Significance
Preeclampsia, a leading cause of maternal and perinatal morbidity and mortality worldwide, is accompanied by shallow placentation and deficient remodeling of the uterine spiral arteries by invasive placental trophoblast cells during the first trimester of pregnancy. Here, we generated induced pluripotent stem cells from umbilical cords of normal pregnancies and ones complicated by early onset preeclampsia (EOPE) and converted them to trophoblast to recapitulate events of early pregnancy. Parameters disturbed in EOPE, including trophoblast invasiveness, were assessed. Under low O2, both sets of cells behaved similarly, but, under the more stressful 20% O2 conditions, the invasiveness of EOPE trophoblast was markedly reduced. Gene expression changes in EOPE trophoblast suggested a dysregulation invasion linked to high O2.
Keywords: bone morphogenetic protein-4, cell migration, induced pluripotent stem cells, oxidative stress, transcriptome
Abstract
We describe a model for early onset preeclampsia (EOPE) that uses induced pluripotent stem cells (iPSCs) generated from umbilical cords of EOPE and control (CTL) pregnancies. These iPSCs were then converted to placental trophoblast (TB) representative of early pregnancy. Marker gene analysis indicated that both sets of cells differentiated at comparable rates. The cells were tested for parameters disturbed in EOPE, including invasive potential. Under 5% O2, CTL TB and EOPE TB lines did not differ, but, under hyperoxia (20% O2), invasiveness of EOPE TB was reduced. RNA sequencing analysis disclosed no consistent differences in expression of individual genes between EOPE TB and CTL TB under 20% O2, but, a weighted correlation network analysis revealed two gene modules (CTL4 and CTL9) that, in CTL TB, were significantly linked to extent of TB invasion. CTL9, which was positively correlated with 20% O2 (P = 0.02) and negatively correlated with invasion (P = 0.03), was enriched for gene ontology terms relating to cell adhesion and migration, angiogenesis, preeclampsia, and stress. Two EOPE TB modules, EOPE1 and EOPE2, also correlated positively and negatively, respectively, with 20% O2 conditions, but only weakly with invasion; they largely contained the same sets of genes present in modules CTL4 and CTL9. Our experiments suggest that, in EOPE, the initial step precipitating disease is a reduced capacity of placental TB to invade caused by a dysregulation of O2 response mechanisms and that EOPE is a syndrome, in which unbalanced expression of various combinations of genes affecting TB invasion provoke disease onset.
Preeclampsia (PE), whether of the early or later onset form (1, 2), is characterized by gestational hypertension and proteinuria, with onset of symptoms in the second half of pregnancy. The more severe, early onset form of PE (EOPE) can be diagnosed as early as 20 weeks of gestation and occurs in ∼0.4% of pregnancies and often leads to fetal growth restriction (3). The origins of either form remain enigmatic, as the causes are likely multifactorial, with multiple proposed risk factors and complex inheritance patterns (4, 5). Removing the placenta is the only known cure for either form of the disease, suggesting that factors released by trophoblast (TB) acting on a susceptible mother are responsible for disease symptoms (6).
EOPE, in particular, has been attributed to deficient remodeling of the uterine spiral arteries by the invasive extravillous TB (EVTB) (7), which begins about midway through the first trimester of pregnancy before disease symptoms are evident (8, 9). In turn, the unmodified arteries cause erratic perfusion of the placenta as it matures, with ischemia−reperfusion leading to oxidative stress (10). EOPE TB has been proposed to have an inherently impaired response to oxidative stress (11), which causes an increased release (by placental TB cells) of antiangiogenic factors that provoke endothelial dysfunction and inflammation in the maternal vessels. In a normal pregnancy, up-regulation of vascular endothelial growth factor (VEGF) and placental growth factor (PGF) are important for proper angiogenesis and vasodilation (12, 13), while, in EOPE, in particular, PGF is released in reduced quantities (14) and an antagonist of VEGF, known as placenta-derived soluble FMS-like tyrosine kinase-1 (sFLT1), is typically up-regulated (15).
Studying the etiology of all forms of PE, including EOPE, has been hampered by lack of model systems. While rodent models have demonstrated features of EOPE, none encompass the full range of symptoms and nearly all lack the expected disease progression to eclampsia (16). In vitro models that use primary cells from placenta are probably inadequate for several reasons. The insults leading to EOPE almost certainly arise early in the first trimester when EVTB is colonizing the endometrium and before onset of extensive maternal blood perfusion, whereas term placentae lack an invasive component. Additionally, term placentae from PE pregnancies show signs of secondary dysfunction and structural damage resulting from the disease (17). On the other hand, while it is possible to obtain primary cells from the first trimester of pregnancy, PE cannot be diagnosed at that stage.
As an alternative to animal models or primary tissues derived from placentae, our laboratory has developed a model system for studying TB in which pluripotent stem cells are exposed to bone morphogenetic protein 4 (BMP4) in combination with signaling inhibitors of ACTIVIN-A (A83-01) and FGF2 (PD173074) (BAP treatment) (18, 19). These BAP-derived TBs are thought to represent highly invasive cells of the primitive placenta (20, 21), and therefore provide an advantageous model to study EOPE. To capture potential genetic or epigenetic features that might characterize EOPE, fibroblast cells were cultured from explants from umbilical cords (UC) of babies born to mothers who had experienced EOPE during their pregnancies as well as homologous cells from UC of infants born to mothers following a normal pregnancy to act as controls (CTLs) (22). It was noted that establishing cultures under 20% O2 conditions proved significantly more challenging from EOPE than CTL UC explants and that the cells were more susceptible to oxidative stress, suggesting that there were possibly genetic differences that distinguished the two (22).
For the present study, both the EOPE and CTL fibroblast cultures generated from UC were reprogrammed to create induced pluripotent stem cell (iPSC) lines, with the goal of converting the latter to early-stage placental TB by the BAP protocol described above. In this manner, we sought to determine whether hypersensitivity to high O2 remained evident in the TB derived from EOPE pregnancies and whether such cells demonstrated other disparities relative to CTL TB, especially in gene expression. The goal was, therefore, to attempt to recapitulate events as they occur in the early stages of TB development when EOPE is believed to arise.
Results
Phenotypes of the Primary Fibroblast and iPSCs.
Primary UC fibroblasts were reprogrammed under low O2 (4 to 5% by volume) to provide 29 iPSC lines (10 CTL, MRuc1-10; 19 EOPE, MRucA-S) (Table 1). Cell line authentication by short tandem repeat (STR) profiling (SI Appendix, Table S1) verified that each iPSC line exhibited an STR profile identical to that obtained from the DNA of the original progenitor cells. Each also possessed a transcriptome characteristic of a pluripotent stem cell (23) (SI Appendix, Table S2).
Table 1.
