Abstract
Aims
Nuclear factor kappa light chain enhancer of activated B cells (NFkB) is a ubiquitous transcription factor well known for its role in the innate immune response. As such, NFkB is a transcriptional activator of inflammatory mediators such as cytokines. It has recently been demonstrated that alcohol and other drugs of abuse can induce NFkB activity and cytokine expression in the brain. A number of reviews have been published highlighting this effect of alcohol, and have linked increased NFkB function to neuroimmune-stimulated toxicity. However, in this review we focus on the potentially non-immune functions of NFkB as possible links between NFkB and addiction.
Methods
An extensive review of the literature via Pubmed searches was used to assess the current state of the field.
Results
NFkB can induce the expression of a diverse set of gene targets besides inflammatory mediators, some of which are involved in addictive processes, such as opioid receptors and neuropeptides. NFkB mediates complex behaviors including learning and memory, stress responses, anhedonia and drug reward, processes that may lie outside the role of NFkB in the classic neuroimmune response.
Conclusions
Future studies should focus on these non-immune functions of NFkB signaling and their association with addiction-related processes.
INTRODUCTION
Nuclear factor kappa light chain enhancer of activated B cells (NFkB) is a ubiquitous transcription factor with varied roles within the mammalian cell. While best known for its regulation of inflammation and innate immunity, NFkB has a wide range of gene targets and can influence complex behaviors such as learning and memory, addiction and depression. This review will highlight these diverse functions, and draw links between specific gene targets and the development of addictive-like behaviors. Some excellent reviews on similar topics have been published, but these have focused primarily on the neuroimmune response as a causal link between NFkB activation and substance abuse (Crews and Vetreno, 2011; Crews et al., 2011). This review will highlight the role of NFkB from a different angle. Specifically, we will put forth the idea that even though NFkB is involved in inflammation, this does not necessarily mean that NFkB is involved in addiction solely via the activation of inflammatory mediators. We will describe the role of NFkB in behavioral, cellular and molecular processes that may be mediated by gene targets not related to the innate immune response. While various drugs of abuse will be mentioned, the primary focus of this review will be on the role of NFkB in alcohol use disorders.
NFkB: HISTORY AND REGULATORY PROCESSES
Many excellent reviews on NFkB, its regulation and molecular effects have been published over the last several years (Neumann and Naumann, 2007; Oeckinghaus and Ghosh, 2009). These topics will be described briefly here, but the reader is referred to these reviews for more in-depth information concerning the general functions of this transcription factor.
NFkB, first identified by Sen and Baltimore in the 1980s (Sen and Baltimore, 1986), is widely expressed in many mammalian cell types and its binding elements are found in a vast range of genes with diverse functions. NFkB is most well known for its role in innate immunity and has gained much attention for being stimulated by inflammatory mediators such as cytokines and receptors that respond to bacterial infection, such as the toll-like receptors (TLRs). There are a number of subunits that can comprise a transcription factor dimer of the NFkB family, including p65, RelB, c-Rel, p50 and p52 (Oeckinghaus and Ghosh, 2009). These subunits form heterodimer and homodimer complexes, resulting in up to 15 dimers with varying functions within the cell. Each of the NFkB subunits contains a Rel homology domain, a conserved sequence 300 amino acids in length. The two most common subunits are p65 (RelA gene) and p50 (NFkB1 gene). Generally, p65/p50 heterodimers are thought to trigger active transcription while p50/p50 homodimers act as transcriptional repressors.
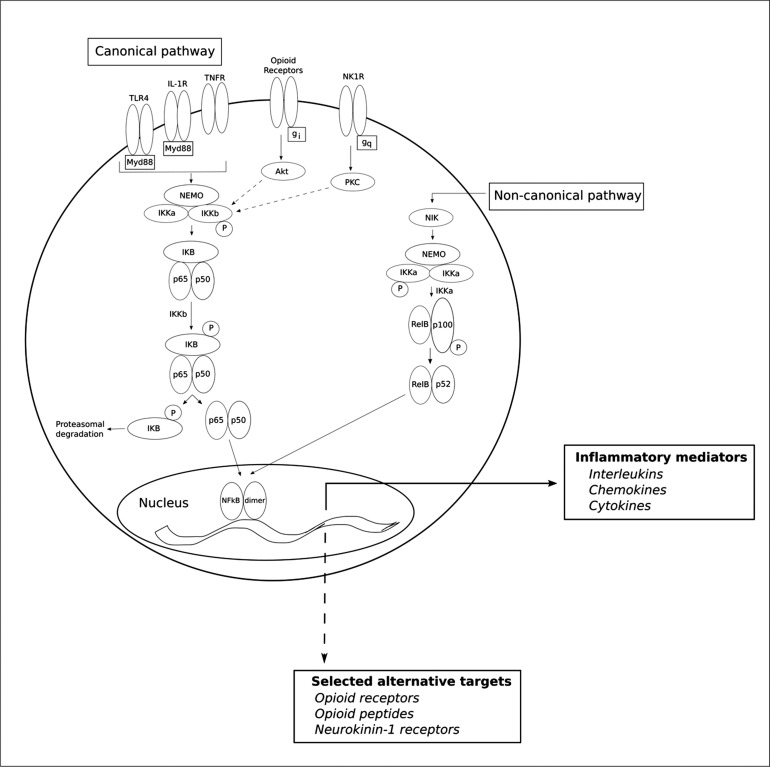
Under baseline conditions, NFkB subunit dimers are bound in the cytosol by an inhibitory protein known as inhibitor of NFkB (IkB). IkB kinase (IKK) complex consists of two catalytic subunits (IKKα and IKKβ) and a regulatory subunit (NEMO). When activated, IKK phosphorylates IkB, thus tagging it for proteasomal degradation and freeing the NFkB subunit dimers. TLR4, TNF-α receptor (TNFR) and interleukin-1 receptor (IL-1R) are known to stimulate NFkB through this pathway, as activation of these receptors leads to phosphorylation of IKK (Gerondakis et al., 2014), and this process is mediated by the adapter protein MyD88 (Laird et al., 2009). This pathway is considered the classical, or canonical, pathway of NFkB. In the alternative, or non-canonical, pathway, NFkB-inducing kinase (NIK) activates IKKα, which in turn phosphorylates p100, the precursor protein of p52. p100 is then processed into p52/RelB heterodimers (Gerondakis et al., 2014). After freeing from the IkB complex, NFkB dimers translocate to the nucleus where they bind to specific DNA sequences in the promoter region of a wide array of genes (Hayden and Ghosh, 2004; Oeckinghaus and Ghosh, 2009). Most prominently, NFkB stimulation triggers the expression of inflammatory mediators including cytokines, chemokines and cell adhesion molecules, thus creating a positive feedback loop (see Fig. 1 for schematic of NFkB regulation).
