Abstract
Health concerns have been pronounced for cosmetologists and manicurists, who are exposed daily to cosmetic products containing known or suspected human carcinogens and endocrine disruptors. In this retrospective cohort study, the authors used probabilistic record linkage between California's statewide cosmetology licensee and cancer surveillance files to identify newly diagnosed invasive cancers among female workforce members during 1988–2005. Rate ratios and 95% confidence intervals for cancer among workforce members compared with the general female population in California were estimated via Poisson regression. For comparison, site-specific proportional incidence ratios were computed. The authors identified 9,044 cancer cases in a cohort of 325,228 licensees. Rate ratios for all sites combined suggested lower incidence among both cosmetologists (rate ratio = 0.84, 95% confidence interval (CI): 0.82, 0.86) and manicurists (rate ratio = 0.87, 95% CI: 0.84, 0.90). Proportional incidence ratios were modestly elevated for thyroid cancer among all licensees (proportional incidence ratio = 1.13, 95% CI: 1.04, 1.23) and for lung cancer among manicurists (proportional incidence ratio = 1.21, 95% CI: 1.07, 1.36). Although there did not appear to be a cancer excess, these findings may be artifactually influenced by limitations in demographic information available from the licensee files. Additionally, the relatively young ages of cohort members and demographic shifts in the industry composition in recent years suggest a need for further follow-up.
Keywords: cohort studies, cosmetics, environment and public health, incidence, lung neoplasms, occupational exposure, thyroid neoplasms
In recent years, the safety of cosmetic products has garnered considerable public attention because of the potentially harmful compounds included in them, some of which are known or suspected carcinogens and endocrine disruptors. Public concerns were raised after the European Union passed laws restricting the use of potentially harmful compounds in cosmetic products (1). Despite the heavy use of personal care products, the cosmetic industry is largely unregulated in the United States (2, 3). Health concerns have been particularly pronounced for cosmetology workers who provide hair and nail care services because they are exposed daily to an array of potentially hazardous compounds associated with nearly every hair and nail care service they provide.
The cosmetology industry in the United States is composed mainly of cosmetologists (who provide hair and nail care services) and manicurists (who provide nail care services only) and has been one of the fastest growing professions in the nation. California has led the way with over 300,000 technicians licensed to perform hair and nail care services since 1970 (4). Recent industry estimates also show that a vast majority of cosmetology workers, particularly manicurists, are women (5).
Hair and nail care products may contain toxic and potentially hazardous ingredients in varying amounts, including solvents, plasticizers, resins, and acids (6). Known and suspected carcinogenic compounds found in these cosmetic products include titanium dioxide, formaldehyde, benzoyl peroxide, and 1,4-dioxane (7, 8). Formaldehyde and titanium dioxide, found in low levels in nail care products, are known or suspected carcinogens (9). Other compounds, such as benzoyl peroxide found in artificial nail products, are potentially linked to certain cancers on the basis of evidence from animal studies (7, 10). Hair products, such as dyes, relaxers, and removers, also contain impurities, such as 1,4-dioxane, a possible carcinogen (9). Furthermore, acetone, toluene, paraben, and dibutyl phthalates are commonly found in hair and nail care products and have been shown to affect a woman's endocrine system, which raises concerns regarding hormonally mediated cancers such as breast and ovarian cancer (2, 11).
Many of these chemicals are highly volatile, and most beauty salons are small workplaces with inadequate ventilation, serving to exacerbate workers’ occupational exposures (12–14). The fact that cosmetic products are largely unregulated contributes to inadequate product labeling and limited safety information for cosmetology workers, which may in turn lead to higher exposure. Furthermore, the presence of numerous chemical compounds in beauty salons is likely to be continuous and mixed, the synergistic effects of which are largely unknown. Although exposure levels for individual compounds may be generally low by legal or recommended standards (many of which were established several decades earlier), multiple chemical and multiple routes of exposure (often via inhalation and skin absorption) combined with the inadequate ventilation underscore the need for systematic health assessments in this workforce.
