Significance
Glycosylation is important for the proper folding and function of glycoproteins. However, glycosylation on viral surface protein may mask the conserved epitopes and causes poor host immune responses and protection against viral infection. To expose more antigenic sites shielded by glycans, we developed a strategy to remove the glycans on the viral surface by modification of the traditional egg-based influenza vaccine production procedure. By means of immunization with the monoglycosylated split virus vaccine, mice were induced with broader immune responses, and thus better protection against cross-strain H1N1 virus infections. The results showed that the monoglycosylated split virus vaccine is a broadly protective vaccine and can be produced through simple modifications of the current vaccine manufacturing process.
Keywords: influenza vaccines, N-glycosylation, hemagglutinin stem-specific antibody, monoglycosylated influenza split virus vaccine
Abstract
Each year influenza virus infections cause hundreds of thousands of deaths worldwide and a significant level of morbidity with major economic burden. At the present time, vaccination with inactivated virus vaccine produced from embryonated chicken eggs is the most prevalent method to prevent the infections. However, current influenza vaccines are only effective against closely matched circulating strains and must be updated and administered yearly. Therefore, generating a vaccine that can provide broad protection is greatly needed for influenza vaccine development. We have previously shown that vaccination of the major surface glycoprotein hemagglutinin (HA) of influenza virus with a single N-acetylglucosamine at each of the N-glycosylation sites [monoglycosylated HA (HAmg)] can elicit better cross-protection compared with the fully glycosylated HA (HAfg). In the current study, we produced monoglycosylated inactivated split H1N1 virus vaccine from chicken eggs by the N-glycosylation process inhibitor kifunensine and the endoglycosidase Endo H, and intramuscularly immunized mice to examine its efficacy. Compared with vaccination of the traditional influenza vaccine with complex glycosylations from eggs, the monoglycosylated split virus vaccine provided better cross-strain protection against a lethal dose of virus challenge in mice. The enhanced antibody responses induced by the monoglycosylated vaccine immunization include higher neutralization activity, higher hemagglutination inhibition, and more HA stem selectivity, as well as, interestingly, higher antibody-dependent cellular cytotoxicity. This study provides a simple and practical procedure to enhance the cross-strain protection of influenza vaccine by removing the outer part of glycans from the virus surface through modifications of the current egg-based process.
Influenza pandemics have played a devastating role in human history. The most deadly pandemic was the 1918 Spanish flu that killed 50 to 100 million people (1). In addition, seasonal influenza viruses cause infections of more than 5% of adults and 20% of children every year, from which more than 200,000 people die worldwide (2). For the prevention of influenza virus infection, vaccination is the most effective approach.
Although several influenza vaccines have been approved for human use, influenza viruses still cause epidemics and pandemics continuously around the world (2, 3). Due to antigenic drift and antigenic shift of the major surface antigens of influenza virus, mainly hemagglutinin (HA), influenza vaccines lose their protection against newly circulating virus strains (3). Because of this, the WHO annually predicts the vaccine strains for the upcoming season based on the results from the influenza surveillance network. After the vaccine strains are predicted, preparing the seed viruses, certifying reagents, and vaccine manufacturing require more than 6 mo (4). Influenza vaccine is the only human vaccine of which the ingredients need to be reformulated every year. Furthermore, if a mismatch occurs between the vaccine strains and the circulating strains, reduced efficacy of influenza vaccine can cause poor protection (5). To mitigate the shortcomings caused by the mismatch between the vaccine and circulating strains, as well as the time-consuming process of reformulating vaccines, the main goal for influenza vaccine research is to develop a more effective vaccine that can induce wide antiinfluenza immunity.
Novel approaches to increase the spectrum of antiinfluenza immunity have been reported recently; these include the following: a prime-boost combination by using HA DNA vaccine as a priming agent, followed by the seasonal inactivated vaccine as a boosting agent (6); a chimeric recombinant HA immunization (i.e., a sequential immunization of chimera HAs with a conserved stem domain but different globular domains of various subtypical HAs) (7); a stem HA nanoparticle (i.e., a structure-based design of the HA stem domain expressed and fused with ferritin that can self-assemble into nanoparticles) (8); and new live-attenuated vaccines, which eliminate the replication ability by abolishing the function of neuraminidase (NA) or matrix protein 2 (M2) (9, 10). In addition, we have previously shown that the monoglycosylated HA (HAmg) protein vaccine is a cross-strain influenza vaccine in which the HAmg still keeps its structural integrity and provides better and wider protection against H1N1 influenza virus infections in both mouse and ferret animal models (11). The vaccination with HAmg elicited a more diverse B cell repertoire, stimulated better dendritic cell maturation, and induced more CD8+ memory T cells and IgG-secreting plasma cells (11).
In this study, we report the procedure to produce the monoglycosylated influenza split virus vaccine, which is a simple modification of the regular trivalent or quadrivalent split virus vaccine production procedure, and evaluate the vaccine efficacy in a mouse model. Immunization of the monoglycosylated split virus vaccine induced more neutralizing antibody and provided better protection against a lethal dose of virus infection from strain-specific and cross-strain H1N1 viruses. Furthermore, our data also showed that the monoglycosylated split virus vaccine induced more HA stem-specific antibodies, and, interestingly, an enhancement of antibody-dependent cellular cytotoxicity (ADCC) against influenza-infected cells was observed. Based on our studies, the monoglycosylated split virus vaccine is an improved influenza vaccine that provides better antiinfluenza immune responses than the current inactivated virus vaccine and is practical to produce.
