Significance
Many bacteria encode ferrous iron transporters to obtain essential iron. The ferrous iron transporter FeoB has a GTPase domain that closely resembles eukaryotic G proteins; however, the role of the GTPase and mechanistic details of Feo iron transport are unclear. In this report, we show that Vibrio cholerae FeoB possesses ATP, as well as GTP, hydrolysis activity. The ATPase activity was sufficient for functional ferrous iron transport. The demonstration of simultaneous ATPase and GTPase activities of FeoB provides important insights into both iron transport and the complexity of bacterial G proteins.
Keywords: Feo, iron transport, Vibrio cholerae, GTPase, ATPase
Abstract
The Feo ferrous iron transporter is widely distributed among bacteria and archaea, but its mechanism of transport has not been fully elucidated. In Vibrio cholerae, the transport system requires three proteins: the small cytosolic proteins FeoA and FeoC and a large cytoplasmic-membrane–associated protein FeoB, which has an N-terminal G-protein domain. We show that, in contrast to Escherichia coli FeoB, which is solely a GTPase, the V. cholerae and Helicobacter pylori FeoB proteins have both GTPase and ATPase activity. In V. cholerae, mutation of the G4 motif, responsible for hydrogen bonding with the guanine base, abolished the GTPase activity but not ATPase activity. The ATPase activity of the G4 motif mutants was sufficient for Feo function in the absence of GTPase. We show that the serine and asparagine residues in the G5 motif likely play a role in the ATPase activity, and substitution of these residues with those found in the corresponding positions in E. coli FeoB resulted in similar nucleotide hydrolysis activity in the E. coli protein. These results add significantly to our understanding of the NTPase domain of FeoB and its role in Feo function.
Iron is an essential element for most living organisms, and its acquisition presents challenges for human pathogens competing with their host for the element. Ferrous iron (II) is more prevalent than ferric iron (III) in anoxic environments and at acidic pH, environments that gastrointestinal bacteria may encounter while colonizing the human host (1). The bacterial ferrous iron transport system Feo is an evolutionarily ancient ferrous iron transporter, widely distributed among archaea and bacteria. The Feo system plays an important role in bacterial survival or virulence in mammalian hosts, as has been demonstrated by assessing the effects of deletion or mutation of the feo genes in several bacterial species (2–5).
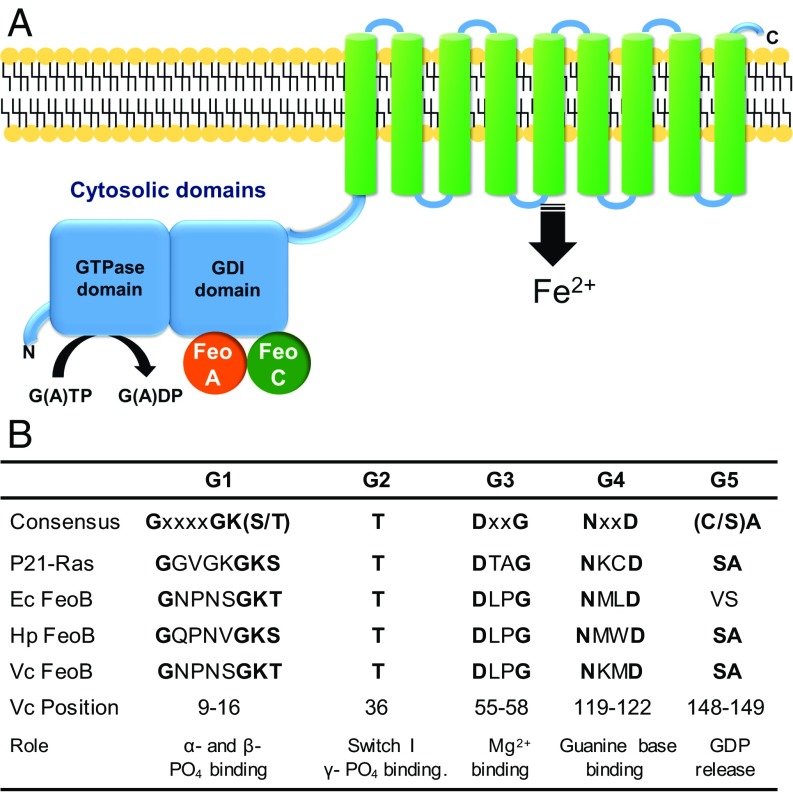
The Feo system was first described in Escherichia coli K-12 (6), and the feo locus includes three genes encoding the FeoA, FeoB, and FeoC proteins (Fig. 1A). FeoB is a multidomain transmembrane protein with an N-terminal cytoplasmic GTPase and GDP dissociation inhibitor (GDI) domain and a C-terminal transmembrane domain. The transmembrane domain is predicted to consist of 8–12 transmembrane helices and to be the region that physically transports ferrous iron from the periplasm into the cytoplasm. FeoB was initially predicted to hydrolyze ATP based on its sequence homology with nucleotide-binding sites of ATPases (6). This was supported by the evidence showing that addition of ATP hydrolysis inhibitors resulted in abrogation of ferrous iron transport in Helicobacter pylori (2). Other studies, however, demonstrated that the N terminus of E. coli FeoB contains the G1–G4 and Switch I/II signature sequences characteristic of G proteins (7, 8) and that it hydrolyzes GTP but does not bind or hydrolyze ATP (7) (Fig. 1B). Subsequently, FeoB has been considered to be a GTPase.
Fig. 1.
Schematic organization of FeoABC complex and conserved residues of the FeoB GTPase. (A) Schematic representation of FeoABC. FeoB consists of two cytosolic domains (blue) and a polytopic membrane domain (green). The cytosolic proteins FeoA and FeoC are shown in orange and green, respectively. (B) Consensus sequences of conserved GTPase sequence elements compared with human p21-Ras and the FeoB proteins from E. coli, H. pylori, and V. cholerae. Conserved residues are shown in boldface, and the positions of amino acid residues of each motif in V. cholerae FeoB are indicated.
Compared with other G proteins, FeoB possesses a slow intrinsic GTPase turnover rate and weak nucleotide-binding affinity, prompting questions regarding its function as an active iron transporter or a channel. The GTP hydrolysis activity of Streptococcus thermophilus FeoB was shown to be activated by potassium, similar to the effect of GTPase-activating proteins (GAPs), supporting the idea of an active transporter (9). However, such stimulation was not observed for Pseudomonas aeruginosa FeoB (10). In addition, GTP hydrolysis is not potassium-activated in TEES GTPases, among which FeoB might be classified (11). Seyedmohammad et al. (10) proposed that FeoB may function as a GTP-gated channel, regulated by slow hydrolysis of GTP. Thus, fundamental questions, including the role of the FeoB GTPase domain, the source of energy for the iron transport, and mechanistic details of the process, remain unanswered.
