Significance
Regulatory T cells (Tregs) protect cancer cells from immune attack. Tregs express CD25 on their surface and can be killed by antibodies and immunoconjugates targeting CD25. These agents have not been effective in controlling tumor growth, probably because they also kill cytotoxic CD8 T cells expressing CD25 that are needed for antitumor activity. To overcome these deficiencies, we have directly injected tumors with a potent anti-CD25 immunotoxin, 2E4-PE38, that kills CD25-expressing Treg cells in the tumor, causes injected and distant tumors to regress, and induces antitumor immunity. Because 2E4-PE38 is present at very low concentrations in the blood, it does not kill CD25-expressing cells at distant sites. This strategy could be employed for treating tumors in people.
Keywords: immunotherapy, cancer therapy, intratumoral injection, regulatory T cells, mesothelioma
Abstract
The tumor microenvironment plays a critical role in controlling tumor progression and immune surveillance. We produced an immunotoxin (2E4-PE38) that kills mouse cells expressing CD25 by attaching the Fv portion of monoclonal antibody 2E4 (anti-mouse CD25) to a 38-kDa portion of Pseudomonas exotoxin A. We employed three mouse cancer tumor models (AB1 mesothelioma, 66c14 breast cancer, and CT26M colon cancer). Tumors were implanted at two sites on BALB/c mice. On days 5 and 9, one tumor was directly injected with 2E4-PE38, and the other was not treated; 2E4-PE38 produced complete regressions of 85% of injected AB1 tumors, 100% of 66c14 tumors, and 100% of CT26M tumors. It also produced complete regressions of 77% of uninjected AB1 tumors, 47% of 66c14 tumors, and 92% of CT26M tumors. Mice with complete regressions of 66c14 tumors were immune to rechallenge with 66c14 cells. Mice with complete regressions of AB1 or CT26M tumors developed cross-tumor immunity rejecting both tumor types. Injection of anti-CD25 antibody or a mutant inactive immunotoxin were generally ineffective. Tumors were analyzed 3 days after 2E4-PE38 injection. The number of regulatory T cells (Tregs) was significantly reduced in the injected tumor but not in the spleen. Injected tumors contained an increase in CD8 T cells expressing IFN-γ, the activation markers CD69 and CD25, and macrophages and conventional dendritic cells. Treatment with antibodies to CD8 abolished the antitumor effect. Selective depletion of Tregs in tumors facilitates the development of a CD8 T cell-dependent antitumor effect in three mouse models.
The concept of suppressor T cells was proposed in the 1970s (1). However, the existence of suppressor T cells as a distinct lineage of T cells was controversial (2). In the mid-1990s, the concept of regulatory T cells (Tregs) was proposed, and since then Tregs have been extensively studied in mice and in humans (3). It is now well established that Tregs are a distinct lymphocyte lineage endowed with regulatory properties that affect a variety of immune cells (4). Tregs play an important role in immune escape by suppressing antitumor immunity, thereby providing an environment of immune tolerance. T cells that recognize cancer cells are often present in large numbers in tumors, but their cytotoxic function is suppressed by nearby immune-suppressor cells. Tregs are abundant in many different cancers (5), are highly enriched in the tumor microenvironment, and are well known for their role in tumor progression.
It has been demonstrated that Tregs contribute to the early establishment and progression of tumors in murine models and that their absence results in delay of tumor progression (6–9). High tumor infiltration by Tregs and a low ratio of effector T cells (Teffs) to Tregs is associated with poor outcome in solid tumors (10). Conversely, a high Teff/Treg cell ratio is associated with responses to immunotherapy (11). To date, most studies support the notion that targeting Tregs, either by depletion or functional modulation, offers a significant therapeutic benefit, particularly in combination with other immune modulatory interventions such as vaccines and checkpoint blockade (12–15). Defining appropriate targets for selective interference with Tregs is a critical step in the development of effective therapies. In this regard, CD25, also known as the interleukin-2 high-affinity receptor alpha chain (IL-2Rα), was the first surface marker used to identify Tregs (3) before the discovery of their master regulator, transcription factor fork-head box p3 (Foxp3). CD25 is also the most extensively studied target for inhibiting or eliminating Tregs and is absent on naive Teffs. However, transient up-regulation of CD25 has been observed upon activation of Teffs (16).
A number of preclinical studies in mice have used an anti-CD25 antibody, which partially depletes Tregs in the blood and peripheral lymphoid organs (9, 17). When the antibody was administered before tumor challenge, there was inhibition of tumor growth and improved survival (7–9, 14, 18, 19). However, the administration of anti-CD25 antibody against established tumors has failed to delay tumor growth (7–9, 19). This has been attributed to several factors, including poor T cell infiltration of the tumor (14) and potential depletion of activated effector CD8+ and CD4+ T cells that up-regulate CD25 (9). Clinical studies exploring the use of vaccines in combination with daclizumab, a humanized IgG1 anti-human CD25 antibody, or denileukin diftitox, a recombinant fusion protein combining human IL-2 and a fragment of diphtheria toxin, or LMB-2, a recombinant fusion protein combining anti-human CD25 Fv and a fragment of Pseudomonas exotoxin A (PE) had a variable impact on the number of circulating Tregs and vaccine-induced immunity (20–24). Assessment of Foxp3 transcript levels in the tumors provides no clear evidence that Tregs in the tumor microenvironment were effectively reduced, and antitumor activity has been disappointing across all studies, with no survival benefit (20–24).
It is widely recognized that immune modulatory antibody-based therapies can affect the level of Tregs and that the antibody isotype is important (25–29). We have now re-evaluated CD25 as a target for Treg depletion in vivo, but instead of using a monoclonal antibody (mAb), we have used a locally injected recombinant immunotoxin (RIT) with potent cytotoxic activity for killing Tregs. Systemic blockade of Tregs has produced life-threatening and dose-limiting autoimmune side effects in patients because the expression of CD25 is not limited to Tregs; Teffs also express CD25 (30). To maintain self-tolerance and proper control of adaptive immune responses, there must be a balance between Tregs and Teffs. Consequently, systemic depletion of Tregs may not be a good choice for cancer treatment, and we have used local therapy to avoid systemic side effects.