Results
Preparation of Monoglycosylated Virus with Glycosylation Process Inhibitor and Endoglycosidase.
To explore whether the findings of the HAmg protein vaccine can be applied to the traditional egg-based vaccine, monoglycosylated influenza split virus vaccine was generated. The schematic diagram in Fig. 1A shows the strategy for monoglycosylated split virus vaccine production, and the different glycosylation states of HA on viral membranes along the process are depicted. After the screening of various glycosylation processing inhibitors that may work in the egg-based procedure, kifunensine was chosen to inhibit the glycosylation process to keep the glycan modification on HA at high-mannose type (12). Kifunensine is a potent inhibitor of α-mannosidase I and has been used to block the glycosylation process in cell cultures (12, 13). To test whether kifunensine is able to arrest the glycosylation state of viral surface glycoproteins at high-mannose type in the egg-based procedure, different concentrations of kifunensine were applied during virus inoculation in embryonated chicken eggs. With 100 μg/mL or higher concentrations of kifunensine treatment, viral HA from the allantoic fluid shifted to lower molecular weights compared with that from no or 10 μg/mL kifunensine-treated samples (Fig. 1B). The gel shifts indicated that the glycan modifications on kifunensine-treated HA may be high-mannose types but not complex-type glycans, which have higher molecular weights. Several glycosylation inhibitors that have the ability to arrest the glycan modification of glycoproteins at different glycosylated states (14) (SI Appendix, Fig. S1A) were also tested during virus inoculation (e.g., deoxymannojirimycin, swainsonine). However, they either needed to work at high concentrations (SI Appendix, Fig. S1B) or arrested the glycan modifications at unusual hybrid-type glycans, which cannot be easily modified into simplified glycans by endoglycosidase treatment (SI Appendix, Fig. S1C). For the production of monoglycosylated virions, endoglycosidase H (Endo H) was used to trim down the high-mannose-type glycans on viral proteins to a single N-acetylglucosamine (GlcNAc) (15). The molecular weight of HA in the kifunensine-treated allantoic fluid was further decreased by Endo H digestion (Fig. 1C). The molecular weights of HA1 and HA2 both shifted again to lower positions after being treated with 10 μg/mL or higher concentrations of Endo H. With Western blotting analysis of the viral proteins (HA) on influenza virions, the data suggested that the monoglycosylated progeny virions were obtained by utilizing the glycosylation inhibitor, kifunensine, and the endoglycosidase, Endo H, from the virus-inoculated allantoic fluid.
Fig. 1.
Preparation of monoglycosylated virus in the embryonated chicken egg. (A) Schematic overview of monoglycosylated split virus vaccine production and viral HA with different glycan states. Viral HAfg, HA with the typical complex type N-glycans; viral HAhm, HA with high mannose type N-glycans; viral HAmg, HA with GlcNAc at its N-glycosylation sites. Models were created with Protein Data Bank (PDB) ID code 3LZG by adding glycan using GlyProt (www.glycosciences.de/modeling/glyprot/php/main.php) and PDB files of lipid bilayer from Lipid Bilayer Membranes for RasMol (https://www.umass.edu/microbio/rasmol/bilayers.htm). The images are displayed using the PyMOL (https://pymol.org/2/) program. (B) Western blot analysis of viral HA in allantoic fluids that were inoculated with different concentrations of kifunensine. (C) Western blot analysis of viral HA in kifunensine-treated allantoic fluids with different concentration of Endo H digestion. In B and C, viral HA was detected by rabbit anti-HA polysera in Western blot analysis.
Characterization of the HAmg on Virus.
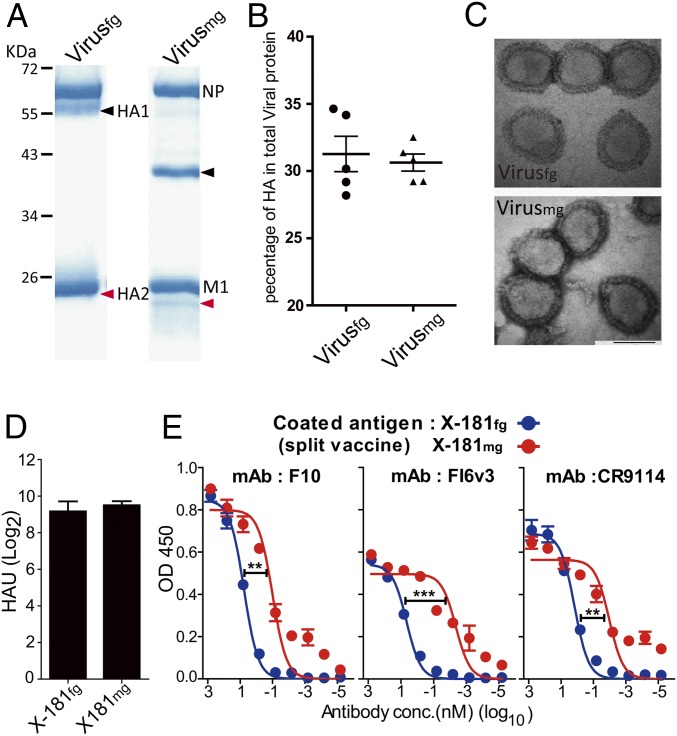
The sucrose gradient-purified viruses were analyzed by SDS/PAGE (Fig. 2A). Compared with fully glycosylated HA1, the band of monoglycosylated viral HA1 shifted to a lower molecular weight, from 55 kDa to 37 kDa. Similarly, the band of fully glycosylated HA2 originally overlapping with matrix protein 1 (M1) now shifted to the lower position (25 kDa) in the monoglycosylated state. The molecular weight of HAmg was similar to the predicted molecular weight of the HA polypeptide chain, implying that the majority of glycans on viral HA have been removed.
