry CL. Discordance in degree of right and left ventricular dilation in patients with dilated cardiomyopathy: recognition and clinical implications. Journal of the American College of Cardiology 1993. 21 649–654. ( 10.1016/0735-1097(93)90097-K) [DOI] [PubMed] [Google Scholar]
- 10.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. European Heart Journal 2014. 35 3452–3462. ( 10.1093/eurheartj/ehu193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sylva M, van den Hoff MJB, Moorman AFM. Development of the human heart. American Journal of Medical Genetics Part A 2014. 164A 1347–1371. ( 10.1002/ajmg.a.35896) [DOI] [PubMed] [Google Scholar]
- 12.Harvey RP. Patterning the vertebrate heart. Nature Reviews Genetics 2002. 3 544–556. ( 10.1038/nrg843) [DOI] [PubMed] [Google Scholar]
- 13.Hoffman D, Sisto D, Frater RW, Nikolic SD. Left-to-right ventricular interaction with a noncontracting right ventricle. Journal of Thoracic and Cardiovascular Surgery 1994. 107 1496–1502. [PubMed] [Google Scholar]
- 14.Le Tourneau T, Deswarte G, Lamblin N, Foucher-Hossein C, Fayad G, Richardson M, Polge AS, Vannesson C, Topilsky Y, Juthier F, et al Right ventricular systolic function in organic mitral regurgitation: impact of biventricular impairment. Circulation 2013. 127 1597–1608. ( 10.1161/CIRCULATIONAHA.112.000999) [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European Heart Journal: Cardiovascular Imaging 2015. 16 233–271. ( 10.1093/ehjci/jev014) [DOI] [PubMed] [Google Scholar]
- 16.Lancellotti P, Pellikka PA, Budts W, Chaudhry FA, Donal E, Dulgheru R, Edvardsen T, Garbi M, Ha JW, Kane GC, et al The clinical use of stress echocardiography in non-ischaemic heart disease: recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Journal of the American Society of Echocardiography 2017. 30 101–138. ( 10.1016/j.echo.2016.10.016) [DOI] [PubMed] [Google Scholar]
- 17.Kaul S, Tei C, Hopkins JM, Shah PM. Assessment of right ventricular function using two-dimensional echocardiography. American Heart Journal 1984. 107 526–531. ( 10.1016/0002-8703(84)90095-4) [DOI] [PubMed] [Google Scholar]
- 18.D’Oronzio U, Senn O, Biaggi P, Gruner C, Jenni R, Tanner FC, Greutmann M. Right heart assessment by echocardiography: gender and body size matters. Journal of the American Society of Echocardiography 2012. 25 1251–1258. ( 10.1016/j.echo.2012.08.013) [DOI] [PubMed] [Google Scholar]
- 19.Tamborini G, Pepi M, Galli CA, Maltagliati A, Celeste F, Muratori M, Rezvanieh S, Veglia F. Feasibility and accuracy of a routine echocardiographic assessment of right ventricular function. International Journal of Cardiology 2007. 115 86–89. ( 10.1016/j.ijcard.2006.01.017) [DOI] [PubMed] [Google Scholar]
- 20.Innelli P, Esposito R, Olibet M, Nistri S, Galderisi M. The impact of ageing on right ventricular longitudinal function in healthy subjects: a pulsed tissue Doppler study. European Journal of Echocardiography 2009. 10 491–498. ( 10.1093/ejechocard/jen313) [DOI] [PubMed] [Google Scholar]
- 21.Anavekar NS, Gerson D, Skali H, Kwong RY, Kent Yucel EK, Solomon SD. Two-dimensional assessment of right ventricular function: an echocardiographic–MRI correlative study. Echocardiography 2007. 24 452–456. ( 10.1111/j.1540-8175.2007.00424.x) [DOI] [PubMed] [Google Scholar]
- 22.Nass N, McConnell MV, Goldhaber SZ, Chyu S, Solomon SD. Recovery of regional right ventricular function after thrombolysis for pulmonary embolism. American Journal of Cardiology 1999. 83 804–806, A10 ( 10.1016/S0002-9149(98)01000-5) [DOI] [PubMed] [Google Scholar]
- 23.Zornoff LAM, Skali H, Pfeffer MA, St John Sutton M, Rouleau JL, Lamas GA, Plappert T, Rouleau JR, Moyé LA, Lewis SJ, et al Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. Journal of the American College of Cardiology 2002. 39 1450–1455. ( 10.1016/S0735-1097(02)01804-1) [DOI] [PubMed] [Google Scholar]