We have previously published that injection of an immunotoxin that targets mesothelin directly into tumors induces antitumor immunity when combined with anti–CTLA-4 (31). We have also reported that systemic injection of an immunotoxin targeting CD25 intravenously causes transient depletion of Tregs in the circulation, but no antitumor activity was observed (23). In the current study, we have taken advantage of our observation that intratumor injection of an immunotoxin can help initiate systemic immunity, and that injected immunotoxin (2E4-PE38) selectively kills T cells in the tumor, but does not deplete T cells outside the tumor. Using only the anti-CD25 immunotoxin, we have treated three different types of mouse cancers: 66c14 breast cancer, AB1 mesothelioma, and CT26M colon cancer. We have found that the immunotoxin caused complete regressions of the injected tumors, as well as most distal uninjected tumors and induced systemic antitumor immunity.
Results
Production of the Anti-Mouse CD25 Immunotoxin 2E4-PE38.
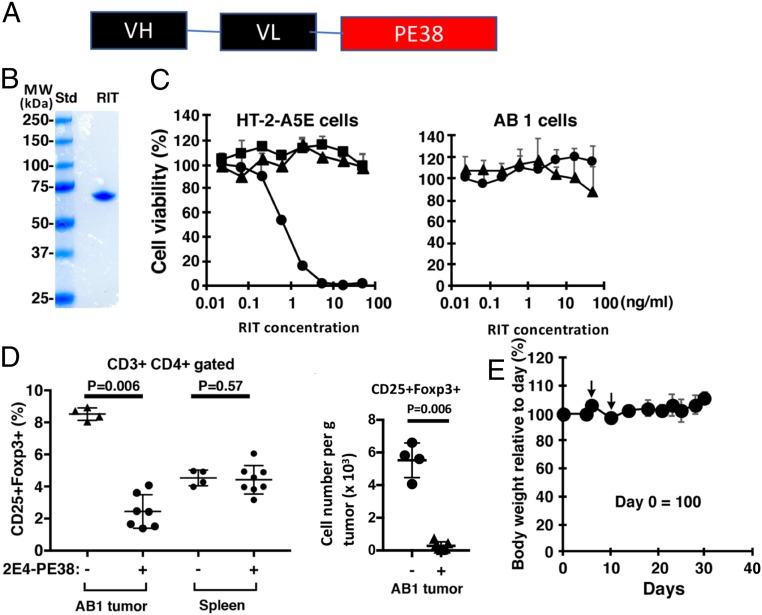
To test our hypothesis that local injection of an immunotoxin that targets mouse CD25 will selectively deplete Tregs within the tumor microenvironment and induce activation of Teffs, we produced an immunotoxin that kills cells expressing mouse CD25 as described in Materials and Methods. The characteristics of 2E4-PE38 are shown in Fig. 1. Fig. 1A illustrates that 2E4-PE38 is composed of a single chain Fv linked to a 38-kDa fragment of PE. The protein elutes as a monomer upon TSK gel-filtration chromatography and migrates as a single band of about 62 kDa in SDS/PAGE (Fig. 1B). To confirm that the 2E4-PE38 specifically kills mouse CD25 (mCD25) positive cells, we measured cytotoxic activity using mCD25-positive and -negative cells. Fig. 1C shows the specific cytotoxic activity of 2E4-PE38 against the CD25-expressing mouse T-lymphocyte cell line, HT-2-A5E. Using a CCK-8 viability assay, 2E4-PE38 has an IC50 of 0.8 ng/mL, whereas SS1P, an immunotoxin-targeting human mesothelin that is not expressed on HT2-A5E cells, has no cytotoxic activity (32). Furthermore, 2E4-PE38 does not kill the AB1 mesothelioma cells that do not express CD25. We also made an inactive mutant with a D mutation at position 553 of PE and found that 2E4-PE38-E553D has no cell-killing activity (Fig. 1C).
Fig. 1.
2E4-PE38 kills target cells and is nontoxic to mice. (A) Composition of 2E4(scFv)-PE38. (B) PAGE of purified 2E4-PE38. The purified protein (1 μg) was loaded on the SDS/PAGE gels. Molecular mass standard was loaded on the left lane. (C) Cytotoxic activity of 2E4-PE38 (circle), SS1P (triangle), and 2E4-PE38-E553D inactive mutant (square) toward the HT-2-A5E, CD25-positive line and AB1 mouse mesothelioma cells, CD25-negative line. (D) Intratumoral injected 2E4-PE38 (10 μg) depleted intratumoral CD4+CD25+Foxp3+ Tregs but did not significantly deplete Tregs in spleen. AB1 tumors were analyzed 3 d after RIT injection by flow cytometry (Left). (Right) CD25+Foxp3+ Treg cell number per gram of tumor. P values were calculated with Mann–Whitney test. (E) Body weight of mice bearing tumors with 2E4-PE38 treatment. 2E4-PE38 was injected into the tumor, which was implanted on the dorsal skin of BALB/c mice bearing AB1 tumors on days 5 and 9. Body weight was monitored. Mice kept body weight (n = 7). Day 0 = 100. Arrows: 2E4-PE38 intratumoral injection. Error bars represent SD.
CD3+CD4+CD25+Foxp3+ Tregs Are Abundant in Tumors.
Tregs are abundant in many cancers (5). To determine the number of Tregs present in AB1 tumors, we used flow cytometry. Analysis of the CD3+CD4+ lymphocytes in AB1 tumors shows that 8% are CD25+Foxp3+ Tregs, compared with 4% in the spleen (SI Appendix, Fig. S1). These data confirm that CD3+CD4+CD25+Foxp3+ Tregs are common within AB1 tumors and are a good target for an immunotoxin targeting CD25.
Targeting Tregs Induces Regression of Injected and Uninjected Distant Tumors.
To evaluate the efficacy and safety of intratumor injections, we compared the effect of injecting two doses of 10, 5, or 1.25 μg of 2E4-PE38 and found that only the highest dose produced complete regressions (CRs) of all injected tumors (SI Appendix, Fig. S2). Therefore, this dose was chosen for all subsequent studies. This dose is safe because it does not cause weight loss or damage the skin lying over the tumor (Fig. 1E). In all subsequent experiments, mice were treated with 100 μL of a 100 μg/mL solution of 2E4-PE38 given twice on days 5 and 9 after tumor implantation.