Fig. 2.
Characterization of the fully glycosylated and monoglycosylated viruses and split vaccine products. (A) SDS/PAGE analysis of the purified fully glycosylated and monoglycosylated viruses. Arrowheads indicate the viral HA protein (black, HA1; red, HA2). NP, nucleoprotein. (B) Ratio of the amount of HA to total viral proteins of the purified viruses. Proteins deglycosylated by peptide-N-glycosidase F (PNGase F) of the purified viruses were quantified with SDS/PAGE analysis. (C) Transmission electron microscopic images of the fully glycosylated and monoglycosylated viruses. (D) Hemagglutination assay of X-181fg and X-181mg split vaccine. HAU, hemagglutination unit. (E) Binding of FI6v3, F10, and CR9114 to X-181fg or X-181mg split vaccine. *P < 0.05; **P < 0.01; ***P < 0.001. The P value was calculated with Prism software using the Student’s t test and two-way ANOVA. Values are mean ± SEM. conc., concentration.
To evaluate whether the protein composition of the monoglycosylated virus is altered when treated with kifunensine, the HA content of total viral protein was calculated (SI Appendix, Fig. S2). The percentages of HA in total viral proteins have no significant difference between fully glycosylated and monoglycosylated viruses; in either case, they are about 30% of total viral proteins, which is similar to what has been reported previously (16) (Fig. 2B). The result demonstrated that kifunensine treatment does not affect the protein composition of progeny virus in the viral assembly. Images of negatively stained virions taken by transmission electron microscopy also showed that there are no morphological changes between the fully glycosylated and monoglycosylated viruses (Fig. 2C). To confirm that the glycan at each glycosylation site of HAmg on the virus is indeed a single GlcNAc residue, the site-specific glycan analysis was performed by MS (SI Appendix, Table S1). In the glycopeptide analysis of the original fully glycosylated viral HA, the glycans on four of six glycosylation sites are the complex-type glycans and the other two are the high-mannose types. Indeed, after the modification, 99% of glycans at each glycosylation site of viral HAmg contain only a single GlcNAc.
To test whether the antigenicity of viral HA has been altered by changing the N-glycosylation form to mono-GlcNAc, a hemagglutination assay was performed, and the result showed that there is no significant difference in the hemagglutination ability of viral HA between the monoglycosylated vaccine and the fully glycosylated split vaccine (Fig. 2D). To ensure that the epitopes of the stem region exhibit the correct conformation, both of the split vaccines were coated as antigens on ELISA plates and binding with the known broadly neutralizing human monoclonal antibodies F10, FI6v3, and CR9114 was evaluated. The data showed that the binding of these stem-specific antibodies to the monoglycosylated split vaccine is tighter than that of the fully glycosylated ones (Fig. 2E), indicating that the viral HA in the monoglycosylated split vaccine has similar structural integrity of the epitopes, which are recognized by broadly neutralizing anti-HA stem antibodies, and removal of the glycan shields increased binding by these broadly neutralizing antibodies. The results of the hemagglutination assays and ELISA strongly suggest that the monoglycosylation treatment does not disrupt the native conformation and the function of viral HA of the monoglycosylated split vaccine.
The Immune Responses Elicited by Fully Glycosylated X-181, Monoglycosylated X-181, and Trivalent Inactivated Influenza Vaccine Split Virus Vaccines in Mice.
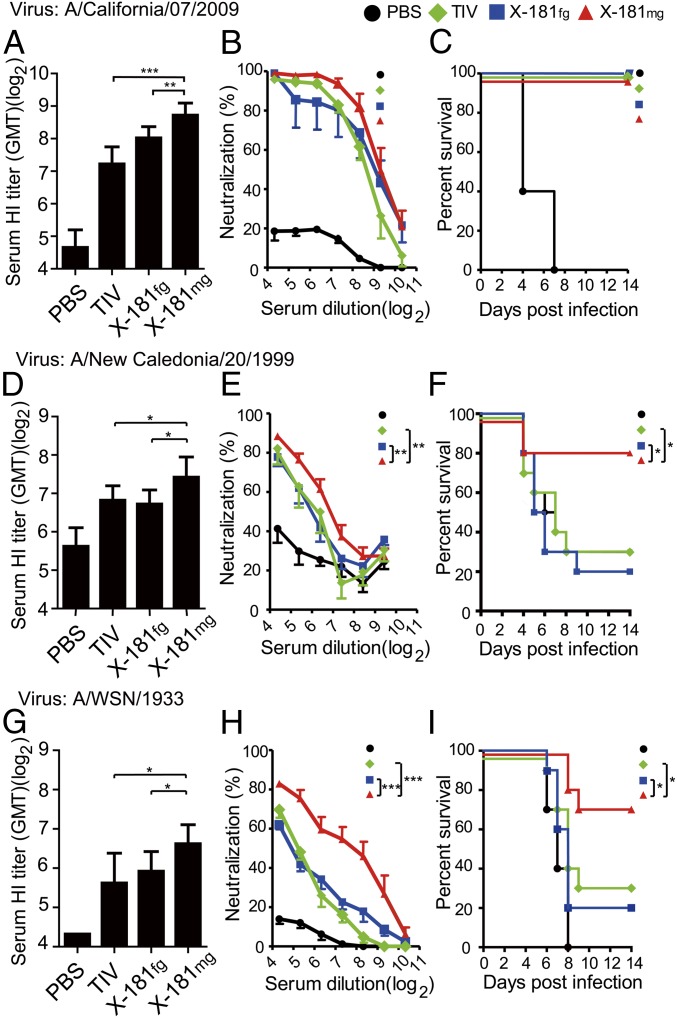
