ABSTRACT
Background: Ghrelin, which is a stomach-derived hormone, increases with fasting and energy restriction and may influence eating behaviors through brain hedonic reward-cognitive systems. Therefore, changes in plasma ghrelin might mediate counter-regulatory responses to a negative energy balance through changes in food hedonics.
Objective: We investigated whether ghrelin administration (exogenous hyperghrelinemia) mimics effects of fasting (endogenous hyperghrelinemia) on the hedonic response and activation of brain-reward systems to food.
Design: In a crossover design, 22 healthy, nonobese adults (17 men) underwent a functional magnetic resonance imaging (fMRI) food-picture evaluation task after a 16-h overnight fast (Fasted-Saline) or after eating breakfast 95 min before scanning (730 kcal, 14% protein, 31% fat, and 55% carbohydrate) and receiving a saline (Fed-Saline) or acyl ghrelin (Fed-Ghrelin) subcutaneous injection before scanning. One male subject was excluded from the fMRI analysis because of excess head motion, which left 21 subjects with brain-activation data.
Results: Compared with the Fed-Saline visit, both ghrelin administration to fed subjects (Fed-Ghrelin) and fasting (Fasted-Saline) significantly increased the appeal of high-energy foods and associated orbitofrontal cortex activation. Both fasting and ghrelin administration also increased hippocampus activation to high-energy- and low-energy-food pictures. These similar effects of endogenous and exogenous hyperghrelinemia were not explicable by consistent changes in glucose, insulin, peptide YY, and glucagon-like peptide-1. Neither ghrelin administration nor fasting had any significant effect on nucleus accumbens, caudate, anterior insula, or amygdala activation during the food-evaluation task or on auditory, motor, or visual cortex activation during a control task.
Conclusions: Ghrelin administration and fasting have similar acute stimulatory effects on hedonic responses and the activation of corticolimbic reward-cognitive systems during food evaluations. Similar effects of recurrent or chronic hyperghrelinemia on an anticipatory food reward may contribute to the negative impact of skipping breakfast on dietary habits and body weight and the long-term failure of energy restriction for weight loss.
INTRODUCTION
Eating behaviors involve the integration of peripheral signals of energy balance to alter hunger and food reward. Hedonic and motivational responses involve a network of corticolimbic brain regions including the orbitofrontal cortex (OFC)4, amygdala, nucleus accumbens (NAcc), caudate, and insula (1, 2). Homeostatic and hedonic systems interact directly because fasting or energy restriction enhances hedonic responses, memory recall of food, and the activation of brain-reward systems to food (3–7). Skipping breakfast is associated with increased dietary fat intake, binge eating, activation of brain-reward systems to food, and longitudinal weight gain (8, 9). Gastroenteropancreatic hormones mediate homeostatic responses to acute food intake or fasting including anorexigenic insulin, peptide YY (PYY), glucagon-like peptide-1 (GLP-1) and cholecystokinin, and orexigenic ghrelin (10, 11). Appetitive hormones act through the hypothalamus and brainstem (10) but may also influence brain reward-cognitive systems to alter hunger and satiety and food hedonic responses (5, 12–18).
Ghrelin is a 28–amino acid stomach-derived hormone, of which the posttranslational acylation by the ghrelin O-acyltransferase (GOAT) enzyme instills biological activity (11). Plasma ghrelin concentrations rise with acute fasting and a chronic negative energy balance, fall after food intake, and are reduced in obesity (11, 19). Acyl ghrelin acts as a growth-hormone (GH) secretagogue through the growth-hormone secretagogue receptor 1a (GHSR1a) in the hypothalamus and pituitary gland. Ghrelin also stimulates hunger and food intake (20, 21) through hypothalamic feeding neuropeptides (10, 11). In rodents, central ghrelin GHSR1a signaling also has roles in reward-based eating, responses to nonfood rewards (eg, drugs of abuse and alcohol), food anticipatory behavior, foraging, hoarding and olfactory responses, stress-induced overeating, motivation, learning, memory, anxiety, and mood via the stimulation of the mesolimbic dopaminergic circuitry, hypothalamus, hippocampus, dorsal raphe serotonergic neurons, and other pathways (17, 18, 22–24). Thus, ghrelin may mediate many of the effects of food restriction on reward responses to food. A limited number of interventional or purely correlative neuroimaging studies have investigated such a role for ghrelin in humans by using fMRI (13, 25, 26). Ghrelin altered the activation in a number of brain regions involved in reward, cognition, visual, and taste processing either at rest or in response to visual food cues assessed by changes in the BOLD signal.
However, these fMRI studies have not reported any direct measures of food hedonics or directly compared effects of ghrelin administration and fasting or examined the influence of rewarding properties (eg, energy density) of the food in the response to food cues (13, 26). Therefore, we compared situations where plasma acyl ghrelin concentrations were raised endogenously (by fasting) or exogenously (by acyl ghrelin administration to fed subjects) with a situation in which plasma concentrations were endogenously low (after the consumption of a meal) in the same subjects. We hypothesized that both conditions associated with hyperghrelinemia would increase hedonic-reward system responses to anticipatory food cues. As a secondary outcome, we examined whether these effects were influenced by the energy density of food cues.
SUBJECTS AND METHODS
See Supplemental Methods under “Supplemental data” in the online issue for additional details.
Participants
Subjects were recruited by using a public advertisement. The study was approved by the local research ethics committee (07/Q0406/19) and was performed in accordance with the principles of the Declaration of Helsinki. All participants provided written informed consent. The initial recruitment date for the study was 2 April 2008.
Exclusion criteria were as follows: 1) history of an eating disorder; 2) being vegetarian, vegan, or gluten or lactose intolerant; 3) consumption of a non-Western diet (assessed by a dietary record); 4) significant dietary restraint (Dutch Eating Behavior Questionnaire-Restraint score >3.0); 5) missing breakfast >3 d/wk; 6) recent change in weight (>5% change in the past 3 mo); 7) BMI (in kg/m2) <18.0 or >30.0; 8) smoking or alcohol excess; 9) any history of significant neurologic, psychiatric, endocrine, metabolic, or cardiovascular disease including addiction, diabetes, stroke, or epilepsy; 10) severe depression indicated by a Beck Depression Inventory II score >28 (27); 11) claustrophobia; 12) pregnancy or breastfeeding; 13) any metallic objects excluding MRI scanning (eg, pacemaker, magnetic implant); and 14) inability to use a right-handed button box.
Study protocol
Healthy, weight-stable, nonobese adults (BMI: 18.0–29.9; age: 18–50 y) of either sex were studied. Subjects attended 4 separate study visits ≥5 d apart [median (IQR): 16 d (7–35 d)] after an overnight fast (Figure 1, Table 1; see Supplemental Table 1 under “Supplemental data” in the online issue). At each visit, subjects performed an fMRI picture-evaluation task in which they rated the appeal of different food and control pictures. There was an initial practice visit, when subjects were not given breakfast, and received a saline injection (Fasted-Initial) and, then in a randomized, crossover design, another fasted visit when they received a saline injection (Fasted-Saline) and 2 fed visits when, after eating breakfast, subjects received a saline (Fed-Saline) or ghrelin (Fed-Ghrelin) injection. No Fasted-Ghrelin visit was performed because endogenous ghrelin concentrations are high when fasted. Women were always scanned in days 1–14 of their menstrual cycle to attenuate variations in reward responses including food over the menstrual cycle (28).
FIGURE 1.
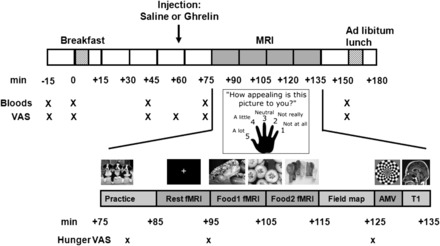
Study-visit protocol. Timings of blood sampling and VAS ratings and detail of scanning, including food picture and AMV fMRI runs and anatomical T1. AMV, auditory-motor-visual; T1, T1 MRI brain scan; VAS, visual analog scale.
TABLE 1.