Vibrio cholerae is a human pathogen that causes a severe diarrheal disease associated with devastating epidemics. V. cholerae has an absolute requirement for iron and has evolved a variety of mechanisms to acquire iron from the different environments, such as the intestine of the human host or marine waters, that the pathogens inhabit (12). Feo is the major ferrous iron transport system in V. cholerae (1, 13, 14). In previous studies, we showed that V. cholerae FeoA, FeoB, and FeoC interact to form a large complex in the inner membrane (15), and each of these components is required for in vivo ferrous iron transport (14). V. cholerae FeoB has all five of the G-protein signature motifs in its N-terminal domain, suggesting that it is a functional G protein.
In this study, we show that V. cholerae FeoB not only has GTPase activity, but also functions as an ATPase. Mutants of the G4 motif, responsible for guanine nucleotide binding, lack GTPase activity; however, these mutants retain ATP hydrolysis activity and active ferrous iron transport, indicating that the ATP hydrolysis is sufficient for iron transport. We suggest that serine and asparagine residues in the G5 motif are likely to be involved in the ATPase activity, and in support of this, we show that H. pylori FeoB, which has the same residues as V. cholerae in its G5 motif, also has ATPase activity. Finally, we demonstrate that FeoA and FeoC affect the nucleotide hydrolysis activity of FeoB.
Results
The N-Terminal Domain of V. cholerae FeoB Possesses ATPase Activity.
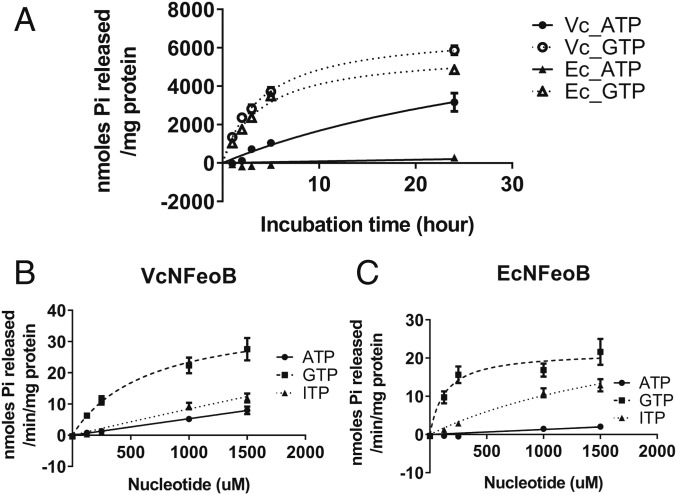
The N terminus of V. cholerae FeoB (VcNFeoB), has the characteristic motifs of a GTPase, and mutations in the putative G1, G2, and G3 motifs, as well as in the Switch I and II domains, e.g., the G2/Switch I mutation T36K, eliminated FeoB iron transport function, indicating a requirement for nucleotide hydrolysis (16). However, mutations such as D122N in the highly conserved G4 domain, which was shown to be required for GTPase activity and Feo function in E. coli (7), had no effect on V. cholerae Feo function in vivo (16). A second possible G4 domain was identified based on sequence homology, but mutation of this region also failed to affect Feo function (15). Because the G4 domain is responsible for guanine nucleotide specificity, retention of FeoB function in these mutants suggested the possibility that nucleotides other than GTP might be used by V. cholerae FeoB. To determine the nucleotide specificity of VcNFeoB, the N-terminal 272 amino acids of the protein encompassing the entire cytoplasmic region was purified, and nucleotide hydrolysis activity was measured and compared with the activity of the N-terminal domain of E. coli FeoB (EcNFeoB) (Fig. 2A). Like EcNFeoB, VcNFeoB catalyzed GTP hydrolysis. Unexpectedly, VcNFeoB also catalyzed ATP hydrolysis, whereas EcNFeoB showed no significant ATP hydrolysis.
Fig. 2.
NTPase activity of V. cholerae and E. coli NFeoB. (A) Incubation of VcNFeoB with 1,000 µM ATP or GTP shows hydrolysis of ATP (●) as well as GTP (○). EcNFeoB showed GTPase activity (Δ) but no significant ATPase activity (▲). (B and C) The reaction rates for VcNFeoB (B) and EcNFeoB (C) with ATP (●), ITP (▲), and GTP (■) as substrate were measured as described in Materials and Methods.
To further characterize the nucleotide specificity of VcNFeoB, we compared hydrolysis activities with the purine nucleotides ATP, GTP, and ITP (Fig. 2 B and C). VcNFeoB catalyzed GTP hydrolysis with the most efficient rate (kcat = 2.0 × 10−2 ± 3.3 × 10−3 s−1), but it was also able to catalyze hydrolysis of ATP and ITP at significant levels (Fig. 2B). It is noted that catalytic efficiencies for ATP and ITP could not be determined at saturating conditions due to the high absorbance of substrate. In contrast, as reported previously (7), EcNFeoB catalyzed hydrolysis of GTP with a kcat of 1.1 × 10−2 ± 1.2 × 10−3 s−1, but did not show significant hydrolysis of ATP (Fig. 2C). Like VcNFeoB, it was also able to catalyze hydrolysis of ITP, suggesting that there is some flexibility in purine recognition by EcNFeoB. Because VcNFeoB also hydrolyzes ATP, it has greater steric flexibility for nucleotide binding than EcNFeoB, especially for the N6 position of the purine bases. We also tested dATP and dGTP, as well as pyrimidine deoxynucleotides. dATP and dGTP were efficient substrates, but neither dTTP nor dCTP was hydrolyzed (SI Appendix, Fig. S1), indicating specificity for purine nucleotides.
Mutants of the V. cholerae G4 Motif Retain ATP Hydrolysis Activity Sufficient for Iron Transport.