The vaccine strain H1N1 influenza virus [A/reassortant/New York Medical College (NYMC) X-181] was chosen as the seed virus to prepare the fully glycosylated (X-181fg) or monoglycosylated (X-181mg) X-181 virions for producing the X-181fg and X-181mg split virus vaccines, respectively. To ensure that an equal HA content between different vaccines was applied, the HA of (X-181fg and X-181mg) split virus vaccine was quantified by SDS/PAGE (SI Appendix, Fig. S2) and adjusted to 1 μg of HA per dose for immunization. BALB/c mice were immunized twice with X-181fg, X-181mg, or commercially available trivalent inactivated influenza vaccine (TIV) split virus vaccine, the seasonal trivalent flu vaccine also containing X-181, at a 2-wk interval, and mice antisera were collected and tested for hemagglutinin inhibition (HI) and microneutralization assays against the strain-specific [A/California/07/2009 (Cal/09)] and the cross-strain [A/New Caledonia/20/1999 (NC/99) and A/Wilson Smith Neorotropic (WSN)/1933 (WSN/33)] viruses. NC/99 virus was selected as the H1N1 vaccine strain in the year 2000, which exhibits more than 97% sequence identity to the vaccine strains from 1999 to 2008 and shares cross-reactivity of HI with the other vaccine strains (17). WSN/33 is a mouse-adapted human H1N1 influenza strain that is widely used in influenza animal experiments (18). Both of them have low sequence identity (NC/99 = 80%, WSN/33 = 82%) to the 2009 H1N1 pandemic-like strain NYMC-X-181. Compared with X-181fg and TIV, the X-181mg antisera were found to have the highest HI titer (≥100) against all three viruses (Fig. 3 A, D, and G). In addition, the X-181mg antisera provided significantly better neutralization ability against the cross-strain viruses (Fig. 3 B, E, and H). To examine whether X-181mg vaccination provides cross-protection against different H1N1 viruses, the vaccinated mice were intranasally inoculated with lethal doses of Cal/09, NC/99, and WSN/33 viruses, and the vaccine efficacy was monitored for 14 d based on body weight loss and survival (Fig. 3 C, F, and I). In the strain-specific virus challenge study, all three split virus vaccines showed good protection (100%) against Cal/09 viruses. The TIV split virus vaccine only offered 30% protection against NC/99 and WSN/33 challenges. The X-181fg split virus vaccine has similar low protection against these two viruses. Notably, X-181mg split virus vaccine offered more than 70% protection against both cross-strain viruses, NC/99 and WSN/33 (Fig. 3 F and I). These data suggested that vaccination of the monoglycosylated split virus induces better antibody responses and provides better protection against cross-strain influenza challenges. The virus challenge assays were also performed with two additional influenza vaccine strains A/Michigan (MC)/45/2015 and A/Solomon Islands (SI)/3/2006, of which the HA sequence identity to X-181 HA is 96% and 80%, respectively. Compared with TIV and X-181fg split vaccine, the monoglycosylated split vaccine provided comparable protection (100%) against A/MC/45/2015, and better protection (around 60% protection) against A/SI/3/2006 (SI Appendix, Fig. S3). Also, the X-181mg–vaccinated mice contained significantly lower A/SI/3/2006 virus in their lungs compared with that in X-181fg– or TIV-vaccinated mice (SI Appendix, Fig. S4). The mouse weight changes of all of the virus challenges were also recorded (SI Appendix, Fig. S5). During the virus challenges, the X-181mg–vaccinated mice showed lower or comparable body weight loss compared with other vaccination groups. In addition, when H5N1 (NIBRG-14) was used in the neutralization study, X-181mg vaccine induced better neutralization activity (SI Appendix, Fig. S6).
Fig. 3.
Protection against Cal/09, NC/99, and WSN/33 viruses with monoglycosylated split virus vaccine. X-181 split virus vaccines (X-181fg and X-181mg) and commercial TIV were used as vaccines. The sera of vaccinated mice were assayed by HI and microneutralization (MN) against X-181 virus (A and B), NC/99 virus (D and E), and WSN/33 virus (G and H), respectively. For virus challenge, the immunized mice were inoculated with a lethal dose of X-181 (10-fold LD50) (C), NC/99 (2.5-fold LD50) (F) and WSN/33 (10-fold LD50) (I) viruses, and the efficacy was evaluated by survival over 14 d. GMT, geometric mean titer. *P < 0.05; **P < 0.01; ***P < 0.001. The P value of HI and MN was calculated with Prism software using the Student’s t test and two-way ANOVA. The statistical significances of mice survival data were determined using log-rank tests.
Vaccination with Monoglycosylated Split Virus Vaccine Induces More Stem-Specific Antibody and Increased ADCC Activity.
