Significance
Multiple myeloma is a deadly disease for which new therapies are urgently needed. B cell maturation antigen (BCMA) is an excellent target for therapy of myeloma because it is a lineage restricted differentiation protein that is expressed on myeloma cells and plasma cells but not on essential organs. We have produced recombinant immunotoxins that target and kill myeloma cells expressing BCMA. We have examined two mouse models of myeloma and show that the anti-BCMA immunotoxins produce long-term complete regressions in both models. These data support further preclinical development of these agents.
Keywords: LMB-75, LMB-70, H929, MM.1S, BM306
Abstract
Multiple myeloma (MM) is a B cell malignancy for which new treatments are urgently needed. The B cell maturation antigen (BCMA) is a lineage-restricted differentiation protein highly expressed on myeloma. Recombinant immunotoxins (RITs) are proteins composed of the Fv or Fab portion of an antibody fused to a bacterial toxin. We previously treated H929 myeloma s.c. tumors with anti-BCMA immunotoxins, very active on killing cultured cells, and observed tumor growth inhibition but not complete tumor responses. To determine if immunotoxins were more active against cells growing in the bone marrow (BM), the normal location of myeloma cells, we developed a BM mouse model that is more relevant to human disease. H929 cells were transfected with luciferase and GFP, enriched by flow, recycled through the BM of a mouse, and injected IV into nonobese diabetic scid γ mice (NSG) mice. A second myeloma mouse model used the MM.1S-GFP-luc cell line. Mice were treated IV with immunotoxins, and the tumor burden was assessed using bioluminescence imaging. We achieved complete durable remissions when treating mice with H929-GFP-luc cells with anti-BCMA RITs both leptomycin B-75 (LMB-75) [anti-BCMA-disulfide-stabilized (ds)-Fv-PE24] (where PE represents Pseudomonas exotoxin A) or LMB-70 (anti-BCMA-Fab-PE24) given every other day for 5-d (QOD×5) doses beginning on day 4 or day 8. Mice were disease free at 3 months; untreated mice became moribund around day 40. We also achieved long-term responses using the MM.1S-GFP-luc myeloma cell line. Treatment with an 1.5 mg/kg LMB-75 QOD×5 anti-BCMA RIT beginning on day 4 caused the complete disappearance of tumors for 80 days. To summarize, LMB-75 and LMB-70, our anti-BCMA RITs, induced complete durable responses in two myeloma models.
Multiple myeloma (MM) is a B cell malignancy that originates in the bone marrow (BM). For many decades, the traditional therapy for this cancer included a combination of alkylating agents and corticosteroids and eventually autologous stem cell transplantation (1). The past two decades, however, have brought major advances for the treatment of MM with the introduction of immunomodulatory drugs, proteasome inhibitors, and histone deacetylase inhibitors (1). These advances have nearly doubled the 5-y survival rate for MM patients since the late 1980s (1). Still, MM remains incurable with most patients eventually relapsing (2). Resistance to therapy is also a significant problem for MM patients as MM has a high propensity for clonal heterogeneity and has complex interactions with the BM environment (2). There were ∼30,000 new cases of MM and 13,000 deaths in the United States in 2018 according to data from the American Cancer Society (3).
The first two monoclonal antibodies (mAbs) for the treatment of MM, daratumumab and elotuzumab, were approved in 2015 and indicate that immunotherapeutic approaches have great promise in the treatment of this disease (4). Although cancers are sometimes resistant to mAbs alone, recombinant immunotoxins (RITs) kill cells with the help of a cytotoxic agent that is attached to an antibody (5). RITs are composed of an antibody variable fragment fused to a portion of the bacterial toxin PE, and several are in preclinical development or in clinical trials (5). Moxetumomab pasudotox, which targets CD22 on B cell malignancies, has a very high response rate in hairy cell leukemia, has produced complete and durable responses in many patients, and was approved by the Food and Drug Administration for the treatment of relapsed or refractory hairy cell leukemia patients (6). An immunotoxin targeting mesothelin has also caused major tumor regressions in patients with chemotherapy-resistant malignant mesothelioma (7).
New effective immunotherapeutic treatment strategies rely on the identification of specific highly expressed antigen targets. One such target, CD46, showed promising results for antibody-based treatment of MM in a recent preclinical study (8). Another very attractive immunotherapy target for MM is the B-cell maturation antigen (BCMA) (4, 9). The BCMA, a member of the tumor necrosis receptor superfamily, is a lineage-restricted differentiation antigen present on normal and malignant plasma cells (10). Given its high expression on malignant plasma cells and lack of expression on essential organs, the BCMA is an attractive MM target, and we decided to pursue it in making immunotoxins for MM.
We recently published that the BCMA-targeted RIT LMB-70, which is composed of the anti-BCMA Fab of mAb BM306 fused to domain III of PE, has high cytotoxic activity in vitro against the BCMA expressing cell lines and myeloma cells from patients (11). In mice with myeloma cells implanted s.c., LMB-70 as a single agent caused the shrinkage of s.c. tumors but did not produce complete responses (11). Here, we employ the cell line used in this s.c. mouse model to produce a BM myeloma mouse model that is more relevant to human disease. We determine the efficacy of LMB-70 in this model as well as LMB-75, which uses an anti-BCMA dsFv fused to domain III of PE (12). Both produced complete remissions when injected into mice with H929 cells growing in their BM.
Results
Generation of H929 BM Model.
