Significance
A major challenge in biology is to understand how complex traits important for ecological interactions between species coevolve and diversify across contrasting ecosystems. Floral scents are complex, and are often composed of a diverse array of chemicals important for interactions between plants and pollinators, herbivores, and microbial symbionts. We studied diversification of floral scents among populations of all woodland star species (Lithophragma) across far-western North America. Floral scent variation was structured not only phylogenetically among species and geographically among populations, but some of the divergence was driven by local differences in the presence of coevolved Greya moth pollinators. These results highlight the importance of conserving multiple populations of species if we are to maintain the evolutionary potential of coevolving interactions.
Keywords: geographic mosaic of coevolution, floral volatiles, geographic variation, floral parasitism, pollination
Abstract
A major challenge in evolutionary biology is to understand how complex traits of multiple functions have diversified and codiversified across interacting lineages and geographic ranges. We evaluate intra- and interspecific variation in floral scent, which is a complex trait of documented importance for mutualistic and antagonistic interactions between plants, pollinators, and herbivores. We performed a large-scale, phylogenetically structured study of an entire plant genus (Lithophragma, Saxifragaceae), of which several species are coevolving with specialized pollinating floral parasites of the moth genus Greya (Prodoxidae). We sampled 94 Lithophragma populations distributed across all 12 recognized Lithophragma species and subspecies, and four populations of related saxifragaceous species. Our results reveal an unusually high diversity of floral volatiles among populations, species, and clades within the genus. Moreover, we found unexpectedly major changes at each of these levels in the biosynthetic pathways used by local populations in their floral scents. Finally, we detected significant, but variable, genus- and species-level patterns of ecological convergence in the floral scent signal, including an impact of the presence and absence of two pollinating Greya moth species. We propose that one potential key to understanding floral scent variation in this hypervariable genus is its geographically diverse interactions with the obligate specialized Greya moths and, in some species and sites, more generalized copollinators.
Heritable trait variation among and within populations provides the raw material for evolution. This variation is filtered through genetic drift and local selection from the abiotic environment and from intra- and interspecific interactions in each local population (1–4). The complexity of species interactions, gene flow, and genomic variation among populations and the ever-changing composition of local networks of interacting species generate geographic mosaics of adaptation, maladaptation, evolution, and coevolution (5), which, in turn, can remix and generate new trait variation to be molded by natural selection. A current major challenge in evolutionary biology is therefore to understand how complex traits of multiple functions have diversified and codiversified across interacting evolutionary lineages and geographic ranges (6, 7). In working toward that goal, the combined effects of a variable composition and intensity of species interactions, a shifting environment, and the impact of historical gene flow can make it difficult to detect local adaptation and coadaptation within individual populations. Instead, many of these processes must be studied using large-scale multipopulation and multispecies approaches (1, 7, 8). Such studies, however, are rare because it is challenging to collect data on complex trait variation in relation to ecological variation across multiple populations.
Among the most diverse species interactions on Earth are those between plants and plant-feeding insects (5, 9). Although many complex traits shape these interactions, floral scent is often pivotal in molding gene flow in plants and specialization in many insects (10–12). Single volatiles can mediate interactions between plants and pollinators (13, 14), but complex floral scent bouquets are common in many taxa (15). These bouquets may heighten the attraction of preferred pollinators, but they may function simultaneously as cues for resource detection by seed predators or herbivores (16–19) and mediate interactions with microbes (20, 21). Hence, floral scent should be sensitive to selection imposed by the local assemblage of mutualist and antagonist insects, and it may vary among populations within species due to selection from each local insect assemblage. It is unclear, however, whether divergence in volatile composition should involve small changes among populations within chemical pathways or major shifts to volatiles produced by different pathways. Few plant lineages have been analyzed systematically for patterns of divergence in floral scent (22, 23), and there are no studies in which multiple populations across multiple taxa within an entire lineage have been studied for geographic variation in floral volatiles.
At an interspecific level, several lines of evidence indicate a strong impact of pollinator-driven floral scent diversification. First, unrelated plant species that interact with the same pollinator group often converge in their composition of floral volatiles, forming so-called pollination syndromes (24–27). Conversely, closely related plant species pollinated by different pollinator types have been shown to emit divergent scent bouquets (28, 29), supporting a role for floral scent during species divergence and/or in niche partitioning (30). In some highly specialized brood-site plant–pollinator interactions, such as figs and fig wasps or yuccas and yucca moths, evidence suggests that the pollinator-specific matching of the floral scent signal is taken to the extreme through the evolution of private channels of uncommon or unique compounds that specifically target the obligate pollinator (10, 14, 31). In these interactions, the pollinating insects lay eggs in the same flowers that they pollinate. Hence, reproduction (and, as a consequence, fitness) of the insect and host plant is intimately linked. If floral scents and insect responses are locally coadapted, these chemical cues could function as important isolating traits among populations, and thus function as drivers of diversification (32).
Some plants involved in brood-site pollination mutualisms interact both with obligate specialists and with generalist pollinators. This is the case for many species in the genera Lithophragma (Saxifragaceae) and Silene (Caryophyllaceae) (33–38), which contrasts with plant–pollinator mutualisms that are reciprocally obligate in all populations and species, such as figs and fig wasps and yucca and yucca moths. In woodland stars (Lithophragma), there are multiple levels of geographic and phylogenetic variation in the small networks of plants and insects involved in the interaction (7). Some Lithophragma species are self-pollinating or are involved in generalized pollination systems, but most species are obligately outcrossing and are involved in tight coevolutionary interactions with Greya moths (Prodoxidae) (6, 7). Within those species, Greya moths are generally the primary pollinators (37, 39).
There is, however, considerable geographic variation in the interactions between woodland star plants and their pollinators. Floral morphology of Lithophragma varies geographically within and among species, depending on whether flower-visiting Greya moth species are present locally and also on which of two Greya moth species are present (7). Greya politella oviposits into floral ovaries by inserting its abdomen into the corolla tube, thereby efficiently pollinating the host plant through a close mechanical fit (6). In contrast, Greya obscura oviposits into the floral wall or scape tissue from a posture external to the flower (40). Although G. obscura is a much less efficient pollinator than G. politella, because it pollinates only while drinking nectar, detailed studies at a site where both moths are present have shown that G. obscura can contribute significantly to pollination during years when G. politella numbers are low (39). At least one of these two Greya species is present in most populations of most Lithophragma species (7). Each of these pollinating moth species exhibits phylogeographic and morphological divergence across the geographic range of its interactions with woodland stars (6, 7, 41, 42).
Under these conditions, natural selection could favor strong divergence in floral scents among Lithophragma populations and species, and adaptation of Greya moths to the scent of their local host plants. Indeed, several Lithophragma species strongly differ in floral volatile emission rates and chemical composition (43, 44), and local populations of G. politella and G. obscura moths preferentially orient toward the scent of their local Lithophragma host species (40, 44). Also, for populations that have been tested experimentally, G. politella moths preferentially oviposit in flowers of the local host population, indicating that floral scent can be important for mediating local specificity in the Greya–Lithophragma interaction (44), and that Greya moths could potentially favor local convergence of floral scent in co-occurring Lithophragma species. In addition to selection on plants imposed by Greya moths, generalist bees and bombyliid flies are present as copollinators in some populations. In a few populations studied at the northern geographic limits of these woodland stars, these interactions can sometimes be so common that they swamp the mutualism between the plants and the moths (34, 37). Even when less common, these other floral visitors could contribute to divergent selection among Lithophragma populations in floral scent.
This rich set of previous studies on Lithophragma biology allowed us to test the hypothesis that the local presence of Greya moth pollinators has contributed significantly to the phylogenetic and geographic divergence of floral scent within and among Lithophragma species. We addressed that goal by performing a systematic, standardized sampling of floral scent from multiple populations of all species of Lithophragma and from closely related outgroups. Our study included 94 populations across the entire latitudinal distribution of the genus in western North America. We then combined phylogenetic and geographic analyses of floral scent for populations with one, both, or neither of the pollinating Greya moth species (7), and evaluated the extent to which among-population scent variation within Lithophragma can be partitioned into components attributable to (i) phylogenetic distance, (ii) geographic distance, and (iii) differences in interactions with Greya moths. These results provide the most comprehensive study so far of the magnitude and structure of floral scent variation within and among plant species of any genus.
Results
Phylogenetic Divergence in Floral Scent Profiles.
Floral scent within Lithophragma varied substantially at every level of the biological hierarchy among the samples of the 94 populations of all recognized Lithophragma species and subspecies, and four outgroup populations of other saxifragaceous species (SI Appendix, Table S1 and Dataset S1). Gas chromatography/mass spectrometry (GC/MS) analysis, combined with solid-phase microextraction (SPME), allowed the identification of 132 different floral volatile compounds, of which 120 could be identified using cochromatography with synthetic standards, concordance with published retention indices and mass spectra, or both criteria (SI Appendix, Dataset S1). The mass spectra of seven of the remaining 12 compounds allowed tentative identification based on library matches but were insufficient to suggest likely identities for five other compounds, which were left undetermined (SI Appendix, Dataset S1). Identified compounds were distributed among multiple compound groups and included aliphatics, monoterpenoids, sesquiterpenoids, irregular terpenoids, and aromatic compounds with or without N-atoms.