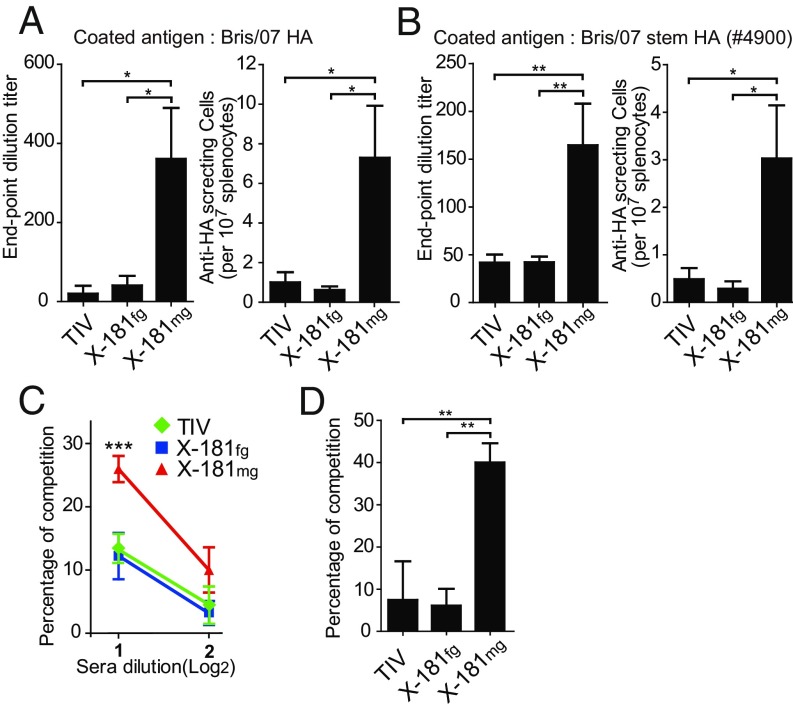
To analyze whether the better efficacy of the monoglycosylated split virus vaccine in cross-strain protection comes from cross-reactive antibodies, the activity of antibodies and antibody-secreting splenocytes from immunized mice to recognize cross-strain A/Brisbane/59/2007 (Bris/07) HA was estimated by ELISA and enzyme-linked immune absorbent spot (ELISpot) assays. Results of ELISA showed that sera from X-181mg–vaccinated mice contain significantly higher amounts of cross-reactive HA-specific antibodies (Fig. 4A). Results of ELISpot analysis also demonstrated that cross-reactive antibody-secreting splenocytes are considerably increased after X-181mg vaccination (Fig. 4A).
Fig. 4.
More cross-strain immune responses elicited by vaccination with monoglycosylated split virus vaccine. (A) ELISA and ELISpot of antibody bindings to a cross-strain Brisbane (Bris/07) HA. (B) ELISA and ELISpot of antibody bindings to stem-HA. (C) Competition ELISA binding to Brisbane HA with the biotinylated F10 antibody. The percentage of competition showed the amount of anti-HA stem antibodies in immunized sera. (D) Alternative competition ELISA binding to Brisbane HA with or without F10 preblocking. The percentage of competition in the alternative competition ELISA showed the ratio of anti-HA stem antibodies (F10-competitive antibodies) to total HA-specific antibodies in immunized sera. *P < 0.05; **P < 0.01; ***P < 0.001. The P value was calculated with Prism software using the Student’s t test. Values are mean ± SEM.
The stem region of HA (stem HA) has been shown by recent studies to be the major target recognized by broadly neutralizing antibodies (19–21). To evaluate whether the amount of anti-HA stem antibodies is different after X-181mg vaccination, stem HA #4900 (22) was employed as the antigen to analyze the amount of stem-specific antibody in the immunized sera and of stem-specific antibody-secreting splenocytes by ELISA and ELISpot, respectively (Fig. 4B). In addition, HA stem-specific competition ELISA was performed to evaluate the contents of anti-HA stem antibodies in mice sera that compete with stem-binding antibody F10 (21). Antisera of X-181mg–vaccinated mice had significantly higher competitiveness against F10 than that of X-181fg and TIV (Fig. 4C and SI Appendix, Fig. S7). These data indicate that X-181mg split virus vaccine-immunized mice induced more stem-specific antibody and antibody-secreting splenocytes. Next, to further evaluate whether the increase of the total amount of anti-HA stem antibodies also altered the ratio of those antibodies to the total antibody in the sera, the ratio of stem-specific antibodies was determined by the alternative competition ELISA analysis (Fig. 4D and SI Appendix, Fig. S7). The HA was blocked by F10 antibody, and the mice antisera were then added to interact with the remaining binding sites. By comparing the ELISA results of antisera against F10 preblocked or free HA, we can evaluate the ratio of anti-HA stem antibodies to total HA-specific antibodies in the antisera. The results showed that the HA binding of X-181mg antisera was reduced about 40% with F10 preblocking, which is much higher than that of X-181fg and TIV antisera, both with nearly a 10% reduction (Fig. 4D). The reduction of HA binding indicates that X-181mg antisera contain more antistem antibodies (F10-competitive antibody) in the total antibody compared with X-181fg and TIV antisera. Alternatively, a similar result was shown in chimeric HA ELISA. The chimeric HA, which had an H5 head (48% identity with the H1 head region) and H1 stem region, and H1N1 HA were used for analysis of stem-specific antibody in immunized mice sera (SI Appendix, Fig. S8). The result of the chimeric HA binding assay also agreed with the result of alternative competition ELISA to show that sera from X-181mg–vaccinated mice contain more stem-specific antibodies in the total HA-specific antibody. The results of the different competition and binding experiments strongly suggested that X-181mg vaccination can induce not only a larger amount but also a higher ratio of anti-HA stem antibodies against the stem region of HA.