Subject characteristics1
| Average (n = 22) | Range | |
| Age (y) | 25.9 ± 1.72 | 19–44 |
| Sex (M:F) | 17:5 | |
| Ethnicity (white:nonwhite) | 15:7 | |
| Height (m) | 1.78 ± 0.02 | 1.61–1.93 |
| Weight (kg) | 76.0 ± 2.9 | 51.6–100.9 |
| BMI (kg/m2) | 23.9 ± 0.6 | 19.1–29.9 |
| Body fat (%) | 16.1 ± 1.4 | 7.8–30.7 |
| BDI-II (of 63) | 0 (0–3)3 | 0–8 |
Age and anthropometric data are taken from the Fasted-Initial visit. BDI-II, Beck Depression Inventory II; Fasted-Initial, first scanning visit with 16-h overnight fast and given subcutaneous saline injection.
Mean ± SEM (all such values).
Median; IQR in parentheses. Not normally distributed.
On the day before each study visit, subjects were instructed to avoid exercise and alcohol intake, eat a similar supper at 2000, and attend the Sir John McMichael Centre Clinical Investigation Unit at Hammersmith Hospital, London, United Kingdom, in the morning having eaten nothing since supper the evening before. At each visit, subjects had measurements of height, weight, percentage of body fat by bioelectrical impedance analysis (Bodystat 1500), completed the Positive and Negative Affect Schedule to measure mood over the past week because brain responses to food pictures have been reported to vary depending on affect (29), and had an insertion of a cannula into an antecubital vein ≥30 min before the start of serial blood sampling. Pregnancy was excluded at each visit by using a urinary human chorionic gonadotropin test.
The administration of ghrelin or saline was double blinded at each fed visit and single-blinded at each fasted visit. The order of random assignment used a sequential list of visits. Subjects did not know whether they would be given breakfast until the appropriate time point (t = 0 min). The median time since the last meal until the fMRI scanning was 16.3 h at the Fasted-Initial visit and 15.7 h at the Fasted-Saline visit compared with 1.6 h at both Fed-Saline and Fed-Ghrelin visits (see Supplemental Table 1 under “Supplemental data” in the online issue). The full scanning and study protocol was otherwise identical at each of the 4 visits.
At fed visits, subjects consumed a 730-kcal fixed breakfast (14% protein, 31% fat, and 55% carbohydrate) at ~1000–1100, t = 0 min, which consisted of 220 mL orange juice, 40 g bran flakes, 170 mL semiskimmed milk, and 2 slices of whole-meal bread, each slice with 10 g margarine, one slice with 10 g strawberry jam, and the other slice with an 8-g slice of cheese. Subjects finished all given foods.
Visual analog scales
Visual analog scale (VAS) ratings (0–10 cm) were recorded over the study day to measure hunger, pleasantness to eat, volume of food wanting to eat, fullness, sickness, anxiety, and stress (Figure 1). Subjects were also asked to rate their hunger at 3 time points while in the MRI scanner, twice before and once after viewing food pictures (+80, +95, and +125 min) by using a 5-finger keypad and rating from 1 to 5, whereby 1 denoted not at all and 5 denoted a lot.
Ghrelin injection
At +55 min, subcutaneous saline or human acyl ghrelin (Bachem UK) was injected into the abdomen at a dose of 3.6 nmol/kg (21).
Metabolic and hormone assays
Serial venous blood samples were taken at −15, 0, +40, +70, and +150 min for the assay of plasma glucose, PYY, GLP-1, and acyl ghrelin and serum insulin, GH, and triglycerides to ascertain whether differences in food reward between fasted and fed visits were explained by changes in these appetitive hormones and metabolites (14–16, 30).
Blood samples for gut-hormone analysis were collected into chilled lithium heparin polypropylene tubes that contained 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (A8456; Sigma-Aldrich) and aprotinin (Nordic Phama UK) protease inhibitor to give final concentration of 1 mg/mL and 200 kIU/mL whole blood, respectively. Blood samples were centrifuged at 4°C, 4000 rpm, for 10 min. Aliquots of separated plasma were immediately mixed with HCl (final concentration of 0.05 mol/L) for the subsequent assay of acyl ghrelin and separate unacidified aliquots for the assay of other gut hormones (GLP-1 and PYY). All plasma samples were stored at −80°C until assay. Other metabolic and hormonal assays were done on plain serum or fluoride-oxalate plasma samples sent immediately to the routine clinical laboratory.
Plasma glucose, serum insulin, GH, and triglycerides were measured in the Department of Clinical Biochemistry, Imperial College Healthcare National Health Service Trust by using either an Abbott Architect ci8200 analyzer (Abbott Diagnostics) or an Axsym analyzer (Abbott Diagnostics). Intraassay CVs of all measurements were from 1.0 to 5.0%. Plasma GLP-1 (GLP-11–36 amide, GLP-17–36 amide, and GLP-19–36 amide) and PYY (total PYY1–36 and PYY3–36) were assayed by using established in-house radioimmunoassays in a single run (31, 32). Plasma acyl ghrelin was measured by using a 2-site sandwich ELISA in a single run (33). Intraassay CVs for gut hormones were <10%.
MRI scanning protocol
After the injection, each subject had a 60-min MRI session, which started at +75 min (3T Achieva whole-body system; Philips) as previously described (34). After an initial practice run, subjects had a resting-state fMRI scan that lasted 10 min starting at +85 min (data will be reported in a future article because of space limitations) and was followed by the picture fMRI paradigm at +95 min (Figure 1), an auditory-motor-visual (AMV) fMRI task at +125 min, and the collection of structural MR brain scans including high-resolution T1-weighted scans (Figure 1).
Food-picture evaluation fMRI paradigm
During the fMRI food-picture paradigm, 4 types of color photographs were presented in a block design split across 2 9-min, 192-vol runs as follows: 1) 60 high-energy-dense foods (HEs) (eg, pizza, cakes, and chocolate), 2) 60 low-energy-dense foods (LEs) (eg, salads, vegetables, and fish), 3) 60 non–food-related household objects (eg, furniture and clothing), and 4) 180 Gaussian blurred images of the other pictures (as a low-level baseline) as previously described (34). Food images were selected to represent familiar foods that are typical in the modern Western diet. Pictures were obtained from freely available web sites and the International Affective Picture System (National Institute of Mental Health Center for the Study of Emotion and Attention, University of Florida). Food and object pictures were of similar luminosity and resolution.
Each run contained different pictures in 5 blocks each of high- and low-energy foods and objects interleaved with 16 blocks of blurred pictures (6 pictures/18 s) by using one of 4 pseudorandom block orders (randomized for each subject across study visits) with a randomized picture order within each block. Every image was displayed for 2500 ms and followed by a 500-ms interstimulus interval of a fixation cross. Each high-energy food block consisted of equal numbers of foods that contained chocolate, nonchocolate sweet, and savory nonsweet foods (2 of each).
Images were viewed via a mirror mounted above an 8-channel radiofrequency head coil that displayed images from a projector by using the Integrated Functional Imaging System image-presentation system (In Vivo) and ePrime 2 software (Psychology Software Tools Inc). While each image was on display to subjects in the scanner, they were asked to immediately and simultaneously rate how appealing each picture was to them by using a 5-button handheld keypad (1 = not at all, 2 = not really, 3 = neutral, 4 = a little, and 5 = a lot) (6, 34). Thus, the appeal rating was made and recorded simultaneously with the stimulus presentation used for the measurement of the BOLD signal.
The total energy load, energy density, and macronutrient composition of food pictures used in the fMRI task were assessed with Dietplan6 software (Foresfield Software Ltd) (mean ± SEM for high-energy foods: 834 ± 100 kcal, 321 ± 13 kcal/100g, 42 ± 2% fat, 48 ± 1% carbohydrate, and 10 ± 1% protein; low-energy foods: 157 ± 18 kcal, 64 ± 5 kcal/100g, 35 ± 3% fat, 35 ± 3% carbohydrate, and 29 ± 3% protein; and high- compared with low-energy foods: P < 0.001 for energy content, density, percentage of protein, and percentage of carbohydrate, and P = 0.03 for percentage of fat.
AMV control fMRI paradigm
A-6 min, 114-vol AMV control task was performed to look for nonspecific changes in the BOLD signal between visits. Over nine 33-s blocks, subjects performed 2 of each of the following tasks simultaneously: 1) listening to a story, 2) tapping their right index finger once every 1 s, or 3) watching a 4-Hz color (yellow/blue) flashing checkerboard with each task performed in 6 blocks and instructions about whether to start or stop the motor task displayed for 3 s before each block (34).