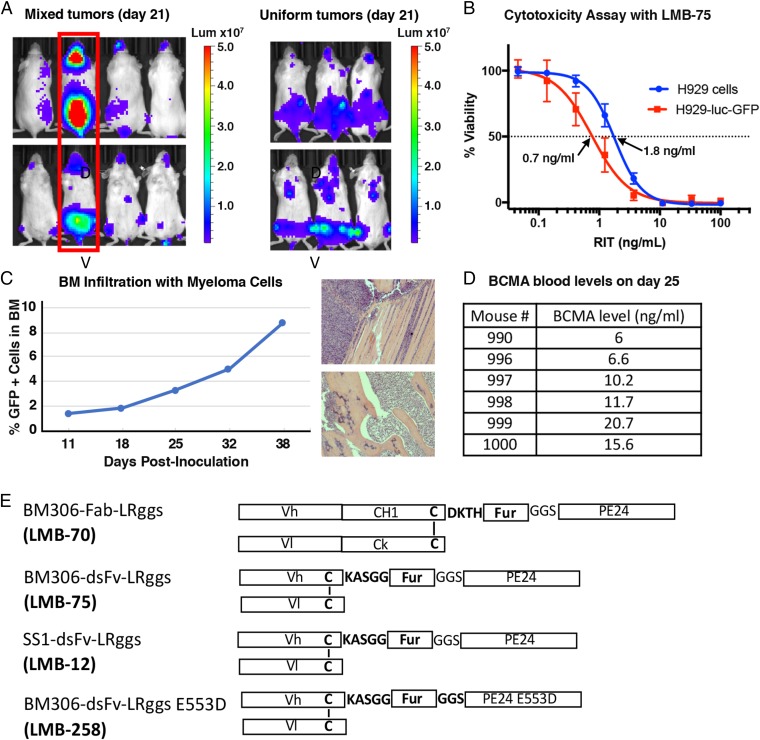
To generate a BM myeloma mouse model, the H929 cells were transfected with a lentiviral vector containing luciferase and GFP. Cells with high GFP expression were isolated as described in the Materials and Methods. To evaluate how the tumor cells grew in mice, 1 × 107 H929-luc-GFP cells were injected IV into female NSG mice, and the tumor burden was assessed by bioluminescence imaging. At 21 d post-tumor inoculation, one out of four mice showed a very high tumor burden (Fig. 1A). We harvested BM cells from the spine and femurs of this mouse, grew the cells in culture, and verified that the cells had high GFP expression using a fluorescent microscope. We also verified the sensitivity of the recovered cells to the immunotoxin using a cytotoxicity assay (Fig. 1B). The H929-luc-GFP cells are twofold more sensitive to the immunotoxin than the original H929 cell line with an IC50 value of 0.76 ng/mL compared with 1.78 ng/mL (Fig. 1B). After expanding and freezing the recovered H929-luc-GFP cells, we analyzed cell growth in mice by injecting 1 × 107 cells IV into female NSG mice. At day 21, bioluminescence imaging demonstrated that the tumor growth was rapid and uniform (Fig. 1A). We used these cells for all future experiments.
Fig. 1.
Generation of the H929 BM mouse model. (A) Growth of H929-luc-GFP cells in NSG mice. H929-luc-GFP cells were injected IV into four NSG mice, resulting in nonuniform tumor growth among the mice, based on bioluminescence imaging 21 d after tumor inoculation (Left). The mouse with the highest tumor burden (encircled in red) was killed, and BM cells were harvested from its spine and femurs. These recovered cells were used to inoculate tumors IV in three NSG mice. The bioluminescence images of these mice demonstrate uniform tumor growth at day 21 (Right). Dorsal (D) and ventral (V) images are shown for each mouse. (B) Recovered H929-luc-GFP cells are sensitive to LMB-75. Representative WST8 assay of the original H929 cell line compared with the luciferase transfected cells that were recovered from the mouse in A (abbreviated H929-luc-GFP). (C) BM infiltration of H929-luc-GFP cells. The mice bearing H929 tumors were killed, and the femurs harvested for BM cell isolation on days 11, 18, 25, 32, and 38. BM cells were sorted by flow cytometry for GFP+ cells on these days, indicating that the percentage of myeloma cells in the femurs at the time points listed were 1.4, 1.8, 3.3, 5.0, and 8.8, respectively (Left). Histological analysis (Right) of H929 mice on day 38 demonstrates infiltration of the femur (Top) and hind limb (Bottom) with myeloma cells. Invading tumor cells marked by asterisks. (D) H929-tumor bearing mice shed the BCMA in the serum. Soluble BCMA levels were measured from the sera of H929-tumor bearing mice at day 25 by BCMA-detection ELISA. The BCMA levels ranged from 6 to 21 ng/mL. (E) Schematics of various immunotoxins used in this paper. BM306 Fab RIT (LMB-70), BM306 dsFv RIT (LMB-75), SS1 dsFv RIT (LMB-12), and BM306 dsFv with an E553D mutation in PE24 (LMB-258).
To study the BM infiltration, we killed mice on days 11, 18, 25, 32, and 38 post-tumor inoculation and harvested the femurs to determine what percentage of the BM cells were myeloma cells. The percentage of cells that were myeloma cells in the femurs at the days indicated was 1.4, 1.8, 3.3, 5.0, and 8.8, respectively (Fig. 1C). This corresponds to 7 × 106, 9 × 106, 1.7 × 107, 2.5 × 107, and 4.4 × 107 myeloma cells, respectively. To determine where the myeloma cells were growing in the mice, a necropsy was performed on day 38. Pathology indicated the presence of myeloma cells in the capsular surface of one kidney, the inguinal canal region, the ovary, the urinary bladder, the sternal BM, the tibial and femoral BM, the spinal column BM, the spinal cord, the olfactory region of the nasal cavity, the skull BM, the meninges of the brain, the pituitary region, the inner ear, and around the incisor tooth roots. Lesions were not observed in the heart, liver, spleen, pancreas, and lungs. Histological analysis demonstrates infiltration of the femur and hind limb with myeloma cells (Fig. 1C and SI Appendix, Fig. S1).