Fig. 1.
NFkB activation mediates both inflammatory mediators and selected alternative targets. Activation of NFkB via the canonical and non-canonical pathways. After freeing from the IkB complex, NFkB dimers translocate to the nucleus and bind to specific DNA sequences within the promoter region of vast array of genes. These targets include genes involved in the inflammatory response; however, this review will focus on select targets not related to inflammation. The gene targets discussed here include opioid receptors, opioid peptides and neurokinin-1 receptors.
Other cell-surface receptors can serve as potential activators of NFkB including opioid receptors and the neurokinin-1 receptor (NK1R; Lieb et al., 1997; Wang et al., 2004; Sun et al., 2008; Sawaya et al., 2009; Xu et al., 2011). Opioid receptors are G protein-coupled receptors that are linked to inhibitory G-proteins (Gi). Chemokine activation of receptors coupled to the inhibitory Gi subunit has been found to activate NFkB through phosphorylation of protein kinase B (Akt) (Chandrasekar et al., 2003). Akt is known to mediate IKK phosphorylation and subsequent disinhibition of the p50/p65 dimer (Bai et al., 2009), evidence that may link the Gi-coupled opioid receptors to this pathway. The NK1R is coupled to stimulatory Gq subunits that activate protein kinase C (PKC). In vitro, PKC induces NFkB activation and translocation to the nucleus, potentially linking activation of these receptors to the NFkB pathway (Shirakawa and Mizel, 1989).
NFkB AND INFLAMMATION IN ALCOHOL ABUSE
Much research has focused on the role of NFkB in alcohol-induced neurotoxicity and inflammation. This is reviewed elsewhere and will be briefly discussed in this space. For example, high concentrations of alcohol increase NFkB activity in human astroglial cultures (Davis and Syapin, 2004) and rat brain hippocampal slices (Zou and Crews, 2010). Furthermore, chronic binge alcohol exposure increases cytokine expression and enhances the immune response to lipopolysaccharide (LPS) injection in rodents (Qin et al., 2008). There is evidence to suggest that ethanol-induced NFkB activation mediates neurotoxicity that is caused by in vivo binge models (Crews et al., 2006). However, little is known about the effect of lower, non-neurotoxic doses of alcohol on NFkB function. TLR4 is also known to play a critical role in the neuroimmune response and consequent NFkB activation induced by ethanol. TLR4s have been shown to directly influence alcohol-induced inflammation, neurotoxicity and some behavioral effects (Alfonso-Loeches et al., 2010; Pascual et al., 2011; Wu et al., 2012). This signaling pathway plays a critical role in the production of cytokines and pro-inflammatory mediators induced by ethanol, as evidenced in binge-exposed adolescent mice (Montesinos et al., 2015).
There are some interesting findings on sex differences of NFkB activity, with most focusing on hepatic activity. In one study, ovariectomized female rats receiving constant estrogen administration had decreased hepatic IKB mRNA expression and increased NFkB activity following alcohol consumption compared with controls and rats receiving testosterone (Lee et al., 2012). In another study, chronic ethanol administration was found to increase hepatic levels of NFkB in female rats to a greater extent than males, a finding that may explain why alcohol-induced liver injuries are more prevalent in the females (Kono et al., 2000). These differences may be due to a sexually dimorphic NFkB signaling response found by Wilhelm and colleagues during peak ethanol withdrawal (Wilhelm et al., 2014).
In addition to these preclinical findings, NFkB dysfunction has been observed in postmortem samples from chronic alcoholics (Okvist et al., 2007). Moreover, polymorphisms in the NFkB1 gene associate with increased risk of alcoholism (Edenberg et al., 2008). It is unclear if alterations in NFkB activity and related signaling cascades are induced by genetic factors that predispose individuals to alcohol abuse, if chronic alcohol exposure impacts NFkB functioning, or both. These relationships should be examined in future studies and will provide valuable mechanistic insight for therapeutic development.
The prevailing wisdom suggests that NFkB contributes to alcohol seeking via the stimulation of inflammatory mediator gene expression. While it seems likely that inflammatory cytokines contribute in some capacity to the induction of compulsive alcohol consumption, it should not be overlooked that NFkB has many targets beyond these, such as opioid receptors, glutamate receptors, and neuropeptides, which can mediate alcohol seeking. LPS is often used as a stimulus to induce NFkB activity, and this treatment can increase alcohol consumption. Specifically, a single LPS injection induces a long lasting increase in alcohol intake in the two bottle choice paradigm (Blednov et al., 2011). While LPS treatment is known to both increase cytokine production and result in increased alcohol consumption, it has not been definitively demonstrated that LPS-induced escalations in alcohol intake are specifically mediated by cytokines themselves.