Ad libitum test lunch
After MRI scanning at +150 min, each subject received an excess amount of a savory lunch and were instructed to “eat as much as they wanted until they felt comfortably full,” as previously described (16). The lunch meal was macaroni and cheese (per 100 g: 205 kcal, 6.5 g protein, 18.9 g carbohydrate, and 11.5 g fat), or if this meal was not liked at least moderately on a VAS at their screening visit, an alternative of chicken tikka masala (per 100 g: 150 kcal, 6.6 g protein, 13 g carbohydrate, and 8 g fat). Men were presented with 2000 g, and women were presented with 1500 g, of lunch together with ad libitum water. The total energy eaten and energy per kilogram of lean body mass (LBM) were calculated from the formula
Image preprocessing
fMRI data processing was carried out with FEAT software (FMRI Expert Analysis Tool, version 5.98), which is part of the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL), version 4.1, as previously described (see Supplemental Methods under “Supplemental data” in the online issue for references) (34). Subjects were removed from the fMRI analysis if there was excess head movement (average relative motion across both food runs >0.5 mm/vol). The following preprocessing was applied: motion correction by using MCFLIRT software, field-map-based EPI unwarping by using PRELUDE+FUGUE software, nonbrain removal by using BET software, spatial smoothing by using a Gaussian kernel of full-width half-maximum 6.0 mm, and grand-mean intensity normalization of the entire 4-dimension dataset by using a single multiplicative factor and high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting with σ = 100.0 s).
A time-series statistical analysis was carried out by using FILM software with local autocorrelation correction including picture onsets (high- and low-energy food and objects) integrated with the γ hemodynamic response function with temporal derivative and motion variables as covariates. Registration to high-resolution T1 structural images was carried out by using FLIRT software. Registration from high-resolution structural to standard spaces was then further refined by using FNIRT nonlinear registration software. For food pictures, a higher level analysis was carried out by using a fixed-effect model to combine the 2 runs by forcing the random-effects variance to zero in FLAME (FMRIB’s Local Analysis of Mixed Effects) software to determine the BOLD signal for the following contrasts: high-energy or low-energy food (FOOD) greater than object pictures, HE greater than object pictures, LE greater than object pictures, and high- greater than low-energy food (HE greater than LE) pictures (34).
A similar time-series statistical analysis was performed for the single-run AMV paradigm including onsets of each task (auditory, motor, and visual) to contrast the BOLD signal during the performance of each task with that when other tasks were being performed (34). All higher-level analyses were carried out with FLAME stage 1 software.
Whole-brain analysis
A whole-brain analysis to determine the spatial extent of differences in the BOLD signal between study visits for FOOD, HE, and LE contrasts was examined by 1-factor repeated-measures ANOVA within FSL with FEAT version 5.98 software with both statistical voxel-wise threshold family-wise error P < 0.05 and false discovery rate (FDR) P < 0.05 corrections for multiple comparisons.
fMRI regions of interest
The following functional regions of interest (fROIs) were determined from the average group activation at the Fasted-Initial visit for the FOOD contrast: OFC, hippocampus, NAcc, caudate, anterior insula, and amygdala (Table 1; see Supplemental Figure 1 under “Supplemental data” in the online issue). These 6 regions of interest (ROIs) were chosen on the basis of preclinical studies (17, 35–37) and published human fMRI studies that used food pictures after ghrelin, PYY, or GLP-1 administration (13, 16) or an identical fMRI paradigm after bariatric surgery for obesity (34). Additional ROIs were not examined to avoid type 1 errors because no Bonferroni correction was made for multiple comparisons. For the control AMV task, ROIs were the superior temporal gyrus posterior division for secondary auditory cortex, the precentral gyrus for the primary motor cortex, and the lingual gyrus for the primary visual cortex (see Supplemental Figure 2 under “Supplemental data” in the online issue) as previously described (34).
The hypothalamus was not chosen as an a priori ROI because neither exogenous ghrelin nor fasting altered hypothalamic BOLD responses to food pictures in previous human fMRI studies (13, 38), and fasting was not associated with hypothalamic activation during the evaluation of food pictures by using an identical evaluative fMRI paradigm to our study (34). The interpretation of midbrain ventral tegmental area (VTA) signal in fMRI task paradigms is also complicated by physiologic noise and registration issues (39).
fROIs were determined from the Fasted-Initial visit by a higher level whole-brain analysis with a mixed-effects analysis to identify voxels that were significantly more activated at the group level, for the FOOD contrast in the food evaluation paradigm, and for the control AMV task with FEAT version 5.98 software. Correction for multiple comparisons was made by using a voxel-wise FDR at P < 0.05.
The fROIs were obtained by masking these group activation maps with an a priori anatomical ROI with fslmaths software within FSL. As previously described (34), these fROIs were defined by the relevant bilateral ROIs from the cortical and subcortical structural Harvard FSL atlas threshold at 10% probability. The OFC fROI included regions in the OFC and frontal pole with y greater than 22 and z less than −6 because the analysis of functional activation in this region showed distinct bilateral clusters that overlapped anatomical Harvard atlas regions (see Supplemental Figure 1 under “Supplemental data” in the online issue.). The insula was further subdivided into the anterior insula (y greater than +4).
Comparison of BOLD signals between visits
The average (median) magnitude of the bilateral BOLD signal within each a priori fROI was extracted for each individual subject separately for the Fasted-Saline, Fed-Saline, and Fed-Ghrelin visits for FOOD, HE, LE, and HE greater than LE contrasts by using Featquery software in FSL to measure differences in the BOLD signal between visits for different picture categories or control tasks (34). An analysis of the fROI BOLD signal was performed in all subjects and, in a subanalysis, was performed just in men (the number of women was too small to examine between-sex differences or effects in women alone).
Correlations of individual changes in the magnitude of the BOLD signal between visits were also made with individual changes in the picture appeal rating between visits. A similar analysis was performed to compare auditory, motor, and visual task BOLD signals in relevant fROIs between visits. The average BOLD signal for each of these contrasts within each ROI was compared between visits outside FSL.
Statistics
Results are presented as means ± SEMs or medians (IQRs) if data were not normally distributed. Comparisons between 3 visits were made by using a 1-factor repeated-measures ANOVA with the post hoc Student-Newman-Keuls test (or Dunnett’s test if a single control group was used). If not normally distributed, data were log10 transformed or analyzed by using Friedman’s ANOVA on ranks with the post hoc Student-Newman-Keuls test. Comparison within visits or between 2 visits were made by using the paired Student’s t test or Wilcoxon’s signed rank test if not normally distributed.
When the ANOVA for the effect of the visit for picture-appeal ratings and BOLD signal was nonsignificant, a comparison was also performed by using a 1-factor repeated-measures ANCOVA including BMI alone or BMI, age, and sex as covariates. Adjustment for BMI alone or for BMI, age, and sex did not render nonsignificant differences between visits to be significant (P > 0.05).
A linear regression analysis (to determine Pearson’s r) was performed to examine relations between the ROI BOLD signal (dependent variable) and food-appeal scores or plasma ghrelin concentrations (independent variable). This analysis was done for each individual visit and differences between visits, and only for those ROIs that showed an overall difference between visits (to avoid issues around multiple comparisons). For correlations of plasma ghrelin concentrations with the BOLD signal, the AUC from +40 to +150 min was used to cover the time of MRI scanning and improve the accuracy with repeated measurements. Adjustment for BMI, age, and sex by using multiple linear regression with BMI, age, and sex as additional independent variables did not render nonsignificant correlations to be significant (P = 0.11–0.89). Significance was set at P < 0.05. We used SigmaStat 2.03 (Systat Software) and SPSS v21.0 (IBM) programs for analyses.
RESULTS
Participants
Twenty-two healthy, nonobese adults completed the study. Subject characteristics are given in Table 1. None of the subjects had mild, moderate, or severe depression (all Beck Depression Inventory II scores <14) (27). The median time since the last meal until the fMRI scanning was 16.3 h at the Fasted-Initial visit and 15.7 h at the Fasted-Saline visit compared with 1.6 h at both Fed-Saline and Fed-Ghrelin visits (see Supplemental Table 1 under “Supplemental data” in the online issue). One subject (20-y-old man with BMI of 21.2) was removed from the fMRI BOLD signal analysis because of excess head motion. An additional 3 subjects did not complete all study visits.