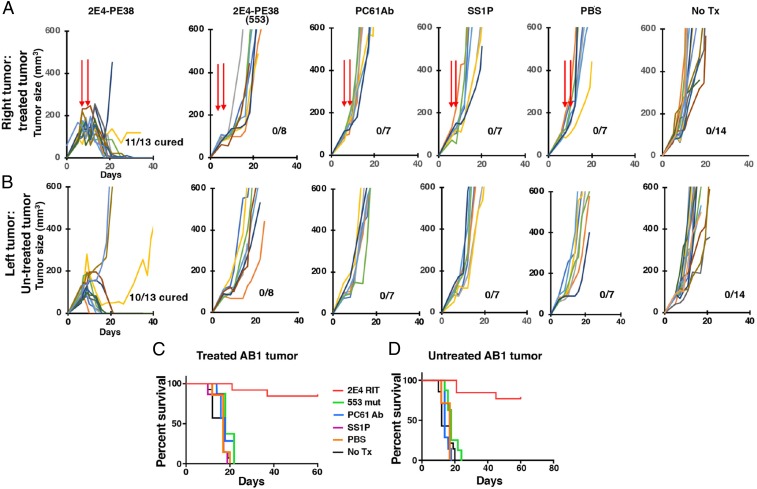
To determine if the 2E4-PE38 could cause tumor remissions and induce antitumor immunity, we implanted mice with AB1 tumors at two sites and allowed 5 d for the tumors to become established and reach about 100 mm3. We then injected 2E4-PE38 on days 5 and 9 into only one tumor. This interval was chosen because immunotoxins of this type have a short half-life and are gone within a few hours, allowing other CD25-positive cells like cytotoxic CD8 T cells to enter and kill tumor cells. The animals were monitored for tumor growth at both the injected and the distant uninjected sites. As shown in Fig. 2, 11/13 tumors injected with 2E4-PE38 underwent CRs as did 10/13 untreated tumors in the same mouse. Several different control experiments were performed. We injected tumors with 2E4-PE38 containing a E553D mutation, which destroys its cytotoxic activity. It had no effect on tumor growth. We also injected tumors with the PC61 antibody, which has been shown to prevent tumor growth by other researchers (9). We found that direct injection of PC61 had very little effect on tumor growth (Fig. 2). To assess for nonspecific effects of the toxin, we injected SS1P, an immunotoxin targeting human mesothelin not expressed on these tumors and saw no effect on tumor growth. Also, injection of PBS was ineffective. To investigate the possibility that some of the 2E4-PE38 leaked out of the tumor and reached the second tumor via circulation, we injected 2E4-PE38 subcutaneously close to the tumors and observed no antitumor activity (SI Appendix, Fig. S3). In summary, direct injection of two doses of an immunotoxin targeting CD25 into a tumor causes long-lasting regressions of injected and uninjected tumors.
Fig. 2.
Local 2E4-PE38 therapy is effective for AB1 mesothelioma. BALB/c mice were implanted subcutaneously with AB1 (5 × 106) tumors on both the right and left of their dorsal side. Tumor growth was monitored with a caliper. (A) 2E4-PE38 (10 μg), 2E4-PE38-E553D inactive mutant (10 μg), PC61 anti-CD25 antibody (10 μg), SS1P (10 μg), or PBS (100 μL) were injected into one right tumor site on days 5 and 9. Tumors were between 50 and 100 mm3 on day 5. (B) Left-side untreated tumors were also monitored. Mice survivals, defined as the tumor volume failing to reach 400 mm3, are shown in C and D.
Regulatory T Cell Changes.
To determine if tumor regressions were accompanied by a decrease in regulatory T cells, we analyzed tumor-infiltrating lymphocytes and splenic lymphocytes on day 8, 3 d after the first 2E4-PE38 injection. Analysis of tumor-infiltrating lymphocytes and splenic lymphocytes 3 d after 2E4-PE38 treatment showed that depletion of CD4+CD25+Foxp3+ Tregs was limited to the tumor and that it did not occur in the spleen (Fig. 1D). Immunotoxins containing PE38 have a half-life of 20 min when injected intravenously into mice and are undetectable by 3 h (33). We measured the levels of 2E4-PE38 in the blood of four mice after intratumor injection. In two mice, it could not be detected (<100 ng/mL); in the other two mice, the levels were less than 800 ng/mL at 3 min and undetectable at 60 min (SI Appendix, Table S1). Therefore, after 3 d, immunotoxin had been absent for several days, and we were observing the consequences of its injection.
Other Tumor Models.
Targeting Tregs using anti–CD25-PE38 was evaluated in two other tumor models: CT26M colon cancers and 66c14 breast cancers. CT26M cells are CT26 cells engineered to express human mesothelin. As shown in SI Appendix, Fig. S4, 2E4-PE38 produced complete regressions of 12/12 injected CT-26M tumors and 11/12 uninjected tumors. As expected, two of seven tumors injected with SS1P had CRs because of expression of human mesothelin in these cells. CT26M cells are moderately sensitive to SS1P treatment; IC50 = 3 ng/mL (SI Appendix, Fig. S5). SS1P treatment did not cause CRs in untreated tumors, although two tumors did stop growing for several days. Injection of mice with 66c14 breast cancers with 2E4-PE38 was also effective; 15/15 injected tumors underwent CRs as did 7/15 untreated tumors (SI Appendix, Fig. S6). None of the controls produced any effect, including mAb 2E4 that was used to make 2E4-PE38. Because all of the injected tumors completely regressed we could not evaluate the effect of additional injections on the second tumor.
Mice Show Persistent Antitumor Immunity.
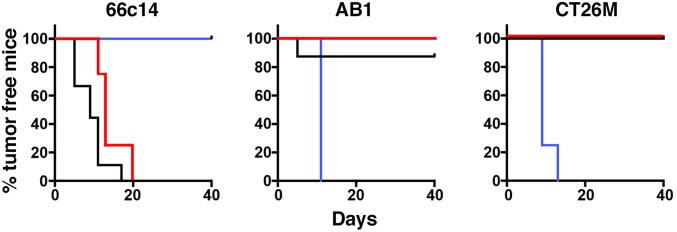
The regression of the uninjected tumors indicates the development of systemic antitumor immunity. To evaluate if mice that had CRs would reject a tumor challenge, we waited more than 50 d and then challenged mice with three different types of tumors growing at different locations. Fig. 3 shows that in mice that had CRs of 66c14 tumors, 66c14 cells would not grow, but AB1 and CT26M cells grew normally (SI Appendix, Fig. S7). In mice that had CRs of AB1 tumors, AB1 cells were rejected by six of six mice, but unexpectedly seven of eight mice rejected CT26M cells; no mice rejected 66c14 cells. Conversely, mice that had CRs with CT26M tumors rejected CT26M and AB1 tumors, but not 66c14 tumors. These studies show that the antitumor immunity persists for almost 2 mo. They also show that CT26M and AB1 cells share one or more tumor antigens. To be sure that the AB1 and CT26M cells were really different cells and not a result of some laboratory contamination, we performed short-tandem repeat analysis (Cell Check, IDEXX BioResearch) and demonstrated that the two cell lines were indeed different (SI Appendix, Table S2).