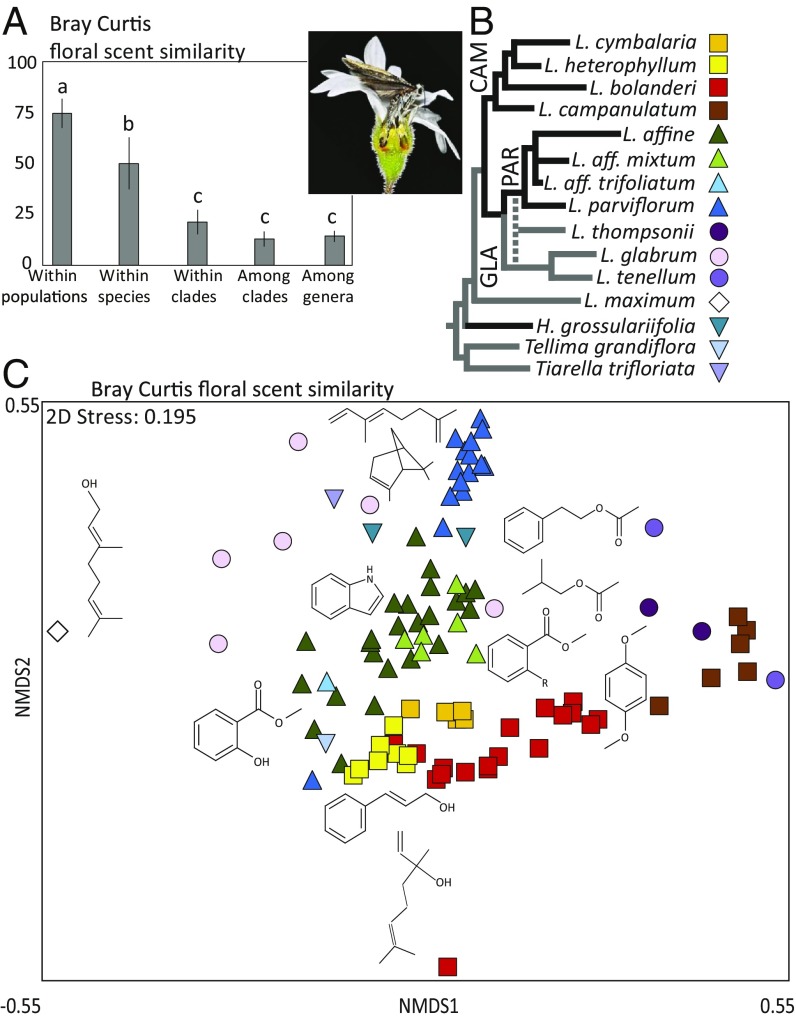
The three major Lithophragma clades, the Lithophragma campanulatum (CAM) clade, the Lithophragma parviflorum (PAR) clade, and the Lithophragma glabrum (GLA) clade, differed significantly in the combination of chemical compounds they emitted, based on analysis of pairwise Bray–Curtis distances in multivariate space [permutational multivariate analysis of variance (PERMANOVA): F2,90 = 11.45, R2 = 0.21, P < 0.001; Fig. 1]. Species in the CAM and PAR clades are pollinated by Greya moths, whereas the GLA clade does not interact with pollinating Greya (Fig. 1B). Phylogenetic differences also were apparent in a cluster analysis, because floral scent variation largely mirrored the species-level phylogeny (SI Appendix, Fig. S1). Analyses of all species represented by five or more populations showed that species differed significantly from each other in the scent combinations they produced (PERMANOVA: F7,80 = 14.0, R2 = 0.55, P < 0.001; all pairwise contrasts, P < 0.05 after sequential Bonferroni correction; Fig. 1C).
Fig. 1.
(A) Average floral scent similarity (1 − Bray–Curtis distance) of Lithophragma samples from the same population, from different conspecific populations, from different species of the same Lithophragma clade, and from populations of different clades (ANOVA: F4,32 = 65.95, P < 0.001). Different letters above bars indicate significantly different pairwise comparisons (Tukey’s honest significant difference). (B) G. politella female oviposits into the ovary and simultaneously pollinates a Lithophragma flower. Eight of the 12 Lithophragma taxa (black lines) and one outgroup taxon (H. grossulariifolia) are pollinated in this way. Within Lithophragma, two paraphyletic clades, the PAR clade and the CAM clade, are pollinated by Greya moths (phylogeny from refs. 70, 71). Dotted line indicates that L. thompsoni is a hybrid species between ancestors in the PAR and GLA clades. (C) Phylogenetic distribution of floral scent variation among populations shown as an MDS plot illustrating the 2D distance between all populations sampled.
Regional Patterns of Chemical Profiles Within Clades and Species.
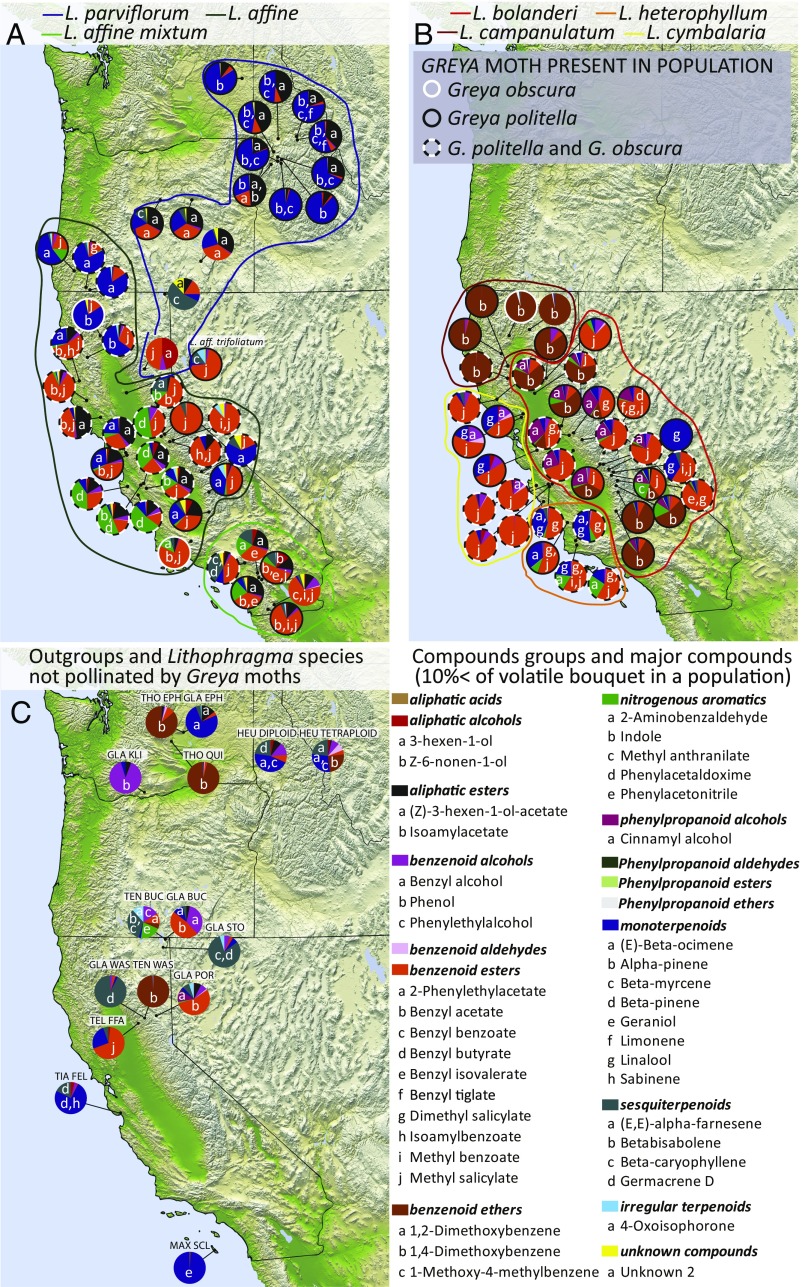
Not only did the compound groups dominating floral scent composition differ among clades and species, but the dominant compounds and compound groups also differed regionally among populations of the same species (Fig. 2 and SI Appendix, Dataset S1). For example, although scent bouquets of several populations of the closely related species L. parviflorum and Lithophragma affine ssp. affine were dominated by monoterpenoids (Fig. 2A), the predominant monoterpene was α-pinene for L. parviflorum and often was (E)-β-ocimene for L. affine ssp. affine (Fig. 2A and SI Appendix, Fig. S2). Farther south, L. affine ssp. affine populations often were dominated by nonterpenoid compounds, including benzenoid esters and nitrogenous aromatics (Fig. 2A and SI Appendix, Fig. S3). Similar regional clustering occurred in the CAM clade (Fig. 2B and SI Appendix, Figs. S2–S4). The outgroups and the species not associated with Greya moths also exhibited considerable biosynthetic variation among species and, where available, among populations (Fig. 2C). These taxa produced volatile compounds from all of the major biosynthetic pathways found within Lithophragma. Hence, the large variation in floral scent found within Lithophragma derives, in part but not solely, from the versatile biosynthetic potential common to this clade of the Saxifragaceae.
Fig. 2.
Geographic distribution of floral scent variation for the entire floral scent bouquet at the compound group level in the PAR clade (blue line, L. parviflorum; dark green line, L. affine ssp. affine; light green line, L. affine spp. mixtum; no color, L. affine spp. trifoliatum) (A), the CAM clade (brown line, L. campanulatum; red line, L. bolanderi; orange line, L. cymbalaria; yellow line, L. heterophyllum) (B), and the non–Greya-pollinated Lithophragma species (GLA, L. glabrum; MAX, L. maximum; TEN, L. tenellum; THO, the hybrid species L. thompsoni) and outgroups (HEU, H. grossulariifolia; TEL, T. grandiflora; TIA, T. trifoliata) (C). Pies show the approximate location of each population, and colors within pies show the proportional contribution of different volatile compound groups to the population scent signal. Letters in pie sections indicate compounds that contribute more than 10% of the total scent variation in each population. Rings around pies indicate the presence of G. politella (black), G. obscura (white ring), or both G. politella and G. obscura (black and white ring).