Food-appeal ratings
During the fMRI task, all food pictures (high or low energy) were rated on average as significantly more appealing at the Fasted-Saline visit than Fed-Saline visit with a similar trend for the Fed-Ghrelin visit (P = 0.057) (Figure 2A; see Supplemental Table 2 under “Supplemental data” in the online issue). When we looked at the different food categories, high-energy foods were rated significantly more appealing at both Fasted-Saline and Fed-Ghrelin visits than the Fed-Saline visit (Figure 2, A and B; see Supplemental Table 2 under “Supplemental data” in the online issue). The appeal of low-energy foods was not significantly different between visits (P = 0.14). By contrast, appeal ratings of objects were similar between visits (see Supplemental Table 2 under “Supplemental data” in the online issue). The reaction time for subjects to rate any food or high-energy food pictures was significantly less at Fasted-Saline and Fed-Ghrelin visits than the Fed-Saline visit (see Supplemental Table 2 under “Supplemental data” in the online issue). The reaction time for low-energy food pictures was not significantly different between visits (P = 0.13–0.14).
FIGURE 2.
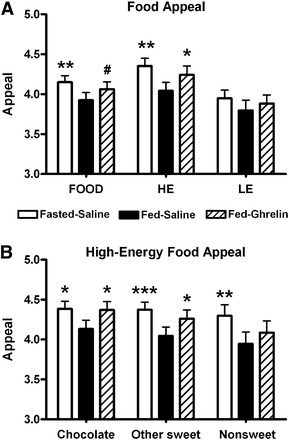
Mean (±SEM) food-appeal ratings at study visits. Appeal rating of food pictures (1 = not at all; 5 = a lot) at Fasted-Saline, Fed-Saline, and Fed-Ghrelin visits for FOOD, HE, and LE (A) and different categories of high-energy foods (chocolate, other sweet, and nonsweet) (B). n = 22. #P = 0.057, *P < 0.05, **P < 0.01, ***P < 0.005 compared with Fed-Saline using a 1-factor repeated-measures ANOVA with post hoc Student Newman-Keuls test. Fasted-Saline, 16-h overnight fast and given subcutaneous saline injection; Fed-Ghrelin, given breakfast and subcutaneous ghrelin injection; Fed-Saline, given breakfast and subcutaneous saline injection; FOOD, high- or low-energy foods; HE, high-energy foods; LE, low-energy foods.
BOLD signal in food-evaluation task
Group activation maps from the Fasted-Initial visit showed a number of brain regions including corticolimbic reward systems with activation to food pictures, including the amygdala, caudate, NAcc, hippocampus, inferior and middle frontal gyrus, insula, OFC, posterior cingulate cortex, putamen, thalamus, and occipital cortex (see Supplemental Figure 1 under “Supplemental data” in the online issue). These activation maps were used to generate fROIs for the OFC, hippocampus, NAcc, caudate, anterior insula, and amygdala (Table 2).
TABLE 2.
Coordinates of functional ROIs for the food-evaluation functional MRI task1
| L/R | ROI | Voxels | Z 2 | x | y | z |
| R | Orbitofrontal cortex | 89 | 3.38 | 22 | 32 | −22 |
| L | Orbitofrontal cortex | 86 | 3.70 | −26 | 34 | −18 |
| R | Hippocampus | 184 | 3.72 | 30 | −30 | −8 |
| L | Hippocampus | 152 | 4.04 | −22 | −30 | −6 |
| R | Nucleus accumbens | 45 | 3.36 | 10 | 16 | −4 |
| L | Nucleus accumbens | 175 | 4.37 | −8 | 10 | −8 |
| R | Caudate | 32 | 3.36 | 10 | 16 | −4 |
| L | Caudate | 134 | 4.11 | −12 | 12 | −6 |
| R | Anterior insula | 463 | 5.24 | 36 | 8 | −16 |
| L | Anterior insula | 204 | 4.93 | −38 | 6 | −12 |
| R | Amygdala | 64 | 3.65 | 22 | −2 | −16 |
| L | Amygdala | 20 | 3.05 | −20 | 2 | −20 |
Activation within functional ROIs at the second level group mixed-effects analysis for FOOD minus object picture contrast at the Fasted-Initial visit (n = 21). Results represent the L or R hemisphere, number of voxels within a cluster (2 mm3; minimum cluster size: 10), Z statistic using a statistical threshold voxel-wise FDR P < 0.05, and coordinates of peak statistical voxel (x, y, z in Montreal Neurological Institute 152 space) in each ROI. Fasted-Initial, first scanning visit with 16-h overnight fast and given subcutaneous saline injection; FDR, false discovery rate; FOOD, high- or low-energy foods; L, left hemisphere; R, right hemisphere; ROI, region of interest.
2All FDRs, P < 0.05.
Whole-brain fMRI analysis
In the whole-brain analysis within the FSL, there were no voxels that displaying significant differences in the BOLD signal for any food, high-energy or low-energy foods, or between Fasted-Saline, Fed-Saline and Fed-Ghrelin visits by using voxel-wise correction at a family-wise error P < 0.05 or FDR P < 0.05 in all subjects.
Comparison of ROI BOLD signals between study visits
OFC activation to any of the foods and only high-energy foods was significantly higher at both Fasted-Saline and Fed-Ghrelin visits than the Fed-Saline visit (Figure 3A; see Supplemental Table 3 under “Supplemental data” in the online issue). A similar trend was seen for OFC activation to low-energy foods to be higher at the Fasted-Saline visit than the Fed-Saline visit (P = 0.09).
FIGURE 3.
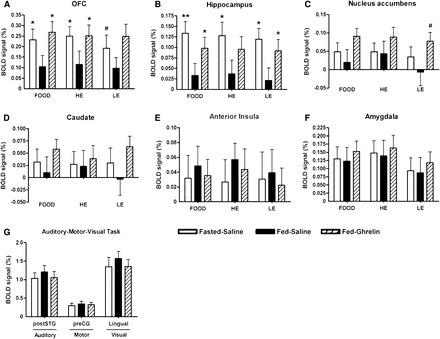
Mean (±SEM) group activation of brain-reward systems to food pictures and auditory, motor, and visual cortex during a control task at study visits. Magnitude of group activation (percentage of BOLD signal change) for FOOD, HE, or LE minus object picture contrast at Fasted-Saline, Fed-Saline, and Fed-Ghrelin visits in the bilateral OFC (A), hippocampus (B), nucleus accumbens (C), caudate (D), anterior insula (E), and amygdala (F). Magnitude of group activation to a control auditory task (listening to a story) in the bilateral postSTG, motor task (button press) in the left preCG, and visual task (flashing checkerboard) in the lingual gyrus (G). n = 21 (both sexes). #P = 0.08–0.09, *P < 0.05, **P< 0.005 compared with Fed-Saline by using a 1-factor repeated-measures ANOVA with the post hoc Student Newman-Keuls test. Fasted-Saline, 16-h overnight fast and given subcutaneous saline injection; Fed-Ghrelin, given breakfast and subcutaneous ghrelin injection; Fed-Saline, given breakfast and subcutaneous saline injection; FOOD, high- or low-energy foods; HE, high-energy foods; LE, low-energy foods; OFC, orbitofrontal cortex; postSTG, posterior division of the superior temporal gyrus; preCG, precentral gyrus.
The hippocampus BOLD signal to any food was significantly higher at both Fasted-Saline and Fed-Ghrelin visits than the Fed-Saline visit (Figure 3B; see Supplemental Table 3 under “Supplemental data” in the online issue). Similar significant patterns were seen for high-energy and low-energy foods when examined separately, although the difference in the hippocampus BOLD signal to high-energy foods between Fed-Ghrelin and Fed-Saline visits was not statistically significant.
There was no significant difference between visits in the BOLD signal in the NAcc, caudate, anterior insula, or amygdala to all foods and high- or low-energy foods (Figure 3, C–F; see Supplemental Table 3 under “Supplemental data” in the online issue), although the BOLD signal in the NAcc to low-energy foods showed a trend to be greater at the Fed-Ghrelin visit than Fed-Saline visit (P = 0.08)
There was no significant correlation between the OFC or hippocampus BOLD signal to any food and high- or low-energy foods (compared with objects) and the respective food appeal (compared with objects) at individual Fasted-Saline, Fed-Saline, or Fed-Ghrelin visits (P = 0.11–0.93) other than a negative correlation between the OFC BOLD signal to low-energy foods and appeal of low-energy foods at the Fasted-Saline visit (r = −0.49, P = 0.03). There was also no significant correlation between the increase in the OFC or hippocampus BOLD signal and increase in food appeal (compared with objects) from Fed-Saline to Fasted-Saline visits (P = 0.19–0.78) or from Fed-Ghrelin to Fed-Saline visits (P = 0.19–0.98).