- 24.Simsek E, Nalbantgil S, Ceylan N, Zoghi M, Kemal HS, Engin C, Yagdi T, Ozbaran M. Assessment of right ventricular systolic function in heart transplant patients: correlation between echocardiography and cardiac magnetic resonance imaging. Investigation of the accuracy and reliability of echocardiography. Echocardiography 2017. 34 1432–1438. ( 10.1111/echo.13650) [DOI] [PubMed] [Google Scholar]
- 25.Tei C, Dujardin KS, Hodge DO, Bailey KR, McGoon MD, Tajik AJ, Seward JB. Doppler echocardiographic index for assessment of global right ventricular function. Journal of the American Society of Echocardiography 1996. 9 838–847. ( 10.1016/S0894-7317(96)90476-9) [DOI] [PubMed] [Google Scholar]
- 26.Sebbag I, Rudski LG, Therrien J, Hirsch A, Langleben D. Effect of chronic infusion of epoprostenol on echocardiographic right ventricular myocardial performance index and its relation to clinical outcome in patients with primary pulmonary hypertension. American Journal of Cardiology 2001 88 1060–1063. ( 10.1016/S0002-9149(01)01995-6) [DOI] [PubMed] [Google Scholar]
- 27.Cheung MM, Smallhorn JF, Redington AN, Vogel M. The effects of changes in loading conditions and modulation of inotropic state on the myocardial performance index: comparison with conductance catheter measurements. European Heart Journal 2004. 25 2238–2242. ( 10.1016/j.ehj.2004.07.034) [DOI] [PubMed] [Google Scholar]
- 28.Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, Vogel-Claussen J, Turkbey EB, Williams R, Plein S, Tee M, Eng J, Bluemke DA. Normal values for cardiovascular magnetic resonance in adults and children. Journal of Cardiovascular Magnetic Resonance 2015. 17 29 ( 10.1186/s12968-015-0111-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khoo NS, Young A, Occleshaw C, Cowan B, Zeng ISL, Gentles TL. Assessments of right ventricular volume and function using three-dimensional echocardiography in older children and adults With congenital heart disease: comparison With cardiac magnetic resonance imaging. Journal of the American Society of Echocardiography 2009. 22 1279–1288. ( 10.1016/j.echo.2009.08.011) [DOI] [PubMed] [Google Scholar]
- 30.Niemann PS, Pinho L, Balbach T, Galuschky C, Blankenhagen M, Silberbach M, Broberg C, Jerosch-Herold M, Sahn DJ. Anatomically oriented right ventricular volume measurements with dynamic three-dimensional echocardiography validated by 3-tesla magnetic resonance imaging. Journal of the American College of Cardiology 2007. 50 1668–1676. ( 10.1016/j.jacc.2007.07.031) [DOI] [PubMed] [Google Scholar]
- 31.Kim H-K, Kim Y-J, Park E-A, Bae J-S, Lee W, Kim K-H, Kim K, Sohn D, Ahn H, Park J, et al Assessment of haemodynamic effects of surgical correction for severe functional tricuspid regurgitation: cardiac magnetic resonance imaging study. European Heart Journal 2010. 31 1520–1528. ( 10.1093/eurheartj/ehq063) [DOI] [PubMed] [Google Scholar]
- 32.Fine NM, Chen L, Bastiansen PM, Frantz RP, Pellikka PA, Oh JK, Kane GC. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circulation: Cardiovascular Imaging 2013. 6 711–721. ( 10.1161/CIRCIMAGING.113.000640) [DOI] [PubMed] [Google Scholar]
- 33.Alghamdi MH, Mertens L, Lee W, Yoo S-J, Grosse-Wortmann L. Longitudinal right ventricular function is a better predictor of right ventricular contribution to exercise performance than global or outflow tract ejection fraction in tetralogy of Fallot: a combined echocardiography and magnetic resonance study. European Heart Journal: Cardiovascular Imaging 2013. 14 235–239. ( 10.1093/ehjci/jes137) [DOI] [PubMed] [Google Scholar]