As another marker of disease burden, we measured the level of soluble BCMA in the sera of several mice. The BCMA blood levels were undetectable at day 18 but were detectable at day 25 with levels ranging from 6.0 to 20.7 ng/mL among six mice (Fig. 1D). At their end points, the mice in this model developed hind limb paralysis and/or rapidly lost greater than 15% of their body weight. The mice became moribund around day 41.
Immunotoxin Constructs.
We tested two different anti-BCMA RITs in this model. We began with LMB-70 (BM306-Fab-LRggs), which contains a Fab fused to domain III of PE (Fig. 1E). LMB-70 was used in previous experiments in which H929 cells were grown s.c (11). We also tested RIT LMB-75 (BM306-dsFv-LRggs), which contains the dsFv of the BM306 mAb fused to domain III of PE (Fig. 1E). The dsFv format was used for RITs in the clinical trials previously described, although the payload in those proteins was PE38 instead of PE24, which lacked almost all of domain II of PE except for an 11-amino acid furin cleavage site. Domain II removal eliminates many B and T cell epitopes which contribute to antidrug antibody formation and is not needed for the cytotoxic activity of most immunotoxins except those targeting CD25 (13, 14).
Efficacy of LMB-70 in H929 BM Mouse Model.
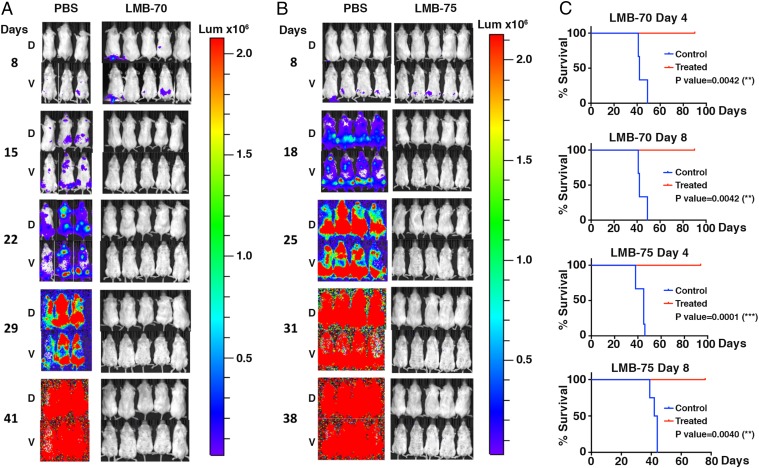
Since our previous studies with H929 cells growing as s.c. tumors were carried out with LMB-70 (BM306-Fab-LRggs), which contains the Fab portion of the BM306 mAb (Fig. 1E), we used it for the first series of experiments. Fig. 2A and SI Appendix, Fig. S2A show two experiments in which mice were injected IV with 1 × 107 H929-GFP-luc cells. Imaging shows the tumor was present in the femoral region of all mice when treatment began. Groups of five mice received five doses of LMB-70 IV at 1.5 mg/kg QOD×5 beginning 4 or 8 d post-tumor inoculation (Fig. 2A and SI Appendix, Fig. S2A). The mice were imaged weekly and monitored for weight loss (SI Appendix, Fig. S3). The images indicate that the tumor rapidly disappeared and could not be detected 1 wk after treatment was begun. In the untreated mice, the luciferase signal became progressively stronger involving the entire mouse by day 40 when mice were killed because of poor health. In contrast, the treated mice remained healthy, did not lose significant weight, and had no signal on day 90 when the experiment was terminated. Kaplan-Meier plots in Fig. 2C show a very significant difference between treated and untreated groups [P value for day 4 and day 8 treatment groups = 0.0042 (**)].
Fig. 2.
Efficacy of LMB-70 and LMB-75 in the H929 BM mouse model. H929-luc-GFP cells were injected IV into NSG mice. The mice were treated IV beginning on day 8 with PBS or 1.5 mg/kg LMB-70 (A) or LMB-75 (B) QOD×5, and bioluminescence imaging was used to assess the tumor burden. All mice were imaged with identical camera settings. Dorsal (D) and ventral (V) images are shown for each mouse. (C) Kaplan-Meier curves for mice treated with LMB-70 beginning on day 4 or 8 [P value = 0.0042 (**) for both] or with LMB-75 beginning on day 4 or 8 [P value = 0.0001(***) or 0.004 (**), respectively]. A logarithmic-rank (Mantel-Cox) test was used to determine P values.
Efficacy of LMB-75 in H929 BM Mouse Model.
We also tested LMB-75, which contained the dsFv of the BM306 mAb fused to domain III of PE, in the H929 model. Fig. 2B and SI Appendix, Fig. S2B show two experiments in which mice were injected IV with 1 × 107 H929-GFP-luc cells. All mice showed detectable signals at days 4 and 8, primarily in the femoral region based on ventral images. The signal generally became visible in dorsal images of the control mice by day 11 and increased thereafter. Mice were treated beginning on day 4 or 8 with 1.5 mg/kg LMB-75 QOD×5 (Fig. 2B and SI Appendix, Fig. S2B). The mice were imaged weekly and monitored for weight loss. Bioluminescence imaging results demonstrate that both treatment groups experienced complete regressions of their tumors, and the tumors did not return (Fig. 2B and SI Appendix, Fig. S2B). Treated animals lived without detectable tumor relapse until the studies were terminated around 3 mo. Kaplan-Meier end point survival curves in Fig. 2C demonstrated that the mice treated beginning on day 4 lived significantly longer than the control mice as did mice treated beginning on day 8 with P values of 0.0001 (***) and 0.0040 (**), respectively. The treated mice appeared healthy and did not lose weight (SI Appendix, Fig. S3).