BOLD signals in the secondary auditory cortex, primary motor cortex, and primary visual cortex were similar between visits during the control AMV fMRI task (Figure 3G; see Supplemental Figure 2 under “Supplemental data” in the online issue).
ROI BOLD signal in men only
When we restricted the fMRI analysis to men only (n = 16), the comparison of the ROI BOLD signal between study visits gave broadly similar results to that seen when women were included (see Supplemental Table 4 under “Supplemental data” in the online issue). Despite the smaller number of subjects, the BOLD signal to low-energy foods in the OFC and NAcc and high-energy foods in the hippocampus was now significantly greater at the Fed-Ghrelin visit than Fed-Saline visit (see Supplemental Table 4 under “Supplemental data” in the online issue). However, the BOLD signal to low-energy foods in the OFC and NAcc was not significantly different between Fasted-Saline and Fed-Saline visits.
Direct comparison of high- with low-energy foods
High-energy foods were of significantly greater appeal than low-energy foods at both Fasted-Saline (P = 0.006) and Fed-Ghrelin (P = 0.025) visits (see Supplemental Table 2 under “Supplemental data” in the online issue). At the Fasted-Saline visit, each category of high-energy food was rated as significantly more appealing than low-energy foods (chocolate, sweet, and nonsweet: P < 0.05 compared with low energy). At the Fed-Ghrelin visit, only chocolate and sweet high-energy foods were rated significantly more appealing than low-energy foods (P < 0.05). By contrast, high-energy foods were not significantly different in appeal to low-energy foods at the Fed-Saline visit (P = 0.22). Consistent with this result, there was a trend for the appeal rating of high- minus low-energy foods to be higher at the Fasted-Saline visit than Fed-Saline visit (P = 0.056) and Fed-Ghrelin visit than Fed-Saline visit (P = 0.096) visits (see Supplemental Table 2 under “Supplemental data” in the online issue).
However, there was no significant differences in the reaction time (P = 0.32; see Supplemental Table 2 under “Supplemental data” in the online issue) or BOLD signal in any ROI (P = 0.55–0.95; see Supplemental Tables 3 and 4 under “Supplemental data” in the online issue) for high minus low-energy foods between Fasted-Saline, Fed-Saline and Fed-Ghrelin visits.
VASs and food intake
VAS ratings outside the MRI scanner (10-cm scale) for hunger, pleasantness to eat, and volume able to eat were significantly lower and, for fullness, significantly greater at Fed-Saline and Fed-Ghrelin visits than the Fasted-Saline visit both before and after the injection (Figure 4; see Supplemental Figure 3 under “Supplemental data” in the online issue). However, there was no significant difference in the appetite VAS outside the scanner between Fed-Saline and Fed-Ghrelin visits after the injection.
FIGURE 4.
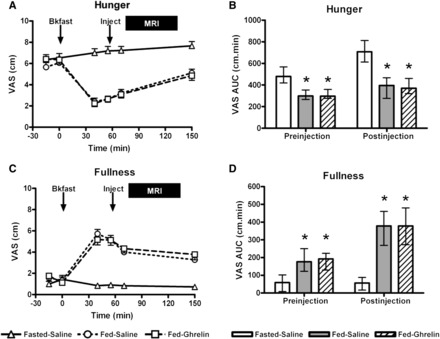
Hunger and fullness VASs at study visits. Mean (±SEM) VAS ratings plotted against time for hunger (A) and fullness (C) at Fasted-Saline, Fed-Saline, and Fed-Ghrelin visits. Arrows indicate the time of breakfast (0 min) and subcutaneous injection (+55 min), and the solid bar denotes the time of the MRI scan (from +75 to +135 min). n = 22. Median (IQR) AUC VAS ratings for hunger (B) and fullness (D) at Fasted-Saline, Fed-Saline, and Fed-Ghrelin visits. The preinjection AUC was for the time period from −15 to +55 min (maximum value: 700 cm/min). The postinjection AUC was for the time period from +55 to +150 min and covered the period of the MRI scan (maximum value: 950 cm/min). *P < 0.05 compared with Fasted-Saline for post hoc Student Newman-Keuls test in a 1-factor repeated-measures ANOVA on ranks (both P < 0.001 for the overall ANOVA). n = 22. Bkfast, breakfast; Fasted-Saline, 16-h overnight fast and given subcutaneous saline injection; Fed-Ghrelin, given breakfast and subcutaneous ghrelin injection; Fed-Saline, given breakfast and subcutaneous saline injection; Inject, injection; VAS, visual analog scale.
Mean hunger ratings (maximum 5) inside the scanner were significantly lower at Fed-Saline (3.46 ± 0.20; P < 0.001) and Fed-Ghrelin (3.74 ± 0.15; P < 0.001) visits than the Fasted-Saline visit (4.73 ± 0.09), but the difference between the 2 fed visits was not significant (P = 0.21). Energy intake at lunch after scanning was significantly greater at the Fasted-Saline visit [median (IQR): 1314 kcal (1143–1848 kcal); 23.1 kcal/kg LBM (19.8–27.5 kcal/kg LBM)] than at both Fed-Saline [1184 kcal (975–1502 kcal); 19.2 kcal/kg LBM (16.5–23.3 kcal/kg LBM), P < 0.01] and Fed-Ghrelin [1067 kcal (974–1703 kcal; 18.6 kcal/kg LBM (15.5–23.7 kcal/kg LBM); P < 0.01] visits (overall ANOVA on ranks, both P = 0.001), but there was no significant difference in food intake between Fed-Ghrelin and Fed-Saline visits (both P > 0.05).
Confounding variables
There was no significant difference in potential confounding variables between study visits such as the duration of sleep, mood, body composition, time since last visit, time since supper, energy consumed at supper, time to eat breakfast, or head motion during fMRI scanning (see Supplemental Table 1 under “Supplemental data” in the online issue). VAS ratings outside the MRI scanner for side effects such as sickness, anxiety, and stress were also similar at all 3 study visits (see Supplemental Table 5 under “Supplemental data” in the online issue).
Blood hormones and metabolites
Serum GH concentrations after the injection at +55 min were significantly higher at both Fed-Ghrelin and Fasted-Saline visits than the Fed-Saline visit (Figure 5A; see Supplemental Table 6 under “Supplemental data” in the online issue). As expected, plasma acyl ghrelin concentrations were significantly higher over the fMRI scan at the Fasted-Saline visit than Fed-Saline visit because of a postprandial decline after breakfast (Figure 5B; see Supplemental Table 6 under “Supplemental data” in the online issue) and were higher at the Fed-Ghrelin visit than both Fasted-Saline and Fed-Saline visits.
FIGURE 5.
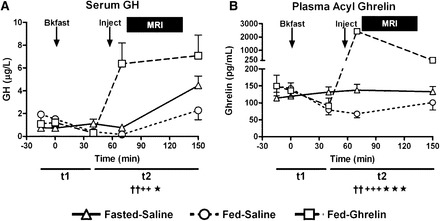
Blood growth hormone and acyl ghrelin concentrations at study visits. Mean (±SEM) concentrations over time of serum GH (A) and plasma acyl ghrelin (B) at Fasted-Saline, Fed-Saline, and Fed-Ghrelin visits. Arrows indicate the time of breakfast (0 min) and subcutaneous injection (+55 min), and the solid bar denotes the time of the MRI scan (from +75 to +135 min). n = 22. AUC for t1 (from −15 to +40 min) or t2 (from +40 to +150 min). ††P < 0.01, Fasted-Saline compared with Fed-Saline; ++P < 0.01, +++P < 0.005, Fed-Ghrelin compared with Fed-Saline; *P < 0.05, ***P < 0.001, Fed-Ghrelin compared with Fasted-Saline (see Supplemental Table 6 under “Supplemental data” in the online issue). Comparisons were made by using a 1-factor repeated-measures Friedman’s ANOVA on ranks with post hoc Student-Newman-Keuls test. Bkfast, breakfast; Fasted-Saline, 16-h overnight fast and given subcutaneous saline injection; Fed-Ghrelin, given breakfast and subcutaneous ghrelin injection; Fed-Saline, given breakfast and subcutaneous saline injection; GH, growth hormone; Inject, injection.