- 34.Vitarelli A, Cortes Morichetti M, Capotosto L, De Cicco V, Ricci S, Caranci F, Vitarelli M. Utility of strain echocardiography at rest and after stress testing in arrhythmogenic right ventricular dysplasia. American Journal of Cardiology 2013. 111 1344–1350. ( 10.1016/j.amjcard.2013.01.279) [DOI] [PubMed] [Google Scholar]
- 35.Lisi M, Cameli M, Righini FM, Malandrino A, Tacchini D, Focardi M, Tsioulpas C, Bernazzali S, Tanganelli P, Maccherini M, et al RV longitudinal deformation correlates with myocardial fibrosis in patients with end-stage heart failure. JACC: Cardiovascular Imaging 2015. 8 514–522. ( 10.1016/j.jcmg.2014.12.026) [DOI] [PubMed] [Google Scholar]
- 36.Muraru D, Onciul S, Peluso D, Soriani N, Cucchini U, Aruta P, et al Sex- and method-specific reference values for right ventricular strain by 2-dimensional speckle-tracking echocardiography. Circulation: Cardiovascular Imaging 2016. 9 e003866 ( 10.1161/CIRCIMAGING.115.003866) [DOI] [PubMed] [Google Scholar]
- 37.Voigt J-U, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, et al Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. European Heart Journal: Cardiovascular Imaging 2015. 16 1–11. ( 10.1093/ehjci/jeu184) [DOI] [PubMed] [Google Scholar]
- 38.Sallach JA, Tang WHW, Borowski AG, Tong W, Porter T, Martin MG, Jasper SE, Shrestha K, Troughton RW, Klein AL. Right atrial volume index in chronic systolic heart failure and prognosis. JACC: Cardiovascular Imaging 2009. 2 527–534. ( 10.1016/j.jcmg.2009.01.012) [DOI] [PubMed] [Google Scholar]
- 39.Gan CT-J, Holverda S, Marcus JT, Paulus WJ, Marques KM, Bronzwaer JGF, Twisk JW, Boonstra A, Postmus PE, Vonk-Noordegraaf A. Right ventricular diastolic dysfunction and the acute effects of sildenafil in pulmonary hypertension patients. Chest 2007. 132 11–17. ( 10.1378/chest.06-1263) [DOI] [PubMed] [Google Scholar]
- 40.Guazzi M, Villani S, Generati G, Ferraro OE, Pellegrino M, Alfonzetti E, Labate V, Gaeta M, Sugimoto T, Bandera F. Right ventricular contractile reserve and pulmonary circulation uncoupling during exercise challenge in heart failure. JACC: Heart Failure 2016. 4 625–635. ( 10.1016/j.jchf.2016.03.007) [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto K, Tanaka H, Onishi A, Motoji Y, Tatsumi K, Sawa T, Miyoshi T, Imanishi J, Mochizuki Y, Hirata K. Bi-ventricular contractile reserve offers an incremental prognostic value for patients with dilated cardiomyopathy. European Heart Journal: Cardiovascular Imaging 2015. 16 1213–1223. ( 10.1093/ehjci/jev069) [DOI] [PubMed] [Google Scholar]
- 42.Chia EM, Lau EMT, Xuan W, Celermajer DS, Thomas L. Exercise testing can unmask right ventricular dysfunction in systemic sclerosis patients with normal resting pulmonary artery pressure. International Journal of Cardiology 2016. 204 179–186. ( 10.1016/j.ijcard.2015.11.186) [DOI] [PubMed] [Google Scholar]
- 43.D’Alto M, Pavelescu A, Argiento P, Romeo E, Correra A, Di Marco GM, D’Andrea A, Sarubbi B, Russo MG, Naeije R. Echocardiographic assessment of right ventricular contractile reserve in healthy subjects. Echocardiography 2017. 34 61–68. ( 10.1111/echo.13396) [DOI] [PubMed] [Google Scholar]
- 44.Guihaire J, Haddad F, Noly P-E, Boulate D, Decante B, Dartevelle P, Humbert M, Verhoye J, Mercier O, Fadel E. Right ventricular reserve in a piglet model of chronic pulmonary hypertension. European Respiratory Journal 2015. 45 709–717. ( 10.1183/09031936.00081314) [DOI] [PubMed] [Google Scholar]
- 45.Almeida AR, Loureiro MJ, Lopes L, Cotrim C, Lopes L, Repolho D, Pereira H. Echocardiographic assessment of right ventricular contractile reserve in patients with pulmonary hypertension. Revista Portuguesa de Cardiologia 2014. 33 155–163. ( 10.1016/j.repc.2013.09.015) [DOI] [PubMed] [Google Scholar]
- 46.Vitel E, Galli E, Leclercq C, Fournet M, Bosseau C, Corbineau H, Bouzille G, Donal E. Right ventricular exercise contractile reserve and outcomes after early surgery for primary mitral regurgitation. Heart 2018. 104 855–860. ( 10.1136/heartjnl-2017-312097) [DOI] [PubMed] [Google Scholar]