Samples used in the study
| CTL or EOPE | Sample ID | Sex | Gestational age, wk | Clinical condition* | Mode of delivery† | Betamethasone (B), (× no. of injections) | Microarray analysis of cells grown in 4%, 20% O2 |
| CTL | 1 | M | 39 3/7 | Normal | SVD | No | No, no |
| CTL | 2 | F | 37 | Normal | CD | No | Yes, yes |
| CTL | 3 | M | 39 2/7 | Normal | SVD | No | Yes, yes |
| CTL | 4 | M | 40 | Normal | CD (breech) | No | Yes, yes |
| CTL | 5 | F | 39 3/7 | Normal | (Repeat) CD | No | Yes, yes |
| CTL | 6 | M | 40 | Normal | SVD | No | Yes, yes |
| CTL | 7 | M | 34 | Preterm Premature Rupture of Membranes (PPROM) | SVD | B (× 2) | Yes, yes |
| CTL | 8 | F | 33 | PPROM | SVD | no | Yes, yes |
| CTL | 9 | F | 39 | Normal | CD | no | Yes, yes |
| CTL | 10 | F | 39 1/7 | Normal | CD | no | Yes, yes |
| EOPE | A | F | 30 5/7 | HELLP syndrome | CD | B (× 2) | Yes, yes |
| EOPE | B | M | 29 5/7 | Severe PE | CD | No, due to emergent delivery | Yes, yes |
| EOPE | C | M | 32 3/7 | Severe PE | CD | B (× 2) | Yes, yes |
| EOPE | D | M | 32 4/7 | Severe PE | CD | B (× 2) | Yes, yes |
| EOPE | E | M | 34 1/7 | HELLP syndrome | CD | No, due to age > 34 wk | No, no |
| EOPE | F | M | 32 6/7 | Severe PE, IUGR | CD | B (× 2) | Yes, yes |
| EOPE | G | M | 30 | Severe PE superimposed on maternal hypertension | (Repeat) CD | B (× 2) | Yes, yes |
| EOPE | H | M | 31 | Severe PE | (Repeat) CD | B (× 3) | Yes, yes |
| EOPE | I | M | 29 | Severe PE, dichorionic/diammniotic twins | (Repeat) CD | B (× 2) | Yes, yes |
| EOPE | J | M | 29 | Severe PE, dichorionic/diammniotic twins | (Repeat) CD | B (× 2) | Yes, yes |
| EOPE | K | M | 27 3/7 | Severe PE | CD | B (× 2) | Yes, yes |
| EOPE | L | M | 26 6/7 | Severe PE | CD (breech) | B (× 2) | No, yes |
| EOPE | M | M | 33 5/7 | Severe PE | CD (breech) | B (× 2) | Yes, yes |
| EOPE | N | M | 28 5/7 | Severe PE | CD | B (× 2) | Yes, yes |
| EOPE | O | M | 28 5/7 | Severe PE | CD | B (× 2) | Yes, yes |
| EOPE | P | F | 31 5/7 | Severe PE, IUGR | CD | B (× 1) | Yes, yes |
| EOPE | Q | F | 27 | Severe PE | CD | B (× 2) | No, no |
| EOPE | R | F | 34 1/7 | Severe PE | SVD | B (× 2) | No, no |
| EOPE | S | F | 32 6/7 | HELLP syndrome | SVD | B (× 4) | No, no |
Patient information for UC samples collected from CTLs (1 to 10) and from infants whose mothers suffered EOPE (A to S). Note that the majority of EOPE lines were derived from infants after Cesarian delivery. Half of CTL lines and two EOPE lines were generated following spontaneous vaginal delivery of the infants. All mothers in the EOPE group, with the exception of B and E, received from one to four injections of betamethasone before delivery. Of the CTLs, only the mother of infant 7 received betamethasone.
HELLP, haemolysis, elevated liver enzymes and low platelets; IUGR, intrauterine growth restriction; PPROM, preterm premature rupture of membranes.
CD, Cesarean delivery; SVD, spontaneous vaginal delivery.
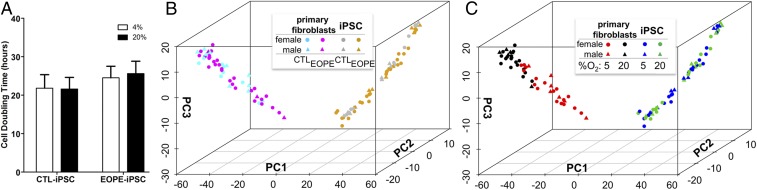
The population doubling times of selected iPSC lines were measured under both 5% and 20% O2 conditions of culture. Under low O2 conditions (4 to 5% O2), the oxygen concentrations in the medium were ∼3.5 ppm; in air/5% CO2, they were ∼7.8 ppm (partial O2 pressures of ∼58 mm of Hg and ∼159 mm of Hg, respectively). Population growth did not differ between these two conditions (Fig. 1A). This experiment provided a control to ensure that high O2 did not greatly influence mean population doubling times of CTL versus EOPE lines and hence initial cell numbers before inducing differentiation to TB. Further, a principal component analysis (PCA) based on microarray data from 29 iPSC lines (10 CTL, 19 EOPE) also could not readily distinguish the CTL versus EOPE iPSCs either relative to disease status (CTL versus EOPE) (Fig. 1B, right) or to the responses of the cell lines to culture under 20% O2 (Fig. 1C, right). This mingling of transcriptome data in response to 20% O2 was in contrast to the primary fibroblast cells from which the iPSCs had been generated, where there was a clear separation according to the O2 atmosphere under which the cell lines had been cultured (Fig. 1C, left). Together, these experiments suggest that the O2 susceptibility of the primary fibroblast cultures, which were used as the progenitors of the iPSCs, might have been lost as a result of reprogramming (22). Finally, there was no indication that male (triangles) and female (circles) fibroblast or iPSC lines could be readily distinguished as assessed by PCA of global gene expression under either O2 condition (Fig. 1 B and C).
Fig. 1.
(A) Cell doubling times performed on randomly selected cell lines (four CTL iPSC lines: MRuc4, MRuc7, MRuc8, MRuc10; six EOPE iPSC lines: MRucB, MRucE, MRucI, MRucJ, MRucK, MRucN). Values are the means ± SE for three individual experiments in each line. The O2 concentrations employed are represented by open (4%) and closed (20%) bars. (B and C) PCA of transcriptomes of UC fibroblasts from EOPE and CTL pregnancies and iPSC lines derived from them. The sexes of the samples are designated by circles (female) and triangles (male). (B) Neither primary UC fibroblasts (left; CTL, cyan; EOPE, magenta) nor the iPSC lines (right; CTL, silver; EOPE, gold) show separation by disease status. (C) UC fibroblasts cluster separately according to whether they were cultured under either 20% O2 (black) or 5% (red) O2. The iPSC lines (right; green and blue, respectively) did not separate by O2 condition. Data were generated with microarrays (GSE54400).
Invasive Features of iPSC-Derived TB Under Low and High O2.
The O2 sensitivity observed in primary cultures from EOPE patients (22) was lost when the fibroblasts were reprogrammed to iPSCs (Fig. 1C), prompting the question as to whether measurable differences existed between CTL and EOPE iPSC lines after reprogramming. One of the hallmarks of EOPE is reduced EVTB invasion. Therefore, the ability of TB derived from CTL and EOPE iPSC lines to invade through Matrigel was assessed under both 5% O2, more closely mimicking concentrations in the female reproductive tract (24), and oxidative stress conditions (20% O2).
To address this point, CTL- and EOPE-derived iPSC lines, all of which had been generated and expanded under 5% O2 conditions before cryopreservation, were cultured under nondifferentiating conditions in both 5% and 20% O2 for at least three passages to acclimate them to the respective gas atmospheres and to minimize acute responses to initial O2 shock. Next, these CTL and EOPE iPSCs were plated on Matrigel-coated, porous membranes in invasion chambers and exposed to BAP conditions for 6 d to induce TB differentiation under the contrasting O2 conditions.