At individual Fasted-Saline, Fed-Saline, and Fed-Ghrelin visits, there was no significant correlation between plasma ghrelin concentrations (AUC from +40 to +150 min) and the BOLD signal to any food or just high-energy or low-energy foods in the OFC (P = 0.44–0.97) or hippocampus (P = 0.11–0.85) other than a trend for the hippocampal BOLD signal to low-energy foods to be positively correlated with ghrelin concentrations at the Fed-Ghrelin visit (r = +0.42, P = 0.058). Similarly, there was no significant correlation between increases in the OFC or hippocampal BOLD signal for any food category and increases in plasma ghrelin concentrations from Fed-Saline to Fasted-Saline visits (P = 0.23–0.56) or from Fed-Ghrelin to Fed-Saline visits (P = 0.12–0.99).
Plasma glucose concentrations rose initially after breakfast at both Fed-Saline and Fed-Ghrelin visits to become higher than at the Fasted-Saline visit (see Supplemental Figure 4A and Table 6 under “Supplemental data” in the online issue). However, over the fMRI scanning period, glucose concentrations did not differ significantly between Fed-Saline and Fasted-Saline visits but were significantly higher at the Fed-Ghrelin visit than both Fed-Saline and Fasted-Saline visits (see Supplemental Figure 4A and Table 6 under “Supplemental data” in the online issue).
Serum insulin and triglycerides were significantly higher over both Fed-Saline and Fed-Ghrelin visits than the Fasted-Saline visit because of postprandial increases after breakfast (see Supplemental Figure 4D and Table 6 under “Supplemental data” in the online issue). However, serum insulin and triglycerides did not differ significantly between Fed-Ghrelin and Fed-Saline visits.
Plasma GLP-1 and PYY concentrations were not significantly different between Fasted-Saline and Fed-Saline visits (see Supplemental Figure 4, E and F, and Table 6 under “Supplemental data” in the online issue). However over the fMRI scanning period, both plasma GLP-1 and PYY were significantly and unexpectedly higher at the Fed-Ghrelin visit than both Fed-Saline and Fasted-Saline visits (see Supplemental Figure 4, E and F, and Table 6 under “Supplemental data” in the online issue).
DISCUSSION
In this fMRI study of nonobese adults, ghrelin administration after breakfast mimicked the effects of fasting to increase the appeal of and speed of rating for high-energy foods, markers of both explicit and implicit wanting (40), and the associated OFC BOLD signal. There were no significant effects of the visit on these measures for low-energy foods, but there were no significant differences between high-energy and low-energy foods. In men, ghrelin administration but not fasting significantly increased OFC and NAcc BOLD signals to low-energy foods. Both ghrelin and fasting increased the hippocampal activation to food, irrespective of the energy density. Thus, ghrelin and fasting have been shown, for the first time to our knowledge, to display similar stimulatory effects on hedonic food ratings and the activation of corticolimbic reward-cognitive systems during food evaluation in the same subjects. These effects of fasting and ghrelin can be envisaged as an appropriate counter-regulatory response to a negative energy balance, as with the GH secretagogue action of ghrelin to maintain plasma glucose concentrations during food restriction (41).
Effects of exogenous and endogenous hyperghrelinemia to increase the OFC BOLD signal during the evaluation of high-energy food pictures supported a stimulatory effect of ghrelin on food hedonics, which was consistent with results of preclinical studies (22, 42, 43). The OFC plays a role in the encoding of value and the salience of rewards, including food (6, 34, 44, 45). These findings are in partial agreement with those of previous fMRI studies. Acute intravenous ghrelin administration to fed, nonobese subjects increased the BOLD signal to the viewing of food pictures in several brain regions including the OFC and hippocampus but also insula, amygdala, and caudate (13). A purely associative fMRI study showed that fasting plasma ghrelin concentrations in normal-weight subjects were positively correlated with the BOLD signal to passive viewing of highly palatable foods in regions related to visual processing, reward, and taste including in the caudate, pallidum, rolandic operculum, amygdala, and anterior cingulate gyrus (as well as the hypothalamus and midbrain) but showed no correlation with the OFC or hippocampus BOLD signal (26). In a recent pharmacological MRI study, ghrelin administration in nonobese subjects in the postprandial state decreased the resting (nontask-related) BOLD signal in a number of brain regions including the brainstem, hypothalamus, and hippocampus but not the OFC (25). Rodent studies have shown that the amygdala is a neurobiological target of ghrelin (35).
However, we were unable to show increases in the BOLD signal of the amygdala, caudate, or anterior insula to food pictures by ghrelin. This result may have been related to differences in study designs, fMRI paradigms (passive compared with. active evaluation) (46), statistical methods (whole brain compared with an ROI approach) and thresholds, and, perhaps, a masking effect from the suppression of activation with habituation to previously viewed images. NAcc, caudate, and anterior insula BOLD signals to identical food pictures tended to decrease between Fasted-Initial and Fasted-Saline visits (see Supplemental Table 4 under “Supplemental data” in the online issue), consistent with a role for a hippocampus-NAcc network in the encoding of a stimulus novelty (47). Indeed ghrelin enhances novelty-seeking behavior (48). Despite ghrelin’s stimulatory effect on VTA-NAcc dopaminergic neurons via GHSR1a in the VTA, dorsolateral tegmental area, and hypothalamic-midbrain orexin neurons in preclinical studies (17, 18, 49–51), no influence of ghrelin on the NAcc BOLD signal to food or at rest has been reported in any previous ghrelin fMRI studies to our knowledge (13, 25, 26). We detected a significant increase in the NAcc BOLD signal to low-energy foods with ghrelin administration in a subanalysis of men, which suggested possible sex differences (52, 53), although our study was not powered to examine this possibility directly.
The increase in the hippocampus BOLD signal to food pictures by endogenous and exogenous hyperghrelinemia was consistent with results of preclinical (36, 37) and human fMRI (13, 26) studies. Reward motivation is also thought to promote memory formation via hippocampal dopamine release before learning (54), and ghrelin administration increases food-picture recognition at recall (13), as does fasting, where enhanced food memory has also been linked to OFC activation (3).
Consistent with a previous study of fasting (6), there was a trend for both fasting and ghrelin to increase the appeal of high-energy foods relative to low-energy foods. However, when directly subtracted, we were unable to find any significant influence of the energy content on the effect of ghrelin or fasting on appeal rating, reaction time, and the OFC or hippocampal BOLD signal. These results differed from previous studies that have reported interactions of food-picture energy density and fasting on the activation in several brain regions, including the OFC (6, 52, 53). These differences may be explicable because of different fMRI analytic approaches, the use of a fixed breakfast meal at fed visits (compared with subjects eating from a choice of foods to satiation), the preponderance of men (52, 53), and the inclusion of only nonobese subjects who may have different responses than those of obese subjects (55).
Effects of ghrelin and fasting did not appear related to absolute or relative hypoglycemia because plasma glucose concentrations over scanning were not lower at the Fasted-Saline or Fed-Ghrelin visits than the Fed-Saline visit (30). Similarly, their effects were not related to consistent changes in plasma insulin, PYY, and GLP-1. Although insulin concentrations were reduced by fasting which could have increased hedonic responses (14, 15), insulin concentrations were not altered by ghrelin administration. Breakfast consumption did not increase plasma PYY and GLP-1 concentrations, as also reported for PYY3–36 or active GLP-1 (GLP-17–36 amide and GLP-17–37) when using an identical meal (16). Furthermore, the stimulatory effect of ghrelin on anticipatory food reward occurred despite unexpected increases in anorexigenic GLP-1 and PYY concentrations, probably because of a prokinetic effect of ghrelin on gastric emptying after breakfast (56), which would be predicted to have reduced BOLD signals in our ROIs (16).
The relatively small number of subjects may have contributed to a type 2 error in the lack of effects on appeal rating, reaction time, and the OFC BOLD signal to low-energy foods and precluded any examination of sex differences. This limitation might also explain why no effect of exogenous ghrelin to increase hunger VAS ratings or ad libitum food intake was seen (20, 21). Alternatively, effects of ghrelin to increase plasma glucose and anorexigenic plasma GLP-1 and PYY concentrations may have counteracted a stimulatory effect of ghrelin on hunger or food intake. The lack of a buffet choice may also have hindered the ability to see an effect of exogenous ghrelin on food intake (20). Nevertheless, differential effects of fasting and ghrelin on hunger and food intake but identical effects on food hedonics and brain activation would also have been consistent with ghrelin’s action on food reward being dissociable from effects of hunger and consumptive aspects of eating behavior, as shown in preclinical studies (43).