Fig. 3.
Tumors undergoing complete regression are immune to rechallenge with other tumor cells. (Left) Mice with cured 66c14 tumors (more than 50 d after complete remission of the tumor) were rechallenged with 66c14 (blue), AB1 (red), and CT26M (black) cells with 5 × 106 at three separate locations. Mice with cured AB1 (Middle) and CT26M tumors (Right) were also rechallenged with three cell lines. The percentage of tumor-bearing mice, that is defined as the tumor volume failing to reach 150 mm3, in each group is shown. Size was measured by caliper. Rechallenged tumor growth of each mouse is shown in SI Appendix, Fig. S7.
2E4-PE38 Induces Rapid Activation of Tumor-Infiltrating CD8 T Cells and Increases the Number of Antigen-Presenting Cells.
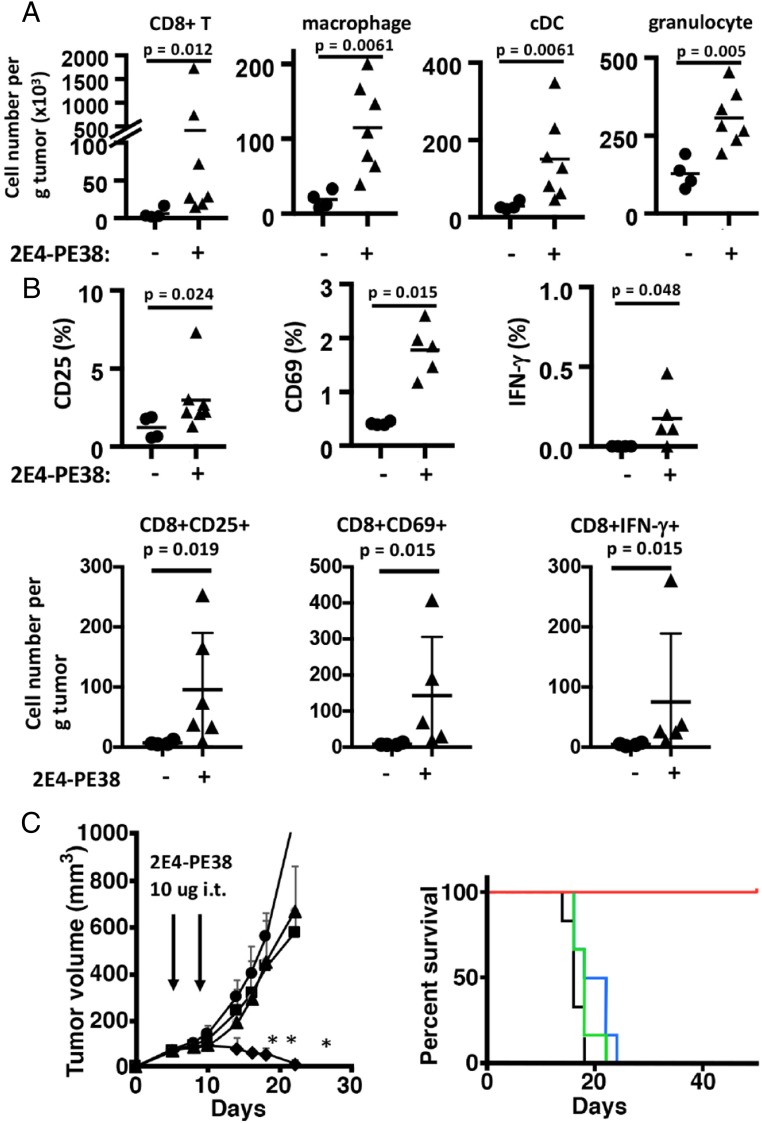
To elucidate how the specific depletion of tumor-infiltrating Tregs with local 2E4-PE38 stimulated tumor regression, we determined if the number of immune cells increased in the tumor microenvironment after 2E4-PE38 treatment. Three days after treatment with 2E4-PE38, immune cells in the tumor were analyzed by flow cytometry (Fig. 4A). The number of CD8 T cells in untreated tumors averaged 2.8 × 103/g and increased markedly to 3.8 × 105/g after 2E4-PE38 treatment (P = 0.012). The number of macrophages increased about sixfold from 1.9 × 104/g in untreated tumors to 1.2 × 105/g (P = 0.0061), and conventional dendritic cells (cDCs) increased from 2.9 × 103 to 1.5 × 105/g (P = 0.0061). The increase in the number of antigen-presenting cells may have been caused by their exposure to products released by dead or dying Tregs or to tumor cells killed by CD8 T cells. An increase of granulocytes in treated tumors was also detected (2.9 × 103 to 1.3 × 105, P = 0.005), suggesting that 2E4-PE38 treatment may affect the innate immune system. We also determined if the CD8 T cells in the tumor were activated by 2E4-PE38 treatment. CD3+CD8+ T cells were analyzed for CD25- and CD69-expressing cells (Fig. 4B, Upper). In the untreated tumor, CD8 T cells contained 1.2% CD25-positive cells, which increased to 3.0% after treatment (P = 0.024). CD69-positive cells also increased from 0.37 to 1.8% (P = 0.015). Also, CD8 T cells producing IFN-gamma (IFN-γ) rose from 0 to 0.18% (P = 0.048). We also calculated the cell number per gram of tumor (Fig. 4B, Lower). In the untreated tumor, CD8 T cells contained 7.2/g CD25-positive cells, which increased to 95.5/g after treatment (P = 0.019). CD69-positive cell also increased from 8.1/g to 143/g (P = 0.015). CD8 T cells producing IFN-γ rose from 4.6/g to 75/g (P = 0.015). Thus, Treg depletion by 2E4-PE38 results in rapid activation of tumor-infiltrating CD8 T cells that accompanies tumor regressions.