Negative Controls in H929 BM Mouse Model.
We evaluated two immunotoxins as negative controls in this model. LMB-12 is an off-target immunotoxin that targets mesothelin (Fig. 1E). Mice were treated with one of the treatment schedules used with LMB-75 and LMB-70, beginning on day 8 with 1.5 mg/kg LMB-12 QOD×5. Bioluminescence imaging results demonstrated that the treatment group did not respond to LMB-12 (SI Appendix, Fig. S4A). The median survival for the mice treated with LMB-12 was 39 d, compared with 41 d for the control mice (Table 1). The results were the same with LMB-258, which is similar to LMB-75, but contains an inactive cytotoxic payload (Fig. 1E). Bioluminescence imaging demonstrated that the mice did not respond to treatment (SI Appendix, Fig. S4B). The median survival for mice treated with LMB-258 was 42 d, compared with 41 d for the control mice (Table 1). The median survival for mice treated with LMB-12 and LMB-258 was the same as that for the mice treated with PBS. Table 1 shows a summary of mice treated with various immunotoxins. Five mice were treated beginning on day 4 with 1.5 mg/kg LMB-70 QOD×5, and all achieved complete responses as did the 14 mice treated beginning on day 8 with 1.5 mg/kg LMB-70 QOD×5. These mice remained tumor free until the studies were discontinued around day 90. Eleven mice were treated beginning on day 4 with 1.5 mg/kg LMB-75 QOD×5, and all achieved complete responses up to day 75–90. The results were the same for the 11 mice treated beginning on day 8 with 1.5 mg/kg LMB-75. None of the five mice responded to treatment with 1.5 mg/kg LMB-12 QOD×5 beginning on day 8. The same is true for the five mice treated with 1.5 mg/kg LMB-258 beginning on day 8. These data indicate the immunotoxins containing anti-BCMA antibody fragments specifically cause the regression of H929 tumors.
Table 1.
Summary of responses and survival in the H929 model
| Treatment outcome | PBS | LMB-75 1.5 mg/kg QOD×5 | LMB-70 1.5 mg/kg QOD×5 | LMB-12 1.5 mg/kg QOD×5 | LMB-258 1.5 mg/kg QOD×5 |
| Responses (treatment starting on day 4) | 0/20 CR | 11/11 CR | 5/5 CR | ND | ND |
| Responses (treatment starting on day 8) | 0/19 CR | 11/11 CR | 14/14 CR | 0/5 CR | 0/5 CR |
| Median survival (in days) | 41 | >90 | >90 | 39 | 42 |
CR, complete response; ND, not done.
Efficacy of LMB-75 in MM.1S Mouse Model.
After achieving complete responses with LMB-70 and LMB-75 in the H929 model, we set out to show that the immunotoxins were effective in an additional BM mouse model and used MM.1S cells to do that. To achieve uniform tumor growth with a high luminescence signal, we isolated single clones from the MM.1S myeloma cell line. We identified clones that had high GFP expression as determined by flow cytometry. The original cell population had an IC50 value of 9.5 ng/mL when treated with LMB-75, whereas pure clones with high GFP expression 14, 15, 19, and 21 had IC50 values of 13.9, 13.3, 6.6, and 6.3 ng/mL, respectively (SI Appendix, Fig. S5). We chose clone 21 to use in our mouse model.
This clone was expanded, and 1 × 107 or 5 × 106 cells were injected IV into four mice each (SI Appendix, Fig. S6). The mice showed strong signals 4 d after inoculation with both cell numbers with the signals covering almost the entirety of each mouse (SI Appendix, Fig. S6). The signals in the upper part of the control mice decreased at days 11, 18, and 25 with cells that presumably initially went to the lungs and other organs but ultimately failed to engraft. The signals in the lower portion of the mice remained and began increasing starting at day 30 and increased thereafter. Two mice in each group were treated beginning on day 4 with 1.5 mg/kg LMB-75 QOD×5 (SI Appendix, Fig. S6). All four treated mice had no signal on day 45. The mice injected with 5 × 106 cells that were treated with LMB-75 showed no detectable signal at day 11 and onwards, remaining tumor free until the end of the study at day 80. The treated mice experienced no toxicity or weight loss from immunotoxin treatment.