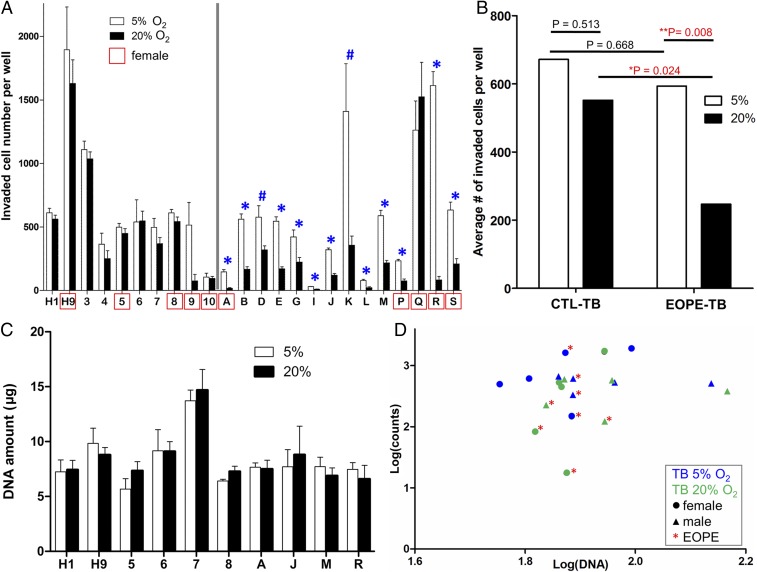
In total, invasion assays employed two human embryonic stem cell (hESC) lines (H1 & H9), 8 CTL TB lines, and 14 EOPE TB lines (Fig. 2A). Some cell lines were omitted from the invasion experiments because they did not readily make the transition from the mTeSR1 culture medium to the medium employed in the differentiation protocol (DME/F12 with knockout serum replacement). Others failed to attach firmly to the Matrigel-coated membranes in the invasion chambers. Each of the cell lines that could be differentiated consistently were tested on at least three separate occasions, and, on each occasion, the invasion assays were run in triplicate or quadruplicate (SI Appendix, Table S4). Before statistical analysis was performed, the data were log-transformed to account for the unequal variance among the cell lines. While the magnitude of invasion varied among cell lines, reproducibility was generally consistent across experimental replicates (SI Appendix, Table S4).
Fig. 2.
(A) Comparison of TB invasion of 10 CTLs (CTL-TB, left: H1 and H9, lines 3 to 10) and 14 EOPE-TB (right: lines A to S) after exposure to BAP conditions for 6 d. Each letter or number corresponds to the sample ID in Table 1. Values are the mean number of invaded cells ± SE in 12 randomly selected fields for three or four independent experiments, each in triplicate wells. For statistical comparisons, a complete randomized design was performed as a 2 × 2 × 2 factorial with CTL TB and EOPE TB, O2, and sex as factors on log-transformed invaded cell counts to control for unequal variance across cell lines. Open bars indicate physiological (5%) O2, and closed bars represent 20% O2 conditions. Significance is highlighted (*P < 0.05; #P ≤ 0.054; pairwise comparison 5% vs. 20%). Cell lines outlined in red (5, 8, 9, and 10 CTL; A, P, Q, R, and S EOPE) are female. (B) Combined TB invasion results for all CTL TB and EOPE TB lines. The log-transformed least-square means were used to determine significance. (C) TB cell proliferation as assessed by total DNA content. Two ESC (H1, H9*; *female line), four CTL TB (5*, 6, 7, 8*), and four EOPE TB (A*, J, M, R*) were measured for total DNA concentration after 6 d of BAP treatment. Values are the mean DNA amount ± SE for three individual experiments. A two-way ANOVA indicated no significant differences in DNA concentration in response to O2 concentration, and Bonferroni posttests determined no significance in pairwise comparisons. Overall DNA concentration was significantly affected by cell line (P < 0.0001). (D) The DNA content of TB cells (C) was log-transformed and plotted to determine whether there was a correlation with invasion cell counts (log-transformed values; counts) from A (values also shown in SI Appendix, Table S4). There was no significant correlation between DNA concentration and the number of invaded cells of each line (Pearson r = 0.16, P = 0.50). Such lack of correlation is also observed in CTL lines (Pearson r = −0.02, P = 0.94) and EOPE lines (Pearson r = 0.07, P = 0.88) when analyzed separately.
As illustrated in Fig. 2 A and B, (i) under 5% O2, invasiveness of CTL TB and EOPE TB lines was not different (P = 0.668); (ii) under 20% O2, invasiveness of EOPE TB was significantly lower than that of CTL TB (P = 0.024); (iii) invasiveness of CTL TB was not influenced by 20% O2 (P = 0.513); and (iv) invasiveness of EOPE TB as a group was significantly inhibited by 20% O2 (P = 0.008). No sex differences were noted. In pairwise comparisons (Fig. 2A), all but three of the EOPE TB lines displayed a significant reduction in invasion under 20% O2 conditions compared with 5% conditions. Of those three unaffected by high O2, lines D and K (MRucDi and MRucKi) approached significance (P = 0.053 and P = 0.051, respectively), while line Q (MRucQi) lacked any indication of the loss-of-invasion phenotype seen in the other EOPE TB lines. One CTL TB line (9; MRuc9i) showed reduced invasion under 20% O2 conditions, which approached significance (P = 0.08) (Fig. 2A and SI Appendix, Table S4). Of note is that this CTL displayed reduced growth in the invasion chambers under conditions designed to maintain pluripotency before initiating differentiation and attached less well to the Matrigel substratum than most other iPSC lines. This unpredictability probably led to uneven results in the invasion assays and suggests that the decreased invasion of BAP-differentiated line MRuc9i might have been an anomaly unrelated to O2 responsiveness. Also of note is that CTL lines 7 and 8 (MRuc7i and MRuc8i) were generated from infants delivered prematurely at 34 and 33 weeks, respectively, and the mother of the infant who provided line 7 had received betamethasone, similar to the delivery conditions of the EOPE cell donors (Table 1). The responses of these cell lines to 20% O2 were typical of the rest of the CTL group; namely, invasion was not impaired (Fig. 2A and SI Appendix, Table S4).
Taken together, however, these results from a heterogeneous population of infants indicated a remarkably consistent phenotypic difference in TB invasiveness between CTL and EOPE cell lines when the assays were performed under 20% O2. Additionally, it was clear that sensitivity to O2 displayed by the progenitor EOPE fibroblasts (22) was at least partially regained when the iPSCs were differentiated into TB.
Effect of Low and High O2 on Relative Growth Rates of Differentiating CTL iPSC and EOPE iPSC.
To assure that the reduced number of invading TB cells displayed by EOPE TB under 20% O2 conditions was not an indirect effect of reduced proliferation, the total DNA concentration of CTL and EOPE lines cultured under low and high O2 conditions was measured at day 6 of differentiation, the equivalent time point used for the invasion assays. DNA concentration was selected as a gauge of cell proliferation, because counting cells at day 6 is complicated by fusion of TB into multinucleated syncytia. Two ESC lines (H1 and H9), four CTL lines (MRuc5, MRuc6, MRuc7, and MRuc8), and four EOPE lines (MRucA, MRucJ, MRucM, and MRucR) were selected. Except to ensure that there was equal sex representation within groups, the selection of lines was made arbitrarily. The cell colonies were differentiated to TB by 6-d BAP treatment in an identical manner to that described in the invasion assays except that the cells were cultured on six-well dishes rather than in the invasion chambers. In pairwise comparisons, no cell line exhibited a significant difference in total DNA amount when grown under the two contrasting O2 conditions (Fig. 2C). However, the DNA content, and hence population doubling time, was significantly affected by cell line (P < 0.0001, two-way ANOVA) (Fig. 2C). As cell lines differed in the number of invading cells, we sought to determine whether this feature was correlated with the DNA content of the cultures. No such correlation was observed (Pearson r = 0.16, P = 0.50) (Fig. 2D). Overall, these results indicate that the differences in invaded cell numbers of the CTL TB and EOPE TB at 20% O2 were not explained by the overall proliferation rates of individual iPSC lines under BAP differentiation conditions.
Production of hCG and Progesterone by CTL TB and EOPE TB Under 5% and 20% O2 Conditions.