- 47.Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. European Heart Journal: Cardiovascular Imaging 2013. 14 611–644. ( 10.1093/ehjci/jet105) [DOI] [PubMed] [Google Scholar]
- 48.Velayudhan DE, Brown TM, Nanda NC, Patel V, Miller AP, Mehmood F, et al Quantification of tricuspid regurgitation by live three-dimensional transthoracic echocardiographic measurements of vena contracta area. Echocardiography 2006. 23 793–800. ( 10.1111/j.1540-8175.2006.00314.x) [DOI] [PubMed] [Google Scholar]
- 49.Topilsky Y, Nkomo VT, Vatury O, Michelena HI, Letourneau T, Suri RM, Pislaru S, Park S, Mahoney DW, Biner S, et al Clinical outcome of isolated tricuspid regurgitation. JACC: Cardiovascular Imaging 2014. 7 1185–1194. ( 10.1016/j.jcmg.2014.07.018) [DOI] [PubMed] [Google Scholar]
- 50.Chen T-E, Kwon SH, Enriquez-Sarano M, Wong BF, Mankad SV. Three-dimensional color Doppler echocardiographic quantification of tricuspid regurgitation orifice area: comparison with conventional two-dimensional measures. Journal of the American Society of Echocardiography 2013. 26 1143–1152. ( 10.1016/j.echo.2013.07.020) [DOI] [PubMed] [Google Scholar]
- 51.Go YY, Dulgheru R, Lancellotti P. The conundrum of tricuspid regurgitation grading. Frontiers in Cardiovascular Medicine 2018. 5 164 ( 10.3389/fcvm.2018.00164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hahn RT, Zamorano JL. The need for a new tricuspid regurgitation grading scheme. European Heart Journal: Cardiovascular Imaging 2017. 18 1342–1343. ( 10.1093/ehjci/jex139) [DOI] [PubMed] [Google Scholar]
- 53.Antunes MJ, Rodríguez-Palomares J, Prendergast B, De Bonis M, Rosenhek R, Al-Attar N, Barili F, Casselman F, Folliguet T, Iung B, et al Management of tricuspid valve regurgitation. European Journal of Cardio-Thoracic Surgery 2017. 52 1022–1030. ( 10.1093/ejcts/ezx279) [DOI] [PubMed] [Google Scholar]
- 54.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, et al 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 2014. 63 e57–e185. ( 10.1016/j.jacc.2014.02.536) [DOI] [PubMed] [Google Scholar]
- 55.Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, et al Guidelines on the management of valvular heart disease, 2012 version: the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). European Heart Journal 2012. 33 2451–2496. ( 10.1093/eurheartj/ehs109) [DOI] [PubMed] [Google Scholar]
- 56.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, et al 2017 ESC/EACTS Guidelines for the management of valvular heart disease. European Heart Journal 2017. 38 2739–2791. ( 10.1093/eurheartj/ehx391) [DOI] [PubMed] [Google Scholar]
- 57.Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, et al 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. European Heart Journal 2016. 37 67–119. [DOI] [PubMed] [Google Scholar]
- 58.Vachiéry JL, Adir Y, Barberà JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galiè N, Ghio S, Gibbs JSR, et al Pulmonary hypertension due to left heart diseases. Journal of the American College of Cardiology 2013. 62 (25 Supplement) D100–D108. ( 10.1016/j.jacc.2013.10.033) [DOI] [PubMed] [Google Scholar]
- 59.Matsunaga A, Duran CMG. Progression of tricuspid regurgitation after repaired functional ischemic mitral regurgitation. Circulation 2005. 112 (9 Supplement) I453–I457. [DOI] [PubMed] [Google Scholar]
- 60.Song H, Kim MJ, Chung CH, Choo SJ, Song MG, Song JM, Kang DH, Lee JW, Song JK. Factors associated with development of late significant tricuspid regurgitation after successful left-sided valve surgery. Heart 2009. 95 931–936. ( 10.1136/hrt.2008.152793) [DOI] [PubMed] [Google Scholar]