Fig. 4.
Local 2E4-PE38 therapy affects the tumor microenvironment, and the CD8 T cell has the main role for antitumor activity. (A) Local therapy of 2E4-PE38 increased the number of immune cells in the treated tumors 3 d after the treatment. (Left to Right) CD8+ T cells, macrophage (CD11b+ LY6G− CD11clow/neg), conventional DC (MHC-II+ CD11chigh), and granulocyte (CD11b+ Ly6G+). Bars represent an average. (B) Local 2E4-PE38 induced up-regulation of CD25, CD69, and IFN-γ on CD8 T cells 3 d after the treatment. All graphs were CD3+ CD8+-gated. (Upper) Percentage of CD8 T cells. (Lower) Cell number per gram of tumor (C) The depletion of T cells reduced the effect of 2E4-PE38. Anti-CD8 T cell depletion antibody (100 μg) was injected intraperitoneally on days 4, 8, 12, and 16. PBS (triangle) and nontreated (circle) tumors grew fast. But 2E4-PE38 injection (diamond) suppressed tumor growth. Anti-CD8 antibody combined with 2E4-PE38 treated tumor (square) grew fast, indicating that an antitumor effect of local 2E4-PE38 depends on CD8 T cells. Survival curves are shown on the Right. Treatment groups are 2E4-PE38 (red), PBS (blue), no treatment (black), and 2E4-PE38 with CD8 T cell depletion (green). n = 5–7; *P < 0.01 combined therapy vs. 2E4-PE alone, Mann–Whitney test.
Antitumor Effects of 2E4-PE38 Depend on CD8 T Cells.
To elucidate the role of effector T cells in the therapeutic efficacy of local RIT therapy, we depleted CD8 T cells using systemic administration of an anti-CD8 antibody (Fig. 4C, Left). In a preliminary study, we used three BALB/c mice bearing AB1 tumor and injected anti-CD8 antibody alone. The tumor growth was pretty similar to the control group. However, T cell depletion attenuated the efficacy of 2E4-PE38 treatment, as demonstrated by tumor growth and mouse survival data (Fig. 4C, Right). These data indicate that the antitumor activity of 2E4-PE38 treatment is mediated by CD8 T cells.
CD8 T Cell Expression Activation Markers Are Present in the Nontreated Tumor After Treatment with 2E4-PE38.
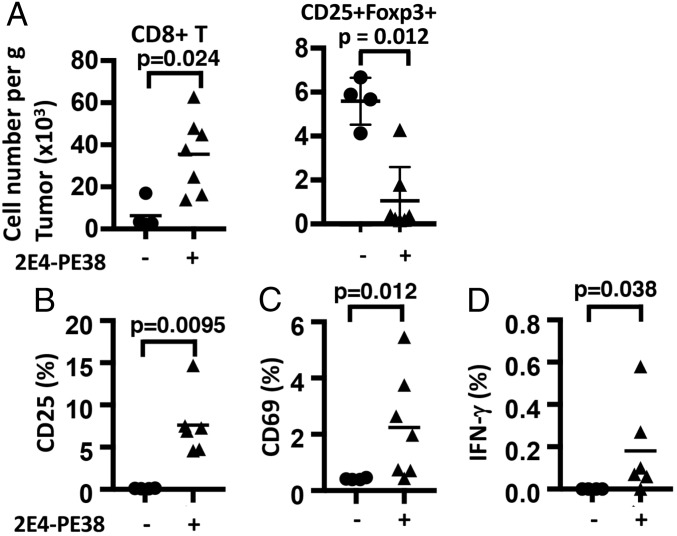
To determine if the distant nontreated tumors contained activated CD8 T cells after 2E4-PE38 treatment, 3 d after 2E4-PE38 treatment of one tumor, the untreated tumor was resected and analyzed by flow cytometry (Fig. 5). The number of CD8 T cells was increased in nontreated tumors from 6.3 × 103 to 3.5 × 104 (P = 0.024) (Fig. 5A, Left). The number of CD25+Foxp3+ Tregs was decreased in nontreated tumors from 5.5 × 103 to 1.0 × 103 (Fig. 5A, Right). CD8+ T cells expressing the activation marker CD25 increased from 0.12 to 7.6% (P = 0.0095), and CD69 increased from 0.41 to 2.2% (P = 0.012) (Fig. 5 B and C). IFN-γ rose from 0 to 0.15% (P = 0.038) (Fig. 5D). In summary, the immune responses triggered by 2E4-PE38 injection in tumors induced similar changes in uninjected tumors.
Fig. 5.
Activated CD8 T cells are present in the untreated tumor after intratumor 2E4-PE38 treatment. CD8 T cells collected from left untreated dorsal tumors in mice receiving local 2E4-PE38 treatment of the right dorsal tumors were analyzed for their expression of activation markers 3 d after the treatment. (A) 2E4-PE38 induced the number of cells per gram in untreated tumors (P = 0.024). CD8 T cells with up-regulated CD25 (B, P = 0.0095), CD69 (C, P = 0.012), and IFN-γ (D, P = 0.038) were present in the left nontreated tumor after 2E4-PE38 treatment of the right-dorsal tumor (n = 4–8). (+) 2E4-PE38 injected group; (−) nontreated group, bar: average. P value was calculated using Mann–Whitney test.
Discussion
We show here that direct injection of an immunotoxin targeting CD25 into tumors causes tumor regressions and the development of antitumor immunity in three tumor models. Systemic administration of drugs inhibiting Tregs or otherwise modulating immune responses often provides a therapeutic benefit, but these agents can also produce life-threatening side effects, such as autoimmune disease (30). To prevent systemic side effects and at the same time decrease the number of Tregs in tumors, we chose to inject a potent immunotoxin targeting CD25 directly into tumors. We find this approach a very effective way to treat tumors without producing systemic toxicity. We believe that this therapeutic approach is successful because it targets only cells in the tumor microenvironment. Other researchers have successfully employed intratumor injection to treat cancer. Sagiv-Barfi et al. (34) employed intratumor injection of CpG and agonistic anti-OX40 antibody to trigger T cell responses. For superficial tumors, Sato et al. (35) used CD25-targeted near-infrared photoimmunotherapy to selectively deplete Tregs, activate CD8 T and natural killer cells, and produce local antitumor immunity.