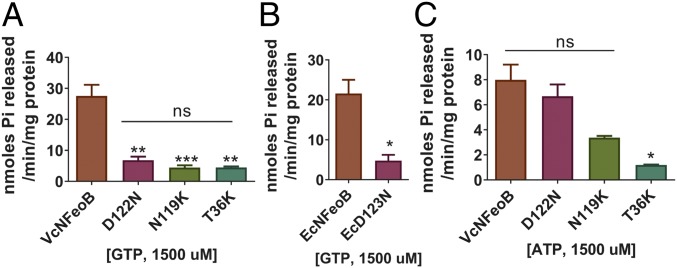
Because the G4 motif (NXXD, residues 119–122 in VcFeoB) is responsible for nucleotide specificity and has been shown in E. coli to be essential for GTPase activity and Feo function (7), we tested the effect of mutations in the V. cholerae G4 motif on nucleotide hydrolysis activity (Fig. 3A and SI Appendix, Fig. S2). Both N119K and D122N showed loss of GTPase activity. The corresponding mutant of D122N in EcNFeoB (EcD123N) also showed loss of GTPase activity (Fig. 3B). However, the ATPase activity of the VcNFeoB D122N mutant was not significantly different compared with that of wild-type VcNFeoB, indicating that it retains ATP hydrolysis despite loss of GTPase activity (Fig. 3C). The N119K mutant protein was purified from inclusion bodies, and the solubilized and refolded protein had lower activity overall, but it still retained ATP hydrolysis activity (Fig. 3C). In contrast, the G2/Switch I mutant T36K lacked both GTPase and ATPase activity, as expected (Fig. 3 and SI Appendix, Fig. S3).
Fig. 3.
Mutants of the G4 motif (D122N and N119K) still possess ATPase activity. (A and B) GTP hydrolysis activities of the mutants were compared using 1,500 µM GTP. Mutants of VcNFeoB (A) and EcNFeoB (B) showed significantly decreased GTPase activity compared with the wild-type VcNFeoB and EcNFeoB, respectively, similar to the negative control G2/switch I mutant T36K. (C) ATP hydrolysis activities of the mutants were compared using 1,500 µM of ATP. While the T36K mutant was deficient in ATP hydrolysis, the ATPase activities of the G4 motif mutants of VcNFeoB were not significantly different from the wild type. The N119K protein required solubilization and refolding, reducing its activity compared with the other proteins. Asterisks (*, **, or ***) represent statistical significance by t test (B) or one-way ANOVA with Dunnett’s multiple comparison test (A and C) at the levels of 0.05, 0.01, or 0.001, respectively.
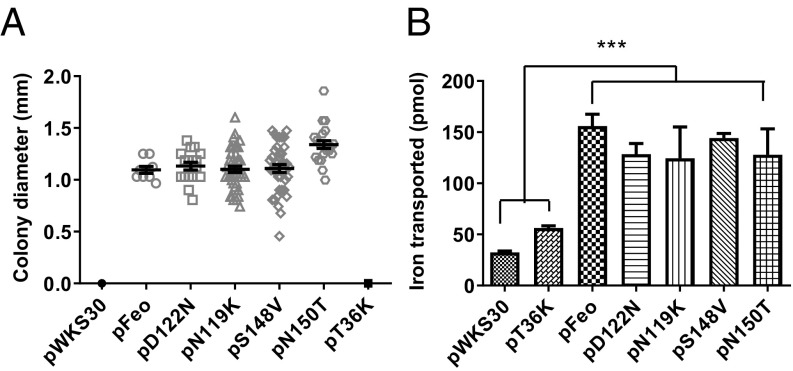
To investigate the effect of the mutations on in vivo ferrous iron transport, we carried out functional assays to test the importance of nucleotide hydrolysis for iron transport in vivo. V. cholerae EPV6, which has mutations in all of the iron transport systems except heme uptake (1), is able to grow in the absence of heme supplementation only when a functional iron transport system, such as Feo, is supplied (1, 15). EPV6 harboring wild-type Feo grew well in the absence of heme supplementation, while the strain carrying the empty vector or pFeo with the T36K mutation failed to grow, as expected (Fig. 4A). EPV6 carrying pFeo with the D122N or N119K mutations grew as well as EPV6 carrying the wild-type pFeo in the absence of heme, and there was no significant difference between colony sizes of the wild-type and mutants (Fig. 4A). The results of the colony size assays were further confirmed by radioactive ferrous iron transport assays, showing that mutations in the G4 domain did not affect ferrous iron transport (Fig. 4B). These results demonstrate that the VcNFeoB ATPase activity alone is sufficient for Feo iron transport function.
Fig. 4.
Either ATP or GTP hydrolysis activity is required for in vivo ferrous iron transport. (A) For colony size assays, colony diameters of EPV6 carrying either empty vector (pWKS30) or a plasmid encoding Feo (pFeo) or its mutants were measured using ImageJ after 24 h of incubation at 37 °C on unsupplemented LB agar. The strains carrying empty vector (pWKS30) or the negative control construct (pT36K) did not grow, and their growth was indicated as zero. (B) The EPV6 strains were grown to midexponential phase in LB and resuspended in M9 medium containing 50 µg/ml of ethylene diamine diorthohydroxyphenyl acetic acid. The assay was begun with the addition of 55Fe in the presence of 5 mM sodium ascorbate, and samples were removed after 10 min and filtered to determine the amounts of cell-associated radioactivity. The data are the averages from three replicates. The error bars represent SEM. Asterisks (***) represent statistical significance by one-way ANOVA with Dunnett’s multiple comparison test at the level of 0.001.
GTPase and ATPase Activities of VcNFeoB Are Not Stimulated by Potassium.