- 61.Kwak J-J, Kim Y-J, Kim M-K, Kim H-K, Park J-S, Kim K-H, Kim K, Ahn H, Sohn D, Oh B, et al Development of tricuspid regurgitation late after left-sided valve surgery: a single-center experience with long-term echocardiographic examinations. American Heart Journal 2008. 155 732–737. ( 10.1016/j.ahj.2007.11.010) [DOI] [PubMed] [Google Scholar]
- 62.Matsuyama K, Matsumoto M, Sugita T, Nishizawa J, Tokuda Y, Matsuo T. Predictors of residual tricuspid regurgitation after mitral valve surgery. Annals of Thoracic Surgery 2003. 75 1826–1828. ( 10.1016/S0003-4975(03)00028-6) [DOI] [PubMed] [Google Scholar]
- 63.Shiran A, Sagie A. Tricuspid regurgitation in mitral valve disease. Journal of the American College of Cardiology 2009. 53 401–408. ( 10.1016/j.jacc.2008.09.048) [DOI] [PubMed] [Google Scholar]
- 64.Guenther T, Noebauer C, Mazzitelli D, Busch R, Tassani-Prell P, Lange R. Tricuspid valve surgery: a thirty-year assessment of early and late outcome. European Journal of Cardio-Thoracic Surgery 2008. 34 402–409; discussion 409. ( 10.1016/j.ejcts.2008.05.006) [DOI] [PubMed] [Google Scholar]
- 65.Schleifer JW, Pislaru SV, Lin G, Powell BD, Espinosa R, Koestler C, Thome T, Polk L, Li Z, Asirvatham SJ, et al Effect of ventricular pacing lead position on tricuspid regurgitation: a randomized prospective trial. Heart Rhythm 2018. 15 1009–1016. ( 10.1016/j.hrthm.2018.02.026) [DOI] [PubMed] [Google Scholar]
- 66.Lee RC, Freidman SE, Kono AT, Greenberg ML, Palac RT. Tricuspid regurgitation following implantation of endocardial leads: incidence and predictors. Pacing and Clinical Electrophysiology 2015. 38 1267–1274. [DOI] [PubMed] [Google Scholar]
- 67.Epstein AE, Kay GN, Plumb VJ, Dailey SM, Anderson PG. Gross and microscopic pathological changes associated. Circulation 1998. 98 1517–1524. [DOI] [PubMed] [Google Scholar]
- 68.Robboy SJ, Harthorne JW, Leinbach RC, Sanders CA, Austen WG. Autopsy findings with permanent pervenous pacemakers. Circulation 1969. 39 495–501. [DOI] [PubMed] [Google Scholar]
- 69.Uehara K, Minakata K, Watanabe K, Sakaguchi H, Yamazaki K, Ikeda T, Sakata R. Tricuspid valve repair for severe tricuspid regurgitation due to pacemaker leads. Asian Cardiovascular and Thoracic Annals 2016. 24 541–545. [DOI] [PubMed] [Google Scholar]
- 70.Chang JD, Manning WJ, Ebrille E, Zimetbaum PJ. Tricuspid valve dysfunction following pacemaker or cardioverter-defibrillator implantation. Journal of the American College of Cardiology 2017. 69 2331–2341. ( 10.1016/j.jacc.2017.02.055) [DOI] [PubMed] [Google Scholar]
- 71.Al-Bawardy R, Krishnaswamy A, Bhargava M, Dunn J, Wazni O, Murat Tuzcu E, Stewart W, Kapadia SR. Tricuspid regurgitation in patients with pacemakers and implantable cardiac defibrillators: a comprehensive review. Clinical Cardiology 2013. 36 249–254. ( 10.1002/clc.22104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cho JY, Kim KH, Kim JY, Sim DS, Yoon HJ, Yoon NS, Hong YJ, Park HW, Kim JH, Ahn Y, et al Predictors of reversible severe functional tricuspid regurgitation in patients with atrial fibrillation. Journal of Cardiology 2016. 68 419–425. ( 10.1016/j.jjcc.2015.11.010) [DOI] [PubMed] [Google Scholar]
- 73.Di Mauro M, Bezante GP, Di Baldassarre A, Clemente D, Cardinali A, Acitelli A, Salerni S, Penco M, Calafiore AM, Gallina S. Functional tricuspid regurgitation: an underestimated issue. International Journal of Cardiology 2013. 168 707–715. ( 10.1016/j.ijcard.2013.04.043) [DOI] [PubMed] [Google Scholar]
- 74.Taramasso M, Hahn RT, Alessandrini H, Latib A, Attinger-Toller A, Braun D, Brochet E, Connelly KA, Denti P, Deuschl F, et al The international multicenter TriValve registry: which patients are undergoing transcatheter tricuspid repair? JACC: Cardiovascular Interventions 2017. 10 1982–1990. ( 10.1016/j.jcin.2017.08.011) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a