Antibody-mediated protection against virus infection has been traditionally emphasized the neutralization ability of virus; however, recent investigations have also shown that Fc receptor-mediated antiviral activities of antibodies are also important for viral clearance and provide broader protection against HIV or influenza virus infections (23–27). Furthermore, studies showed that the broad protection of HA stem-specific antibody is partially contributed to by the Fc-mediated immune response (20, 28, 29). Here, the ADCC assay was performed to analyze the Fc receptor-mediated immune response that is induced by monoglycosylated split virus vaccine immunization. To evaluate the ADCC activity of monoglycosylated split virus vaccine-immunized sera, serially diluted antisera from X-181mg–, X-181fg–, or TIV-vaccinated mice were incubated with mouse peripheral blood mononuclear cells (effector cells) and Bris/07 or Cal/09 HA-expressed HEK293 cells (target cells), mimics of Bris/07 and Cal/09 virus-infected cells, respectively. The X-181mg–vaccinated antisera exhibited modest yet statistically significant higher ADCC activity in both 80- and 40-fold dilutions compared with X-181fg (Fig. 5). This result indicated that the antibodies induced by X-181mg vaccination possess better ADCC activity against both strain-specific and cross-strain HA-expressing cells. The broader protection provided by the monoglycosylated split virus vaccine immunization may include contributions by more HA stem-specific antibodies and better ADCC activity against influenza virus-infected cells.
Fig. 5.
ADCC assay of immunized sera against HA-expressed HEK cells. ADCC-mediated antibodies of X-181fg–, X-181mg–, and TIV-vaccinated sera were detected by ADCC assay using Bris/07 (A) or Cal/09 (B) HA protein expressing HEK293T cells as target cells. The cytotoxicity was measured and indicated the ADCC effect of vaccinated mice sera. *P < 0.05; **P < 0.01. The P value was calculated with Prism software using the Student’s t test. Values are mean ± SEM.
Discussion
The glycosylation pattern of influenza HA protein has been shown to play an important role in modulating immune responses to infection (30). The mice vaccinated with the previous seasonal H1N1 strains whose HA has more glycosylation sites failed to withstand 2009 H1N1 pandemic virus infection (31). When recombinantly adding more glycosylation sites onto HA, vaccination with the mutant strain cannot induce an effective immune response to protect the infected mice against the original virus strain (32–34). In contrast, if the number of glycosylation sites on HA is reduced, the vaccination elicits an immune response with broader HI or neutralization ability against strain-specific or cross-strain infection (32, 34–36). These studies suggest that the removal of the glycan mask on influenza virus may be a good simple strategy in designing a better flu vaccine. However, the removal of all glycans from HA by genetic mutation may cause improper structure folding (37–39), and the proper HA structure is important for inducing a good immune response against influenza virus infection (11). Also, due to a preferred low-pH requirement, the deglycosylation treatments by some endoglycosidases can trigger irreversible conformational changes of HA, which may have a detrimental effect on the maintenance of the structural integrity of HA (40–42), and a weak immune response has been demonstrated by immunization of low pH-treated whole-virus particles (43). In this study, we used Endo H to digest the kifunensine-treated influenza virus in the egg allantoic fluid without altering the pH for monoglycosylated influenza split virus vaccine production. We showed that there is no significant difference in the epitope integrity of the stem region and hemagglutination ability between the monoglycosylated split vaccine and the wild-type fully glycosylated split virus vaccine (Fig. 2 D and E). Furthermore, the first GlcNAc of N-glycosylation is the most important glycan, which provides most of the energy to stabilize the native state of the protein (44). The HAmg in the current study is thus a rational design to trim down the glycan on HA to monoglycosylated GlcNAc for removing the glycan mask of HA without affecting the structural integrity.
The stem region of influenza HA has been considered the most conserved antigen to induce broadly neutralizing antibodies against different influenza strains (19–21, 45). However, the stem-specific antibodies were not easily induced by vaccination or viral infection in either humans or mice (46, 47), and this may be due to the glycan mask around the stem region. Without the protection of the glycan shields, the epitopes of the HA stem region can be exposed to professional antigen-presenting cells, such as naive B cells and dendritic cells, which then can be processed and presented for B cell maturation. We speculate that this may be the underlying mechanism that resulted in the higher antistem-specific antibodies in antisera from monoglycosylated split virus vaccine-immunized mice.
Influenza-specific ADCC of isolated human peripheral blood leukocytes was described 40 y ago (26). Recent studies in mice have shown that ADCC is necessary for in vivo protection against influenza virus infection, and the stem-specific antibody not only had the neutralizing ability but also contributed to ADCC activity (20, 28, 29). In this study, we showed that vaccination with monoglycosylated split virus vaccine induced better influenza-specific ADCC activity (Fig. 5), and this may partly come from the increase of the antistem antibodies in monoglycosylated split virus vaccine-immunized mice. Moreover, ADCC activity can be induced by the broad binding antibodies against the HA globular region (29). Vaccination with the monoglycosylated split virus vaccine may also elicit more diverse antibodies by exposing new epitopes on the HA globular region. The enhancement in ADCC activity from the sera of monoglycosylated split virus vaccine-immunized mice may come from these newly generated antistem or broad binding antibodies.