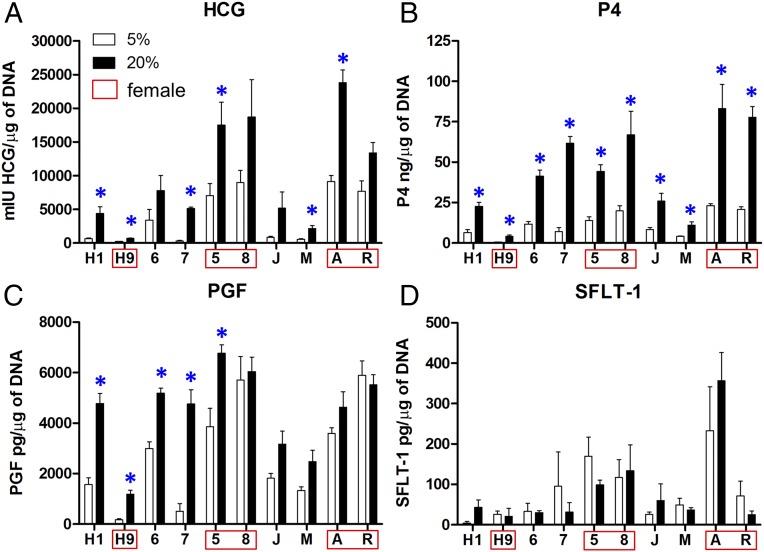
A feature of BAP-treated iPSCs/ESCs is their ability to secrete the placental hormones hCG and progesterone (P4) (18). These hormones are also secreted in greater amounts under 20% O2 conditions than under the lower O2 conditions (25). Additionally, serum concentrations of hCG (26, 27) and P4 (28, 29) have been reported to be higher in PE patients relative to CTLs as pregnancy progresses. To assess whether hCG and P4 production differed between cell lines, media samples from the 24 h of culture between day 5 and day 6 were collected, and the concentrations of hCG and P4 produced was determined, with all values normalized to the DNA concentrations of the respective cultures (Fig. 3 A and B). The CTL TB and EOPE TB displayed no significant differences in hCG production. As anticipated (18), 20% O2 significantly increased hCG and P4 production relative to 5% conditions (P = 0.02 and P = 0.008, respectively). Additionally, sex affected hCG production in the iPSC-derived TB, with female cells releasing more than males under 20% O2 conditions (P = 0.007).
Fig. 3.
Expression of (A) hCG, (B) P4, (C) PGF, and (D) sFLT-1 in 24-h culture between day 5 and day 6 following initiation of BAP-induced differentiation. The experiment used two ESCs (H1, H9*; *female line), four CTL TB (5*, 6, 7, 8*), and four EOPE TB (A*, J, M, R*) lines. The concentrations of the various proteins measured by ELISA were normalized to DNA content of the cultures. Values are means ± SE for three individual experiments. Pairwise comparisons were determined by using the Student t test, with significance (P < 0.05) shown by asterisks.
Consistent with the results observed for hCG, colonies from female cell lines demonstrated higher mean production of P4 than males (P = 0.02), but this inference was complicated by an interaction between sex and disease status (P = 0.02). More studies based on additional cell lines will be needed to determine whether these relationships to sex and disease are borne out. Collectively, however, the hCG and P4 data indicate that EOPE iPSC display no obvious defect in BAP-directed syncytioTB differentiation relative to CTLs, as they produce placental hormones at a comparable magnitude and respond to O2 concentrations similarly (Fig. 3 A and B).
Production of Angiogenic Factors PGF and FLT1 by CTL TB and EOPE TB Under 5% and 20% O2 Conditions.
As EOPE is commonly associated with a dysregulation of angiogenic factors and specifically an increased ratio of sFLT1 to PGF (30), the production of PGF and the soluble form of FLT1 (sometimes known as VEGFR-1) was determined in the same cell lines as used for hCG and P4 assays in Production of hCG and Progesterone by CTL TB and EOPE TB Under 5% and 20% O2 Conditions (Fig. 3 C and D). While, as a group, EOPE TB and CTL TB cultures did not significantly differ in their overall PGF production, PGF was slightly increased in CTL TB in response to high O2 (P = 0.05), while EOPE TB lacked such a response to O2 (P = 0.55) (Fig. 3C). Individual pairwise comparisons indicated that three out of the four CTL TB and TB from both ESC lines up-regulated PGF in response to high (20%) O2 conditions, while PGF production was not responsive to high O2 in any of the EOPE cultures (P = 0.13).
In contrast to PGF, there were no differences between the EOPE and CTL cultures in sFLT1 production (Fig. 3D). Further, there were no significant differences in sFLT1 associated with culture in high O2 in either EOPE TB or CTL TB. However, there was a slight effect of sex (P = 0.04), with females producing more sFLT1 than males.
Gene Expression Profiles of EOPE TB and CTL TB.
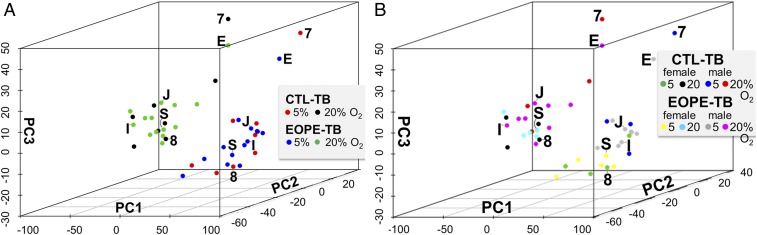
Next, we asked whether there were differences in gene expression that distinguished EOPE TB and CTL TB after culture under 5% and 20% O2 conditions. Each cell line (8 CTL and 14 EOPE) (plus the H1 and H9 ESC CTLs, which were not included in the subsequent PCA analyses) was differentiated to TB under each O2 condition (5% and 20%). After 6 d of BAP treatment, RNA was extracted and processed for RNA sequencing (RNAseq). PCA was performed on the top quartile of genes based on their variance across all of the samples. The first three principal components, comprising ∼67% of the total variance, were then plotted (Fig. 4). This PCA analysis indicated that the samples clustered by O2 condition rather than by CTL TB and EOPE TB identity, an outcome not dissimilar to that displayed by the progenitor UC primary fibroblast cells but not by the iPSC lines (Fig. 1C). Next, differential expression analysis was performed by using EdgeR to compare EOPE TB and CTL TB under each O2 condition. Two genes [RIBOSOMAL PROTEIN S17 (RPS17), false discovery rate (FDR) = 0.0005; HUMANIN-LIKE 2, MTRNR2L2, FDR = 0.005] appeared to be differentially expressed (SI Appendix, Table S5). However, further examination of the data found that this effect was largely a consequence of their up-regulation in two CTL lines, MRuc3 and MRuc7. Without these two samples, the genes would not have been assessed as differentially expressed, indicating that there was no evidence that a particular gene or set of genes distinguished CTL TB and EOPE TB at 20% O2.
Fig. 4.
PCA of RNAseq data from CTL TB and EOPE TB (GSE119265). Data are based on the log-transformed TPM from the top quartile of the most variable genes. (A) Each dot represents an individual sample from either CTL TB 5% O2 (red), CTL TB 20% (black), EOPE TB 5% (blue), or EOPE TB 20% (green). The first three principal components show a separation by O2 treatment but not by disease (CTL vs. EOPE). (B) The same analysis as in A but showing the sex of each line. Each dot represents an individual sample from either female CTL TB 5% O2 (green), female CTL TB 20% (black), male CTL TB 5% O2 (blue), male CTL TB 20% (red); female EOPE TB 5% (yellow), female EOPE TB 20% (cyan), male EOPE TB 5% (gray), or male EOPE TB 20% (magenta). Shown on both plots are the locations of the CTL cultures derived from patients 7 and 8 (Table 1), where the infant was born prematurely, and EOPE cultures E and S, which represent their nearest neighbors in the PCA plot. Also shown are locations of two male EOPE lines, J and I, derived from UC of dichorionic twins (Table 1).