Direct injection of 2E4-PE38 is very toxic to CD25-expressed T cells, such as Tregs, because the concentration of 2E4-PE38 in the tumor environment is around 100 μg/mL after the injection of 2E4-PE38 (10 μg/100 μL injection into the tumor). Because the IC50 of 2E4-PE38 is 8 ng/mL, 100 μg/mL in the tumor microenvironment is more than 1,000-fold higher than the IC50. The killing of Tregs in the tumor can activate CD8 T cells present in the tumor or that have been recruited to the tumor. Before 2E4-PE38 administration, the CD8 T cells in the tumor do not express CD25 (Fig. 4B, Left), allowing 2E4-PE38 to selectively kill Tregs. After 2E4-PE38 injection, naive CD8 T cells are activated and can kill tumor cells. Because intratumorally injected 2E4-PE38 disappeared from the tumor in a few hours, injected 2E4-PE38 did not inhibit the activation of CD8 T cells expressing CD25 (SI Appendix, Table S1). This sequence of events differs from systemic Treg depletion therapy with i.v. injection of CD25-targeting agents because Tregs can be depleted without affecting Teffs (20–22). In most previous reports using anti-CD25 antibody given systemically, engraftment was prevented but complete regressions of established tumors were not noted (9). In our local injection model, anti-CD25 antibody treatment did not affect the growth of established tumors because PC61 antibody is a rat IgG1 that may have low effector function in mice (29). 2E4-PE38 was very effective for the treatment of established tumors (Fig. 2).
Local 2E4-PE38 Therapy Induces Immune Responses in Injected Tumor.
The efficacy of the 2E4-PE38 treatment is explained by the fact that 2E4-PE38 kills Tregs and changes the Teff/Treg balance and activating CD8 T cells present in the tumor microenvironment that were suppressed by the Tregs. At the time of 2E4-PE38 injection, the CD8 T cells in the tumor do not express CD25 (Fig. 3, Middle) so that 2E4-PE38 cannot kill these CD8 T cells. After local 2E4-PE38 treatment, CD8 T cells express CD25 and become activated and can kill tumor cells. Because 2E4-PE38 has a short half-life and within a few hours is gone from the tumor and the circulation, it cannot kill activated T cells (SI Appendix, Table S1). CD8 cells play a major role in the antitumor responses to 2E4-PE38 treatment because the antitumor effect is abrogated by anti-CD8 antibody. 2E4-PE38 also increased the number of cDCs, probably by their exposure to components of dead Tregs and/or tumor cells killed by activated CD8 T cells (Fig. 3, Left). Increased cDCs can further activate naive T cells against various antigens derived from killed tumor cells. Also, the increase in the number of macrophages may play a role in initiating an immune response against killed tumor cells. The immune response after 2E4-PE38 treatment was induced by DCs that recognize mutated neoantigens and/or cancer germline antigens. Local therapy of 2E4-PE38 may be effective for such immunogenic tumors. For nonimmunogenic tumors, this therapy is likely to be less effective. Fortunately, many tumors have a substantial number of mutated proteins.
Local 2E4-PE38 Therapy Induces Immune Responses in Uninjected Tumor.
Three days after local 2E4-PE38 therapy, analysis of the untreated tumors shows similar changes to those observed in the treated tumors. The number of CD8 T cells increased, and CD8+ T cells expressing CD25 and CD69 and IFN-γ also increased (Fig. 5 B–D). Since these changes are not due to exposure of the second tumor to 2E4-PE38, it is likely that they are initiated by cells from the treated tumor migrating to the untreated tumor. How this occurs is not yet known.
Local 2E4-PE38 Therapy Induces Long-Lasting Antitumor Immunity.
We observed that mice cured of 66c14 tumors using anti-CD25 RIT became specifically resistant to rechallenge with 66c14 cells (Fig. 3 and SI Appendix, Fig. S7). Also, mice with CRs of AB1 tumors became resistant to AB1 and CT26M cells, and mice cured of CT26M tumors became resistant to CT26M and AB1 cells. Cross-resistance indicates that the target antigen(s) of the immune response induced in 2E4-PE38–treated mice shared tumor rejection antigens. Cross-protective immunity was previously reported by Golgher et al. (7) who found that immunity to CT26 colon cancer tumors due to depletion of Tregs using anti-CD25 antibody was accompanied by immunity to B-cell lymphomas and a renal cell carcinoma.
An immunotoxin targeting human CD25 has been extensively used in patients and has an excellent safety profile. Therefore, we believe that this immunotoxin could be employed in clinical trials to deplete Tregs in human tumors. RITs are used in cancer immunotherapeutics, which were originally destined to directly kill cancer cells, and one of these that targets CD22 has recently been approved by the Food and Drug Administration for treatment of drug-resistant hairy cell leukemia. In the current study, we have taken advantage of potent cell-killing activity of an immunotoxin to kill normal cells that regulate tumor growth. The agent used in this study kills mouse CD25-expressing cells. Selective depletion of Tregs in tumors facilitates the development of a CD8 T cell-dependent antitumor effect in three mouse models and could complement other immunotherapies such as checkpoint inhibitors or adoptive T cell therapy or cancer vaccines.
Materials and Methods
RIT Construction and Production.
To produce an immunotoxin that would kill cells expressing CD25, we used previously published conditions to construct the RIT shown in Fig. 1A (33). The sequence of the variable region of anti-CD25 was resolved from the 2E4 hybridoma by rapid amplification of cDNA ends and cloned into our conventional PE38 expression vector (36, 37). 2E4 (scFv)-PE38 is a single-chain RIT in which the Fv portion of Rat mAb 2E4 is genetically fused to PE38, a truncated form of PE (Fig. 1A). The RIT was produced in Escherichia coli, and the protein was purified by ion-exchange and size-exclusion chromatography after renaturation from inclusion bodies as described previously (37). The RITs produced eluted as a monomer on TSK gel filtration chromatography and migrated as a single band of about 62 kDa in SDS/PAGE (Fig. 1B). 2E4-PE38-E553D is an inactive mutant with a D mutation at position 553 of PE, which was introduced by PCR overlap extension (38). SS1P, an anti-human mesothelin RIT, was manufactured by ABL (32).