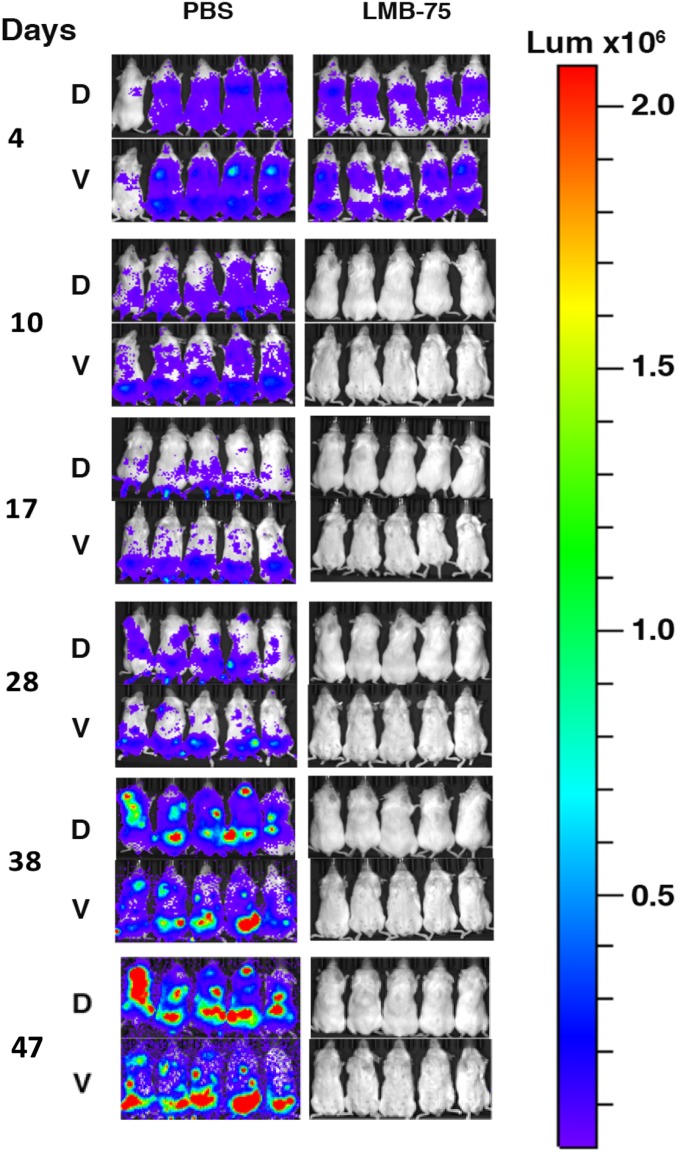
Fig. 3 shows a second experiment with five mice in each group in which the mice were implanted with 5 × 106 cells. The mice showed strong signals on day 4, which was completely eliminated with treatment of 1.5 mg/kg LMB-75 QOD×5 beginning on day 4, validating our previous result.
Fig. 3.
Efficacy of LMB-75 in the MM.1S BM mouse model. 5 × 106 MM.1S-luc cells were injected IV into 10 mice. The mice were treated IV beginning on day 4 with PBS or with 1.5 mg/kg LMB-75. Bioluminescence imaging was used to assess tumor burden with identical camera settings used for all images. Dorsal (D) and ventral (V) images are shown for each mouse.
SI Appendix, Table S1 shows a summary of mice in the MM.1S model. Two mice injected with 1 × 107 cells and seven mice injected with 5 × 106 were treated beginning on day 4 with 1.5 mg/kg LMB-75 QOD×5, and all mice achieved complete responses.
Pharmacokinetic Studies.
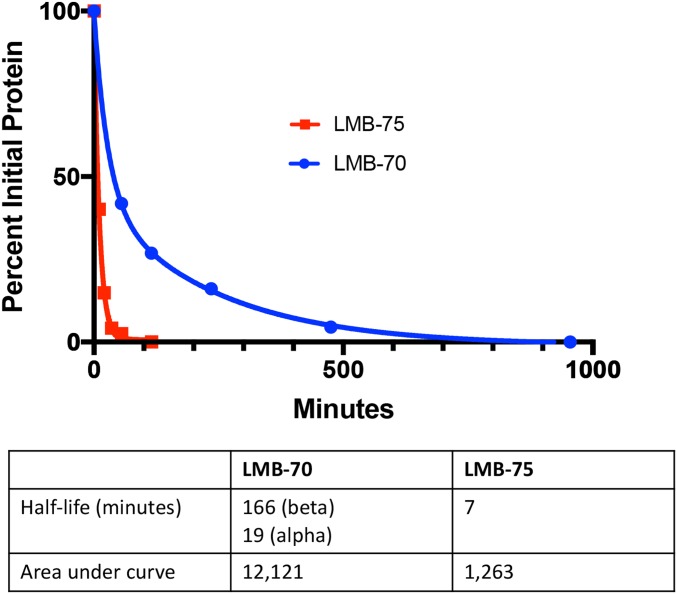
The pharmacokinetic behaviors of LMB-70 and LMB-75 were compared by injecting mice with 25 μg of LMB-75 or LMB-70. For LMB-75, blood was collected at 5, 15, 25, 40, 60, and 120 min. For LMB-70, blood was collected at 5, 60, 120, 240, 480, and 960 min. The decay curves in Fig. 4 show that LMB-70 has a much longer half-life than LMB-75. The decay of LMB-75 is essentially monoexponential with a half-life of 7.4 min and an area under the curve (AUC) of 1,263. The behavior of LMB-70 is very different with much slower removal from the circulation. The decay curve fits a biexponential decay curve with an α of 19 min and a β of 166 min. The AUC is 12,121 or 10-fold more than LMB-75.
Fig. 4.
Pharmacokinetics of LMB-70 and LMB-75 in the mice. Half-life studies were performed by injecting 25 μg of LMB-70 into the mice and bleeding the mice at 5, 60, 120, 240, 480, and 960 min. For LMB-75, 25 μg of protein were injected into the mice, and the mice were bled at 5, 15, 25, 40, 60, and 120 min. We assume the α phase is due to equilibration with the extracellular compartment, and the β phase is due to metabolism by the kidney and liver. LMB-75 displayed a one-phase decay with a half-life of 7 min. We assume it is rapidly filtered and degraded by proximal tubular cells in the kidney. The AUCs for LMB-70 and LMB-75, respectively, are 12, 121, and 1,263.