DIVERSE ROLES AND GENE TARGETS OF NFkB
In the sections that follow we will describe the role of NFkB in the following processes that influence the development of addiction: drug reward, emotional stress/anhedonia and learning/memory. Within each section, we will present evidence for the involvement of NFkB in these behaviors. In a fourth section under this heading, we will present potential gene targets that contain NFkB responsive elements and mediate the behavioral processes discussed. After examining the multitude of NFkB gene targets, we decided to focus on a few candidates that have strong evidence of NFkB activation, are known to be related to addiction, and are not primarily thought of as inflammation-related transcripts.
NFkB in drug reward and response to chronic drug exposure
NFkB activity can be induced by environmental stimuli not directly related to innate immunity, such as drugs of abuse. Because NFkB activity is induced by drugs of abuse and NFkB-regulated genes influence drug seeking, NFkB may be a critical cellular mediator of the neuroadaptations that are induced following long-term exposure to drugs. For example, expression and/or phosphorylation of NFkB subunits, and functional activity of NFkB, is upregulated by chronic cocaine administration in vivo (Ang et al., 2001; Russo et al., 2009). In addition, chronic morphine and other µ-opioid receptor (MOR) agonists increase NFkB function in vitro (Hou et al., 1996; Wang et al., 2004; Sawaya et al., 2009), and influence the phosphorylation state of NFkB subunits in vivo (Zhang et al., 2011).
While it has been recognized that chronic drug exposure activates NFkB for some time, more recent evidence has indicated a functional role of NFkB in the rewarding properties of drugs of abuse. For example, NFkB inhibition in the nucleus accumbens attenuates the development of morphine conditioned place preference in rats (Zhang et al., 2011). Furthermore, Russo et al. (2009) demonstrated that knockdown of NFkB activity using viral vectors decreased sensitivity to the rewarding properties of cocaine and attenuated the reward-sensitizing effects of repeated cocaine injection. In addition to influencing the rewarding effects of these drugs, NFkB also mediates withdrawal from chronic drug administration, at least for opiates. Specifically, NFkB inhibitors attenuate precipitated withdrawal behavior (using naloxone administration) in rodents chronically injected with morphine (Rehni et al., 2008). Similar results were observed in an in vitro model which measured the contraction of guinea pig isolated ileum (Capasso, 2001).
As outlined above, the evidence for the stimulation of NFkB by alcohol exposure is extensive. For example, Crews and colleagues have shown in a series of studies that chronic alcohol administration can trigger the activity of NFkB, as evidenced by gel shift assays as well as quantitative PCR for NFkB target genes (Crews et al., 2006; Qin et al., 2008; Zou and Crews, 2010). Furthermore, the induction of NFkB activity by a single injection of LPS induces a long lasting (weeks to months) increase in voluntary alcohol consumption in mice (Blednov et al., 2011). This is quite intriguing, as it is unlikely that inflammatory processes actively continue for weeks following stimulation by LPS.
NFkB in response to emotional stressors and anhedonia
Stress can be a major driver of drug intake and can trigger relapse to drug seeking in abstinent addicts. Additionally, alterations in the hedonic set point which are induced by chronic drug administration or emotional stressors contribute to escalating drug intake. The rapid response of NFkB to immune and cellular stress has been well documented. However, an emerging literature has demonstrated a significant role of NFkB in response to emotional stressors as well.
Multiple studies by Russo and colleagues have demonstrated a role of NFkB activity in the susceptibility to depressive-like symptoms following exposure to chronic social defeat stress. These effects have been demonstrated through manipulation of NFkB activity in the ventral striatum using viral vector strategies (Christoffel et al., 2011, 2012). Specifically, this group demonstrated that viral vector-driven knockdown of NFkB activity attenuates the behavioral response to chronic social defeat stress, and conversely that upregulation of NFkB function increases sensitivity to a subthreshold exposure to this stressor. NFkB can also mediate neurological responses to acute stressors. For example, inhibition of NFkB attenuates the suppression of hippocampal neurogenesis that is typically observed following exposure to acute restraint stress (Koo et al., 2010). This study also showed a similar effect of NFkB signaling in the behavioral phenotypes induced by chronic unpredictable stress. In fear conditioning models, NFkB activation is involved in consolidation and retrieval of contextual fear memories (Lubin and Sweatt, 2007). Furthermore, increased expression of NFkB is observed in the hippocampus following predator-scent stress, an effect that is blunted with a selective NFkB inhibitor (Cohen et al., 2011). Thus, targeting NFkB activity may be relevant for potential treatment of many neuropsychiatric disorders that involve stress as one of its prominent symptoms.
NFkB stimulation has been found to have complex effects on the reward pathways, which may alter the baseline reward state and affect subsequent reward processing following drug administration. For example, LPS injection decreases the basal firing rate of dopaminergic neurons in the VTA (Blednov et al., 2011). Furthermore, anhedonia as measured by reduced sucrose preference is a consistently observed phenotype induced by chronic stressors such as social defeat and chronic unpredictable stress, and this behavior is influenced by NFkB (Koo et al., 2010). In clinical studies, endotoxin exposure, a potent stimulator of NFkB, induces anhedonia-like responses in brain imaging studies as evidenced by reduced striatal activation in response to reward, as well as increased self-reported depressed mood (Eisenberger et al., 2010).