Reagents.
Anti-mouse CD25 antibody (PC-61.5.3, Rat IgG1) and anti-mouse CD8a antibody (clone 2.43) were obtained from BioXCell. Anti-CD25 antibody (2E4, Rat IgG) was purified with protein G from the culture supernatant of 2E4 hybridoma and was a gift from Ethan Shevach (National Institute of Allergy and Infectious Diseases, NIH, Bethesda, MD) (36).
Cell Culture.
The AB1 mouse mesothelioma cell line was from Sigma-Aldrich. The CT26M mouse colon cancer cell line transfected with human mesothelin was obtained from Roche. The 66c14 luc2 mouse breast cancer cell line was obtained from C. L. Jorcyk (Boise State University, Boise, ID) (39). IL-2–dependent CD25-expressing mouse T lymphocyte HT-2 clone A5E cells (HT-2-A5E) were purchased from ATCC. The 66c14 cells were cultured in Iscove’s modified Dulbecco’s medium (ThermoFisher Scientific). AB1 and CT26M cells were cultured in RPMI-1640 medium (ThermoFisher Scientific). All media was supplemented with 10% FBS and penicillin–streptomycin (ThermoFisher Scientific). For HT-2-A5E culture, 0.05 mM 2-mercaptoethanol and 0.1 nM human IL-2 (Roche) were also added.
Cytotoxic Activity.
The specific cytotoxic activity of each RIT was assessed by a cell viability assay. Cells were seeded in 96-well plates overnight and treated with various concentrations of RIT in triplicate. Viability was assessed 72 h later using the Cell Counting Kit-8 (Dojindo Molecular Technologies) in accordance with the manufacturer’s instructions. Color change was evaluated at an optical density of 450 nm. Staurosporine (Sigma-Aldrich) was used as a positive control.
Animal and Tumor Models.
All in vivo procedures were performed in accordance with a protocol approved by the NIH Animal Care and Use Committee. All mice were purchased from The Jackson Laboratory. BALB/c mice 8–10 wk old were inoculated with 5 million AB1 cells, 4 million 66c14 luc cells, or 3 million CT26M cells into the right and left dorsum. Mice with tumors measuring 80–100 mm3 were used for the experiments. Mice were monitored daily, and tumor volumes were measured two or three times a week until tumor volumes reached 500 mm3, when mice were euthanized with carbon dioxide.
Intratumor Treatment.
Intratumor injection of RIT was performed 5 d after tumor inoculation for mice bearing multiple tumors. Tumors on the right side were injected with 10 μg/100 μL of RIT on days 5 and 9. Tumor size was measured twice weekly, and mice were euthanized when tumor volume reached 500 mm3.
Analysis of Tumor-Infiltrating and Splenic Immune Cells.
To determine the effect of 2E4-PE38 administration on various lymphocytes, tumors (treated and untreated) and spleen were harvested 3 d after the intratumor injection of 10 μg of 2E4-PE38. Mice were killed on day 8 with CO2. Spleen and tumors were placed in Hanks’ Balanced salt solution (HBSS), cut up with scissors, digested with a mixture of 0.33 mg/mL DNase (Sigma-Aldrich) and 0.27 mg/mL Liberase TM (Sigma-Aldrich) in HBSS for 30 min at 37 °C, and passed through a 40-μm filter. To identify the T cell population, samples were stained with the anti-mouse antibodies shown in SI Appendix, Table S3. Foxp3 and intracellular cytokine staining were performed using Foxp3/Transcription Factor Fixation/Permeabilization Buffer (ThermoFisher Scientific) and antibodies against Foxp3 and IFN-γ, according to the manufacturer’s instructions. To identify macrophages and granulocytes, samples were stained with antibodies against CD11b, Ly6G, and CD11c (SI Appendix, Table S3). For identifying cDC, antibodies against MHC II and CD11c were used (SI Appendix, Table S3). The stained cells were applied to a spectral analyzer (Sony SA3800; SONY Biotechnology), and data were analyzed with the FlowJo software or Sony SA3800.
Immune Depletion of CD8 T Cells.
Anti-CD8a (2.43) depleting antibody was injected intraperitoneally every 4 d starting from 1 d before the 2E4-PE38 treatment at doses of 100 μg, until the mice were euthanized. Tumors were measured twice weekly, and mice were euthanized when any tumor volume reached 500 mm3.
Immunotoxin Levels in the Blood.
BALB/c mice bearing AB1 tumors were injected intratumorally with 10 μg/100 μL of 2E4-PE38 (n = 4). Blood samples were drawn at 3, 10, 30, 50, 80, and 120 min. The concentration of 2E4-PE38 in the serum was measured by ELISA using the standard curve for 2E4-PE38 as described previously (33). Briefly, ELISA plates were coated with 1 μg/mL of mouse CD25 protein (#50292-M08H; Sino Biological) in PBS at 4 °C overnight. Diluted standards or serum samples were applied, followed by incubation with the anti-PE antibody IP12 (40). After washing, anti-mouse IgG-HRP (Jackson Laboratory) was applied, followed by development using a tetramethylbenzidine substrate kit (ThermoFisher Scientific). Serum concentration was calculated with SoftMax Pro (Molecular Devices).
Statistics.
Data are expressed as mean ± SD from three experiments, unless otherwise indicated. Statistical analyses were performed with a statistics program (GraphPad Prism). The Mann–Whitney test was used for comparison. The cumulative probability of survival, defined as the tumor volume failing to reach 400 mm3, was estimated in each group with the Kaplan–Meier survival curve analysis, and the results were compared with the log-rank test and Wilcoxon test.
Supplementary Material
Acknowledgments
We thank Ms. Karen M. Wolcott (Flow Cytometry Core Facility) for instructions on operating the Sony Spectral Analyzer. This research was supported by the Intramural Research Program of the NIH; the National Cancer Institute; and the Center for Cancer Research (Project ZO1 BC008753).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1820388116/-/DCSupplemental.