Discussion
We previously employed an s.c. mouse model using the H929 myeloma cell line to study the activity of RITs targeting the BCMA in mice and found the RITs caused tumor regressions but not complete responses (11). To assess the activity of anti-BCMA immunotoxins in a clinically relevant model, we engineered H929 cells to express luciferase so that their growth in the BM could be monitored and found that immunotoxins caused complete and durable regressions of H929 cells growing in the marrow. In addition, we studied MM1.S myeloma cells and found the RITs also produced durable complete regressions. These results strongly support the development of anti-BCMA immunotoxins for myeloma therapy.
The BM microenvironment provides the ideal niche for myeloma cell survival and is known to contribute to drug resistance in the treatment of MM, so it is crucial to assess potential therapeutics for myeloma in this context (15). Using this model, we found that the BM niche did not protect cells from immunotoxin action and that both LMB-70 and LMB-75 caused complete and durable tumor regressions lasting more than 90 d. Tumor regressions required targeting the toxin to the myeloma cells with an anti-BCMA Fv as well as an active toxin. An immunotoxin with a mutation E553D that inactivates toxin activity has no antitumor activity. Furthermore, the complete regressions were observed under conditions where there were no adverse effects in the mice as evidenced by lack of weight loss. This occurred even though the Fab-immunotoxin LMB-70 remained in the circulation much longer than LMB-75 immunotoxin (Fig. 4). We do not understand why the immunotoxins are not very effective against s.c. tumors since the cells are very sensitive to RIT killing in culture and in the BM but suspect the stroma may exert a protective effect.
When treating mice at later time points, such as day 11 and day 18, when their tumor burdens were higher, several mice died after one dose of the immunotoxin (SI Appendix, Table S2). Because this dose did not affect the health of the mice with lower tumor burdens, we suspect the mice died from tumor lysis syndrome. This syndrome has been observed in myeloma patients treated with Bortezomib (16). Reducing the dose of the immunotoxin from 1.5 to 0.75 mg/kg prevented the mice from dying and caused durable complete responses in 60% of the mice (SI Appendix, Table S2). The further characterization of possible involvement of tumor lysis syndrome in our H929 model is underway.
To demonstrate that our immunotoxins are effective in another BM myeloma mouse model, we isolated and used a single clone from the MM.1S cell line and treated mice with LMB-75. In this model, the bioluminescence signal was stronger and had a different distribution than the H929 model. In the MM.1S model, the signal was distributed throughout the entirety of the mouse on day 4, whereas in the H929 model, the signal began in the hind limbs and then spread throughout the rest of the mouse with time. The end point for the MM.1S-tumor bearing control mice was also different from that of the H929 model. MM.1S mice experienced hind limb paralysis around day 75, whereas H929 reached this stage at day 40. As such, we are still monitoring the MM.1S control and treated mice from Fig. 3 and SI Appendix, Fig. S6 to evaluate their differing survival outcomes.
MM.1S cells (IC50 of 6 ng/mL) are much less sensitive to LMB-75 than H929 cells (IC50 of 1 ng/mL), yet when we treated mice bearing MM.1S tumors with 1.5 mg/kg LMB-75 beginning on day 4, the MM.1S tumors completely regressed, and the treated mice were tumor free until the end of the study. These mice experienced no toxicity from the immunotoxin. This finding suggests we are using more LMB-75 than is needed in the H929 model. This is borne out by recent experiments in which a single dose of 0.5 mg/kg LMB-70 completely eliminated H929 tumors until day 35. Our laboratory previously showed that LMB-70 killed MM cells from patients with IC50 values ranging from 0.4 to 18.0 ng/mL, which is in the same range as the IC50 values we saw with the MM cell lines used previously (11).
There are currently many new antibody-based therapies under development or already in clinical use. These include naked antibodies, antibody drug conjugates, chimeric antigen receptor-T cells, as well as bispecific and trispecific antibodies (4, 8). Each of these modalities has a specific mechanism of action that could have an impact on the treatment of myeloma. Antibody drug conjugates are composed of monoclonal antibodies covalently linked to cytotoxic agents by chemical linkers and are the fastest growing class of cancer therapeutics (4). One of these, GSK2857916, has shown efficacy in relapsed or refractory MM (17, 18). Immunotoxins kill cells by inhibiting protein synthesis, a mechanism that differs from all agents used to treat myeloma (5). Futhermore, in animal models, immunotoxins have been found to synergize with taxanes that inhibit polymerization of microtubules (11). Drugs with this mechanism of action are a frequent component of antibody drug conjugates and are likely to synergize with immunotoxins targeting the BCMA (4).
In this paper, we have evaluated an immunotoxin containing a Fv fused to domain III of PE (LMB-75) that had a half-life of 7 min and another that had a Fab fused to domain III (LMB-70) with a β half-life of 166 min. Because LMB-70 is only slightly larger than LMB-75, it should penetrate into the tumor just as effectively and because of its long half-life is the molecule we plan to develop for clinical trials.
To summarize, we have shown that anti-BCMA immunotoxins produce complete and durable remissions in two orthotopic myeloma models with a good safety profile. These results support the continued preclinical development of these agents.
Materials and Methods
Cell Lines.
H929 cells were obtained from the American Type Culture Collection and maintained following the guidelines provided by the supplier. The MM.1S-luc cells were a gift from Dr. Constantine Mitsiades (Dana-Farber Cancer Institute) and were maintained following the guidelines provided by the supplier.