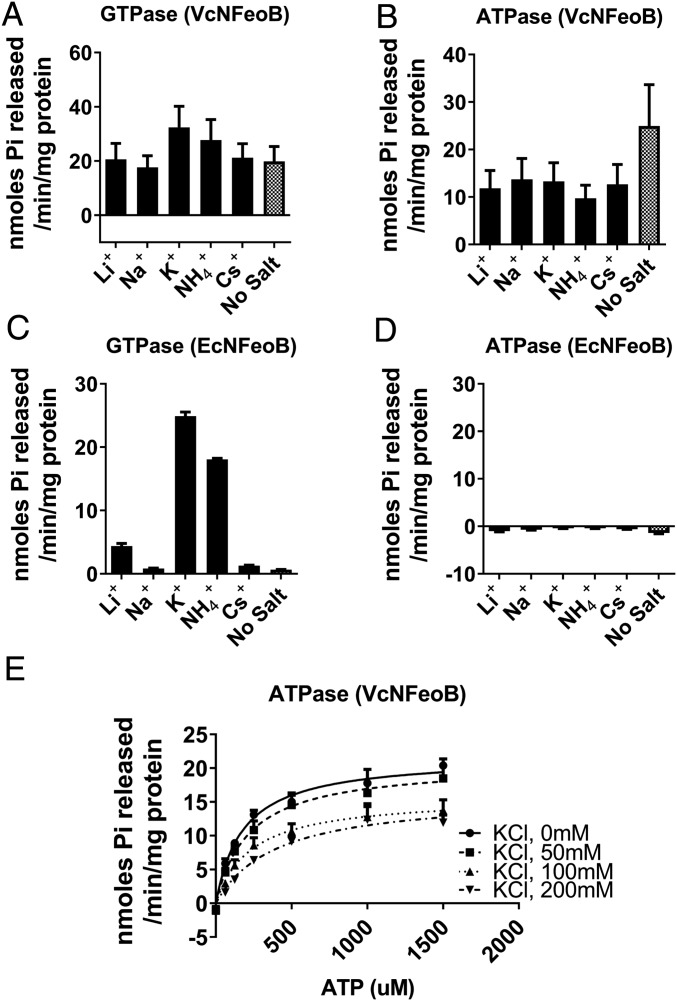
GTPase activity of FeoB in E. coli is stimulated by potassium, which may bind at a site adjacent to the GTP-binding pocket (9, 16). We investigated whether a similar mechanism is operative for GTP and ATP hydrolysis in VcNFeoB. EcNFeoB was included in these assays for comparison. As expected, GTPase activity of EcNFeoB was higher (38-fold) with the addition of 200 mM KCl compared with no additional salt (Fig. 5C). However, the GTPase activity of VcNFeoB did not increase significantly with potassium (Fig. 5A). We tested other monovalent cations, including Li+ and Na+, which have smaller ionic radii than K+; NH4+, which has a similar radius; and Cs+, which is larger, but none had a significant effect compared with no added salt (Fig. 5A). To control for effects of ionic strength, we also tested the addition of a bulky ion, tetraethylammonium ion, which should not interact with a potassium-binding site, and found no difference between it and no added salt (SI Appendix, Fig. S4). ATP hydrolysis activity of VcNFeoB was not accelerated by any monovalent cation. Its activity was highest without additional salts (Fig. 5B and SI Appendix, Fig. S4). Unlike its GTP hydrolysis activity, the ATP hydrolysis activity of VcNFeoB was inhibited by potassium as a mixed inhibition mode (KI = 111.8 ± 42.7 mM, Fig. 5E). No ATPase activity of EcNFeoB in the presence of cations was observed, even with the addition of potassium (Fig. 5D). Thus, VcNFeoB differs from the E. coli protein not only in its possession of ATPase activity, but also in the lack of significant potassium activation of GTPase function. This suggests structural differences in the active sites of the enzymes, and additional residues outside the G1–G4 domains may play important roles in nucleotide specificity and hydrolysis.
Fig. 5.
The ATPase activity of VcNFeoB is not stimulated by potassium. (A and C) While the GTPase activity of VcNFeoB (A) was not stimulated by cations, EcNFeoB (C) was highly stimulated by 200 mM potassium or ammonium ions. VcNFeoB showed high hydrolysis activities of both GTP and ATP without additional cations (no salt), compared with EcNFeoB. (B and D) The ATPase activity of VcNFeoB was not stimulated by potassium or other monovalent cations. EcNFeoB did not show ATPase activity with addition of any cations. (E) The ATPase activity of VcNFeoB was inhibited by potassium in a dose-dependent manner. The curves represent nonlinear fits to the Michaelis–Menten model. The enzyme reaction buffer alone (No Salt) contains 5 mM MgCl2 and 10 mM NaCl.
Ser148 and Asn150 Play a Role in ATP Hydrolysis Activity.
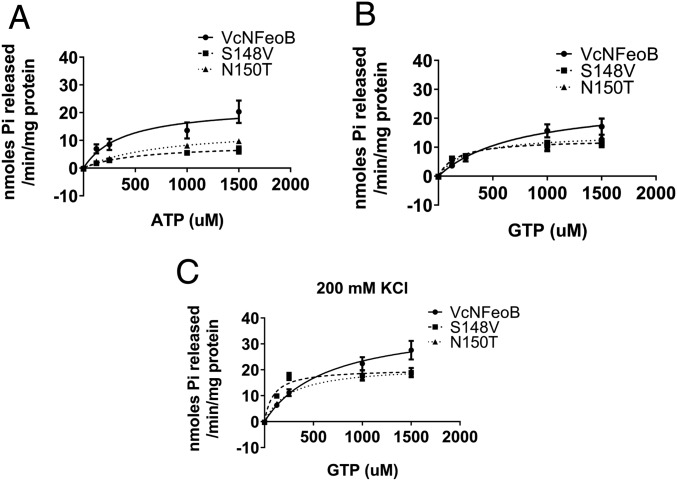
To identify residues with a potential role in ATP hydrolysis activity, we constructed a homology model of VcNFeoB using Klebsiella pneumoniae NFeoB as the template (Protein Data Bank ID code 2WIC; SI Appendix, Fig. S5). From a protein structure alignment of VcNFeoB with EcNFeoB, we identified two distinguishing residues that might contact ATP in the nucleotide-binding site: Ser148 and Asn150 in VcNFeoB, where EcNFeoB possesses Val149 and Thr151, respectively. Ser148 and Asn150 are in the G5 motif, which is a G-protein motif generally composed of six amino acids forming a loop with electrostatic or hydrophobic interactions with the guanine nucleotide base (17). However, compared with other G proteins, this motif has relatively poor sequence conservation in FeoB (SI Appendix, Fig. S6). To determine whether these putative G5 residues played a role in the nucleotide specificity of V. cholerae FeoB, we substituted the residues of VcNFeoB for the corresponding residues in EcNFeoB (S148V and N150T) and compared iron transport function and nucleotide hydrolysis activities. Functional assays for in vivo iron transport showed normal growth of strains carrying the mutant proteins compared with wild-type Feo (Fig. 4). Thus, the mutations did not affect FeoB function. However, both of the substitutions resulted in significantly decreased ATP hydrolysis (Fig. 6A), suggesting that these residues promote ATP binding by VcFeoB. The mutations did not significantly reduce GTP hydrolysis (Fig. 6B). Furthermore, the mutants more closely resembled EcNFeoB in their response to potassium: in the presence of potassium, the S148V and N150T mutants exhibited lower KM for GTP (92.1 ± 32.7 µM and 261 ± 33.0 µM, respectively) than wild-type VcNFeoB (648 ± 258 µM) (Fig. 6C), making them more similar to EcNFeoB (132 ± 57.1 µM, Fig. 2C). These results suggest that Ser148 and Asn150 play a role in ATP binding and hydrolysis and that replacing these residues with those found in E. coli switched their nucleotide specificity to GTP.