Chemical Richness Among and Within Species.
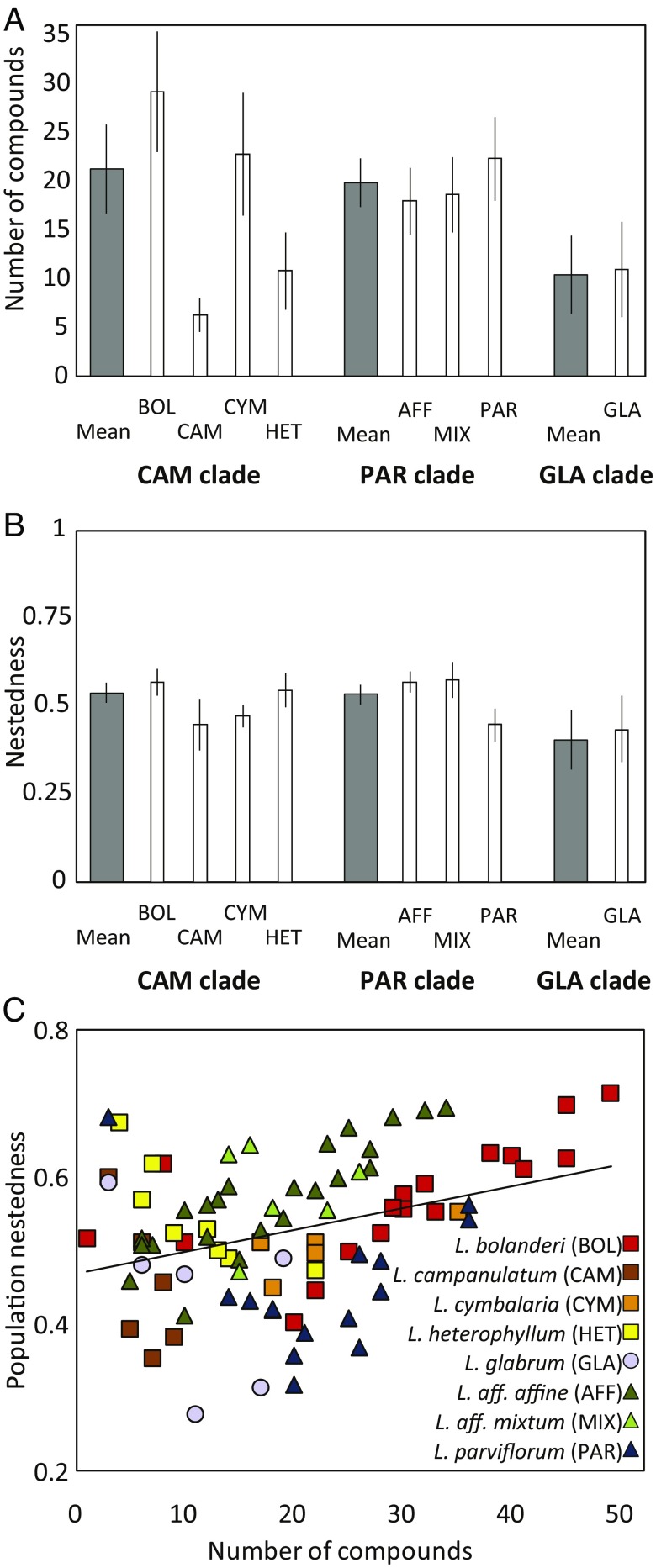
Species differed significantly in the number of volatile compounds emitted by flowers (ANOVA: F7,80 = 7.03, P < 0.001; Fig. 3A). Moreover, none of the 120 total volatile compounds found within Lithophragma was shared by all species of the genus (a detailed description of the scent variation is provided in SI Appendix, Supplementary Results and Dataset S1). To better understand how compounds are shared across populations, we explored patterns of distribution of compounds across populations through nestedness analysis (45). Two populations show high nestedness if the population with fewer compounds emits a subset of the floral scent bouquet of populations with a higher number of compounds. In contrast, a population shows low nestedness if it emits unique sets of compounds of low similarity with other populations. The nestedness analysis of the entire Lithophragma volatile dataset revealed that compounds were hierarchically grouped, showing a nested distribution across populations within species (n = 0.516; higher than the mean level of nestedness expected by matrices generated using a null model: 0.240 ± 0.008, 1,000 null model matrices; additional details on null model analyses are provided in Materials and Methods). At the genus level, populations with fewer compounds tended to be subsets of populations with many compounds.
Fig. 3.
Results of the chemical diversity (A) and nestedness analysis (B) for each of the three major Lithophragma clades (dark bars) and for individual species with more than five sampled populations (white bars). Populations of the GLA clade differed significantly from populations of the CAM and PAR clades both in number of compounds emitted and in nestedness [Tukey’s honest significant difference (HSD): P < 0.05 in both comparisons with the GLA clade], but the moth-pollinated CAM and PAR clades did not differ significantly (Tukey’s HSD: P > 0.05). Error bars in A and B denote 95% confidence intervals. (C) Number of compounds emitted and population nestedness showed a significant positive relationship (R2 = 0.11, F1,86 = 11.26, P = 0.0011).
Species, however, differed greatly in the degree to which populations producing fewer compounds were nested within populations producing more compounds (F7,80 = 5.93, P < 0.001; Fig. 3B). As a result, although mean nestedness per population was positively correlated with the number of compounds recorded in each population, the correlation was weak (Fig. 3C). Much of the overall positive correlation was driven by L. affine (F = 59.84, slope = 0.007, R2 = 0.73, P < 0.001, n = 24 populations) and L. bolanderi (F = 11.57, slope = 0.004, R2 = 0.42, P = 0.004, n = 18 populations). Other species showed either no relationship or, for Lithophragma heterophyllum, a negative relationship (F = 14.66, slope = −0.01, R2 = 0.71, P = 0.009, n = 8 populations). The species-specific variation in how nestedness changes with the number of compounds therefore suggests that there is a diversity of ways by which Lithophragma species diversify chemically. Specifically, populations of L. affine and Lithophragma bolanderi with multiple compounds continue to overlap chemically more with other populations of the Lithophragma species.
Geographic Scale of Chemical Divergence Within Species and Clades.
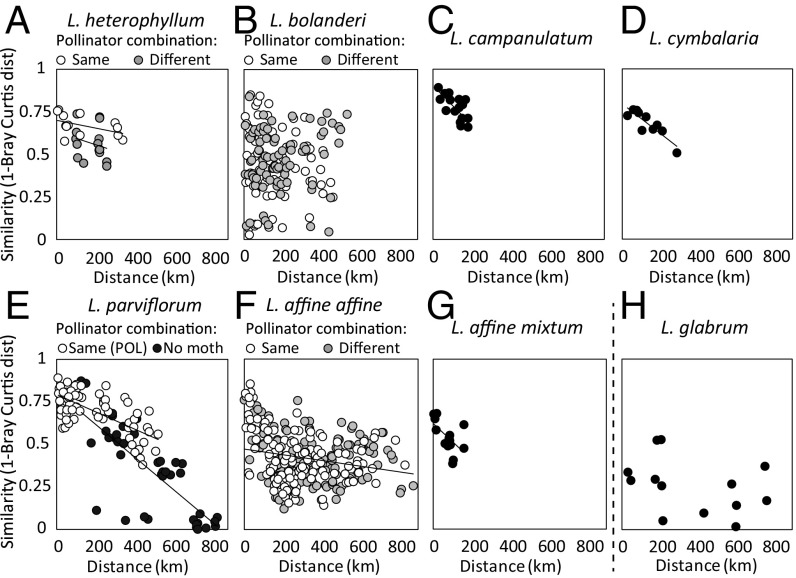
Within most of the species in which more than five populations were sampled, neighboring populations were chemically more similar than distant populations (Fig. 4 A–H and Table 1). Negative relationships between the pairwise population similarity (1 − Bray–Curtis distance) and their geographic distance were strongest in the three taxa with the smallest ranges (L. affine spp. mixtum, Lithophragma cymbalaria, and L. campanulatum) and in the widely distributed L. parviflorum. In L. affine ssp. affine, the negative relationship was significant but weak, and in L. bolanderi and L. glabrum, there were no significant effects of geographic distance on floral scent similarity. The lack of any clear geographic pattern in L. bolanderi was driven, in part, by extensive variation among populations in the presence of the benzenoid ether 1,4-dimethoxybenzene, which varied from dominance to complete absence in a patchy way among populations (SI Appendix, Fig. S4).
Fig. 4.
Relationship between pairwise population-level similarity [1 − Bray–Curtis distance (dist)] and geographic distance within L. heterophyllum (light circles, populations interacting with the same combination of Greya moth pollinators; gray circles, populations with different moth pollinators) (A), L. bolanderi (light circles, same moth pollinator; gray circles, different moth pollinators) (B), L. campanulatum (C), L. cymbalaria (D), L. parviflorum (light circles, same moth pollinator; dark circles, combinations including at least one population that lack moth pollinators) (E), L. affine (light circles, same moth pollinator; gray circles, different moth pollinators) (F), L. affine spp. mixtum (G), and L. glabrum (H).