Inflammatory mediators clearly play a role in the influence of NFkB activity on stress sensitivity. For example, IL-6, a major cytokine target of NFkB, has been shown to mediate defeat stress sensitivity (Hodes et al., 2014). In this study, the investigators correlated IL-6 levels in peripheral blood mononuclear cells with defeat stress sensitivity. Then, by replacing the peripheral immune system of a control mouse with that of an animal that was predicted to be susceptible, they were able to induce susceptibility to defeat stress. While there is clearly a role for neuroimmune reactivity here, it is likely that additional NFkB-regulated target genes outside those inflammatory cytokines could contribute to the role of NFkB in stress-induced phenotypes.
NFkB in behavioral, physiological and neuroanatomical correlates of learning and memory
Another mechanism by which NFkB could influence drug reward and drug seeking is via its role in the behavioral and physiological processes involved in memory formation. Associations between drug properties and certain environments or associated stimuli is a mechanism that can drive continued drug seeking and relapse in addiction.
Extensive research has demonstrated a role of NFkB in learning and memory (Kaltschmidt et al., 2006). For example, NFkB subunit expression is increased during memory formation and is required for normal consolidation of memory (Ahn et al., 2008). NFkB has also been shown to play a functional role in the cellular processes that are believed to underlie memory formation, most notably long-term potentiation (LTP) (Ahn et al., 2008). Interestingly, NFkB mediation of memory function and LTP was influenced by cell type-specific manipulations of IKK activity in microglia or excitatory neurons, with both affecting hippocampal-dependent learning and LTP, although in slightly different ways (Kyrargyri et al., 2015).
Several groups have demonstrated a role of NFkB in spine formation and synaptogenesis (Russo et al., 2009; Boersma et al., 2011; Christoffel et al., 2011, 2012), an important cellular process involved in neuroadaptations induced by learning, drugs of abuse and stress. NFkB plays a pivotal role in processes including differentiation, axon formation and survival, as well as integration of young neurons into neuronal networks (Imielski et al., 2012). With specific connection to drugs of abuse, it has been demonstrated that NFkB function positively regulates spine formation in the nucleus accumbens both at baseline and in response to repeated cocaine administration (Russo et al., 2009). This appears to be a general effect, as it has been demonstrated in other regions of the brain and peripheral nervous system (Gutierrez et al., 2005). Additionally, spine formation is altered by NFkB activation that is induced by either chronic social defeat or viral vector-driven overexpression (Christoffel et al., 2011).
The role of NFkB in spine formation and synaptogenesis is particularly intriguing and is perhaps in most stark contrast to the reputation of NFkB for being a factor related to degeneration and toxicity. Further supporting this contrast are findings that mice lacking the p50 subunit display reduced viability of newly generated neurons within the dentate gyrus of the hippocampus as evidenced through BrdU labeling (Denis-Donini et al., 2008). Furthermore, knockout of the p65 subunit gene results in decreased spine density and spine head volume in cultured hippocampal neurons (Boersma et al., 2011).
Alternative gene targets
Given the diversity of genes whose expression is regulated by NFkB, an argument can be made for further exploration of these non-cytokine targets as mediators of NFkB-influenced drug responses. As stated, it is true that NFkB increases expression of cytokines and other inflammatory mediators, but it also increases the expression of opioids, opioid receptors and other neuropeptides. A few specific candidates for addiction-related NFkB target genes are portrayed in Fig. 1 and discussed below. While the list is certainly not exhaustive, we chose these few candidates based on considerable literature for activation of expression by NFkB, and an involvement in the behavioral and cellular processes outlined above.
Opioid receptors
NFkB activation increases the expression of most major classes of opioid peptides and opioid receptors (Chen et al., 2006), and these signaling systems have been shown to mediate many aspects of alcohol and drug seeking behavior (Gianoulakis, 2009; Le Merrer et al., 2009). For example, alcohol administration has been found to increase endogenous opioid release in the nucleus accumbens and orbitofrontal cortex (Mitchell et al., 2012). Also, naltrexone, a non-specific opioid receptor antagonist that is an FDA-approved treatment for alcoholism, dose-dependently decreases dopamine levels following alcohol administration and influences alcohol seeking behavior (Benjamin et al., 1993). Specific opioid receptors and peptides are discussed in the following paragraphs and the effects of these receptors will focus primarily on alcohol-related behaviors.
The MOR promoter has three NFkB binding elements, suggesting an important role in the regulation of this receptor (Kraus et al., 2003). The gene that transcribes the precursor peptide for β-endorphin, the primary endogenous ligand for the MOR, also contains NFkB elements (Karalis et al., 2004; Asaba et al., 2007). The MOR plays an integral role in behavioral responses to opiate drugs, as it is the primary target of these agents, but can also mediate behavioral responses to other drugs of abuse. For alcohol, microinjections of the MOR agonist (D-Ala2, N-MePhe4, Gly-ol)-enkephalin into the nucleus accumbens shell increases alcohol intake and enhances cue-induced reinstatement (Richard and Fields, 2016). Conversely, MOR antagonists decrease alcohol consumption in rats (Stromberg et al., 1998; Ripley et al., 2015). Stimulation of β-endorphin release and activation of the MOR is thought to be a major mechanism by which alcohol has its rewarding properties.