Relative Up-Regulation of TB Marker Genes During Differentiation of EOPE and CTL iPSCs Under 5% and 20% O2 Conditions.
One possibility is that the differences in invasive responses observed between EOPE TB and CTL TB is the result of altered proportions of TB subtypes, for example, syncytioTB and EVTB. However, there is little evidence for such imbalance under either low or high O2. For example, expression of 11 genes typically up-regulated in syncytioTB (CDH5, CGB5, CGB8, CYP11A1, GATA2, GCM1, LGAL16, MUC15, OVOL1, PGF, and PSG4) were similarly expressed in EOPE and CTL cells at day 6 of differentiation (SI Appendix, Fig. S3A). However, expression of most of these genes was enhanced when cultured in 20% relative to 5% O2, an outcome consistent with previous work from us (18, 19, 31) and others (32) showing that the formation of syncytioTB within colonies of BAP-differentiated hESC was more robust in 20% O2 than in 5% O2. Note that expression of ERVFRD-1 does appear to differ (P < 0.05) between EOPE and CTL samples when comparisons were tested by two-way ANOVA. However, the transcripts per million (TPM) values for ERVFRD had high variance, and differences were insignificant when calculated in EdgeR (FDR = 1), which corrects for multiple comparisons and had a fold-change cutoff of 1.5 for differential expression values.
To determine whether differentiation of cells analogous to EVTB occurred at a similar rate in EOPE and CTL cultures, we also examined the expression of several genes that are typically up-regulated in EVTB. The criterion for selecting these markers was that each was significantly (P < 0.01) and at least twofold up-regulated in HLA-G−positive cells relative to HLA-G−negative cytotrophoblast from a variety of human TB sources, namely, three independent analyses performed on HLA-G+ cells from first trimester placentae (33–35), HLA-G+ cells differentiated from first trimester cytotrophoblast (36), and human TB stem cells directed to an EVTB fate (35). Each of these genes (ADAM19, COL4A1, ERICH5, ITGA5, MCAM, MMP2, SERPINE2, and TGFB2), as well as HLA-G itself, was expressed after 6-d BAP exposure of the EOPE (n = 14) and CTL iPSCs (n = 8). Expression of these genes was not dramatically affected by O2 conditions, although there was a small but significant up-regulation of ADAM19, ITGA3, SERPINE2, and MCAM, and a down-regulation of MMP2, as observed by others (32, 37) in CTL cultures under 20% O2 (SI Appendix, Fig. S3B). Again, these data suggest that the differentiation potential of EOPE and CTL iPSC lines were comparable and that none of these genes, with the possible exception of HLA-G, distinguished the EOPE and CTL cultures under either O2 condition. Like ERVFRD above, the HLA-G expression differences between EOPE and CTL lines were insignificant when they were analyzed in EdgeR.
Coexpressed Gene Modules Associated with Invasion.
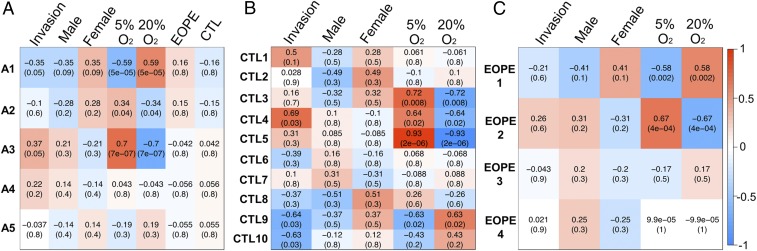
Of the parameters tested to distinguish EOPE and CTL TB, the strongest phenotype was the reduced invasive capacity of EOPE TB under high (20%) O2 conditions (Fig. 2 A and B). As it is clear that this loss of invasiveness under 20% O2 is unlikely to be attributable to differential expression of any single gene, it is plausible to assume that there are multiple genes, most probably working in a combinatorial manner, contributing to this phenotype. Accordingly, a method [weighted gene correlation network analysis (WGCNA)] to identify groups (modules) of coexpressed genes whose expression correlated with cell invasion count (Fig. 2A and SI Appendix, Table S4), disease status (EOPE or CTL), sex, and O2 treatment was employed. In our first analysis, we used all of the datasets, comprising both disease states and both O2 levels, and identified five gene modules (A1 to A5; Fig. 5A). Although there were two modules, A1 and A3, that were strongly linked to O2 sensitivity, the correlation of A1 and A3 with invasion was quite modest (P = 0.05). Moreover, there was no significant association of these two modules with disease.
Fig. 5.
Gene expression modules showing correlations in relation to invasive capacity, sex, O2 treatment, and disease status (EOPE vs. CTL) as determined by WGCNA. (A) A total of 3,845 genes from combined CTL TB and EOPE TB samples identified five coexpressed gene modules. Out of the five modules, two (A1 and A3) showed a significant correlation with invasion and O2 levels, but none correlated with disease status. (B) WGCNA analysis of a total of 3,682 genes for CTL TB. Ten coexpressed gene modules were identified. (C) WGCNA analysis of a total of 3,789 genes for EOPE TB. Four coexpressed gene modules were identified. Each module name is shown on the left, and each column represents the correlation value with the corresponding adjusted P value in parentheses. Positive correlations are shown in red, and negative correlations are shown in blue, with white signifying no correlation.
Because the lack of correlation with disease in all of the five modules could be due to the variance across all of the samples, WGCNA was performed for the CTL TB and EOPE TB datasets separately. This analysis revealed 10 coexpressed gene modules in CTL TB (Fig. 5B; CTL1-10), of which two (CTL4 and CTL9) had a significant correlation with both invasion and O2 conditions. The CTL4 module correlated positively with invasion potential (P = 0.03) and negatively with exposure to 20% O2 (P = 0.02). This module was enriched with ontology terms such as “stem cell differentiation” (q value: 5.19e-3), and “blood vessel morphogenesis” (q value: 7.80e-4). By contrast to the CTL4 module, the CTL9 module showed a negative correlation with invasion potential (P = 0.03) and a positive correlation with exposure to 20% O2 (P = 0.02). This module was enriched with ontology terms such as “blood vessel development” (q value: 7.53e-5), “pre-eclampsia” (q value: 1.23e-3), “regulation of cell-cell adhesion” (q value: 3.88e-3), and “negative regulation of cell migration” (q value: 2.96e-2). The top 100 genes from the CTL9 module, sorted by their module membership value (KME), which calculates the significance of an individual gene within a module, are listed in SI Appendix, Table S6. Contained within this table are a number of genes associated with integrin signaling (SEMA7A and RAP1B), cell migration and adhesion (SDC1 and PVRL3), and genes reported to play a role in the cellular stress response (PPM1D, HSPB1, and DNAJB9). Although not in the top 100, another interesting gene found in the CTL9 module was PGF (KME = 0.947), which, at the protein level, was also up-regulated in response to 20% O2 in CTL TB only (Fig. 3C).