Table 1.
Statistical output from Mantel tests on the relationship between pairwise floral scent similarity (1 − Bray–Curtis distance) and the geographic distance between populations for Lithophragma species with more than five populations sampled
| Plant species | Population, n | r | P |
| L. glabrum | 6 | −0.324 | 0.24 |
| L. affine | 24 | −0.227 | <0.001 |
| L. affine spp. mixtum | 6 | −0.576 | 0.024 |
| L. parviflorum | 15 | −0.832 | <0.001 |
| L. bolanderi | 18 | 0.036 | 0.67 |
| L. campanulatum | 6 | −0.762 | 0.002 |
| L. cymbalaria | 5 | −0.881 | 0.003 |
| L. heterophyllum | 8 | −0.374 | 0.052 |
Significant effects are indicated in bold. Marginally significant effects (0.05 < P < 0.1) are indicated in italics.
The greater similarity between nearby pairs of populations persisted when we held the phylogenetic distance constant by comparing the pairwise similarity of populations across the PAR and CAM clades (Mantel test: r = −0.499, P < 0.001; SI Appendix, Fig. S5). Hence, each pairwise combination included one CAM clade population and one PAR clade population. Consequently, a negative relationship indicates that similarities in scent profiles of adjacent populations were generated by factors other than common descent. However, the negative relationship between population similarity and geographic distance was strongly affected by comparisons involving the allopatric species L. parviflorum in the PAR clade, which is the only moth-pollinated Lithophragma with a northern distribution (compare Fig. 2). When L. parviflorum was removed from the similarity-by-distance analysis, the negative relationship was weak and nonsignificant (Mantel test: r = 0.079, P = 0.062; SI Appendix, Fig. S5).
We used a random forest machine-learning approach to evaluate whether each population’s scent profile fit within the range of scent profiles for each species. The classification algorithm identified six outlier populations in an analysis of the eight species that included samples from more than five populations (n = 88 populations; SI Appendix, Fig. S1). Only two of the six outlier populations grew in microsympatry with other Lithophragma species, and neither of these two was classified as its sympatric congener. Across all 88 populations, differences in scent profiles occurred among species, but microsympatry/microallopatry between species did not significantly affect how often a population was correctly classified in the iteration process (linear model species: F7,73 = 7.29, P < 0.001; sympatry/allopatry: F1,73 = 1.26, P = 0.27; species * sympatry/allopatry: F6,73 = 0.25, P = 0.96; SI Appendix, Fig. S1).
Chemical Profiles Relative to Distribution of Greya Moths.
The average chemical bouquets of populations from the two widely distributed clades that interact with Greya moths (CAM and PAR clades) had significantly more compounds (F2,88 = 3.19, P = 0.046) than the widely distributed clade that does not interact with moths (GLA clade) (Fig. 3A). Moreover, populations from the three Lithophragma clades differed in nestedness of chemical composition (F2,88 = 7.14, P < 0.001), such that populations from the GLA clade overlapped less in their compound profiles than populations of the two clades that include moth-pollinated populations (Fig. 3B).
Within the moth-pollinated CAM and PAR clades, populations that interact with Greya moths had, on average, more compounds (Welsh t test: t8.22 = 3.14, P = 0.013), but not higher nestedness (Welsh t test: t5.37 = 0.72, P = 0.499), than the few (n = 6) populations from the CAM and PAR clades in which Greya are absent. Among moth-pollinated populations (n = 76), different combinations of moths (only G. politella, only G. obscura, or both) did not affect the number of compounds recorded (F2,73 = 0.83, P = 0.44) or the level of nestedness (F2,73 = 2.05, P = 0.14).
Next, we corrected these analyses for moth effects within the CAM and PAR clades by including the geographic proximity of populations. For the four Lithophragma species with eight or more sampled populations, we asked whether populations pollinated by the same moths (G. politella only, G. obscura only, or both species) were more similar in scent bouquets than expected from their geographic proximity. This prediction held for L. heterophyllum, where populations with the same combination of Greya moth pollinators showed higher similarity than population pairs with different combinations of Greya moths (partial Mantel test: r = −0.395, P = 0.037; Fig. 4A). Similarly, in L. parviflorum, the similarity between pairs of populations was higher when both populations interacted with G. politella than when at least one of the compared populations did not interact with G. politella (partial Mantel test: r = −0.374, P = 0.0052; Fig. 4E). No such impact of moth presence on floral scent similarity was detected in L. bolanderi (partial Mantel test: r = −0.029, P = 0.36; Fig. 4B) or L. affine (partial Mantel test: r = 0.0124, P = 0.42; Fig. 4F). Hence, the effect of moths on within-species chemical similarity differed among Lithophragma species within the moth-pollinated clades.
Discussion
The remarkable diversification in floral scent among Lithophragma species and populations shows strong effects of phylogenetic divergence, geographic divergence, and interactions with coevolved pollinators. The high similarity of floral scent samples drawn from the same population in field collections and the laboratory common garden (43, 46) indicates that the detected variation reflects local genetic variation rather than environmentally induced plasticity in volatile production. The results show not only that the biochemical pathways used for floral scent production can diverge strongly within a single plant genus but also that the biochemical pathways can diverge strongly among populations within the same species, mediated at least partially through interactions with coevolved pollinators.
Traits of importance for obligate mutualisms are predicted to be under stabilizing selection due to the increased cost of rare, nonmatching phenotypes (47). Therefore, the Lithophragma scent variation contrasts both with theoretical predictions and with some other studies of plants pollinated by floral parasites as obligate mutualists, for which population-level scent variation in nursery pollination systems is often low (48–51). Thus, the floral scent diversity in Lithophragma provides an opportunity to understand the conditions under which high trait variation, rather than low trait variation, is maintained in coevolving mutualisms. In figs, floral volatiles are often species-specific and attractive to the particular fig wasp pollinator of each species (52), although different fig wasp species may sometimes share fig hosts (53).
At a geographic scale comparable to the distributions of the Lithophragma species in this study, yuccas (Yucca filamentosa and Yucca elata) show minimal within-species variation in floral scent in the subset of species studied to date (48, 49). Yuccas appear to use rare and specific volatiles that generate “private channels” that attract their coevolved yucca moth pollinators (31), in contrast to the complex floral bouquets composed entirely of conventional volatiles produced by Lithophragma species. This disparity in chemical diversity is intriguing in that closely related prodoxid moths pollinate both Lithophragma (Greya spp.) and Yucca (Tegeticula and Parategeticula spp.). Both sets of interaction involve oviposition during pollination, and both appear to be mediated, in part, by floral scent (31, 44). Two aspects of these interactions, however, may differ in ways in that contribute to the differences. One is that Lithophragma species and populations differ in whether they are pollinated locally by one or two Greya moths and also differ among populations in the extent to which copollinators contribute to pollination. The other is that Greya moths may have more restricted dispersal than yucca moths, but only a few species of both groups have been studied so far. Tegeticula yuccasella, which is the main pollinator of Y. filamentosa, shows only weak isolation by distance (54), whereas both G. politella and G. obscura show substantial population structure (41, 42). Difference in population structure in yuccas and Lithophragma could also contribute to the differences, but the available results are still too few to suggest any patterns in the scale of geographic divergence between these two plant genera.
In general, there are only a few examples of plant species scored for intraspecific floral scent variation among multiple populations (55), and most of these studies lack the broader phylogenetic and ecological context available for Lithophragma. In a South African cycad, Encephalartos villosus, scent variation has been tightly linked to geographic variation in the antennal sensitivity and behavioral preferences of the local weevil pollinators (32). Perhaps the most similar system to Lithophragma involves plants of the genus Silene (Caryophyllaceae), which are pollinated both by specialist noctuid moth seed predators of the genus Hadena and by generalized moth pollinators (56). Both Silene latifolia and Silene otites show geographic variation in floral scent bouquets, but the data available thus far do not indicate local variation at the scale that we document for Lithophragma (57, 58).
The combined phylogenetic, geographic, and pollinator-associated results for Lithophragma therefore provide a broader perspective for interpreting the range of species- and population-level variation in floral scent composition found in previous studies of nursery-pollinated plants and flowering plants in general. Although floral scent variation generally has been found in the few other plant genera that have been analyzed, most of that variation has been observed among congeners involved in very different pollination modes or syndromes (59–62). As in other nursery pollination systems, Lithophragma shows low variation within populations in floral scents (43, 44, 46), but differs from these taxa by showing extreme variation among populations and species in floral scent composition and complexity.
Several nonmutually exclusive processes could contribute to the high diversity of floral scent variation found in Lithophragma: relaxed selection; spatially varying selection imposed by the abiotic environment; or spatially varying selection imposed by interacting species, such as Greya moths, and, in some populations, copollinators. The small population sizes of many Lithophragma populations could amplify the effects of genetic drift on plant phenotypes, including floral scent, especially if the cost of emitting floral scent is low. Evidence from other systems, however, suggests that there are likely both ecological (18, 19, 63) and energetic costs (64) involved in the production and emission of floral scent signals. Hence, the likely response to relaxed selection from pollinators on floral scent would be an eventual shutdown of the production of nonnecessary compounds, as is found in some self-pollinating species derived from an insect-pollinated ancestry (65, 66).