The expression of the δ-opioid receptor (DOR) is also influenced by NFkB function (Chen et al., 2007), as is the expression of the gene for the propeptide that produces its primary endogenous ligand, proenkephalin (Rattner et al., 1991). The role of the DOR in addictive behaviors has been reviewed elsewhere (Klenowski et al., 2015). Briefly, DOR antagonists have been found to decrease alcohol consumption in mice (Le et al., 1993). In reinstatement models, DOR antagonists attenuate alcohol seeking that is induced by either yohimbine injection or presentation of alcohol-associated cues (Ciccocioppo et al., 2002; Nielsen et al., 2012). The DOR and enkephalin peptide have been found to modulate LTP formation in the hippocampus (Chavkin et al., 1985), indicating a link between this receptor system and learning and memory. As such, the DOR could be an important alternative target that lies at the intersection of stress, addiction and learning processes that govern pathological drug seeking behavior.
The κ-opioid receptor (KOR) and its endogenous ligand, dynorphin, play a role in stress-induced reward and reinstatement for many drugs of abuse, including alcohol and cocaine (Shippenberg et al., 2007; Bruchas et al., 2010; Wee and Koob, 2010). NFkB regulates the expression of the precursor peptide for dynorphin (Bakalkin et al., 1994). Genetic deletion of the KOR or dynorphin attenuates alcohol intake (Kovacs et al., 2005; Blednov et al., 2006). Both forced swim stress and the KOR agonist U50,488 enhance place preference to cocaine, and this potentiation is blocked by the KOR antagonist norBNI (Schindler et al., 2010). Sperling et al. (2010) confirmed the involvement of the KOR in alcohol reward, as norBNI blocked stress-induced increases in conditioned place preference to alcohol. KOR antagonism has also been shown to mediate alcohol self-administration, withdrawal anxiety and yohimbine-induced reinstatement of alcohol seeking in rats (Walker and Koob, 2008; Schank et al., 2012a; Kissler et al., 2013; Funk et al., 2014). The dynorphin/KOR signaling within the central amygdala is dysregulated in dependent rats, pinpointing a potential neuroanatomical locus that mediates alcohol dependence (Kissler et al., 2013). The KOR system is potentially tied to anhedonia via dynorphin-induced reduction of dopamine release in the nucleus accumbens (Mague et al., 2003). Additionally, KOR activation has been shown to mediate stress responses and monoamine signaling through its actions in the locus coeruleus and dorsal raphe nucleus (Kreibich et al., 2008; Land et al., 2009; Bruchas et al., 2011). Thus, the KOR is linked to addiction via stress and anhedonic responses, two phenomena at the core of addictive behaviors.
Neurokinin-1 receptor
In addition to opioid peptides, NFkB elements activate expression of other neuropeptide receptors including the NK1R (Simeonidis et al., 2003; Ramkissoon et al., 2007), which has been shown to influence drug and alcohol-related behaviors (Schank et al., 2012b; Schank, 2014). For alcohol and psychostimulants, the NK1R has a targeted role in stress-related drug seeking, while for opiate drugs, the NK1R also influences baseline reward and reinforcement (Schank et al., 2011, 2014, 2015; Barbier et al., 2013). There is also a considerable literature supporting the role of the NK1R in stress responsivity, depression-like behavior and anxiety (Ebner and Singewald, 2006). Specifically, substance P is released in nuclei of the limbic circuitry during exposure to stressors and activation of the NK1R increases anxiety-like behavior (Ebner et al., 2004, 2008a, 2008b). Along these lines, the NK1R has been shown to mediate stress-induced release of monoamine transmitters in the cerebral cortex (Hutson et al., 2004; Renoldi and Invernizzi, 2006). An interesting aspect of this NK1R/NFkB relationship is that NK1R also stimulates NFkB activity, and NFkB stimulation by chronic morphine exposure is NK1R dependent (Wang et al., 2004). This may represent a positive feedback loop to amplify cellular responses to NK1R and NFkB activating stimuli, and the resulting stress responses.
Conclusions
It is not the purpose of this review to claim that the neuroimmune response induced by NFkB has no role in addictive processes. It is clear that inflammatory mediators can mediate negative affect, stress responsivity and other factors that influence drug seeking. The primary goal is to expand our thinking about the varied functions of NFkB in the regulation of responses to drugs and alcohol. Divergences between the role of NFkB in spine formation/reward and inflammation/neurotoxicity may be due to activation of differing cell types, gene targets or brain regions. As an example, neuronal NFkB may be recruited in the hippocampus during the formation of LTP, but for response to infection and toxicity, microglial NFkB may be activated. This could be due to differences in the intensity of the stimulus or the upstream receptor target that induces activation of NFkB, for example, NK1R versus IL-1R or TLR4 receptor. Importantly, the pattern, frequency and dose of drug exposure could be a critical parameter that shifts the role of NFkB from reward/neurogenesis promoting to toxicity inducing.
While it has been demonstrated that NFkB alters the expression of the non-inflammatory gene targets discussed above, and that these genes influence behaviors that can mediate addiction, the literature is lacking in studies that demonstrate a causal link between NFkB activation and mediation of drug seeking via these non-immune gene targets. Future research should focus on these complex functions and stimulators of NFkB, and should delve deeply into the specific gene targets that mediate the behavioral effects.
Conflict of Interest Statement
None declared.