Unlike the CTL TB, where 10 modules of coexpressed genes were identified, there were only four coexpressed gene modules identified in the EOPE TB (EOPE1-4; Fig. 5C), of which none displayed a significant correlation with invasion. There were two modules (EOPE1 and EOPE2) that were significantly correlated with 20% O2 and 5% O2, respectively. These modules are composed largely of genes similar to those identified as correlated with O2 levels and invasion in the CTL samples. For example, the EOPE1 gene module in EOPE TB, which had a significant positive correlation with 20% O2, contains 83.74% of the CTL9 module genes (Fig. 5B). Similarly, the EOPE2 module in EOPE TB, which had a significant negative correlation with 20% O2, contains 66.7% of the CTL4 module genes. These data suggest a lack of a controlled, O2-regulated, invasive response in the EOPE TB. Note, however, that there is a higher variance in log-transformed invasion counts across EOPE TB (0.26 at 5% O2 and 0.35 at 20% O2) compared with the variance of log-transformed invasion counts across CTL TB (0.11 at 5% O2 and 0.18 at 20% O2), raising the possibility that the study could have been underpowered despite the use of 14 EOPE cell lines.
Finally, when we added sex as a variable to the WGCNA heatmaps, none of the gene modules identified in the CTL TB and EOPE TB were significantly correlated with sex (Fig. 5). These data, along with the PCA analysis (Fig. 4B) argue that the sex of the infants did not make a major contribution to the increased O2 sensitivity of EOPE cells. A list of genes identified in modules from all WGCNA analyses is provided in Dataset S1.
Discussion
Our goal has been to recapitulate the early stages of placental development as they might occur in a pregnancy complicated with EOPE. To this end, we created 29 independent iPSC lines from UC of EOPE and CTL pregnancies (Table 1) and assessed features of TB differentiation and function for 22 (8 CTL, 14 EOPE) of the 29 lines generated. It should be noted that the data were obtained from whole colonies rather than from individual TB cell types isolated from such colonies, and that the responses to 20% relative to 5% O2 that we followed were not due to acute stress caused by a sudden switch in O2 tensions. Rather, the experiments were performed in cultures acclimated to the two gas atmosphere conditions over three passages. The production of typical syncytioTB products hCG and progesterone by these cell lines (Fig. 3) and up-regulation of genes with normally enriched expression in syncytioTB and EVTB, respectively (SI Appendix, Fig. S3), in response to BAP revealed no apparent differences in the rates at which iPSC representing CTL and EOPE differentiated. Therefore, we sought other, more subtle, differences between the two cell types.
Difference in Invasive Phenotype Between EOPE and CTL TB.
One feature of iPSC differentiation was highly consistent; namely, there were clear differences between the EOPE and CTL lines in their invasive properties when they were cultured in 20% O2. Eleven of the 14 EOPE TB lines (with two of three outliers approaching significance) demonstrated reduced TB invasion under these high O2 conditions (Fig. 2), while the CTLs did not. This outcome is consistent with the central hypothesis that poor TB invasion and subsequent impaired spiral artery remodeling and placental perfusion underpin the fundamental insult (or at least the first “step”) leading to EOPE (38, 39). There may also be an enfeebled ability to counter other stressors.
The TB generated by BAP treatment of iPSC and ESC likely represents that formed during the implantation phase of placentation (21), a period when conceptus loss in humans is thought to be particularly high. Whether these early losses are exaggerated in pregnancies that might otherwise culminate in EOPE is unknown but should be a consideration. As concluded by Burton and Jauniaux (40), “miscarriage, missed miscarriage, and early and late onset PE represent a spectrum of disorders secondary to deficient TB invasion.” Additionally, we noted that the two ESC lines behaved like CTLs, and that male and female lines within each grouping did not differ significantly in invasive behavior.
Production of Angiogenic Factors and Placental Hormones by CTL TB and EOPE TB.
If the first step in establishment of EOPE is inadequate TB invasion, the second is generally recognized to be the responses of the maternal system to this compromised state. Thus, EOPE is recognized by early presentation of maternal symptoms in the form of hypertension, proteinuria, and endothelial dysfunction, even though the only cure is removal of the placenta (41), suggesting that placental TB is the source of the aggravating factors. The maternal endothelial dysfunction may stem from altered balance in the release of proangiogenic and antiangiogenic factors from the affected TB cells (42). Among these factors are PGF and sFLT1. While concentrations of the latter tend to rise in maternal serum, particularly during the second and third trimesters in pregnancies destined to present as PE, PGF levels usually fall, such that the ratio of the two has been exploited as a means to predict disease ahead of the onset of physical symptoms.
Cultures of primary first trimester TB cells have also indicated that PGF and sFLT1 are reciprocally regulated by O2 levels (43). With increasing O2 (2 to 8% to 20%) the levels of secreted PGF increased, whereas sFLT1 production fell. The O2-mediated regulation of PGF appeared to be due to hypoxia-inducible factor 2 alpha (HIF2A) expression, because a siRNA targeted to HIF2A abrogated the hypoxia-induced suppression of PGF (44). In our experiments (Fig. 3C), the output of PGF by CTL TB was also regulated by O2 in five of six CTL lines tested, with the levels significantly increasing under 20% O2 compared with 5%. By contrast, the EOPE TB cells failed to up-regulate PGF under high O2 conditions, suggesting an absence of the anticipated O2 regulation.
A similar O2 regulation to that noted with PGF was not displayed by either CTL TB or EOPE TB with regard to sFLT1 production (Fig. 3D). Nor were sFLT1 levels influenced by the disease origin of the cultures, although the study was relatively underpowered. Of note is that serum sFLT1 and PGF levels have not been useful clinical predictors of EOPE until early in the second trimester and onward, and, even then, cannot provide a completely reliable marker of disease (45–47). Nonetheless, the failure of 20% O2 to stimulate PGF synthesis in cultures of EOPE TB (Fig. 3C), like the reduced invasiveness of EOPE TB under high 20% O2, is consistent with the disease origins of the cultures and suggests that these features of EOPE are patent in the very earliest stages of pregnancy.
Distinctions in Gene Expression Profiles Between CTL TB and EOPE TB.
Attempts to identify differentially expressed genes from the RNAseq data by EdgeR analysis indicated that under both 5% and 20% O2, EOPE TB and CTL TB were essentially indistinguishable. Therefore, given the paucity of differentially expressed genes between EOPE TB and CTL TB, we turned to WGCNA to determine whether particular groups of coexpressed genes were correlated with loss of EOPE TB invasiveness under 20% O2. Even using this approach, we were unable to find a significant correlation between any coexpressed gene modules and invaded cell count for the EOPE TB (Fig. 5C), although there were strong correlations found for CTL TB (Fig. 5B). Importantly, one EOPE TB gene module (EOPE1), which correlated positively with high (20%) O2 level but not significantly with invasion, had high overlap with the CTL9 module. Similarly, the EOPE2 module had high overlap in gene content with CTL4, which had a positive association with invasion count and negative association with high (20%) O2 level (Fig. 5B). We therefore speculate that the regulatory networks that control invasion lose robustness in EOPE TB as O2 levels rise, yet remain strong in CTL TB. Whether this defect is because of an inability of EOPE cells to sense O2 concentrations as they rise or is due to disruption of EOPE TB’s ability to counteract the destructive effects of high O2 and other potential stressors remains elusive.
That EOPE TB produces less of the proangiogenic factor, PGF, in reaction to higher O2 also supports the premise that certain O2 responses are impaired and supports the hypothesis that PGF production and TB invasion of EOPE TB will fall progressively in vivo as EVTB approaches a more oxygenated or otherwise more stressful and challenging environment. It should be recognized, however, that CGA and CGB expression and the genes associated with P4 production during BAP-driven differentiation are both up-regulated by increasing O2 conditions (18) and that these features seem not to distinguish CTL TB and EOPE TB (Fig. 3 A and B), implying that not all O2 response pathways are dysregulated. Together, these findings favor the idea that the EOPE TB cell lines retain some capacity to respond to changing O2 concentrations in a manner similar to CTL TB, despite the fact that other aspects of their phenotype, including their invasive capacity, becomes impaired as O2 concentrations rise to potentially damaging levels.
Sexual Dimorphism.