Development of H929-GFP-Luc Cell Line for Xenograft Mouse Model.
NCI-H929 cells were transfected with GFP-luciferase lentivirus and selected using puromycin at a concentration of 1 μg/mL After several days, the cells were analyzed for GFP expression under a fluorescent microscope. The cells were sorted by flow cytometry to eliminate nontransfected cells. Successfully transfected cells were expanded in culture. Female NSG mice between 5 and 10 wk of age were injected IV with 1 × 107 cells in 200 μL PBS. Mice were imaged weekly using the IVIS Imaging System (described below), and at 21 d post-tumor inoculation, one mouse showed a very high tumor burden compared with the other mice. BM cells were harvested from the spine and femurs of this mouse and grown in culture. Cells were analyzed for GFP expression under a fluorescent microscope. The cytotoxic activity of these cells was verified by a WST8 cytotoxicity assay (described below). These recovered cells, referred to as H929-GFP-luc cells, were used to establish our H929 BM mouse model and for the experiments with the immunotoxins described below.
MM.1S Xenograft Mouse Model.
To develop a BM mouse model with uniform growth and a strong luminescence signal, we isolated single clones from the MM.1S-GL cell line by single cell cloning in 96-well plates. After expansion, cells were analyzed by flow cytometry and clones that had high and uniform GFP expression were retained. The cells were evaluated for their response to LMB-75, and a sensitive clone with high GFP expression was expanded, frozen down, and used for tumor experiments. The 5 × 106 or 1 × 107 MM.1S cells were injected IV into NSG mice between 5 and 8 wk of age. The mice were treated beginning on day 4 with 1.5 mg/kg LMB-75 QOD×5, and the tumor burden was assessed using bioluminescence imaging. All animal experiments were performed in accordance with NIH guidelines and approved by the National Cancer Institute Animal Care and Use Committee.
BM Cell Isolation.
To isolate BM cells from mouse femurs, the heads of the femurs were broken off, and 26G needles connected to 5 mL syringes prefilled with Roswell Park Memorial Institute (RPMI) 10% FBS were inserted into the heads and flushed through. The spines were crushed to release BM cells. The cells from the femurs and spine were filtered through a 70 m cell strainer and spun down at 1,500 rpm for 5 min. Red blood cells were lysed on ice by the addition of 1 mL of ammonium chloride potassium lysis buffer. After a 1 min incubation, 10 mL of RPMI were added, and the resulting cell suspension was spun down at 1,500 rpm for 5 min. The resulting cells were resuspended in complete media.
Bioluminescence Imaging.
The mice were injected intraperitoneally with 150 μg of in vivo grade luciferin in 100 μL saline and imaged using the “automatic” setting on the IVIS Imaging System. The scale of each image was adjusted identically.
Cytotoxicity Assays.
WST-8 assays were used to assess the viability of the cells treated with RITs (11, 13). Briefly, 2 × 104 H929-GFP-luc cells were plated in 96-well round bottom plates in RPMI media containing 10% FBS. The cells were treated with various concentrations of RIT for 72 h. WST-8 was added to the plate and incubated for 3 to 4 h before the plates were read on the spectrophotometer. Then, the concentration of RIT causing a 50% inhibition of cell viability (IC50) was calculated.
Tumor Inoculation.
Female NSG mice between 5 and 10 wk old were injected IV with 1 × 107 of H929-GFP-luc cells or 1 × 107/5 × 106 MM.1S-luc cells. The humane end point for the mice was hind limb paralysis or greater than 15% body weight loss.
Treatment Schedule.
The mice were treated beginning on day 4 or 8 with 1.5 mg/kg LMB-75 and LMB-70 QOD×5. RITs were diluted in PBS to 30 μg/100 μL and adjusted accordingly based on the weights of the mice. As negative controls, mice were treated beginning on day 8 with 1.5 mg/kg LMB-12 and LMB-258 QOD×5. RITs were diluted in PBS to 30 μg/100 μL and adjusted accordingly based on the weights of the mice.
Histology.
Histology analysis was performed by Dr. Matthew Starost (Division of Veterinary Resources, Office of Research Services, NIH).
BCMA Blood Levels.
The control mice in the H929 model were bled 11, 18, and 25 d post-tumor inoculation. A human BCMA-TNFRSF17 DuoSET ELISA kit was used to determine soluble BCMA levels in the blood.
Statistical Analysis and Software.
P values were determined using a logarithmic-rank (Mantel-Cox) test. GraphPad Prism was used to generate Kaplan-Meier survival curves.
Production of Immunotoxins.
The anti-BCMA immunotoxins LMB-70 and LMB-75 used in this study were generated from monoclonal antibody BM306 (11). The binding affinity of BM306 is <1 × 10−10 M. The anti-BCMA RIT LMB-70 (BM306-Fab-LRggs) contained the Fv portions of the BM306 mAb fused to CH1 and Ck domains of human IgG1 followed by domain III of PE. LMB-75 (BM306-dsFv-LRggs) contained the dsFv of the BM306 mAb fused to domain III of PE (12). All immunotoxins used in this study were made following the protocol described earlier from our laboratory (19). Briefly, RITs were expressed as inclusion bodies in BL-21 competent Escherichia coli. The inducible lac promoter was used to express the protein once an OD600 between 2 and 3 was reached. The cell pellets were lysed, and the inclusion bodies were washed with Tris-EDTA-saline buffer (50 mM Tris⋅HCl, pH 8.0; 20 mM EDTA; and 100 mM NaCl) containing 2.5% Triton X-100. Then, 100 mg of the protein were solubilized and denatured in guanidine-Tris-EDTA buffer (6 M guanidine HCl; 100 mM Tris⋅HCl, pH 8.0; and 2 mM EDTA) with 100 mg of dithioerythritol. Next, the protein was refolded for 30–32 h at 4 °C (100 mM Tris⋅HCl; 1 mM EDTA; 0.5 M arginine; and 0.9 mM oxidized glutathione, pH 9.5) and dialyzed for 16–20 h at 4 °C (20 mM Tris⋅HCl, pH 7.4 and 100 mM urea). The dialysate was filter sterilized with a 0.45 μm Millipore filter and purified by anion exchange chromatography (Q Sepharose and Mono Q) followed by size exclusion chromatography (TSK).