References
- Ahn HJ, Hernandez CM, Levenson JM, et al. (2008) c-Rel, an NF-kappaB family transcription factor, is required for hippocampal long-term synaptic plasticity and memory formation. Learn Mem 15:539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, et al. (2010) Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci 30:8285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang E, Chen J, Zagouras P, et al. (2001) Induction of nuclear factor-kappaB in nucleus accumbens by chronic cocaine administration. J Neurochem 79:221–4. [DOI] [PubMed] [Google Scholar]
- Asaba K, Iwasaki Y, Asai M, et al. (2007) High glucose activates pituitary proopiomelanocortin gene expression: possible role of free radical-sensitive transcription factors. Diabetes Metab Res Rev 23:317–23. [DOI] [PubMed] [Google Scholar]
- Bai D, Ueno L, Vogt PK (2009) Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int J Cancer 125:2863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakalkin G, Yakovleva T, Terenius L (1994) Prodynorphin gene expression relates to NF-kappa B factors. Brain Res Mol Brain Res 24:301–12. [DOI] [PubMed] [Google Scholar]
- Barbier E, Vendruscolo LF, Schlosburg JE, et al. (2013) The NK1 receptor antagonist L822429 reduces heroin reinforcement. Neuropsychopharmacology 38:976–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin D, Grant ER, Pohorecky LA (1993) Naltrexone reverses ethanol-induced dopamine release in the nucleus accumbens in awake, freely moving rats. Brain Res 621:137–40. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, et al. (2011) Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun 25:S92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, et al. (2006) Reduced alcohol consumption in mice lacking preprodynorphin. Alcohol 40:73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma MC, Dresselhaus EC, De Biase LM, et al. (2011) A requirement for nuclear factor-kappaB in developmental and plasticity-associated synaptogenesis. J Neurosci 31:5414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C (2010) The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res 1314:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Schindler AG, Shankar H, et al. (2011) Selective p38alpha MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron 71:498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso A. (2001) Involvement of nuclear factor-kB in the expression of opiate withdrawal. Prog Neuropsychopharmacol Biol Psychiatry 25:1259–68. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B, Mummidi S, Perla RP, et al. (2003) Fractalkine (CX3CL1) stimulated by nuclear factor kappaB (NF-kappaB)-dependent inflammatory signals induces aortic smooth muscle cell proliferation through an autocrine pathway. Biochem J 373:547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, Shoemaker WJ, Mcginty JF, et al. (1985) Characterization of the prodynorphin and proenkephalin neuropeptide systems in rat hippocampus. J Neurosci 5:808–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Law PY, Loh HH (2006) Nuclear factor kappaB signaling in opioid functions and receptor gene expression. J Neuroimmune Pharmacol 1:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Law PY, Loh HH (2007) Action of NF-kappaB on the delta opioid receptor gene promoter. Biochem Biophys Res Commun 352:818–22. [DOI] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, et al. (2011) IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci 31:314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Heshmati M, et al. (2012) Effects of inhibitor of kappaB kinase activity in the nucleus accumbens on emotional behavior. Neuropsychopharmacology 37:2615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F (2002) Effect of selective blockade of mu(1) or delta opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology 27:391–9. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kozlovsky N, Matar MA, et al. (2011) The characteristic long-term upregulation of hippocampal NF-kappaB complex in PTSD-like behavioral stress response is normalized by high-dose corticosterone and pyrrolidine dithiocarbamate administered immediately after exposure. Neuropsychopharmacology 36:2286–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, Nixon K, Kim D, et al. (2006) BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcohol Clin Exp Res 30:1938–49. [DOI] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP (2011) Addiction, adolescence, and innate immune gene induction. Front Psychiatry 2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin L (2011) Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun 25:S4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Syapin PJ (2004) Ethanol increases nuclear factor-kappa B activity in human astroglial cells. Neurosci Lett 371:128–32. [DOI] [PubMed] [Google Scholar]
- Denis-Donini S, Dellarole A, Crociara P, et al. (2008) Impaired adult neurogenesis associated with short-term memory defects in NF-kappaB p50-deficient mice. J Neurosci 28:3911–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K, Muigg P, Singewald G, et al. (2008. a) Substance P in stress and anxiety: NK-1 receptor antagonism interacts with key brain areas of the stress circuitry. Ann N Y Acad Sci 1144:61–73. [DOI] [PubMed] [Google Scholar]
- Ebner K, Rupniak NM, Saria A, et al. (2004) Substance P in the medial amygdala: emotional stress-sensitive release and modulation of anxiety-related behavior in rats. Proc Natl Acad Sci USA 101:4280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K, Singewald GM, Whittle N, et al. (2008. b) Neurokinin 1 receptor antagonism promotes active stress coping via enhanced septal 5-HT transmission. Neuropsychopharmacology 33:1929–41. [DOI] [PubMed] [Google Scholar]
- Ebner K, Singewald N (2006) The role of substance P in stress and anxiety responses. Amino Acids 31:251–72. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Xuei X, Wetherill LF, et al. (2008) Association of NFKB1, which encodes a subunit of the transcription factor NF-kappaB, with alcohol dependence. Hum Mol Genet 17:963–70. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, et al. (2010) Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry 68:748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Coen K, Le AD (2014) The role of kappa opioid receptors in stress-induced reinstatement of alcohol seeking in rats. Brain Behav 4:356–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerondakis S, Fulford TS, Messina NL, et al. (2014) NF-kappaB control of T cell development. Nat Immunol 15:15–25. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. (2009) Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem 9:999–1015. [DOI] [PubMed] [Google Scholar]