Further evidence against genetic drift as a sufficient hypothesis for shaping floral scent variation across Lithophragma populations comes from the geographic analyses. Although some Lithophragma species sampled for more than five populations showed negative effects of geographic distance on pairwise similarity, this effect was strongest in three locally endemic species and subspecies, L. affine spp. mixtum, L. campanulatum, and L. cymbalaria, and was less evident or even lacking in the more widespread taxa in which the effects of drift would be expected to be greatest. Furthermore, there was a significant effect of geographic distance on floral scent similarity even when comparing populations belonging to different clades (SI Appendix, Fig. S5). In that analysis, the dissimilarity by distance cannot be explained by genetic drift, since phylogenetic distance among populations is kept constant. This result suggests that at least some of the scent similarity of adjacent populations could be attributed to them evolving in similar ecological settings. The cross-clade effect was, however, most apparent at a regional level, because populations growing in the Californian Floristic Province were more similar to each other than to the allopatric species L. parviflorum that grows further north (SI Appendix, Fig. S5).
The lack of strong dissimilarity by distance in two of the three widespread Lithophragma species (L. affine ssp. affine and L. bolanderi) indicates that the variation in floral scent chemistry is not primarily driven by abiotic selection from, for example, different local climate conditions. If local climate was driving floral scent variation, the strongest effect of geography on floral scent variation would be expected in these widespread species. Other abiotic factors like soil type or nutrient availability could potentially explain selection on floral scent variation. However, we have not detected any direct effects of local conditions on floral scent chemistry, because samples drawn from the field and from the greenhouse common garden are very similar in scent bouquet (43). Moreover, the release of floral scent in L. bolanderi seems canalized and not affected by variation in nutrient availability, whereas plants grown under low nutrient conditions alter several other traits, including the number and color of the leaves produced (46). Experiments on L. bolanderi, L. cymbalaria, and L. parviflorum do show that overall scent production is directly affected by temperature, because these species smell stronger during warm days than under colder nighttime conditions, and experiments showed that a reduced temperature affected floral scent emission also under daytime conditions (44). Therefore, in this study, we only collected scent under warm (>20 °C) conditions.
Furthermore, the experiments that varied daylight and temperature conditions showed that production of aromatic compounds was actively reduced during nighttime, independent of temperature, which implies a cost of signaling at times of low or no pollinator activity (44) and suggests that floral scent signaling is under pollinator-mediated selection. Past studies of Lithophragma have shown that phylogenetic and geographic variation in floral morphological traits correspond at least partially to geographic differences in which Greya moth pollinators are present locally (7). Hence, selection appears to be strong relative to drift for other floral traits important to pollination in Lithophragma. Greya females from different populations prefer to orient toward (40, 44) and oviposit in (44) flowers of the local host species rather than in distant nonlocal hosts of different floral scent composition. It is not yet clear at what geographic scale Greya populations differ in floral scent preference in comparison to variation in these other traits. Even so, the geographic scale of differentiation in floral morphology and chemistry may be partially linked, because different floral volatiles are produced by different floral structures (43). The floral scent variation among species and populations may therefore result from a combination of direct and indirect selection acting on the correlations among morphological and chemical traits.
The geographic pattern of divergence of floral scents further suggests that the observed variation results from selection partially imposed by Greya moths rather than from drift. In the widespread species L. parviflorum, populations pollinated by G. politella are more similar than expected by their geographic distance, whereas the negative relationship between floral scent similarity and distance was elevated in population combinations that included at least one population that lacked Greya moth pollinators (Fig. 4E). Similarly, in L. heterophyllum, populations pollinated by the same combination of moth pollinators (only G. politella, only G. obscura, or both) are more similar than populations pollinated by different moth combinations. Similar patterns, however, were not found in L. affine and L. bolanderi, suggesting that variation in selection imposed by Greya moths is insufficient as a full explanation of variation in floral scent throughout Lithophragma.
It is possible that floral scent variation is affected by a geographically varying selection imposed also by copollinators in some populations. Although Greya moths are the major pollinators in some Lithophragma populations studied in detail, generalized pollinators (e.g., solitary bees, bombyliid flies) have been shown to be important for Lithophragma populations at some sites at the northern geographic boundaries of the genus (34, 37). Lithophragma is widespread and grows in a wide range of habitats, including high-altitude meadows, river valleys, oak woodlands, and open pine forest woodlands. Therefore, both the abundance of Greya and the importance of the network of generalist pollinators are bound to vary among populations. Such ecological variation could select for floral signaling variation (cf. refs. 67, 68), if natural selection favors plants that optimize attraction to the local combination of Greya moths and the generalized pollinators.
The Lithophragma species not pollinated by Greya moths provide further insight into the potential impact of the non-Greya pollinators. The most unusual floral scent was emitted by the basal species in the genus, Lithophragma maximum, which is a rare, self-pollinating species endemic to San Clemente Island. The substantial scent variation detected also in L. glabrum and the lower nestedness of floral scent combinations among L. glabrum and Lithophragma tenellum populations, compared with the moth-pollinated clades, suggest that the moths may favor diversification of floral scents, but only to a subset of the potential universe of floral scent combinations. The pollination systems of L. glabrum and L. tenellum remain largely unknown, and it is unclear whether their evolutionary lineage has ever been involved in a coevolutionary relationship with Greya. Phylogenetically, the GLA clade and the PAR clade are sister lineages (Fig. 1B), which means that a double colonization of moths (to the PAR and CAM clades, respectively) is as parsimonious as a single moth colonization with subsequent termination of interactions with the GLA clade lineage. More detailed studies of the GLA clade and of populations of the PAR and CAM clades, where Greya species are rare or absent, should help to further refine our understanding of how each Greya species, as well as copollinators, contributes to divergence in floral scent.
In conclusion, the floral scent variation among Lithophragma species and populations is extreme relative to most other plant–pollinator systems studied, in the absence of massive pollinator functional group diversification seen, for example, among species in the orchid genus Disa (69). The different Lithophragma species showed large variation in the compounds emitted and in their biosynthetic affinities, but the variation included a certain level of phylogenetic conservatism, because the closely related outgroup taxa collectively comprised much of the variation in biosynthetic pathways found in Lithophragma. The Greya-pollinated species showed an elevated diversification in the floral scent compounds emitted; within species, this variation could be explained only partially by an increasing dissimilarity by distance. These multiple lines of evidence, together with past results, suggest that the variation in floral scent chemistry in Lithophragma plants is fueled by geographically varying selection imposed by Greya moths, possibly augmented by selection imposed by copollinating bees and/or flies. Collectively, these results suggest that the geographic mosaic of interactions between lineages of plants and insects may shape the diversification of traits of importance for the interaction.
Material and Methods
Study System.
The plant genus Lithophragma (Saxifragaceae) comprises 12 recognized species and subspecies, and is distributed across the western United States and southwestern Canada. Eight of these taxa, distributed across two paraphyletic clades (70, 71) (Fig. 1), directly depend on pollination from the moth G. politella (Prodoxidae) (6, 7, 33, 39). The two moth-pollinated clades are the PAR clade (L. parviflorum, L. affine ssp. affine, L. affine ssp. trifoliatum, and L. affine ssp. mixtum) and the CAM clade (L. campanulatum, L. heterophyllum, L. bolanderi, and L. cymbalaria) (Fig. 1). Four Lithophragma species have never been reported to interact with Greya moths and are either self-pollinated (L. maximum) or involved in generalized pollination systems. These are L. glabrum and L. tenellum, which together form the GLA clade, and Lithophragma thompsoni, which is a species of hybrid origin between predecessors in the GLA and PAR clades.
Within the moth-pollinated clades, G. politella is a floral parasite that pollinates the plants while ovipositing into the floral ovaries (43) (Fig. 1). G. politella is subdivided into at least four cryptic subtaxa with geographically nonoverlapping distributions (41). These subtaxa co-occur with different combinations of Lithophragma species, and the cryptic moth taxa show evidence of having gone through morphological coevolution with their main Lithophragma interaction partners (6, 7). Many Californian populations of the moth-pollinated Lithophragma species interact also with G. obscura. This close relative to G. politella oviposits into the floral wall and the stem tissue rather than into the flower (40), and pollinates while nectaring (39). It is a much less effective pollinator than G. politella, but is often more abundant (39). The net effect of the interaction between the plants and G. obscura can be commensalistic or potentially negative during years when the more efficient pollinator, G. politella, is present, but beneficial during years of low G. politella abundance (39). The moths also can be locally absent from local populations (7).
Floral Scent Collection and Analysis.
We used SPME (72) to collect floral scent from plants in 94 Lithophragma populations scattered across all species of the genus, focusing especially on the eight species and subspecies pollinated by the Greya moths. We also collected floral scent from four outgroup populations of other Saxifragaceae species occurring within the Lithophragma geographic range of increasing phylogenetic distance from Lithophragma. The outgroup species were Heuchera grossulariifolia (diploid and tetraploid representatives), Tiarella trifoliata, and Tellima grandiflora. All these outgroup species host one or more nonpollinating Greya species, and some H. grossulariifolia populations are inefficiently pollinated by ovipositing G. politella (73).
Each population sampled was represented by field-sampled flowers, greenhouse-grown flowers, or both (a full list of populations is provided in SI Appendix, Table S1). Previous work has shown that SPME samples collected in the greenhouse common garden and in the field were highly consistent in four populations of four different Lithophragma species (43). Each field sample included a single flower from each of eight different individuals. For each population, we collected two or three such samples depending on plant availability. On a few occasions, when fewer than 16 flowering individuals were available at a site, we collected a single sample from that site. The laboratory samples consisted of four to 16 flowers from available greenhouse-grown individuals. These were planted as seeds or root bulbils in a common garden. Plant growth conditions followed exactly the same protocol outlined by Friberg et al. (43) (SI Appendix).