Despite the growth of this industry and the numerous chemicals of concern, human health studies focused on this workforce are very limited. In the present study, we had an opportunity to conduct a statewide, population-based, retrospective cohort study in the largest cosmetology cohort studied to date to determine whether cosmetology workers have a higher incidence of overall or site-specific cancers compared with the general female population in California.
MATERIALS AND METHODS
Data sources
The study population included female cosmetologists and manicurists who have been licensed in California between January 1, 1970, and December 31, 2005. We obtained registration files of licensees from the California Board of Barbering and Cosmetology that regulates the cosmetology profession in California. Licensure with this state board is required to work as a cosmetologist or manicurist in California. We selected information, such as full name, date of birth, partial Social Security number (last 6 digits), residential address, original date of licensure, and license expiration date (license renewal is required every 2 years, and the expiration date on file is that of the most recent renewal), from these license registration files to use for record linkage and analysis.
We obtained all cases of invasive cancer diagnosed among female residents of the state of California from January 1, 1988, through December 31, 2005, from the California Cancer Registry. A contributor to the National Cancer Institute's Surveillance, Epidemiology, End Results (SEER) Program, the California Cancer Registry maintains high standards for data quality and completeness at the statewide level; their data are estimated to be 98% complete (15). We used information from California death registration files in order to exclude licensees who died before 1988, the first year of the follow-up period, and to right censor (i.e., end follow-up time at the time of death) any deaths occurring after 1988 in the calculation of person-years. Age-, sex-, period-, and site-specific rates for the general population in California were also obtained from the California Cancer Registry, which uses the annual, midyear population estimates for age, race/ethnicity, and gender from the California Department of Finance Demographic Research Unit (15, 16). Because the cancer surveillance program did not begin statewide until 1988, our study period was 1988–2005.
Person-years at risk
In order to define this worker cohort for calculation of person-years at risk, we conducted an evaluation of the completeness of the cosmetology licensee files, removing workers with missing information necessary for analyses (e.g., date of birth). Furthermore, some workers received multiple license types (both as a cosmetologist and as a manicurist); we unduplicated these licensees in order to avoid double counting them in the calculation of person-years. We excluded licensees with a current out-of-state residential address on file because we were uncertain as to whether they are still living in, or when they left, California. The process for constructing the cohort is summarized in Figure 1.
Figure 1.
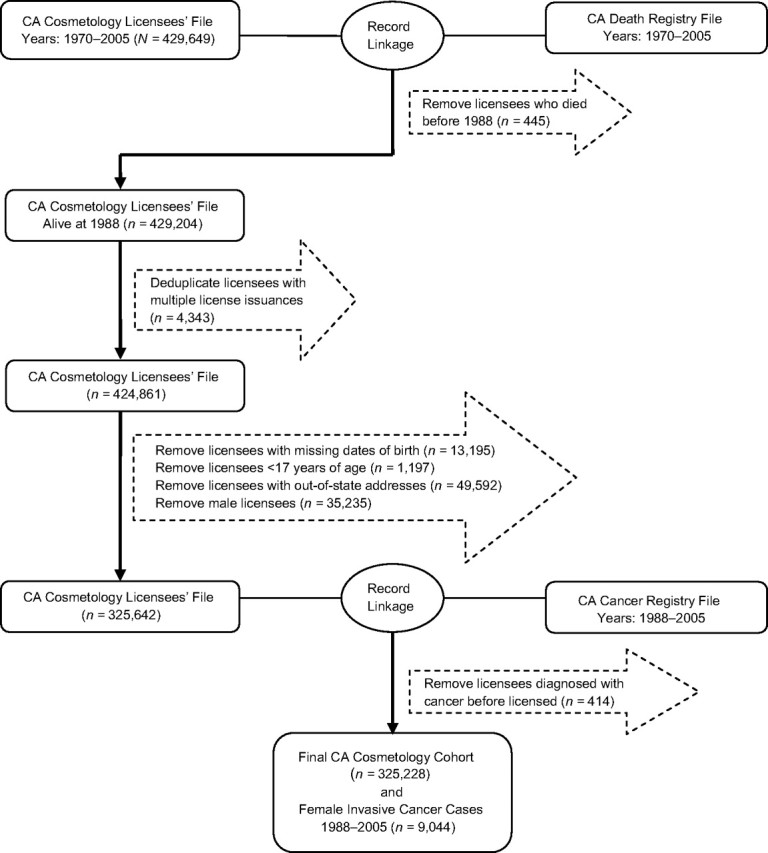
Identification of female cosmetology cohort members with record linkages between the California cosmetology licensee file (1970–2005) and the California Death Registry file (1970–2005) and California Cancer Registry file (1988–2005). CA, California.