- Gutierrez H, Hale VA, Dolcet X, et al. (2005) NF-kappaB signalling regulates the growth of neural processes in the developing PNS and CNS. Development 132:1713–26. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S (2004) Signaling to NF-kappaB. Genes Dev 18:2195–224. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Leboeuf M, et al. (2014) Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci USA 111:16136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou YN, Vlaskovska M, Cebers G, et al. (1996) A mu-receptor opioid agonist induces AP-1 and NF-kappa B transcription factor activity in primary cultures of rat cortical neurons. Neurosci Lett 212:159–62. [DOI] [PubMed] [Google Scholar]
- Hutson PH, Patel S, Jay MT, et al. (2004) Stress-induced increase of cortical dopamine metabolism: attenuation by a tachykinin NK1 receptor antagonist. Eur J Pharmacol 484:57–64. [DOI] [PubMed] [Google Scholar]
- Imielski Y, Schwamborn JC, Luningschror P, et al. (2012) Regrowing the adult brain: NF-kappaB controls functional circuit formation and tissue homeostasis in the dentate gyrus. PLoS One 7:e30838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt B, Ndiaye D, Korte M, et al. (2006) NF-kappaB regulates spatial memory formation and synaptic plasticity through protein kinase A/CREB signaling. Mol Cell Biol 26:2936–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalis KP, Venihaki M, Zhao J, et al. (2004) NF-kappaB participates in the corticotropin-releasing, hormone-induced regulation of the pituitary proopiomelanocortin gene. J Biol Chem 279:10837–40. [DOI] [PubMed] [Google Scholar]
- Kissler JL, Sirohi S, Reis DJ, et al. (2013) The one-two punch of alcoholism: role of central amygdala dynorphins/Kappa-opioid receptors. Biol Psychiatry 75:774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenowski P, Morgan M, Bartlett SE (2015) The role of delta-opioid receptors in learning and memory underlying the development of addiction. Br J Pharmacol 172:297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Wheeler MD, Rusyn I, et al. (2000) Gender differences in early alcohol-induced liver injury: role of CD14, NF-kappaB, and TNF-alpha. Am J Physiol Gastrointest Liver Physiol 278:G652–61. [DOI] [PubMed] [Google Scholar]
- Koo JW, Russo SJ, Ferguson D, et al. (2010) Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci USA 107:2669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs KM, Szakall I, O'brien D, et al. (2005) Decreased oral self-administration of alcohol in kappa-opioid receptor knock-out mice. Alcohol Clin Exp Res 29:730–8. [DOI] [PubMed] [Google Scholar]
- Kraus J, Borner C, Giannini E, et al. (2003) The role of nuclear factor kappaB in tumor necrosis factor-regulated transcription of the human mu-opioid receptor gene. Mol Pharmacol 64:876–84. [DOI] [PubMed] [Google Scholar]
- Kreibich A, Reyes BA, Curtis AL, et al. (2008) Presynaptic inhibition of diverse afferents to the locus ceruleus by kappa-opiate receptors: a novel mechanism for regulating the central norepinephrine system. J Neurosci 28:6516–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrargyri V, Vega-Flores G, Gruart A, et al. (2015) Differential contributions of microglial and neuronal IKKbeta to synaptic plasticity and associative learning in alert behaving mice. Glia 63:549–66. [DOI] [PubMed] [Google Scholar]
- Laird MH, Rhee SH, Perkins DJ, et al. (2009) TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J Leukoc Biol 85:966–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, et al. (2009) Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci USA 106:19168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Quan B, et al. (1993) The effects of selective blockade of delta and mu opiate receptors on ethanol consumption by C57BL/6 mice in a restricted access paradigm. Brain Res 630:330–2. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, et al. (2009) Reward processing by the opioid system in the brain. Physiol Rev 89:1379–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EG, Mickle-Kawar BM, Reinke LA, et al. (2012) Estrogen suppresses heptatic IkappaB expression during short-term alcohol exposure. Inflamm Res 61:1053–61. [DOI] [PubMed] [Google Scholar]
- Lieb K, Fiebich BL, Berger M, et al. (1997) The neuropeptide substance P activates transcription factor NF-kappa B and kappa B-dependent gene expression in human astrocytoma cells. J Immunol 159:4952–8. [PubMed] [Google Scholar]
- Lubin FD, Sweatt JD (2007) The IkappaB kinase regulates chromatin structure during reconsolidation of conditioned fear memories. Neuron 55:942–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, et al. (2003) Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther 305:323–30. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, O'neil JP, Janabi M, et al. (2012) Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med 4:116ra6. [DOI] [PubMed] [Google Scholar]
- Montesinos J, Pascual M, Pla A, et al. (2015) TLR4 elimination prevents synaptic and myelin alterations and long-term cognitive dysfunctions in adolescent mice with intermittent ethanol treatment. Brain Behav Immun 45:233–44. [DOI] [PubMed] [Google Scholar]
- Neumann M, Naumann M (2007) Beyond IkappaBs: alternative regulation of NF-kappaB activity. FASEB J 21:2642–54. [DOI] [PubMed] [Google Scholar]
- Nielsen CK, Simms JA, Bito-Onon JJ, et al. (2012) The delta opioid receptor antagonist, SoRI-9409, decreases yohimbine stress-induced reinstatement of ethanol-seeking. Addict Biol 17:224–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeckinghaus A, Ghosh S (2009) The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 1:a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okvist A, Johansson S, Kuzmin A, et al. (2007) Neuroadaptations in human chronic alcoholics: dysregulation of the NF-kappaB system. PLoS One 2:e930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Balino P, Alfonso-Loeches S, et al. (2011) Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav Immun 25:S80–91. [DOI] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, et al. (2008) Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramkissoon SH, Patel PS, Taborga M, et al. (2007) Nuclear factor-kappaB is central to the expression of truncated neurokinin-1 receptor in breast cancer: implication for breast cancer cell quiescence within bone marrow stroma. Cancer Res 67:1653–9. [DOI] [PubMed] [Google Scholar]