Fig. 6.
Ser148 and Asn150 of VcNFeoB play a role in ATPase, but not GTPase, activity. (A) Mutants of the G5 motif (S148V and N150T) showed decreased ATPase activity compared with wild-type VcNFeoB in the absence of potassium. Both in the absence (B) and presence (C) of potassium, the maximum GTPase activity of the G5 motif mutants was slightly lower compared with wild type, but their GTP-binding affinity was higher, making them more similar to EcNFeoB (Fig. 1C).
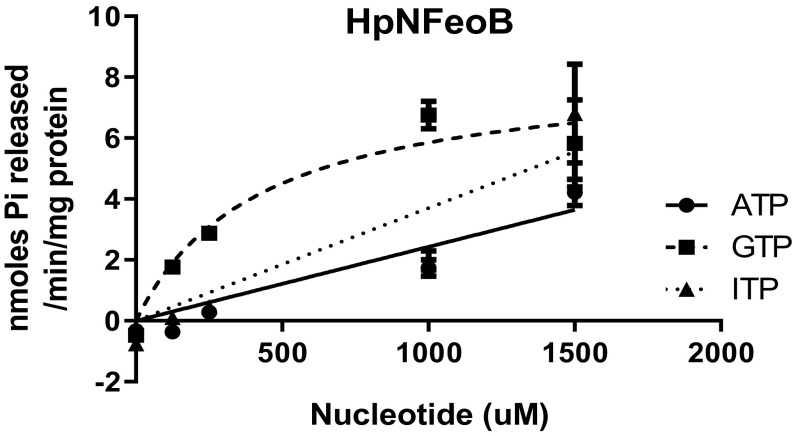
Sequence alignment of prokaryotic FeoB proteins shows weak sequence conservation of the Asn150 residue, although the residue at position 148 in V. cholerae (Ser148) can be separated into either polar (serine, asparagine, and glutamine) or nonpolar (valine and isoleucine) amino acids in the aligned sequences (SI Appendix, Fig. S6). We hypothesized that the polar residue might be crucial for ATP hydrolysis activity of FeoB and tested this using NFeoB from H. pylori, which, like V. cholerae, has serine in this position. We purified the cytosolic domain of H. pylori (HpNFeoB) and analyzed its nucleotide specificity (Fig. 7). Our results clearly show that, like VcNFeoB, HpNFeoB possesses ATP hydrolysis activity. This is consistent with the earlier observation that transport via FeoB was inhibited by ATPase inhibitors in H. pylori (2).
Fig. 7.
H. pylori FeoB possess ATPase activity. HpNFeoB catalyzes hydrolysis of ATP (●) as well as GTP (■) and ITP (▲). It is noted that the HpNFeoB was insoluble in the E. coli expression host and therefore had to be solubilized and refolded, resulting in decreased specific activity.
FeoA and FeoC Affect Nucleotide Hydrolysis Activity.
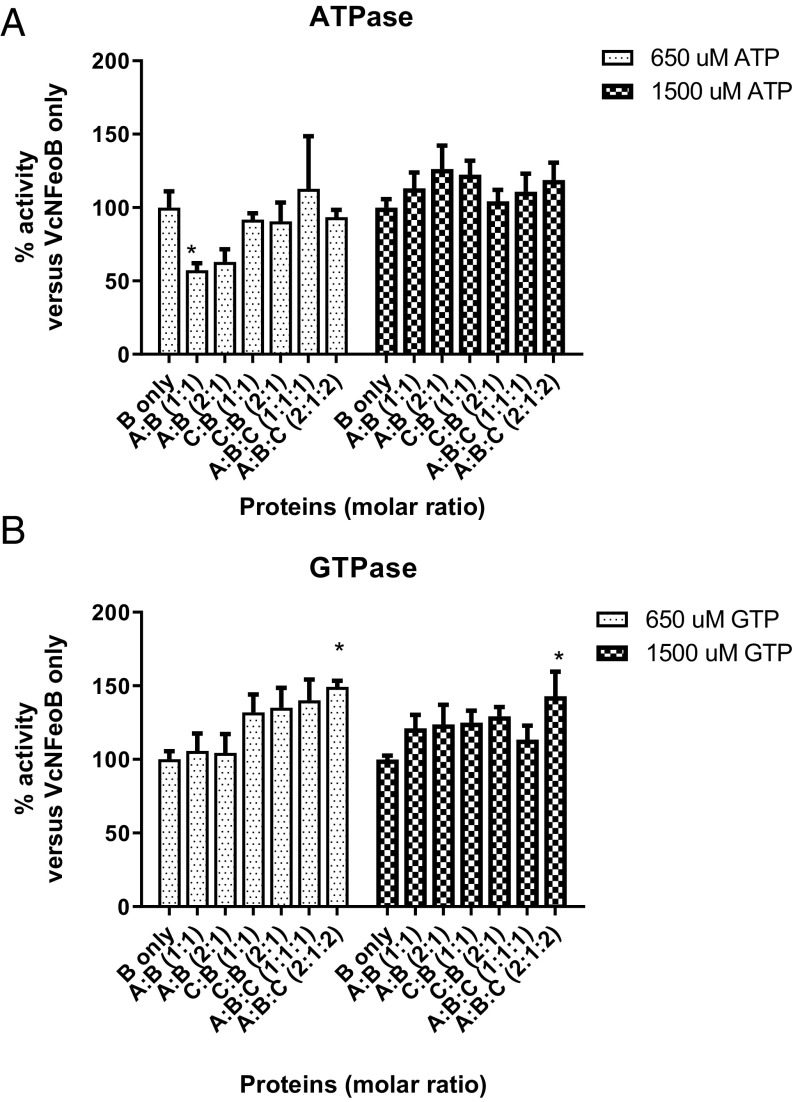
The feo operon in V. cholerae encodes the FeoA, FeoB, and FeoC proteins, which are all required for Feo system function and interact in vivo (14, 15). However, the function of FeoA and FeoC remains unclear. To test possible effects of FeoA and FeoC on nucleotide hydrolysis activity of NFeoB, we purified FeoA and FeoC and measured nucleotide hydrolysis (Fig. 8). FeoA and FeoC were mixed with VcNFeoB at molar ratios of 1:1 or 2:1 (A:B and C:B) and 1:1:1 or 2:1:2 (A:B:C). Nucleotide hydrolysis activities were compared with VcNFeoB alone, using 650 and 1,500 µM ATP or GTP. FeoA inhibited ATP hydrolysis activity (57–63%) at the lower concentration of ATP, but no inhibition was observed at the higher concentration of ATP substrate. The addition of FeoC with FeoA did not affect the ATPase activity. The presence of FeoC and FeoA together modestly stimulated GTPase activity of VcNFeoB (124–149%) at both low- and high-GTP concentrations. These findings suggest that the NTPase activity of FeoB is modestly regulated by FeoA and FeoC.