The use of a fixed breakfast in subjects with different BMI may also have contributed to nonsignificance by increasing variance at fed visits. The inclusion of both lean and overweight subjects was an additional limitation because they may differ in food hedonics and responses to hyperghrelinemia. The inclusion of BMI as a between-subject factor did not render nonsignificant differences in food appeal or the BOLD signal between visits to be significant. Although women were scanned in the first one-half of the menstrual cycle, there could have been differences in food hedonic and reward responses even within this time period, and sex hormones were not measured. The exclusion of females from the analysis gave broadly similar results when ROI BOLD signals between study visits were compared.
Plasma concentrations of acyl ghrelin achieved after the administration of exogenous ghrelin were also much greater than those achieved with endogenous hyperghrelinemia from overnight fasting in our study. Rodent studies have shown an increased brain uptake of and sensitivity to ghrelin when fasted, which might attenuate the biological differences of these dissimilar degrees of hyperghrelinemia (57, 58). However, it was not yet possible to distinguish pharmacological from physiological effects of ghrelin on food-reward systems, which would have required the study of GHSR1a antagonists or GOAT inhibitors in humans. The inclusion of a Fasted-Ghrelin visit would also have enabled the direct examination of different degrees of hyperghrelinemia and any interaction of food intake with hyperghrelinemia.
In conclusion, this study has shown that, compared with when the same individuals have low endogenous plasma ghrelin concentrations (after the consumption of a meal), both endogenous hyperghrelinemia (produced by acute fasting) and exogenous hyperghrelinemia (produced by acute acyl ghrelin administration) increase food hedonic ratings and increase OFC and hippocampus BOLD signals during food evaluation. Dietary manipulations such as recurrent breakfast skipping or food restriction will produce recurrent or chronic hyperghrelinemia (19). There may be limitations of transferring the findings of this acute, experimental study to changes in real-world eating behaviors and weight changes in obese individuals. However, our results support the suggestion that the enhancement of food hedonic-reward responses during such dietary manipulations may contribute to a negative impact on dietary habits and long-term weight loss as part of a homeostatic feedback loop mediated through hyperghrelinemia. This effect suggests that the ghrelin-GOAT-GHSR1a system may be a drug target for reducing food hedonics during dieting.
Supplementary Material
Acknowledgments
We thank Thai Wong, Emer Hughes, Rita Nunes, Jo Hajnal, David Larkman, Joanna Allsop, and the Sir John McMichael Centre/National Institute for Health Research-Wellcome Trust Imperial College Clinical Research Facility staff for support with the study and Susan Carnell for helpful comments.
The authors’ responsibilities were as follows—APG, CGP, GSF, SRB, and JDB: designed the research; DRA: provided essential materials; APG, CGP, SS, ADM, NC, GD, and SSD: conducted the research; APG, CGP, SS, ADM, NC, SSD, CB, MAG, ADW, BDG, and MOT: analyzed data and samples; APG and CGP: wrote the manuscript; APG: took primary responsibility for the final content of the manuscript; and all authors: reviewed the manuscript. None of the authors had a conflict of interest.
ABBREVIATIONS
- AMV
auditory-motor-visual
- Fasted-Initial
first scanning visit with 16-h overnight fast and given subcutaneous saline injection
- Fasted-Saline
16-h overnight fast and given subcutaneous saline injection
- FDR
false discovery rate
- Fed-Ghrelin
given breakfast and subcutaneous ghrelin injection
- Fed-Saline
given breakfast and subcutaneous saline injection
- FMRIB
Functional MRI of the Brain
- FOOD
high-energy or low-energy food
- fROI
functional region of interest
- FSL
FMRIB’s Software Library
- GH
growth hormone
- GHSR1a
growth hormone secretagogue receptor 1a
- GLP-1
glucagon-like peptide-1
- GOAT
ghrelin O-acyltransferase
- HE
high-energy-dense food; Initial-Saline, first fMRI visit, fasted and given subcutaneous saline injection
- LBM
lean body mass
- LE
low-energy-dense food
- NAcc
nucleus accumbens
- OFC
orbitofrontal cortex
- PYY
peptide YY
- ROI
region of interest
- VAS
visual analog scale
- VTA
ventral tegmental area
FOOTNOTES
Supported by grants from the UK Medical Research Council (MRC), the Imperial College Healthcare Charity, and the National Institute for Health Research Imperial Biomedical Research Centre Funding Scheme; a European Union Marie-Curie Fellowship (NuSISCO; to CGP); a Wellcome Trust Research Training Fellowship (to SS); an MRC Clinical Training Fellowship (to ADM); and an Imperial College Healthcare Charity Fellowship (to NC).
REFERENCES
- 1. Berthoud HR.. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol 2011;21:888–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Volkow ND Wang GJ Tomasi D Baler RD.. The addictive dimensionality of obesity. Biol Psychiatry 2013;73:811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morris JS Dolan RJ.. Involvement of human amygdala and orbitofrontal cortex in hunger-enhanced memory for food stimuli. J Neurosci 2001;21:5304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cameron JD Goldfield GS Cyr MJ Doucet E.. The effects of prolonged caloric restriction leading to weight-loss on food hedonics and reinforcement. Physiol Behav 2008;94:474–80. [DOI] [PubMed] [Google Scholar]
- 5. Rosenbaum M Sy M Pavlovich K Leibel RL Hirsch J.. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest 2008;118:2583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldstone AP Prechtl de Hernandez C Beaver JD Muhammed K Croese C Bell G Durighel G Hughes E Waldman AD Bell JD.. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci 2009;30:1625–35. [DOI] [PubMed] [Google Scholar]
- 7. Stice E Burger K Yokum S.. Caloric deprivation increases responsivity of attention and reward brain regions to intake, anticipated intake, and images of palatable foods. Neuroimage 2013;67:322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Merten MJ Williams AL Shriver LH.. Breakfast consumption in adolescence and young adulthood: parental presence, community context, and obesity. J Am Diet Assoc 2009;109:1384–91. [DOI] [PubMed] [Google Scholar]
- 9. Leidy HJ Lepping RJ Savage CR Harris CT.. Neural responses to visual food stimuli after a normal vs. higher protein breakfast in breakfast-skipping teens: a pilot fMRI study. Obesity (Silver Spring) 2011;19:2019–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Field BC Chaudhri OB Bloom SR.. Bowels control brain: gut hormones and obesity. Nat Rev Endocrinol 2010;6:444–53. [DOI] [PubMed] [Google Scholar]
- 11. Scerif M Goldstone AP Korbonits M.. Ghrelin in obesity and endocrine diseases. Mol Cell Endocrinol 2011;340:15–25. [DOI] [PubMed] [Google Scholar]
- 12. Batterham RL1 ffytche DH Rosenthal JM Zelaya FO Barker GJ Withers DJ Williams SC.. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature 2007;450:106–9. [DOI] [PubMed] [Google Scholar]
- 13. Malik S McGlone F Bedrossian D Dagher A.. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab 2008;7:400–9. [DOI] [PubMed] [Google Scholar]
- 14. Figlewicz DP Benoit SC.. Insulin, leptin, and food reward: update 2008. Am J Physiol Regul Integr Comp Physiol 2009;296:R9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guthoff M Grichisch Y Canova C Tschritter O Veit R Hallschmid M Haring HU Preissl H Hennige AM Fritsche A.. Insulin modulates food-related activity in the central nervous system. J Clin Endocrinol Metab 2010;95:748–55. [DOI] [PubMed] [Google Scholar]
- 16. De Silva A. Salem V Long CJ Makwana A Newbould RD Rabiner EA Ghatei MA Bloom SR Matthews PM Beaver JD, et al. The gut hormones PYY 3-36 and GLP-1 7-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab 2011;14:700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dickson SL Egecioglu E Landgren S Skibicka KP Engel JA Jerlhag E.. The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol 2011;340:80–7. [DOI] [PubMed] [Google Scholar]
- 18. Skibicka KP Dickson SL.. Ghrelin and food reward: the story of potential underlying substrates. Peptides 2011;32:2265–73. [DOI] [PubMed] [Google Scholar]