The collected flowers were immediately enclosed in a 4-mL borosilicate glass vial and then capped with a cut gasket of nylon resin oven bagging (Reynolds, Inc.). The sample was equilibrated for 30 min, after which we exposed a 100-μm polydimethylsiloxane fiber of a Supelco (Sigma–Aldrich) SPME field sampler unit to the equilibrated floral headspace for 30 min. During all collection occasions, both under laboratory and field conditions, we collected the volatiles from an empty vial treated in the exact same way (storage, handling) as the floral headspace samples as a negative control. After scent collection under field conditions, SPME units were kept on blue ice and transported to the Marine Analytical Chemistry Laboratory at the University of California, Santa Cruz, for GC/MS.
The GC/MS analysis was performed using the exact same equipment and analytical parameters as used by Friberg et al. (43) (SI Appendix). The volatile peaks were manually integrated using the MS manufacturer’s software (G1034 version C.02.00; Hewlett-Packard), and compounds were tentatively identified based on the MS library suggestions (National Institute of Standards and Technology/Wiley). The identity of most compounds was verified using available literature Kovats retention index values [from columns similar to our polar Econo-Cap (EC-WAX) column], cochromatography of synthetic standards, or both (SI Appendix, Dataset S1).
Comparison Between SPME and Dynamic Headspace Sampling.
We evaluated our use of the SPME collection method by collecting floral scent samples from the greenhouse common garden for one population each of seven different species using dynamic headspace sampling. The populations included L. maximum from San Clemente Island (nSPME = 3, ndynamic headspace = 6), L. campanulatum from Pit River (nSPME = 6, ndynamic headspace = 4), L. bolanderi from Marble Falls (nSPME = 35, ndynamic headspace = 32) (dynamic headspace data from refs. 40, 44), L. affine from Hastings (nSPME = 9, ndynamic headspace = 19), L. cymbalaria from the Sedgwick Reserve (nSPME = 11, ndynamic headspace = 14), L. heterophyllum from Hastings (nSPME = 13, ndynamic headspace = 15), and L. parviflorum from the Turnbull National Wildlife Refuge (nSPME = 12, ndynamic headspace = 23). Dynamic headspace data for the latter four species were obtained from a study by Friberg et al. (43). The dynamic headspace data were sampled and analyzed using GC/MS in accordance with protocols reported elsewhere (43, 44, 46).
Statistical Analyses.
We compared the samples from SPME and dynamic headspace collection techniques of one population each of seven different Lithophragma species by generating Bray–Curtis distances among samples using the package vegan (74) in the statistical software R, version 3.4.1. We generated a subsequent nonmetric multidimensional scaling (MDS) plot (1,000 restarts) to identify consistent variation in how dynamic headspace and SPME samples clustered in multidimensional space. We tested for the effect of species and sampling treatment on the scent variation detected using the function perMANOVA in the R package vegan. Furthermore, for species including more than five SPME samples and five dynamic headspace samples, we asked whether the collection techniques differed in the number of compounds detected. These analyses showed that the samples cluster with species rather than sampling technique, and although the PERMANOVA also reported significant effects of sampling technique (PERMANOVA species: F6,188 = 122.0, P < 0.001; sampling technique: F1,188 = 25.2, P < 0.001; and species * sampling technique: F6,188 = 4.19, P < 0.001), the variance explained by species (75.4%) vastly outweighed the variance explained by sampling technique (2.6%) (SI Appendix, Fig. S6). Much of the variation between sampling techniques is likely driven by an overall tendency for SPME sampling to be more sensitive than dynamic headspace sampling in picking up scent compounds released at low rates of emission, because the SPME samples included a significantly higher number of compounds than the dynamic headspace samples (species: F4,173 = 84.9, P < 0.001; sampling technique: F1,73 = 95.5, P < 0.001; species * sampling technique: F4,173 = 1.41, P = 0.23; SI Appendix, Fig. S6). Collectively, these results show that even though the SPME sampling is more sensitive than the dynamic headspace sampling, the relative contributions of major compounds are consistent among sampling techniques, indicating that the SPME data obtained in this study are suitable for describing proportional differences among floral scent bouquets within and across taxa.
Using the SPME samples, we then investigated if the number of chemical compounds recorded per population varied across distinct species of Lithophragma, using the subset of species in our dataset that were represented by at least five populations per species. We then tested if the number of chemical compounds was correlated with the phylogenetic relatedness and within moth-pollinated clades, with the presence of different moth species. Phylogenetic relatedness was investigated at the clade level, whereas the presence of different moth species was coded in four categories: (i) no moth present at the site, (ii) only G. politella present at the site, (iii) only G. obscura present at the site, or (iv) both G. politella and G. obscura present at the site.
We then explored patterns of chemical overlap across populations of different species using network theory, as well as traditional multivariate analysis of population similarity. In the network analysis, we described the distribution of chemicals across populations as a network formed by two sets of nodes (chemicals and populations). A link between nodes in the network represents the presence of a given compound in a given population. We were particularly interested in determining the population level of nestedness. Nestedness analyses evaluate the extent to which the floral scent variation of each population is structured, such that populations that produce relatively few volatiles have chemical profiles that are subsets of the range of compounds found within the genus, the clade, or particular ecological groups (e.g., populations with moths compared with populations without moths). Nested patterns are frequently observed in multiple biological systems, especially in some types of ecological networks (45). We used tools derived from network theory to first test if the distribution of chemical compounds was nested in the entire dataset. Then, we computed the nestedness of each given population in relation to all populations analyzed. Because we were especially interested in the overlap patterns, we used the nestedness index proposed by Bastolla et al. (75), which allowed us to focus on the patterns of overlap among chemicals of distinct populations without considering other components associated with nestedness, such as the variation in the number of compounds. Nestedness was calculated within populations, between pairs of populations, and across all populations (definitions of nestedness at each of these levels are provided in SI Appendix).
We tested if the matrix of population-level chemical occurrence was more nested than expected by a theoretical benchmark provided by a null model. We used as a theoretical benchmark the null model 2 (45), in which null model matrices are random matrices generated by assuming constraints that preserve the number of populations, the average number of chemical compounds per population, the variation in the number of compounds across populations, and the total number of populations in which one compound was recorded. We then tested whether the level of nestedness for each population was correlated with the phylogenetic relatedness among populations and with the presence of different moth species, with both factors coded as in the analysis of number of compounds.
Thereafter, we generated Bray–Curtis distances to evaluate multivariate similarities among populations using the package vegan (74) in the statistical software R, version 3.4.1. For each population with multiple samples available, we calculated the mean peak area of each compound in the GC/MS chromatogram outputs for each field sample and then repeated this exercise for the laboratory samples. The mean peak area of each compound in the field and laboratory samples then became the consensus sample used in all further analyses. We used the population mean, because initial analyses of all samples revealed a high similarity of samples from the same populations (Fig. 1A and SI Appendix). That analysis corroborated previous work, which has shown a high similarity between field- and laboratory-collected samples at the population level (43), and minor effects of plant age (43) or nutrient availability (46), and allowed us to pool field and greenhouse data. We generated a similarity matrix (1− Bray–Curtis distance) and a subsequent MDS plot (5,000 restarts) and cluster analysis. The main effect of species on floral scent composition was tested in a PERMANOVA using the vegan R package (74), including species with more than five sampled populations. Similar analyses were performed at the level of the clade in three major Lithophragma clades/subclades (the CAM clade, the PAR clade, and the GLA clade; Fig. 1). We further determined the robustness of the proportional dataset by generating Bray–Curtis distances, a subsequent cluster analysis, and an MDS plot also for a dataset with presence vs. absence data for each compound and population. These analyses resulted in very similar patterns as the proportional dataset (SI Appendix, Figs. S1 and S7).
The between-population pairwise similarity matrix was further analyzed in Mantel tests (10,000 permutations) using the Excel plug-in program XLStat (version 2016.01.26040) to determine how the floral scent similarity between two populations varied in relation to the geographic distance between them. The geographic distance between populations was calculated from the field-measured global positioning system (GPS) coordinates using the software ArcMap 10.3. We asked how population similarity related to the geographic distance within each species of more than five populations sampled and among populations of the two moth-pollinated clades (PAR and CAM clades). A negative relationship at the within-species level would indicate similarity by descent, and a negative relationship in the between-clade analyses would indicate that at least parts of this similarity could be due to shared ecology. Finally, in species of more than eight populations sampled (L. affine, L. parviflorum, L. bolanderi, and L. heterophyllum), we tested whether populations that shared the same moth pollinator (G. politella and/or G. obscura) were more similar than expected by their geographic distance. Significance testing in these analyses was performed using partial Mantel tests in the statistical software zt, version 1.1 (76), testing the effect of moth combination (same, different, or at least one population lacking moths) on similarity, with the geographic distance of populations as a covariate.
Finally, we used the “random forests” classification algorithm (77) in R to identify outlier populations of the eight Lithophragma species for which more than five populations were sampled. We asked the machine-learning algorithm to estimate for each population the “out of bag” probability of membership in the eight different species to thereby identify outlier populations (randomForest function in the randomForest package, with 10,000 bootstrap iterations with species as classification categories). We then asked whether misclassified populations and other populations with a low probability of correct classification were typically growing allopatrically or growing sympatrically with other Lithophragma populations.