- Rattner A, Korner M, Rosen H, et al. (1991) Nuclear factor kappa B activates proenkephalin transcription in T lymphocytes. Mol Cell Biol 11:1017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehni AK, Bhateja P, Singh TG, et al. (2008) Nuclear factor-kappa-B inhibitor modulates the development of opioid dependence in a mouse model of naloxone-induced opioid withdrawal syndrome. Behav Pharmacol 19:265–9. [DOI] [PubMed] [Google Scholar]
- Renoldi G, Invernizzi RW (2006) Blockade of tachykinin NK1 receptors attenuates stress-induced rise of extracellular noradrenaline and dopamine in the rat and gerbil medial prefrontal cortex. J Neurosci Res 84:961–8. [DOI] [PubMed] [Google Scholar]
- Richard JM, Fields HL (2016) Mu-opioid receptor activation in the medial shell of nucleus accumbens promotes alcohol consumption, self-administration and cue-induced reinstatement. Neuropharmacology 108:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley TL, Sanchez-Roige S, Bullmore ET, et al. (2015) The novel mu-opioid antagonist, GSK1521498, reduces ethanol consumption in C57BL/6J mice. Psychopharmacology (Berl) 232:3431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Wilkinson MB, Mazei-Robison MS, et al. (2009) Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J Neurosci 29:3529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaya BE, Deshmane SL, Mukerjee R, et al. (2009) TNF alpha production in morphine-treated human neural cells is NF-kappaB-dependent J Neuroimmune Pharmacol 4:140–9. [DOI] [PubMed] [Google Scholar]
- Schank JR. (2014) The neurokinin-1 receptor in addictive processes. J Pharmacol Exp Ther 351:2–8. [DOI] [PubMed] [Google Scholar]
- Schank JR, Goldstein AL, Rowe KE, et al. (2012. a) The kappa opioid receptor antagonist JDTic attenuates alcohol seeking and withdrawal anxiety. Addict Biol 17:634–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, King CE, Sun H, et al. (2014) The role of the neurokinin-1 receptor in stress-induced reinstatement of alcohol and cocaine seeking. Neuropsychopharmacology 39:1093–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Nelson BS, Damadzic R, et al. (2015) Neurokinin-1 receptor antagonism attenuates neuronal activity triggered by stress-induced reinstatement of alcohol seeking. Neuropharmacology 99:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Pickens CL, Rowe KE, et al. (2011) Stress-induced reinstatement of alcohol-seeking in rats is selectively suppressed by the neurokinin 1 (NK1) antagonist L822429. Psychopharmacology (Berl) 218:111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Ryabinin AE, Giardino WJ, et al. (2012. b) Stress-related neuropeptides and addictive behaviors: beyond the usual suspects. Neuron 76:192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AG, Li S, Chavkin C (2010) Behavioral stress may increase the rewarding valence of cocaine-associated cues through a dynorphin/kappa-opioid receptor-mediated mechanism without affecting associative learning or memory retrieval mechanisms. Neuropsychopharmacology 35:1932–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R, Baltimore D (1986) Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell 47:921–8. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI (2007) Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther 116:306–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa F, Mizel SB (1989) In vitro activation and nuclear translocation of NF-kappa B catalyzed by cyclic AMP-dependent protein kinase and protein kinase C. Mol Cell Biol 9:2424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeonidis S, Castagliuolo I, Pan A, et al. (2003) Regulation of the NK-1 receptor gene expression in human macrophage cells via an NF-kappa B site on its promoter. Proc Natl Acad Sci USA 100:2957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RE, Gomes SM, Sypek EI, et al. (2010) Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology (Berl) 210:199–209. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Casale M, Volpicelli L, et al. (1998) A comparison of the effects of the opioid antagonists naltrexone, naltrindole, and beta-funaltrexamine on ethanol consumption in the rat. Alcohol 15:281–9. [DOI] [PubMed] [Google Scholar]
- Sun J, Ramnath RD, Zhi L, et al. (2008) Substance P enhances NF-kappaB transactivation and chemokine response in murine macrophages via ERK1/2 and p38 MAPK signaling pathways. Am J Physiol Cell Physiol 294:C1586–96. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF (2008) Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology 33:643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Douglas SD, Commons KG, et al. (2004) A non-peptide substance P antagonist (CP-96,345) inhibits morphine-induced NF-kappa B promoter activation in human NT2-N neurons. J Neurosci Res 75:544–53. [DOI] [PubMed] [Google Scholar]
- Wee S, Koob GF (2010) The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 210:121–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Hashimoto JG, Roberts ML, et al. (2014) Understanding the addiction cycle: a complex biology with distinct contributions of genotype vs. sex at each stage. Neuroscience 279:168–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Lousberg EL, Moldenhauer LM, et al. (2012) Inhibiting the TLR4-MyD88 signalling cascade by genetic or pharmacological strategies reduces acute alcohol-induced sedation and motor impairment in mice. Br J Pharmacol 165:1319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xu F, Lin Y (2011) Cigarette smoke synergizes lipopolysaccharide-induced interleukin-1beta and tumor necrosis factor-alpha secretion from macrophages via substance P-mediated nuclear factor-kappaB activation. Am J Respir Cell Mol Biol 44:302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cui Y, Jing J, et al. (2011) Involvement of p38/NF-kappaB signaling pathway in the nucleus accumbens in the rewarding effects of morphine in rats. Behav Brain Res 218:184–9. [DOI] [PubMed] [Google Scholar]
- Zou J, Crews F (2010) Induction of innate immune gene expression cascades in brain slice cultures by ethanol: key role of NF-kappaB and proinflammatory cytokines. Alcohol Clin Exp Res 34:777–89. [DOI] [PubMed] [Google Scholar]