References
- 1.Gershon RK, Kondo K. Cell interactions in the induction of tolerance: The role of thymic lymphocytes. Immunology. 1970;18:723–737. [PMC free article] [PubMed] [Google Scholar]
- 2.Möller G. Do suppressor T cells exist? Scand J Immunol. 1988;27:247–250. doi: 10.1111/j.1365-3083.1988.tb02344.x. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 4.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Colombo MP, Piconese S. Regulatory-T-cell inhibition versus depletion: The right choice in cancer immunotherapy. Nat Rev Cancer. 2007;7:880–887. doi: 10.1038/nrc2250. [DOI] [PubMed] [Google Scholar]
- 6.Elpek KG, Lacelle C, Singh NP, Yolcu ES, Shirwan H. CD4+CD25+ T regulatory cells dominate multiple immune evasion mechanisms in early but not late phases of tumor development in a B cell lymphoma model. J Immunol. 2007;178:6840–6848. doi: 10.4049/jimmunol.178.11.6840. [DOI] [PubMed] [Google Scholar]
- 7.Golgher D, Jones E, Powrie F, Elliott T, Gallimore A. Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol. 2002;32:3267–3275. doi: 10.1002/1521-4141(200211)32:11<3267::AID-IMMU3267>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Jones E, et al. Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immun. 2002;2:1. [PubMed] [Google Scholar]
- 9.Onizuka S, et al. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 10.Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci Rep. 2015;5:15179. doi: 10.1038/srep15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bos PD, Plitas G, Rudra D, Lee SY, Rudensky AY. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J Exp Med. 2013;210:2435–2466. doi: 10.1084/jem.20130762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goding SR, et al. Restoring immune function of tumor-specific CD4+ T cells during recurrence of melanoma. J Immunol. 2013;190:4899–4909. doi: 10.4049/jimmunol.1300271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quezada SA, et al. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med. 2008;205:2125–2138. doi: 10.1084/jem.20080099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutmuller RP, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 17.Setiady YY, Coccia JA, Park PU. In vivo depletion of CD4+FOXP3+ Treg cells by the PC61 anti-CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes. Eur J Immunol. 2010;40:780–786. doi: 10.1002/eji.200939613. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: A common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- 19.O’Konek JJ, et al. Differential regulation of T-cell mediated anti-tumor memory and cross-protection against the same tumor in lungs versus skin. OncoImmunology. 2018;7:e1439305. doi: 10.1080/2162402X.2018.1439305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luke JJ, Zha Y, Matijevich K, Gajewski TF. Single dose denileukin diftitox does not enhance vaccine-induced T cell responses or effectively deplete Tregs in advanced melanoma: Immune monitoring and clinical results of a randomized phase II trial. J Immunother Cancer. 2016;4:35. doi: 10.1186/s40425-016-0140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs JF, et al. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: A phase I/II study in metastatic melanoma patients. Clin Cancer Res. 2010;16:5067–5078. doi: 10.1158/1078-0432.CCR-10-1757. [DOI] [PubMed] [Google Scholar]
- 22.Rech AJ, et al. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci Transl Med. 2012;4:134ra62. doi: 10.1126/scitranslmed.3003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powell DJ, Jr, et al. Administration of a CD25-directed immunotoxin, LMB-2, to patients with metastatic melanoma induces a selective partial reduction in regulatory T cells in vivo. J Immunol. 2007;179:4919–4928. doi: 10.4049/jimmunol.179.7.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Attia P, et al. Selective elimination of human regulatory T lymphocytes in vitro with the recombinant immunotoxin LMB-2. J Immunother. 2006;29:208–214. doi: 10.1097/01.cji.0000187959.45803.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bulliard Y, et al. OX40 engagement depletes intratumoral Tregs via activating FcγRs, leading to antitumor efficacy. Immunol Cell Biol. 2014;92:475–480. doi: 10.1038/icb.2014.26. [DOI] [PubMed] [Google Scholar]
- 26.Coe D, et al. Depletion of regulatory T cells by anti-GITR mAb as a novel mechanism for cancer immunotherapy. Cancer Immunol Immunother. 2010;59:1367–1377. doi: 10.1007/s00262-010-0866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selby MJ, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1:32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 28.Simpson TR, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arce Vargas F, et al. Melanoma TRACERx Consortium; Renal TRACERx Consortium; Lung TRACERx Consortium Fc-optimized anti-CD25 depletes tumor-infiltrating regulatory T cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity. 2017;46:577–586. doi: 10.1016/j.immuni.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51–60. doi: 10.1016/j.ctrv.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Leshem Y, et al. Combining local immunotoxins targeting mesothelin with CTLA-4 blockade synergistically eradicates murine cancer by promoting anticancer immunity. Cancer Immunol Res. 2017;5:685–694. doi: 10.1158/2326-6066.CIR-16-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hassan R, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 33.Onda M, et al. Lowering the isoelectric point of the Fv portion of recombinant immunotoxins leads to decreased nonspecific animal toxicity without affecting antitumor activity. Cancer Res. 2001;61:5070–5077. [PubMed] [Google Scholar]
- 34.Sagiv-Barfi I, et al. Eradication of spontaneous malignancy by local immunotherapy. Sci Transl Med. 2018;10:eaan4488. doi: 10.1126/scitranslmed.aan4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato K, et al. Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy. Sci Transl Med. 2016;8:352ra110. doi: 10.1126/scitranslmed.aaf6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ortega G, Robb RJ, Shevach EM, Malek TR. The murine IL 2 receptor. I. Monoclonal antibodies that define distinct functional epitopes on activated T cells and react with activated B cells. J Immunol. 1984;133:1970–1975. [PubMed] [Google Scholar]
- 37.Onda M. 2012. Recombinant immunotoxins with low endotoxins for clinical and animal studies. Antibody Engineering. Methods and Protocols, Methods in Molecular Biology, ed Chames P (Humana Press, Totowa, NJ), pp 627–643.
- 38.Onda M, et al. An immunotoxin with greatly reduced immunogenicity by identification and removal of B cell epitopes. Proc Natl Acad Sci USA. 2008;105:11311–11316. doi: 10.1073/pnas.0804851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolin C, Sutherland C, Tawara K, Moselhy J, Jorcyk CL. Novel mouse mammary cell lines for in vivo bioluminescence imaging (BLI) of bone metastasis. Biol Proced Online. 2012;14:6. doi: 10.1186/1480-9222-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onda M, et al. Characterization of the B cell epitopes associated with a truncated form of Pseudomonas exotoxin (PE38) used to make immunotoxins for the treatment of cancer patients. J Immunol. 2006;177:8822–8834. doi: 10.4049/jimmunol.177.12.8822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.