Pharmacokinetic Studies.
Six nude mice were injected IV with 25 μg in 100 μL PBS of LMB-75, and blood was collected by submandibular bleed at 5, 15, 25, 40, 60, and 120 min. Six NSG mice were injected IV with 25 μg in 100 μL PBS of LMB-70, and blood was collected by submandibular bleed at 5, 60, 120, 240, 480, and 960 min. An ELISA was used to measure RIT serum levels at varying time points. The 96-well ELISA plates were coated with BCMA-Fc overnight, and a blocking buffer containing BSA was used. The serum was separated from the blood samples and added at increasing concentrations, and the IP12 antibody was used to determine how much RIT remained in the serum. Pharmacokinetic data were analyzed in Graphpad Prism using a nonlinear regression (curve fit) and either a one- or two-phase decay.
Supplementary Material
Acknowledgments
The authors thank Emily King for her help to isolate cells from mouse BM. This research was supported by the Intramural Research Program of the NIH, the National Cancer Institute, and the Center for Cancer Research.
Footnotes
Conflict of interest statement: I.P. is an inventor on several patents on immunotoxins that have all been assigned to the NIH.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1821733116/-/DCSupplemental.
References
- 1.Abramson HN. The multiple myeloma drug pipeline—2018: A review of small molecules and their therapeutic targets. Clin Lymphoma Myeloma Leuk. 2018;18:611–627. doi: 10.1016/j.clml.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Varga C, Laubach JP, Anderson KC, Richardson PG. Investigational agents in immunotherapy: A new horizon for the treatment of multiple myeloma. Br J Haematol. 2018;181:433–446. doi: 10.1111/bjh.15116. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 4.Cho SF, Anderson KC, Tai YT. Targeting B cell maturation antigen (BCMA) in multiple myeloma: Potential uses of BCMA-based immunotherapy. Front Immunol. 2018;9:1821. doi: 10.3389/fimmu.2018.01821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–565. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 6.Kreitman RJ, et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol. 2012;30:1822–1828. doi: 10.1200/JCO.2011.38.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassan R, et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med. 2013;5:208ra147. doi: 10.1126/scitranslmed.3006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherbenou DW, et al. Antibody-drug conjugate targeting CD46 eliminates multiple myeloma cells. J Clin Invest. 2016;126:4640–4653. doi: 10.1172/JCI85856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laâbi Y, et al. A new gene, BCM, on chromosome 16 is fused to the interleukin 2 gene by a t(4;16)(q26;p13) translocation in a malignant T cell lymphoma. EMBO J. 1992;11:3897–3904. doi: 10.1002/j.1460-2075.1992.tb05482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seckinger A, et al. Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell. 2017;31:396–410. doi: 10.1016/j.ccell.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Bera TK, et al. Recombinant immunotoxins targeting B-cell maturation antigen are cytotoxic to myeloma cell lines and myeloma cells from patients. Leukemia. 2018;32:569–572. doi: 10.1038/leu.2017.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shancer Z, et al. Preclinical development of anti-BCMA immunotoxins targeting multiple myeloma. Antib Ther. 2018;1:19–25. doi: 10.1093/abt/tby004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weldon JE, et al. A recombinant immunotoxin against the tumor-associated antigen mesothelin reengineered for high activity, low off-target toxicity, and reduced antigenicity. Mol Cancer Ther. 2013;12:48–57. doi: 10.1158/1535-7163.MCT-12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan G, et al. Improving the in vivo efficacy of an anti-Tac (CD25) immunotoxin by Pseudomonas exotoxin A domain II engineering. Mol Cancer Ther. 2018;17:1486–1493. doi: 10.1158/1535-7163.MCT-17-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nass J, Efferth T. Drug targets and resistance mechanisms in multiple myeloma. Cancer Drug Resist. 2018 doi: 10.20517/cdr.2018.04. [DOI] [Google Scholar]
- 16.Oiwa K, et al. High risk of tumor lysis syndrome in symptomatic patients with multiple myeloma with renal dysfunction treated with bortezomib. Anticancer Res. 2016;36:6655–6662. doi: 10.21873/anticanres.11274. [DOI] [PubMed] [Google Scholar]
- 17.Tai YT, et al. Novel anti-B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood. 2014;123:3128–3138. doi: 10.1182/blood-2013-10-535088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trudel S, et al. Targeting B-cell maturation antigen with GSK2857916 antibody-drug conjugate in relapsed or refractory multiple myeloma (BMA117159): A dose escalation and expansion phase 1 trial. Lancet Oncol. 2018;19:1641–1653. doi: 10.1016/S1470-2045(18)30576-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pastan I, Beers R, Bera TK. Recombinant immunotoxins in the treatment of cancer. Methods Mol Biol. 2004;248:503–518. doi: 10.1385/1-59259-666-5:503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.