Fig. 8.
VcFeoA and VcFeoC affect nucleotide hydrolysis activity of VcNFeoB. FeoA and FeoC were mixed with VcNFeoB with molar ratios of 1:1 or 2:1 (A:B and C:B) and 1:1:1 or 2:1:2 (A:B:C). Nucleotide hydrolysis activity was compared with VcNFeoB at 650 and 1,500 µM of ATP or GTP, concentrations that are close to the KM of each nucleotide and the maximum concentrations of substrate for the malachite green assay. Percentage of activity is expressed as activity versus its activity without addition of FeoA or FeoC. (A) FeoA inhibited ATPase activity of VcNFeoB at low ATP concentration, but showed no effect at high ATP concentration or when FeoC was present as well. (B) FeoC stimulated GTPase activity of VcNFeoB at both low- and high-GTP concentrations, although only the FeoA, FeoB, and FeoC combination in a 2:1:2 molar ratio showed a statistical difference. Asterisks (*) represent statistical significance by one-way ANOVA with Dunnett’s multiple comparison test at the level of 0.05.
Discussion
The ferrous iron transporter Feo is widely distributed among bacteria and archaea, but it shares little homology with other bacterial metal ion transporters. The C terminus of the protein has multiple membrane-spanning domains that may form the channel for importation of iron, while the cytoplasmic N terminus has homology to eukaryotic small G proteins and possesses GTPase activity. FeoB was initially thought to be an ATPase based on its amino acid sequence (6), and this model was supported by loss of ferrous iron transport with ATPase inhibitors in H. pylori (2). However, Marlovits et al. (7) showed that the purified N-terminal domain of E. coli FeoB specifically binds GTP but not ATP and that mutations that eliminated GTP binding and hydrolysis in vitro also eliminated ferrous iron transport. This led to the conclusion that FeoB was a GTPase, and additional enzymatic characterization showed that potassium activated the GTPase in S. thermophilus and E. coli NFeoB in vitro (5, 9).
Using V. cholerae as a model system, we characterized the enzymic properties of the Feo system and identified its role in the physiological function of ferrous iron transport. Compared with E. coli FeoB, a distinguishing feature of V. cholerae FeoB is that it exhibits hydrolytic activities toward both ATP and GTP. An in vivo role for the ATPase activity is supported by the finding that mutations in the G4 motif that reduce GTPase, but not ATPase, activity still have normal iron transport function. Unlike E. coli FeoB, neither the GTPase nor the ATPase activities were stimulated by K+, indicating differences in the mechanisms for activating hydrolysis in the different FeoB proteins.
Potassium activates the GTPase activity of S. thermophilus FeoB by binding at a site adjacent to GTP (9, 16, 18). The crystal structure of NFeoB from S. thermophilus suggests that the six-coordinate potassium-binding site consists of two backbone carbonyl groups from the Switch I region, three oxygen atoms from the nucleotide phosphates, and the side-chain amide of Asn11 (16, 18). The potassium ion is coordinated by the Switch I region that undergoes a large conformational change in the transition state of the protein. The potassium ion stabilizes GTP binding and acts as a GAP. In Ras G proteins, the transition state is stabilized through an arginine finger in GAPs, lowering the activation energy barrier (11, 19). Potassium may act in a manner analogous to the arginine finger by neutralizing the developing negative charge during catalysis (11, 20). However, potassium-dependent GTP hydrolysis is not a prerequisite in all GTPases, and our data show that potassium is not a requirement for VcNFeoB NTPase function. Although ATP hydrolysis by VcNFeoB is lower than GTP hydrolysis in the presence of potassium, the intracellular concentration of ATP (3 mM) is higher than GTP (1 mM) (21), which may allow similar hydrolysis of these nucleotide species in vivo.
Broader nucleotide specificity is not unique to FeoB. Among bacterial GTPases, YchF (ribosome-binding ATPase) shows dual specificity for ATP and GTP hydrolysis (22, 23), and X-ray crystallography demonstrated that binding of YchF to ATP or GTP was associated with different conformations. Mutation of the noncanonical YchF G4 motif at positions 2 and 4 (underscored) (NMSE to NKSD) precluded ATP binding and hydrolysis (23); however, a human YchF (called hOLA1) mutant (NLSE to NKSD) retained ATP specificity (22). V. cholerae possesses lysine and aspartate (NKXD) in the G4 motif, while most bacteria possess methionine and aspartate at this site (NMXD). Thus, the amino acid sequence of the G4 motif alone does not determine whether GTP or ATP is bound, suggesting that overall orientation of the motif or additional residues outside the G4 motif are responsible for specific nucleotide binding and hydrolysis.
Our data suggest that Ser148 and Asn150 may be candidates for residues in addition to the G4 motif that determines the nucleotide specificity of V. cholerae FeoB. Mutation of these residues resulted in loss of ATPase but not GTPase activity. These residues are in the G5 motif, generally composed of six amino acids forming a loop with electrostatic or hydrophobic interactions with the nucleotide base (17). So far, there is no information reported on the role of these G5 residues (Ser148 in V. cholerae and Val149 in E. coli), except that the V149A mutant was insoluble in E. coli (24). E. coli FeoB has a serine residue next to the valine (Ser150), and this is important for GDP release (17, 24). Likewise, mutation of Thr151 in E. coli FeoB (T151A, corresponding to Asn150 in V. cholerae) also significantly affected the intrinsic GDP release rate (25), suggesting the possible roles of the G5 motif in its interaction with the nucleotide bases. The G5 motif in eukaryotic G proteins has been of interest because mutations in the motif are linked to human disease phenotypes such as Costello syndrome (26, 27), testotoxicosis (28), and pseudohypoparathyroidism (28), characterized by an accelerated GDP release rate. A comparison of the G5 motifs in bacterial FeoB proteins suggests that there may be at least two different classes of FeoB proteins with different nucleotide specificities. Those like V. cholerae that have serine and asparagine in the G5 motif may use either ATP or GTP, while E. coli and other species that have valine and threonine or similar residues in the G5 motif may rely on GTP. Observing ATPase activity for H. pylori, which has a V. cholerae-like G5 motif, supports this hypothesis, but additional analysis is required to establish a link between the presence of specific G5 residues and nucleotide specificity.