Supplementary Material
Acknowledgments
We thank Karin Gross, Malin Undin, Mia T. Waters, Hampus Petrén, Amy Parachnowitsch, and two anonymous reviewers for helpful comments on earlier drafts of this paper and Pamela S. Soltis and Douglas E. Soltis for comments on Lithophragma phylogeny. We thank Rob Franks at the University of California, Santa Cruz (UCSC) Marine Analytical Laboratory for analytical assistance with the GC/MS equipment; Kate McCurdy (Sedgwick Reserve), Michael Rule (Turnbull National Wildlife Refuge), Sylvia Haultain and Erik Frenzel (Sequoia National Park), Margit Sands (Sutter Buttes), Lyndal Laughrin (Santa Cruz Island Reserve), Michael Hamilton (Blue Oak Reserve), Paul Johnson (Pinnacles National Park), and the staff at Hopland Reserve for their hospitality and assistance during field work; Jim Velzy (UCSC Greenhouse) and Galen Pelzmann, Aliya Ingersoll, Jill Piorkowski, Lindsey Roark, Daniela Ruiz, and Mia T. Waters for help during laboratory and field work; and Rancho Santa Ana Botanical Gardens for L. maximum seeds. This work was supported by the Swedish Research Council, the Fulbright Commission, the Royal Swedish Academy of Sciences, the Crafoord Foundation, and the Swedish Foundation for International Cooperation in Research and Higher Education (to M.F.); by UCSC Langenheim Chair funds (to J.N.T.); by National Science Foundation Grant DEB-0839853 (to J.N.T.) and Grants DEB-0746106 and IOS-0923765 (to R.A.R.); and by Fundação de Amparo à Pesquisa do Estado de São Paulo Grant 2017/08406-7 and a Conselho Nacional de Desenvolvimento Científico e Tecnológico grant (to P.R.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1809007116/-/DCSupplemental.
References
- 1.Kawecki TJ, Ebert D. Conceptual issues in local adaptation. Ecol Lett. 2004;7:1225–1241. [Google Scholar]
- 2.Leimu R, Fischer M. A meta-analysis of local adaptation in plants. PLoS One. 2008;3:e4010. doi: 10.1371/journal.pone.0004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandon S, Nuismer SL. Interactions between genetic drift, gene flow, and selection mosaics drive parasite local adaptation. Am Nat. 2009;173:212–224. doi: 10.1086/593706. [DOI] [PubMed] [Google Scholar]
- 4.Hereford J. A quantitative survey of local adaptation and fitness trade-offs. Am Nat. 2009;173:579–588. doi: 10.1086/597611. [DOI] [PubMed] [Google Scholar]
- 5.Thompson JN. The Geographic Mosaic of Coevolution. Univ of Chicago Press; Chicago: 2005. [Google Scholar]
- 6.Thompson JN, Schwind C, Guimarães PR, Jr, Friberg M. Diversification through multitrait evolution in a coevolving interaction. Proc Natl Acad Sci USA. 2013;110:11487–11492. doi: 10.1073/pnas.1307451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson JN, Schwind C, Friberg M. Diversification of trait combinations in coevolving plant and insect lineages. Am Nat. 2017;190:171–184. doi: 10.1086/692164. [DOI] [PubMed] [Google Scholar]
- 8.Thompson JN. The Coevolutionary Process. Univ of Chicago Press; Chicago: 1994. [Google Scholar]
- 9.Schoonhoven LM, van Loon JJA, Dicke M. Insect-Plant Biology. 2nd Ed Oxford Univ Press; Oxford: 2005. [Google Scholar]
- 10.Raguso RA. Wake up and smell the roses: The ecology and evolution of floral scent. Annu Rev Ecol Evol Syst. 2008;39:549–569. [Google Scholar]
- 11.Raguso RA. Floral scent in a whole-plant context: Moving beyond pollinator attraction. Funct Ecol. 2009;23:837–840. [Google Scholar]
- 12.Schiestl FP. Ecology and evolution of floral volatile-mediated information transfer in plants. New Phytol. 2015;206:571–577. doi: 10.1111/nph.13243. [DOI] [PubMed] [Google Scholar]
- 13.Schiestl FP, et al. The chemistry of sexual deception in an orchid-wasp pollination system. Science. 2003;302:437–438. doi: 10.1126/science.1087835. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, et al. Private channel: A single unusual compound assures specific pollinator attraction in Ficus semicordata. Funct Ecol. 2009;23:941–950. [Google Scholar]
- 15.Knudsen JT, Eriksson R, Gershenzon J, Ståhl B. Diversity and distribution of floral scent. Bot Rev. 2006;72:1–120. [Google Scholar]
- 16.Baldwin IT, Preston C, Euler M, Gorham D. Patterns and consequences of benzyl acetone floral emissions from Nicotiana attenuata plants. J Chem Ecol. 1997;23:2327–2343. [Google Scholar]
- 17.Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- 18.Irwin RE, Adler LS, Brody AK. The dual role of floral traits: Pollinator attraction and plant defense. Ecology. 2004;85:1503–1511. [Google Scholar]
- 19.Theis N, Adler LS. Advertising to the enemy: Enhanced floral fragrance increases beetle attraction and reduces plant reproduction. Ecology. 2012;93:430–435. doi: 10.1890/11-0825.1. [DOI] [PubMed] [Google Scholar]
- 20.Huang M, et al. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012;193:997–1008. doi: 10.1111/j.1469-8137.2011.04001.x. [DOI] [PubMed] [Google Scholar]
- 21.Tabata J, De Moraes CM, Mescher MC. Olfactory cues from plants infected by powdery mildew guide foraging by a mycophagous ladybird beetle. PLoS One. 2011;6:e23799. doi: 10.1371/journal.pone.0023799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raguso RA, Schlumpberger BO, Kaczorowski RL, Holtsford TP. Phylogenetic fragrance patterns in Nicotiana sections Alatae and Suaveolentes. Phytochemistry. 2006;67:1931–1942. doi: 10.1016/j.phytochem.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 23.Van der Niet T, Jürgens A, Johnson SD. Pollinators, floral morphology and scent chemistry in the southern African orchid genus Schizochilus. S Afr J Bot. 2010;76:726–738. [Google Scholar]
- 24.Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. Pollination syndromes and floral specialization. Annu Rev Ecol Evol Syst. 2004;35:375–403. [Google Scholar]
- 25.Dobson H. Relationship between floral fragrance composition and type of pollinator. In: Pichersky E, Dudareva N, editors. Biology of Floral Scent. CRC; Boca Raton, FL: 2006. pp. 147–198. [Google Scholar]
- 26.Schiestl FP, Johnson SD. Pollinator-mediated evolution of floral signals. Trends Ecol Evol. 2013;28:307–315. doi: 10.1016/j.tree.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 27.Junker RR, Parachnowitsch AL. Working towards a holistic view on flower traits—How floral scents mediate plant–animal interactions in concert with other floral characters. J Indian Inst Sci. 2015;95:43–68. [Google Scholar]
- 28.Dobson HE, Arroyo J, Bergström G, Groth I. Interspecific variation in floral fragrances within the genus Narcissus (Amaryllidaceae) Biochem Syst Ecol. 1997;25:685–706. [Google Scholar]
- 29.Byers KJRP, Bradshaw HD, Jr, Riffell JA. Three floral volatiles contribute to differential pollinator attraction in monkeyflowers (Mimulus) J Exp Biol. 2014;217:614–623. doi: 10.1242/jeb.092213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber MG, et al. The evolution of floral signals in relation to range overlap in a clade of California Jewelflowers (Streptanthus s.l.) Evolution. 2018;72:798–807. doi: 10.1111/evo.13456. [DOI] [PubMed] [Google Scholar]
- 31.Svensson GP, Pellmyr O, Raguso RA. Pollinator attraction to volatiles from virgin and pollinated host flowers in a yucca/moth obligate mutualism. Oikos. 2011;120:1577–1583. [Google Scholar]
- 32.Suinyuy TN, Donaldson JS, Johnson SD. Geographical matching of volatile signals and pollinator olfactory responses in a cycad brood-site mutualism. Proc Biol Sci. 2015;282:20152053. doi: 10.1098/rspb.2015.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson JN, Pellmyr O. Mutualism with pollinating seed parasites amid co-pollinators: Constraints on specialization. Ecology. 1992;73:1780–1791. [Google Scholar]
- 34.Thompson JN, Cunningham BM. Geographic structure and dynamics of coevolutionary selection. Nature. 2002;417:735–738. doi: 10.1038/nature00810. [DOI] [PubMed] [Google Scholar]
- 35.Westerbergh A. An interaction between a specialized seed predator moth and its dioecious host plant shifting from parasitism to mutualism. Oikos. 2004;105:564–574. [Google Scholar]
- 36.Kephart S, Reynolds RJ, Rutter MT, Fenster CB, Dudash MR. Pollination and seed predation by moths on Silene and allied Caryophyllaceae: Evaluating a model system to study the evolution of mutualisms. New Phytol. 2006;169:667–680. doi: 10.1111/j.1469-8137.2005.01619.x. [DOI] [PubMed] [Google Scholar]
- 37.Thompson JN, Fernandez CC. Temporal dynamics of antagonism and mutualism in a geographically variable plant-insect interaction. Ecology. 2006;87:103–112. doi: 10.1890/05-0123. [DOI] [PubMed] [Google Scholar]
- 38.Giménez-Benavides L, Dötterl S, Jürgens A, Escudero A, Iriondo JM. Generalist diurnal pollination provides greater fitness in a plant with nocturnal pollination syndrome: Assessing the effects of a Silene-Hadena interaction. Oikos. 2007;116:1461–1472. [Google Scholar]