According to at least one large population study, EOPE occurs more frequently (Adjusted Hazard Ratio = 1.10) in women carrying a male fetus (1). Even so, it was a surprise that, of the first 15 EOPE placentae, which were collected between July 2010 and February 2012, 14 (93.3%) were male, while half the CTL placentae were female (Table 1). In an attempt to balance for sex in our subsequent experiments, EOPE placentae P to S were deliberately selected from pregnancies that provided a female child. Although we did not pursue this apparent skewing toward males further, the observation raised the possibility that there might be a bias toward male EOPE fetuses in the population we studied and that sexual dimorphism might exist in our cell lines. Differences in gene expression based on sex of the fetus have been reported for both human (48) and mouse (49) placentas. Certain female iPSCs have also been reported to present a transcriptome distinguishable by PCA relative to male lines and to other female lines (50). However, we obtained no evidence for comparable global sex differences in gene expression in our transcriptome analyses of either primary fibroblast cultures or iPSC lines (Fig. 1 B and C). Moreover, sex showed no significant correlation with gene modules linked to either invasiveness or responsiveness of TB to O2 (Fig. 5). In other words, there was no obvious relationship revealed between sex and EOPE phenotype. To be clear, however, there were certain features of TB physiology, e.g., production of hCG (Fig. 3A), and modules containing differentially expressed genes (Fig. 5 and Dataset S1), that differed between males and females, but we have not pursued this aspect of sexual dimorphism further.
Is the EOPE Phenotype Observed in iPSC-Generated TB Due to Epigenetic Carryover?
It should be emphasized that our studies do not rule out the possibility that the basis of the O2-sensitive phenotype of EOPE TB, which was regained during differentiation from the pluripotent state, is epigenetic rather than genetic. In other words, epigenetic marks associated with the progenitor EOPE fibroblast cells may have been retained through initial explant culture, expansion, reprogramming to iPSCs, and, ultimately, differentiation of those cells to TB. Although it is generally assumed that reprogramming removes the majority of the epigenetic marks characteristic of a differentiated cell, it does not necessarily delete them all (51–53). On the other hand, the relatively low sensitivity of ESC cells to O2, which has been studied previously in this laboratory (54), and the apparent insensitivity of the iPSC lines, suggests, but clearly does not prove, that epigenetic marks associated with responses to oxidative stress had been erased during reprogramming.
Concluding Remarks.
This study demonstrates the value of an in vitro pluripotent stem cell model designed to examine early TB development in pregnancies that had been complicated by EOPE. The basis of this model is the generation of iPSCs from UC of infants born to mothers who experienced EOPE during the course of their pregnancies. These cell lines may be a useful resource for others wishing to study this enigmatic disease. Data generated by using the model are consistent with the concept that, in EOPE, the initial step precipitating disease is a reduced capacity of placental TB to invade, possibly brought about by a dysregulation of either O2 sensing or protection mechanisms. The experiments tend to confirm that EOPE is a syndrome in which unbalanced expression of various combinations of genes affecting TB invasion provokes disease onset. We conclude that no one gene or even a small group of genes can be singled out as causing the disease.
Experimental Procedures
Primary Tissue.
Fresh human UC tissues were collected in an aseptic manner at the Women’s and Children’s Hospital (University of Missouri). Tissue was deidentified at the time of collection; only gestational age at delivery, delivery route, EOPE or CTL, fetal sex, and use of steroids was recorded at collection. A total of 10 CTLs and 19 infants whose mothers suffered EOPE were assessed in these experiments (Table 1). The UC tissue collection (Projects #1201132 and #1209459) was approved by the University of Missouri Health Sciences Institutional Review Board, and specimens were collected after obtaining appropriate informed consent from all participants. The production of primary cultures from these explants, their subsequent reprogramming to iPSCs, authentication of the cell lines, differentiation of the iPSCs to TB, and the processing of RNA for analysis are described in SI Appendix, SI Materials and Methods. The precautions taken to minimize reoxygenation of cultures during passaging and culture medium changes are also described in SI Appendix.
Invasion Assays.
A detailed protocol describing the protocol for the invasion assays is provided in SI Appendix. Briefly, ESC and iPSC cultures were mechanically dispersed and passaged at a density of 5 × 104 cells per well on BD Matrigel-coated membranes with 8.0-μm pores within commercial invasion chambers in hESC medium conditioned by irradiated mouse embryonic fibroblasts (MEF-CM) + FGF2 (4 ng/mL) under either 5 or 20% O2 conditions. The next day, medium was changed to hESC + BAP to induce TB differentiation, while CTLs remained in MEF-CM + FGF2. Medium was changed daily, and after 6 days of BAP treatment, cells were fixed in 4% (wt/vol) paraformaldehyde. The number of invaded cells was quantified by taking 12 random field images of the stained membrane under 10× magnification. The number of nuclei in each field was quantified by using ImageJ software (https://imagej.nih.gov/ij/). Three or four independent experiments each with three internal experimental replicates were run for each cell line under both O2 conditions (SI Appendix, Table S4).
Other Methods.
Standard protocols, including immunoassays (hCG, PGF, progesterone, and sFLT1), statistical treatment of data, RNA library preparation, sequencing methodology, and procedures for analysis of RNAseq data are described in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Susanta K. Behura (Division of Animal Sciences, University of Missouri) for his initial analysis of the transcriptome data and Mr. Dennis Reith for editorial assistance. Dr. John Dodam (Department of Biomedical Sciences, College of Veterinary Medicine) was invaluable in overseeing the measurements of oxygen tensions in the culture media.
Footnotes
The authors declare no conflict of interest.
Data deposition: Microarray data and RNA sequence data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession nos. GSE54400 and GSE119265, respectively).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1816150116/-/DCSupplemental.