In addition to the G1–G5 motifs, the VcNFeoB protein contains the GDI domain, which can interact with the switch regions and influence GDP binding. The effect of the GDI domain is enhanced by the presence of the FeoB membrane domain (8, 29), which is absent from VcNFeoB. Because it is likely that ATP and ITP bind to the same site as GTP, the GDI domain may influence ADP and IDP affinity in a manner similar to GDP, but this has not been experimentally determined.
The Feo system includes two small cytoplasmic proteins, FeoA and FeoC, the functions of which are poorly understood. In V. cholerae, FeoA is required for FeoABC complex formation, and FeoC is required for transport function (15). FeoC is known to have possible roles in regulating transcription in some species, binding iron by forming iron-sulfur clusters, and protecting FeoB from proteolysis (30, 31). However, V. cholerae FeoC lacks residues characteristic of iron-sulfur–binding sites, and protection of FeoB from hydrolysis cannot be the sole function of FeoC; increasing the amount of FeoB in the absence of FeoC does not restore function (13). These proteins may have some role in nucleotide specificity or hydrolysis. However, FeoC does not appear to determine nucleotide specificity since H. pylori has both GTPase and ATPase activity but lacks a FeoC protein. In this study, we show that the addition of both FeoA and FeoC to VcNFeoB resulted in modest stimulation of GTP hydrolysis, while FeoA inhibited ATPase activity. FeoA and FeoC may have greater effects on nucleotide hydrolysis in vivo than in vitro, but their primary roles may be in other aspects of Feo-mediated iron transport.
The Feo system plays an important role in ferrous iron acquisition in numerous bacterial species. Our data indicate that this family of transporters may be more diverse than previously recognized, with some members being able to function as either a GTPase or ATPase, or both, in transporting iron.
Materials and Methods
Additional information is provided in SI Appendix, Materials and Methods.
Plasmids Construction.
Plasmid pFeoAB-V5C carries the entire V. cholerae feo operon, encoding the full-length FeoB protein N-terminally tagged with a V5 tag, in the low-copy pWKS30 vector. pET21a-based expression vectors encoding the cytosolic N terminus of FeoB from V. cholerae (amino acids 1–272, VcNFeoB), E. coli (amino acids 1–274, EcNFeoB), or H. pylori (amino acids 1–250, HpNFeoB) with a hexa-histidine tag were generated. The plasmids pFeoAB-V5C, pVcNFeoB, and pEcNFeoB were used to generate expression vectors encoding amino acid mutants through site-directed mutagenesis.
Protein Purification.
Purification of VcNFeoB, EcNFeoB, and associated mutants (EcNFeoBD123N, VcNFeoBT36K, VcNFeoBD122N, VcNFeoBS148V, and VcNFeoBN150T) was conducted on a Ni2+-charged His-Bind resin (Millipore) by using 20 mM imidazole as the wash buffer and gradually increasing imidazole (40 to 250 mM) as the elution buffer, in 25 mM Tris⋅Cl (pH 7.5) containing 100 mM NaCl, 10% (vol/vol) glycerol, and 10 mM beta-mercaptoethanol. VcNFeoBN119K, HpNFeoB, FeoA, and FeoC were found almost entirely in the insoluble fraction, so we solubilized and refolded the proteins (SI Appendix, Materials and Methods), followed by immobilized Ni2+-affinity chromatography as described above.
GTPase Assay.
A malachite green-based colorimetric assay was used to measure inorganic phosphate released through NFeoB’s nucleotide hydrolysis activity (32). For the determination of kinetic parameters, the initial rate data were fitted to the Michaelis–Menten equation by nonlinear regression using GraphPad Prism 5. Inhibition of ATPase activity by potassium ion was examined by assays at four fixed potassium ion concentrations at varying concentrations of ATP. KI was calculated from a global fit of the data to a mixed inhibition model using GraphPad Prism 5.
Functional Assays.
V. cholerae strains EPV6 harboring the indicated plasmid were grown overnight in LB broth. Colony size assays were performed on LB agar, measuring the diameters of well-isolated colonies on LB agar after 24 h of incubation at 37 °C. Radioactive iron transport assays were conducted by adding 55FeCl2 (Perkin-Elmer Life and Analytical Sciences) to iron-deprived bacterial cell cultures. Cells were collected by filtration, and the amount of radioactive iron was determined by scintillation counting.
Supplementary Material
Acknowledgments
We thank Camilo Gomez for the gift of V. cholerae EPV6 S148V mutant; Charles Midgett for the tobacco etch virus protease expression plasmid; D. Scott Merrell for generously providing H. pylori genomic DNA; Walter L. Fast for suggestions on the protein structure analysis; and Elizabeth E. Wyckoff for pioneering research on the V. cholerae Feo transport system. This work was supported by National Institutes of Health Grant AI091957 (to S.M.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1817964116/-/DCSupplemental.