- 19. Sumithran P Prendergast LA Delbridge E Purcell K Shulkes A Kriketos A Proietto J.. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 2011;365:1597–604. [DOI] [PubMed] [Google Scholar]
- 20. Wren AM Seal LJ Cohen MA Brynes AE Frost GS Murphy KG Dhillo WS Ghatei MA Bloom SR.. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 2001;86:5992–5. [DOI] [PubMed] [Google Scholar]
- 21. Druce MR Neary NM Small CJ Milton J Monteiro M Patterson M Ghatei MA Bloom SR.. Subcutaneous administration of ghrelin stimulates energy intake in healthy lean human volunteers. Int J Obes (Lond) 2006;30:293–6. [DOI] [PubMed] [Google Scholar]
- 22. Perelló M Zigman JM.. The role of ghrelin in reward-based eating. Biol Psychiatry 2012;72:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tong J Mannea E Aime P Pfluger PT Yi CX Castaneda TR Davis HW Ren X Pixley S Benoit S, et al. Ghrelin enhances olfactory sensitivity and exploratory sniffing in rodents and humans. J Neurosci 2011;31:5841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chuang JC Perello M Sakata I Osborne-Lawrence S Savitt JM Lutter M Zigman JM.. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest 2011;121:2684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones RB McKie S Astbury N Little TJ Tivey S Lassman DJ McLaughlin J Luckman S Williams SR Dockray GJ, et al. Functional neuroimaging demonstrates that ghrelin inhibits the central nervous system response to ingested lipid. Gut 2012;61:1543–51. [DOI] [PubMed] [Google Scholar]
- 26. Kroemer NB Krebs L Kobiella A Grimm O Pilhatsch M Bidlingmaier M Zimmermann US Smolka MN.. Fasting levels of ghrelin covary with the brain response to food pictures. Addict Biol 2013;18:855–62. [DOI] [PubMed] [Google Scholar]
- 27. Beck AT Steer RA Ball R Ranieri W.. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess 1996;67:588–97. [DOI] [PubMed] [Google Scholar]
- 28. Frank TC Kim GL Krzemien A Van Vugt DA.. Effect of menstrual cycle phase on corticolimbic brain activation by visual food cues. Brain Res 2010;1363:81–92. [DOI] [PubMed] [Google Scholar]
- 29. Killgore WD Yurgelun-Todd DA.. Affect modulates appetite-related brain activity to images of food. Int J Eat Disord 2006;39:357–63. [DOI] [PubMed] [Google Scholar]
- 30. Page KA Seo D Belfort-DeAguiar R Lacadie C Dzuira J Naik S Amarnath S Constable RT Sherwin RS Sinha R.. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J Clin Invest 2011;121:4161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kreymann B Williams G Ghatei MA Bloom SR.. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet 1987;2:1300–4. [DOI] [PubMed] [Google Scholar]
- 32. Allen JM Fitzpatrick ML Yeats JC Darcy K Adrian TE Bloom SR.. Effects of peptide YY and neuropeptide Y on gastric emptying in man. Digestion 1984;30:255–62. [DOI] [PubMed] [Google Scholar]
- 33. Liu J Prudom CE Nass R Pezzoli SS Oliveri MC Johnson ML Veldhuis P Gordon DA Howard AD Witcher DR, et al. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab 2008;93:1980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth M, Daud NM, Ismail N, Durighel G, Ahmed AR, Olbers T, et al. Obese patients after gastric bypass surgery have lower brain hedonic responses to food than after gastric banding. Gut (Epub ahead of print 20 August 2013). [DOI] [PMC free article] [PubMed]
- 35. Alvarez-Crespo M Skibicka KP Farkas I Molnar CS Egecioglu E Hrabovszky E Liposits Z Dickson SL.. The amygdala as a neurobiological target for ghrelin in rats: neuroanatomical, electrophysiological and behavioral evidence. PLoS ONE 2012;7:e46321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carlini VP Varas MM Cragnolini AB Schioth HB Scimonelli TN de Barioglio SR.. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochem Biophys Res Commun 2004;313:635–41. [DOI] [PubMed] [Google Scholar]
- 37. Kanoski SE Fortin SM Ricks KM Grill HJ.. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol Psychiatry 2013;73:915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fletcher PC1, Napolitano A, Skeggs A, Miller SR, Delafont B, Cambridge VC, de Wit S, Nathan PJ, Brooke A, O’Rahilly S.et al. Distinct modulatory effects of satiety and sibutramine on brain responses to food images in humans: a double dissociation across hypothalamus, amygdala, and ventral striatum. J Neurosci 2010;30:14346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. D’Ardenne K McClure SM Nystrom LE Cohen JD.. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science 2008;319:1264–7. [DOI] [PubMed] [Google Scholar]
- 40. Finlayson G King N Blundell J.. The role of implicit wanting in relation to explicit liking and wanting for food: implications for appetite control. Appetite 2008;50:120–7. [DOI] [PubMed] [Google Scholar]
- 41. Zhao TJ Liang G Li RL Xie X Sleeman MW Murphy AJ Valenzuela DM Yancopoulos GD Goldstein JL Brown MS.. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA 2010;107:7467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Skibicka KP Hansson C Egecioglu E Dickson SL.. Role of ghrelin in food reward: impact of ghrelin on sucrose self-administration and mesolimbic dopamine and acetylcholine receptor gene expression. Addict Biol 2012;17:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Skibicka KP Hansson C Alvarez-Crespo M Friberg PA Dickson SL.. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience 2011;180:129–37. [DOI] [PubMed] [Google Scholar]
- 44. Rolls ET.. Functions of the orbitofrontal and pregenual cingulate cortex in taste, olfaction, appetite and emotion. Acta Physiol Hung 2008;95:131–64. [DOI] [PubMed] [Google Scholar]
- 45. Wang GJ Volkow ND Telang F Jayne M Ma Y Pradhan K Zhu W Wong CT Thanos PK Geliebter A, et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci USA 2009;106:1249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bender G Veldhuizen MG Meltzer JA Gitelman DR Small DM.. Neural correlates of evaluative compared with passive tasting. Eur J Neurosci 2009;30:327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guitart-Masip M Bunzeck N Stephan KE Dolan RJ Duzel E.. Contextual novelty changes reward representations in the striatum. J Neurosci 2010;30:1721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hansson C Shirazi RH Naslund J Vogel H Neuber C Holm G Anckarsater H Dickson SL Eriksson E Skibicka KP.. Ghrelin influences novelty seeking behavior in rodents and men. PLoS ONE 2012;7:e50409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Naleid AM Grace MK Cummings DE Levine AS.. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides 2005;26:2274–9. [DOI] [PubMed] [Google Scholar]
- 50. Abizaid A Liu ZW Andrews ZB Shanabrough M Borok E Elsworth JD Roth RH Sleeman MW Picciotto MR Tschop MH, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest 2006;116:3229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Perello M Sakata I Birnbaum S Chuang JC Osborne-Lawrence S Rovinsky SA Woloszyn J Yanagisawa M Lutter M Zigman JM.. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry 2010;67:880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Siep N Roefs A Roebroeck A Havermans R Bonte ML Jansen A.. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res 2009;198:149–58. [DOI] [PubMed] [Google Scholar]
- 53. Frank S Laharnar N Kullmann S Veit R Canova C Hegner YL Fritsche A Preissl H.. Processing of food pictures: influence of hunger, gender and calorie content. Brain Res 2010;1350:159–66. [DOI] [PubMed] [Google Scholar]
- 54. Adcock RA Thangavel A Whitfield-Gabrieli S Knutson B Gabrieli JD.. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron 2006;50:507–17. [DOI] [PubMed] [Google Scholar]
- 55. Ziauddeen H Farooqi IS Fletcher PC.. Obesity and the brain: how convincing is the addiction model? Nat Rev Neurosci 2012;13:279–86. [DOI] [PubMed] [Google Scholar]
- 56. Falkén Y1 Hellström PM Sanger GJ Dewit O Dukes G Grybäck P Holst JJ Näslund E.. Actions of prolonged ghrelin infusion on gastrointestinal transit and glucose homeostasis in humans. Neurogastroenterol Motil 2010;22:e192–200. [DOI] [PubMed] [Google Scholar]
- 57. Hewson AK Dickson SL.. Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. J Neuroendocrinol 2000;12:1047–9. [DOI] [PubMed] [Google Scholar]
- 58. Schaeffer M Langlet F Lafont C Molino F Hodson DJ Roux T Lamarque L Verdie P Bourrier E Dehouck B, et al. Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc Natl Acad Sci USA 2013;110:1512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