References
- 1.Lisonkova S, Joseph KS. Incidence of preeclampsia: Risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544.e1–544.e12. doi: 10.1016/j.ajog.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Raymond D, Peterson E. A critical review of early-onset and late-onset preeclampsia. Obstet Gynecol Surv. 2011;66:497–506. doi: 10.1097/OGX.0b013e3182331028. [DOI] [PubMed] [Google Scholar]
- 3.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “great obstetrical syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oudejans CB, van Dijk M, Oosterkamp M, Lachmeijer A, Blankenstein MA. Genetics of preeclampsia: Paradigm shifts. Hum Genet. 2007;120:607–612. doi: 10.1007/s00439-006-0259-1. [DOI] [PubMed] [Google Scholar]
- 5.Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357:53–56. doi: 10.1016/s0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- 6.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 7.Pijnenborg R, Vercruysse L, Hanssens M, Brosens I. Endovascular trophoblast and preeclampsia: A reassessment. Pregnancy Hypertens. 2011;1:66–71. doi: 10.1016/j.preghy.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Khong SL, Kane SC, Brennecke SP, da Silva Costa F. First-trimester uterine artery Doppler analysis in the prediction of later pregnancy complications. Dis Markers. 2015;2015:679730. doi: 10.1155/2015/679730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: A review of the literature. Am J Obstet Gynecol. 2002;187:1416–1423. doi: 10.1067/mob.2002.127305. [DOI] [PubMed] [Google Scholar]
- 10.Burton GJ, Yung HW, Cindrova-Davies T, Charnock-Jones DS. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta. 2009;30(Suppl A):S43–S48. doi: 10.1016/j.placenta.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rolfo A, et al. Abnormalities in oxygen sensing define early and late onset preeclampsia as distinct pathologies. PLoS One. 2010;5:e13288. doi: 10.1371/journal.pone.0013288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torry DS, Mukherjea D, Arroyo J, Torry RJ. Expression and function of placenta growth factor: Implications for abnormal placentation. J Soc Gynecol Investig. 2003;10:178–188. doi: 10.1016/s1071-5576(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 13.Burton GJ, Charnock-Jones DS, Jauniaux E. Regulation of vascular growth and function in the human placenta. Reproduction. 2009;138:895–902. doi: 10.1530/REP-09-0092. [DOI] [PubMed] [Google Scholar]
- 14.Tidwell SC, Ho H-N, Chiu W-H, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol. 2001;184:1267–1272. doi: 10.1067/mob.2001.113129. [DOI] [PubMed] [Google Scholar]
- 15.Maynard SE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pennington KA, Schlitt JM, Jackson DL, Schulz LC, Schust DJ. Preeclampsia: Multiple approaches for a multifactorial disease. Dis Model Mech. 2012;5:9–18. doi: 10.1242/dmm.008516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benirschke K, Kaufmann P, Baergen RN. Pathology of the Human Placenta. 5th Ed Springer; New York: 2006. [Google Scholar]
- 18.Amita M, et al. Complete and unidirectional conversion of human embryonic stem cells to trophoblast by BMP4. Proc Natl Acad Sci USA. 2013;110:E1212–E1221. doi: 10.1073/pnas.1303094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yabe S, et al. Comparison of syncytiotrophoblast generated from human embryonic stem cells and from term placentas. Proc Natl Acad Sci USA. 2016;113:E2598–E2607. doi: 10.1073/pnas.1601630113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain A, Ezashi T, Roberts RM, Tuteja G. Deciphering transcriptional regulation in human embryonic stem cells specified towards a trophoblast fate. Sci Rep. 2017;7:17257. doi: 10.1038/s41598-017-17614-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts RM, Ezashi T, Sheridan MA, Yang Y. Specification of trophoblast from embryonic stem cells exposed to BMP4. Biol Reprod. 2018;99:212–224. doi: 10.1093/biolre/ioy070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang P, et al. Abnormal oxidative stress responses in fibroblasts from preeclampsia infants. PLoS One. 2014;9:e103110. doi: 10.1371/journal.pone.0103110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller FJ, et al. A bioinformatic assay for pluripotency in human cells. Nat Methods. 2011;8:315–317. doi: 10.1038/nmeth.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jauniaux E, Hempstock J, Greenwold N, Burton GJ. Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancies. Am J Pathol. 2003;162:115–125. doi: 10.1016/S0002-9440(10)63803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das P, et al. Effects of fgf2 and oxygen in the bmp4-driven differentiation of trophoblast from human embryonic stem cells. Stem Cell Res (Amst) 2007;1:61–74. doi: 10.1016/j.scr.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wenstrom KD, Owen J, Boots LR, DuBard MB. Elevated second-trimester human chorionic gonadotropin levels in association with poor pregnancy outcome. Am J Obstet Gynecol. 1994;171:1038–1041. doi: 10.1016/0002-9378(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 27.Heikkilä A, Makkonen N, Heinonen S, Kirkinen P. Elevated maternal serum hCG in the second trimester increases prematurity rate and need for neonatal intensive care in primiparous preeclamptic pregnancies. Hypertens Pregnancy. 2001;20:99–106. doi: 10.1081/PRG-100104176. [DOI] [PubMed] [Google Scholar]
- 28.Walsh SW. Progesterone and estradiol production by normal and preeclamptic placentas. Obstet Gynecol. 1988;71:222–226. [PubMed] [Google Scholar]
- 29.Salas SP, Marshall G, Gutierrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension. 2006;47:203–208. doi: 10.1161/01.HYP.0000200042.64517.19. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien M, Baczyk D, Kingdom JC. Endothelial dysfunction in severe preeclampsia is mediated by soluble factors, rather than extracellular vesicles. Sci Rep. 2017;7:5887. doi: 10.1038/s41598-017-06178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz LC, et al. Human embryonic stem cells as models for trophoblast differentiation. Placenta. 2008;29(Suppl A):S10–S16. doi: 10.1016/j.placenta.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horii M, et al. Human pluripotent stem cells as a model of trophoblast differentiation in both normal development and disease. Proc Natl Acad Sci USA. 2016;113:E3882–E3891. doi: 10.1073/pnas.1604747113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Telugu BP, et al. Comparison of extravillous trophoblast cells derived from human embryonic stem cells and from first trimester human placentas. Placenta. 2013;34:536–543. doi: 10.1016/j.placenta.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Apps R, et al. Ex vivo functional responses to HLA-G differ between blood and decidual NK cells. Mol Hum Reprod. 2011;17:577–586. doi: 10.1093/molehr/gar022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okae H, et al. Derivation of human trophoblast stem cells. Cell Stem Cell. 2018;22:50–63.e6. doi: 10.1016/j.stem.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 36.DaSilva-Arnold S, James JL, Al-Khan A, Zamudio S, Illsley NP. Differentiation of first trimester cytotrophoblast to extravillous trophoblast involves an epithelial-mesenchymal transition. Placenta. 2015;36:1412–1418. doi: 10.1016/j.placenta.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Wakeland AK, et al. Hypoxia directs human extravillous trophoblast differentiation in a hypoxia-inducible factor-dependent manner. Am J Pathol. 2017;187:767–780. doi: 10.1016/j.ajpath.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sones JL, Davisson RL. Preeclampsia, of mice and women. Physiol Genomics. 2016;48:565–572. doi: 10.1152/physiolgenomics.00125.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts JM, Redman CW. Pre-eclampsia: More than pregnancy-induced hypertension. Lancet. 1993;341:1447–1451. doi: 10.1016/0140-6736(93)90889-o. [DOI] [PubMed] [Google Scholar]
- 40.Burton GJ, Jauniaux E. Placental oxidative stress: From miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11:342–352. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Fisher SJ. Why is placentation abnormal in preeclampsia? Am J Obstet Gynecol. 2015;213(4) Suppl:S115–S122. doi: 10.1016/j.ajog.2015.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173–192. doi: 10.1146/annurev-pathol-121808-102149. [DOI] [PubMed] [Google Scholar]
- 43.Nagamatsu T, et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: An implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145:4838–4845. doi: 10.1210/en.2004-0533. [DOI] [PubMed] [Google Scholar]
- 44.Fujii T, et al. Enhanced HIF2α expression during human trophoblast differentiation into syncytiotrophoblast suppresses transcription of placental growth factor. Sci Rep. 2017;7:12455. doi: 10.1038/s41598-017-12685-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaiworapongsa T, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 46.Levine RJ, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 47.Thadhani R, et al. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004;89:770–775. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez TL, et al. Sex differences in the late first trimester human placenta transcriptome. Biol Sex Differ. 2018;9:4. doi: 10.1186/s13293-018-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mao J, et al. Contrasting effects of different maternal diets on sexually dimorphic gene expression in the murine placenta. Proc Natl Acad Sci USA. 2010;107:5557–5562. doi: 10.1073/pnas.1000440107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anguera MC, et al. Molecular signatures of human induced pluripotent stem cells highlight sex differences and cancer genes. Cell Stem Cell. 2012;11:75–90. doi: 10.1016/j.stem.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shao K, et al. Induced pluripotent mesenchymal stromal cell clones retain donor-derived differences in DNA methylation profiles. Mol Ther. 2013;21:240–250. doi: 10.1038/mt.2012.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim K, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts CL, et al. Preferential lineage-specific differentiation of osteoblast-derived induced pluripotent stem cells into osteoprogenitors. Stem Cells Int. 2017;2017:1513281. doi: 10.1155/2017/1513281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Westfall SD, et al. Identification of oxygen-sensitive transcriptional programs in human embryonic stem cells. Stem Cells Dev. 2008;17:869–881. doi: 10.1089/scd.2007.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.