References
- 1.Peng ED, Wyckoff EE, Mey AR, Fisher CR, Payne SM. Nonredundant roles of iron acquisition systems in Vibrio cholerae. Infect Immun. 2015;84:511–523. doi: 10.1128/IAI.01301-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Velayudhan J, et al. Iron acquisition and virulence in Helicobacter pylori: A major role for FeoB, a high-affinity ferrous iron transporter. Mol Microbiol. 2000;37:274–286. doi: 10.1046/j.1365-2958.2000.01987.x. [DOI] [PubMed] [Google Scholar]
- 3.Aranda J, et al. Contribution of the FeoB transporter to Streptococcus suis virulence. Int Microbiol. 2009;12:137–143. [PubMed] [Google Scholar]
- 4.Pérez NM, Ramakrishnan G. The reduced genome of the Francisella tularensis live vaccine strain (LVS) encodes two iron acquisition systems essential for optimal growth and virulence. PLoS One. 2014;9:e93558. doi: 10.1371/journal.pone.0093558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau CKY, Ishida H, Liu Z, Vogel HJ. Solution structure of Escherichia coli FeoA and its potential role in bacterial ferrous iron transport. J Bacteriol. 2013;195:46–55. doi: 10.1128/JB.01121-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kammler M, Schön C, Hantke K. Characterization of the ferrous iron uptake system of Escherichia coli. J Bacteriol. 1993;175:6212–6219. doi: 10.1128/jb.175.19.6212-6219.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marlovits TC, Haase W, Herrmann C, Aller SG, Unger VM. The membrane protein FeoB contains an intramolecular G protein essential for Fe(II) uptake in bacteria. Proc Natl Acad Sci USA. 2002;99:16243–16248. doi: 10.1073/pnas.242338299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eng ET, Jalilian AR, Spasov KA, Unger VM. Characterization of a novel prokaryotic GDP dissociation inhibitor domain from the G protein coupled membrane protein FeoB. J Mol Biol. 2008;375:1086–1097. doi: 10.1016/j.jmb.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ash M-R, et al. Potassium-activated GTPase reaction in the G protein-coupled ferrous iron transporter B. J Biol Chem. 2010;285:14594–14602. doi: 10.1074/jbc.M110.111914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seyedmohammad S, et al. Structural model of FeoB, the iron transporter from Pseudomonas aeruginosa, predicts a cysteine lined, GTP-gated pore. Biosci Rep. 2016;36:e00322. doi: 10.1042/BSR20160046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rafay A, Majumdar S, Prakash B. Exploring potassium-dependent GTP hydrolysis in TEES family GTPases. FEBS Open Bio. 2012;2:173–177. doi: 10.1016/j.fob.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Payne SM, Mey AR, Wyckoff EE. Vibrio iron transport: Evolutionary adaptation to life in multiple environments. Microbiol Mol Biol Rev. 2015;80:69–90. doi: 10.1128/MMBR.00046-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wyckoff EE, Mey AR, Leimbach A, Fisher CF, Payne SM. Characterization of ferric and ferrous iron transport systems in Vibrio cholerae. J Bacteriol. 2006;188:6515–6523. doi: 10.1128/JB.00626-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weaver EA, Wyckoff EE, Mey AR, Morrison R, Payne SM. FeoA and FeoC are essential components of the Vibrio cholerae ferrous iron uptake system, and FeoC interacts with FeoB. J Bacteriol. 2013;195:4826–4835. doi: 10.1128/JB.00738-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevenson B, Wyckoff EE, Payne SM. Vibrio cholerae FeoA, FeoB, and FeoC interact to form a complex. J Bacteriol. 2016;198:1160–1170. doi: 10.1128/JB.00930-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ash MR, Maher MJ, Guss JM, Jormakka M. The structure of an N11A mutant of the G-protein domain of FeoB. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011;67:1511–1515. doi: 10.1107/S1744309111042965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guilfoyle A, et al. Structural basis of GDP release and gating in G protein coupled Fe2+ transport. EMBO J. 2009;28:2677–2685. doi: 10.1038/emboj.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ash M-R, Maher MJ, Guss JM, Jormakka M. The initiation of GTP hydrolysis by the G-domain of FeoB: Insights from a transition-state complex structure. PLoS One. 2011;6:e23355. doi: 10.1371/journal.pone.0023355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittal R, Ahmadian MR, Goody RS, Wittinghofer A. Formation of a transition-state analog of the Ras GTPase reaction by Ras-GDP, tetrafluoroaluminate, and GTPase-activating proteins. Science. 1996;273:115–117. doi: 10.1126/science.273.5271.115. [DOI] [PubMed] [Google Scholar]
- 20.Scrima A, Wittinghofer A. Dimerisation-dependent GTPase reaction of MnmE: How potassium acts as GTPase-activating element. EMBO J. 2006;25:2940–2951. doi: 10.1038/sj.emboj.7601171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckstein MH, He J, Rubin H. Characterization of nucleotide pools as a function of physiological state in Escherichia coli. J Bacteriol. 2008;190:718–726. doi: 10.1128/JB.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koller-Eichhorn R, et al. Human OLA1 defines an ATPase subfamily in the Obg family of GTP-binding proteins. J Biol Chem. 2007;282:19928–19937. doi: 10.1074/jbc.M700541200. [DOI] [PubMed] [Google Scholar]
- 23.Cheung M-Y, et al. ATP binding by the P-loop NTPase OsYchF1 (an unconventional G protein) contributes to biotic but not abiotic stress responses. Proc Natl Acad Sci USA. 2016;113:2648–2653. doi: 10.1073/pnas.1522966113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guilfoyle AP, et al. Structural and functional analysis of a FeoB A143S G5 loop mutant explains the accelerated GDP release rate. FEBS J. 2014;281:2254–2265. doi: 10.1111/febs.12779. [DOI] [PubMed] [Google Scholar]
- 25.Guilfoyle AP, Deshpande CN, Schenk G, Maher MJ, Jormakka M. Exploring the correlation between the sequence composition of the nucleotide binding G5 loop of the FeoB GTPase domain (NFeoB) and intrinsic rate of GDP release. Biosci Rep. 2014;34:e00158. doi: 10.1042/BSR20140152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gripp KW, et al. Costello syndrome associated with novel germline HRAS mutations: An attenuated phenotype? Am J Med Genet A. 2008;146A:683–690. doi: 10.1002/ajmg.a.32227. [DOI] [PubMed] [Google Scholar]
- 27.Zampino G, et al. Diversity, parental germline origin, and phenotypic spectrum of de novo HRAS missense changes in Costello syndrome. Hum Mutat. 2007;28:265–272. doi: 10.1002/humu.20431. [DOI] [PubMed] [Google Scholar]
- 28.Iiri T, Herzmark P, Nakamoto JM, van Dop C, Bourne HR. Rapid GDP release from Gs alpha in patients with gain and loss of endocrine function. Nature. 1994;371:164–168. doi: 10.1038/371164a0. [DOI] [PubMed] [Google Scholar]
- 29.Hattori M, et al. Structural basis of novel interactions between the small-GTPase and GDI-like domains in prokaryotic FeoB iron transporter. Structure. 2009;17:1345–1355. doi: 10.1016/j.str.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Kim H, Lee H, Shin D. The FeoC protein leads to high cellular levels of the Fe(II) transporter FeoB by preventing FtsH protease regulation of FeoB in Salmonella enterica. J Bacteriol. 2013;195:3364–3370. doi: 10.1128/JB.00343-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hung K-W, Juan T-H, Hsu Y-L, Huang TH. NMR structure note: The ferrous iron transport protein C (FeoC) from Klebsiella pneumoniae. J Biomol NMR. 2012;53:161–165. doi: 10.1007/s10858-012-9633-6. [DOI] [PubMed] [Google Scholar]
- 32.Quan A, Robinson PJ. Rapid purification of native dynamin I and colorimetric GTPase assay. Meth Enzymol. 2005;404:556–569. doi: 10.1016/S0076-6879(05)04049-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.