Although innovative approaches of influenza vaccine research to increase the spectrum of protection have been reported, some of which however are of drastic change from the current form of influenza vaccine. In this work, we have devised a simple method to minimally modify the procedure used in industrial egg-based virus production. This modified vaccine may thus have a similar safety profile, as it is virtually identical to the current influenza vaccine that has been used in humans since 1945, but with the exception of having the simplified glycans. With the monoglycosylated glycan modification, the split virus influenza vaccine can provide stronger immune responses and broader protection against various H1N1 strains. The broader immunity of this influenza split virus vaccine could reduce the requirement of vaccine reformulation and avoid the economic loss and time-consuming process caused by yearly reformulation. It is also noted that the deglycosylation procedure used in this study may cause the removal of glycans from the surface NA, and how the monoglycosylated NA affects the immune response was not investigated in this study. In summary, the monoglycosylation of split influenza virus vaccine is a strategy for approaching universal vaccine design, and its production can also be easily adapted to industrial vaccine production.
Materials and Methods
The vaccine strains of H1N1 influenza viruses A/California/7/2009 NYMC X-181 and A/MC/45/2015 were obtained from the Adimmune Corporation. A/WSN/33, A/NC/99, and A/SI/3/2006 were from the UK National Institute for Biological Standards and Control. All viruses were inoculated into the allantoic cavities of 10-d-old specific pathogen-free embryonated chicken eggs at 35 °C for 48 h. Harvested allantoic fluid was aliquoted and stored at −80 °C. The 50% tissue culture infective dose of viruses in Madin-Darby canine kidney (CCL-34; American Type Culture Collection) cells and LD50 of virus in BALB/c mice were determined before experiments.
All animal experiments were performed in accordance with the guidelines established by the Institutional Animal Care and Use Committee of Academia Sinica (approval no. 11-03-147).
More details about the experimental materials and methods of this study are provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank Chien-Hung Chen for glycopeptide liquid chromatography MS/MS analysis; Dr. Ting-Jen R. Cheng, Hsin-Yu Liao, and Shih-Chi Wang for providing critical reagents; Shih-Hsin Huang, Hsiu-Hua Ma, Yi-Hsin Yeh, Lin-Yun Wei, Chan-Kun Chang and Yen Chu Lin for technical assistance; and Academia Sinica Genomics Research Center Summit Project and Taiwan Ministry of Science and Technology Grant 105-2325-B-001-007 (to C.M.) for financial support.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1819197116/-/DCSupplemental.
References
- 1.Taubenberger JK, Morens DM. 1918 influenza: The mother of all pandemics. Emerg Infect Dis. 2006;12:15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krammer F, Palese P. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov. 2015;14:167–182. doi: 10.1038/nrd4529. [DOI] [PubMed] [Google Scholar]
- 3.Wong SS, Webby RJ. Traditional and new influenza vaccines. Clin Microbiol Rev. 2013;26:476–492. doi: 10.1128/CMR.00097-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tosh PK, Jacobson RM, Poland GA. Influenza vaccines: From surveillance through production to protection. Mayo Clin Proc. 2010;85:257–273. doi: 10.4065/mcp.2009.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dos Santos G, Neumeier E, Bekkat-Berkani R. Influenza: Can we cope better with the unpredictable? Hum Vaccin Immunother. 2016;12:699–708. doi: 10.1080/21645515.2015.1086047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei CJ, et al. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science. 2010;329:1060–1064. doi: 10.1126/science.1192517. [DOI] [PubMed] [Google Scholar]
- 7.Hai R, et al. Influenza viruses expressing chimeric hemagglutinins: Globular head and stalk domains derived from different subtypes. J Virol. 2012;86:5774–5781. doi: 10.1128/JVI.00137-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yassine HM, et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat Med. 2015;21:1065–1070. doi: 10.1038/nm.3927. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe S, Watanabe T, Kawaoka Y. Influenza A virus lacking M2 protein as a live attenuated vaccine. J Virol. 2009;83:5947–5950. doi: 10.1128/JVI.00450-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu CY, et al. Influenza A surface glycosylation and vaccine design. Proc Natl Acad Sci USA. 2017;114:280–285. doi: 10.1073/pnas.1617174114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JR, et al. Vaccination of monoglycosylated hemagglutinin induces cross-strain protection against influenza virus infections. Proc Natl Acad Sci USA. 2014;111:2476–2481. doi: 10.1073/pnas.1323954111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elbein AD, Tropea JE, Mitchell M, Kaushal GP. Kifunensine, a potent inhibitor of the glycoprotein processing mannosidase I. J Biol Chem. 1990;265:15599–15605. [PubMed] [Google Scholar]
- 13.Chang VT, et al. Glycoprotein structural genomics: Solving the glycosylation problem. Structure. 2007;15:267–273. doi: 10.1016/j.str.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esko JD, Bertozzi CR. Chemical tools for inhibiting glycosylation. In: Varki A, et al., editors. Essentials of Glycobiology. 2nd Ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2009. [PubMed] [Google Scholar]
- 15.Tarentino AL, Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974;249:811–817. [PubMed] [Google Scholar]