- 39.Thompson JN, Laine A-L, Thompson JF. Retention of mutualism in a geographically diverging interaction. Ecol Lett. 2010;13:1368–1377. doi: 10.1111/j.1461-0248.2010.01529.x. [DOI] [PubMed] [Google Scholar]
- 40.Friberg M, Schwind C, Thompson JN. Divergence in selection of host species and plant parts among populations of a phytophagous insect. Evol Ecol. 2016;30:723–737. [Google Scholar]
- 41.Rich KA, Thompson JN, Fernandez CC. Diverse historical processes shape deep phylogeographical divergence in the pollinating seed parasite Greya politella. Mol Ecol. 2008;17:2430–2448. doi: 10.1111/j.1365-294X.2008.03754.x. [DOI] [PubMed] [Google Scholar]
- 42.Thompson JN, Rich KA. Range edges and the molecular divergence of Greya moth populations. J Biogeogr. 2011;38:551–563. [Google Scholar]
- 43.Friberg M, Schwind C, Raguso RA, Thompson JN. Extreme divergence in floral scent among woodland star species (Lithophragma spp.) pollinated by floral parasites. Ann Bot. 2013;111:539–550. doi: 10.1093/aob/mct007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friberg M, Schwind C, Roark LC, Raguso RA, Thompson JN. Floral scent contributes to interaction specificity in coevolving plants and their insect pollinators. J Chem Ecol. 2014;40:955–965. doi: 10.1007/s10886-014-0497-y. [DOI] [PubMed] [Google Scholar]
- 45.Bascompte J, Jordano P, Melián CJ, Olesen JM. The nested assembly of plant-animal mutualistic networks. Proc Natl Acad Sci USA. 2003;100:9383–9387. doi: 10.1073/pnas.1633576100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friberg M, Waters MT, Thompson JN. Nutrient availability affects floral scent much less than other floral and vegetative traits in Lithophragma bolanderi. Ann Bot. 2017;120:471–478. doi: 10.1093/aob/mcx069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raimundo RLG, Gibert JP, Hembry DH, Guimarães PR., Jr Conflicting selection in the course of adaptive diversification: The interplay between mutualism and intraspecific competition. Am Nat. 2014;183:363–375. doi: 10.1086/674965. [DOI] [PubMed] [Google Scholar]
- 48.Svensson GP, et al. Chemistry and geographic variation of floral scent in Yucca filamentosa (Agavaceae) Am J Bot. 2005;92:1624–1631. doi: 10.3732/ajb.92.10.1624. [DOI] [PubMed] [Google Scholar]
- 49.Svensson GP, Pellmyr O, Raguso RA. Strong conservation of floral scent composition in two allopatric yuccas. J Chem Ecol. 2006;32:2657–2665. doi: 10.1007/s10886-006-9189-6. [DOI] [PubMed] [Google Scholar]
- 50.Soler C, et al. Geographic variation of floral scent in a highly specialized pollination mutualism. Phytochemistry. 2011;72:74–81. doi: 10.1016/j.phytochem.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 51.Ibanez S, et al. The role of volatile organic compounds, morphology and pigments of globeflowers in the attraction of their specific pollinating flies. New Phytol. 2010;188:451–463. doi: 10.1111/j.1469-8137.2010.03317.x. [DOI] [PubMed] [Google Scholar]
- 52.Grison-Pigé L, Bessière J-M, Hossaert-McKey M. Specific attraction of fig-pollinating wasps: Role of volatile compounds released by tropical figs. J Chem Ecol. 2002;28:283–295. doi: 10.1023/a:1017930023741. [DOI] [PubMed] [Google Scholar]
- 53.Cornille A, et al. Floral volatiles, pollinator sharing and diversification in the fig-wasp mutualism: Insights from Ficus natalensis, and its two wasp pollinators (South Africa) Proc Biol Sci. 2012;279:1731–1739. doi: 10.1098/rspb.2011.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leebens-Mack J, Pellmyr O. Patterns of genetic structure among populations of an oligophagous pollinating yucca moth (Tegeticula yuccasella) J Hered. 2004;95:127–135. doi: 10.1093/jhered/esh025. [DOI] [PubMed] [Google Scholar]
- 55.Delle-Vedove R, Schatz B, Dufay M. Understanding intraspecific variation of floral scent in light of evolutionary ecology. Ann Bot. 2017;120:1–20. doi: 10.1093/aob/mcx055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pettersson MW. Pollination by a guild of fluctuating moth populations: Option for unspecialization in Silene vulgaris. J Ecol. 1991;79:591–604. [Google Scholar]
- 57.Dötterl S, Wolfe LM, Jürgens A. Qualitative and quantitative analyses of flower scent in Silene latifolia. Phytochemistry. 2005;66:203–213. doi: 10.1016/j.phytochem.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Jhumur US, Dötterl S, Jürgens A. Floral odors of Silene otites: Their variability and attractiveness to mosquitoes. J Chem Ecol. 2008;34:14–25. doi: 10.1007/s10886-007-9392-0. [DOI] [PubMed] [Google Scholar]
- 59.Stuurman J, et al. Dissection of floral pollination syndromes in Petunia. Genetics. 2004;168:1585–1599. doi: 10.1534/genetics.104.031138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shuttleworth A, Johnson SD. The missing stink: Sulphur compounds can mediate a shift between fly and wasp pollination systems. Proc Biol Sci. 2010;277:2811–2819. doi: 10.1098/rspb.2010.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farré-Armengol G, Filella I, Llusia J, Peñuelas J. Floral volatile organic compounds: Between attraction and deterrence of visitors under global change. Perspect Plant Ecol Evol Syst. 2013;15:56–67. [Google Scholar]
- 62.Welsford MR, Hobbhahn N, Midgley JJ, Johnson SD. Floral trait evolution associated with shifts between insect and wind pollination in the dioecious genus Leucadendron (Proteaceae) Evolution. 2016;70:126–139. doi: 10.1111/evo.12821. [DOI] [PubMed] [Google Scholar]
- 63.Schiestl FP, Kirk H, Bigler L, Cozzolino S, Desurmont GA. Herbivory and floral signaling: Phenotypic plasticity and tradeoffs between reproduction and indirect defense. New Phytol. 2014;203:257–266. doi: 10.1111/nph.12783. [DOI] [PubMed] [Google Scholar]
- 64.Gershenzon J. Metabolic costs of terpenoid accumulation in higher plants. J Chem Ecol. 1994;20:1281–1328. doi: 10.1007/BF02059810. [DOI] [PubMed] [Google Scholar]
- 65.Doubleday LAD, Raguso RA, Eckert CG. Dramatic vestigialization of floral fragrance across a transition from outcrossing to selfing in Abronia umbellata (Nyctaginaceae) Am J Bot. 2013;100:2280–2292. doi: 10.3732/ajb.1300159. [DOI] [PubMed] [Google Scholar]
- 66.Sicard A, Lenhard M. The selfing syndrome: A model for studying the genetic and evolutionary basis of morphological adaptation in plants. Ann Bot. 2011;107:1433–1443. doi: 10.1093/aob/mcr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parachnowitsch AL, Raguso RA, Kessler A. Phenotypic selection to increase floral scent emission, but not flower size or colour in bee-pollinated Penstemon digitalis. New Phytol. 2012;195:667–675. doi: 10.1111/j.1469-8137.2012.04188.x. [DOI] [PubMed] [Google Scholar]
- 68.Gross K, Sun M, Schiestl FP. Why do floral perfumes become different? Region-specific selection on floral scent in a terrestrial orchid. PLoS One. 2016;11:e0147975. doi: 10.1371/journal.pone.0147975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson S, Linder H, Steiner K. Phylogeny and radiation of pollination systems in DISA (Orchidaceae) Am J Bot. 1998;85:402. [PubMed] [Google Scholar]
- 70.Deng JB, et al. Phylogeny, divergence times, and historical biogeography of the angiosperm family Saxifragaceae. Mol Phylogenet Evol. 2015;83:86–98. doi: 10.1016/j.ympev.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 71.Kuzoff RK, Soltis DE, Hufford L, Soltis PS. Phylogenetic relationships within Lithophragma (Saxifragaceae): Hybridization, allopolyploidy, and ovary diversification. Syst Bot. 1999;24:598–615. [Google Scholar]
- 72.Prosen H, Zupančič-Kralj L. Solid-phase microextraction. Trends Analyt Chem. 1999;18:272–282. [Google Scholar]
- 73.Thompson JN, Merg KF. Evolution of polyploidy and the diversification of plant-pollinator interactions. Ecology. 2008;89:2197–2206. doi: 10.1890/07-1432.1. [DOI] [PubMed] [Google Scholar]
- 74.Oksanen J, et al. 2017 vegan: Community Ecology Package. R Package Version 2.4-4. Available at https://cran.r-project.org/web/packages/vegan/index.html. Accessed October 6, 2017.
- 75.Bastolla U, et al. The architecture of mutualistic networks minimizes competition and increases biodiversity. Nature. 2009;458:1018–1020. doi: 10.1038/nature07950. [DOI] [PubMed] [Google Scholar]
- 76.Bonnet E, Van de Peer Y. zt: A software tool for simple and partial Mantel tests. J Stat Softw. 2002;7:1–12. [Google Scholar]
- 77.Ranganathan Y, Borges RM. Reducing the babel in plant volatile communication: Using the forest to see the trees. Plant Biol (Stuttg) 2010;12:735–742. doi: 10.1111/j.1438-8677.2009.00278.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.