- 16.Skehel JJ, Waterfield MD. Studies on the primary structure of the influenza virus hemagglutinin. Proc Natl Acad Sci USA. 1975;72:93–97. doi: 10.1073/pnas.72.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang SS, et al. Immunity toward H1N1 influenza hemagglutinin of historical and contemporary strains suggests protection and vaccine failure. Sci Rep. 2013;3:1698. doi: 10.1038/srep01698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thangavel RR, Bouvier NM. Animal models for influenza virus pathogenesis, transmission, and immunology. J Immunol Methods. 2014;410:60–79. doi: 10.1016/j.jim.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dreyfus C, et al. Highly conserved protective epitopes on influenza B viruses. Science. 2012;337:1343–1348. doi: 10.1126/science.1222908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corti D, et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333:850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 21.Sui J, et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009;16:265–273. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Impagliazzo A, et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science. 2015;349:1301–1306. doi: 10.1126/science.aac7263. [DOI] [PubMed] [Google Scholar]
- 23.El Bakkouri K, et al. Universal vaccine based on ectodomain of matrix protein 2 of influenza A: Fc receptors and alveolar macrophages mediate protection. J Immunol. 2011;186:1022–1031. doi: 10.4049/jimmunol.0902147. [DOI] [PubMed] [Google Scholar]
- 24.Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001;166:7381–7388. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- 25.Jayasekera JP, Moseman EA, Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol. 2007;81:3487–3494. doi: 10.1128/JVI.02128-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanderven HA, Jegaskanda S, Wheatley AK, Kent SJ. Antibody-dependent cellular cytotoxicity and influenza virus. Curr Opin Virol. 2017;22:89–96. doi: 10.1016/j.coviro.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto G, Wright PF, Karzon DT. Antibody-dependent cell-mediated cytotoxicity against influenza virus-infected cells. J Infect Dis. 1983;148:785–794. doi: 10.1093/infdis/148.5.785. [DOI] [PubMed] [Google Scholar]
- 28.DiLillo DJ, Tan GS, Palese P, Ravetch JV. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo. Nat Med. 2014;20:143–151. doi: 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiLillo DJ, Palese P, Wilson PC, Ravetch JV. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J Clin Invest. 2016;126:605–610. doi: 10.1172/JCI84428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tate MD, et al. Playing hide and seek: How glycosylation of the influenza virus hemagglutinin can modulate the immune response to infection. Viruses. 2014;6:1294–1316. doi: 10.3390/v6031294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei CJ, et al. Cross-neutralization of 1918 and 2009 influenza viruses: Role of glycans in viral evolution and vaccine design. Sci Transl Med. 2010;2:24ra21. doi: 10.1126/scitranslmed.3000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medina RA, et al. Glycosylations in the globular head of the hemagglutinin protein modulate the virulence and antigenic properties of the H1N1 influenza viruses. Sci Transl Med. 2013;5:187ra70. doi: 10.1126/scitranslmed.3005996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wanzeck K, Boyd KL, McCullers JA. Glycan shielding of the influenza virus hemagglutinin contributes to immunopathology in mice. Am J Respir Crit Care Med. 2011;183:767–773. doi: 10.1164/rccm.201007-1184OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun X, et al. N-linked glycosylation of the hemagglutinin protein influences virulence and antigenicity of the 1918 pandemic and seasonal H1N1 influenza A viruses. J Virol. 2013;87:8756–8766. doi: 10.1128/JVI.00593-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, et al. Hemagglutinin glycosylation modulates the pathogenicity and antigenicity of the H5N1 avian influenza virus. Vet Microbiol. 2015;175:244–256. doi: 10.1016/j.vetmic.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Liu WC, Jan JT, Huang YJ, Chen TH, Wu SC. Unmasking stem-specific neutralizing epitopes by abolishing N-linked glycosylation sites of influenza virus hemagglutinin proteins for vaccine design. J Virol. 2016;90:8496–8508. doi: 10.1128/JVI.00880-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao HY, et al. Differential receptor binding affinities of influenza hemagglutinins on glycan arrays. J Am Chem Soc. 2010;132:14849–14856. doi: 10.1021/ja104657b. [DOI] [PubMed] [Google Scholar]
- 38.Gallagher PJ, Henneberry JM, Sambrook JF, Gething MJ. Glycosylation requirements for intracellular transport and function of the hemagglutinin of influenza virus. J Virol. 1992;66:7136–7145. doi: 10.1128/jvi.66.12.7136-7145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts PC, Garten W, Klenk HD. Role of conserved glycosylation sites in maturation and transport of influenza A virus hemagglutinin. J Virol. 1993;67:3048–3060. doi: 10.1128/jvi.67.6.3048-3060.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bullough PA, Hughson FM, Skehel JJ, Wiley DC. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 41.Skehel JJ, et al. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc Natl Acad Sci USA. 1982;79:968–972. doi: 10.1073/pnas.79.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freeze HH, Kranz C. Endoglycosidase and glycoamidase release of N-linked glycans. Curr Protoc Mol Biol. 2010;Chapter 17:Unit 17.13A. doi: 10.1002/0471142727.mb1713as89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quan FS, et al. Immunogenicity of low-pH treated whole viral influenza vaccine. Virology. 2011;417:196–202. doi: 10.1016/j.virol.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanson SR, et al. The core trisaccharide of an N-linked glycoprotein intrinsically accelerates folding and enhances stability. Proc Natl Acad Sci USA. 2009;106:3131–3136. doi: 10.1073/pnas.0810318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Throsby M, et al. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One. 2008;3:e3942. doi: 10.1371/journal.pone.0003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krammer F, Palese P. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol. 2013;3:521–530. doi: 10.1016/j.coviro.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan HX, et al. Subdominance and poor intrinsic immunogenicity limit humoral immunity targeting influenza HA-stem. J Clin Invest. December 6, 2018 doi: 10.1172/JCI123366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.