Preparation of data files for record linkage
Because the cosmetology licensee files were intended for regulatory purposes related to occupational safety standards and tax payment, they lack some important information needed for health research, notably sex and race/ethnicity. We applied several alternative approaches to obtain information on sex. We used the cancer and/or death files to obtain sex designation among those who were diagnosed with cancer or died during the study period. For the remaining licensees, we applied an imputation approach based on a names list-assisted method. We compiled a list of common male first names from different sources, such as vital records on births in California and multiple Web sources to obtain common first names for men.
In order to validate our method for sex imputation, we compared our names list-assisted categorization of sex with reported sex information from the cancer and death files for the cohort members who had cancer or died during the study period (n = 15,816, approximately 5% of the total cohort). Using the listed sex on the cancer or death files as the “gold standard,” we considered a true positive as a licensee categorized accurately as “male” and a true negative as a licensee categorized accurately as “female.” We obtained a sensitivity (coverage or the proportion of males correctly identified) value of 0.87 and a positive predictive value (accuracy or those categorized as males who are correctly identified) of 0.96. The 35,235 licensees who were identified as male by the names imputation screen were excluded from the analysis (Figure 1).
We did not have residential history and thus did not know the rate of out-of-state migration that may result in loss to follow-up. Because all licensees are required to renew their license every 2 years, however, we could identify individuals who remained in California with a small margin of error. We assumed that, up to the time of the license expiration date, the individual remained in California. For those who had a license expiration date before the end of the study period, we were uncertain whether they had moved out of state during the time between her license expiration date and the end of the study period. Thus, we adjusted the denominator using published age-specific annual rates of out-of-state migration from the California Department of Finance (17).
Record linkage
For record linkages among the 3 statewide data files, we used 2 generalized automated probabilistic record linkage programs, Link Plus (18) and AUTOMATCH (for personal computer) (19), which are both designed to evaluate the likelihood that the identifying variables for the 2 records are similar enough to represent the same individual. Both programs allow the user to perform manual review and decision-making regarding the accuracy of the matches (20, 21), and each has different advantages for maximizing linkages between 2 large files. Link Plus allows for more efficient processing of large data files, whereas AUTOMATCH is more conducive to dealing with ethnically diverse names, such as Asian names, where the first, middle, and last names may be reversed, or Latina names with multiple last names. We conducted initial linkages in Link Plus and then used AUTOMATCH to conduct a second linkage on residual records that were not matched in the first linkage in Link Plus, as a way of maximizing the number of cases identified.
Statistical analysis
We used Poisson regression models to compare the difference in cancer incidence between workforce members and the general female population, using the latter as the reference group. We adjusted for age (5-year age groups) because of changes in cancer risk with age and for time period (1988–1994, 1995–1999, and 2000–2005) because of potential changes in rates over time. We also conducted stratified analyses by license type, since cosmetologists and manicurists may have different exposures due to the fact that cosmetologists also perform hair care services.
Because of the uncertainties of sex and race/ethnicity when calculating person-year estimates for the denominator in the Poisson analysis, we also computed proportional incidence ratios as a qualitative check. Because proportional incidence ratios are computed by using only case information, which is complete for sex and race/ethnicity, and do not require calculation of person-years, they are not sensitive to potential errors in denominator estimation. Proportional incidence ratios were calculated by using the observed:expected ratio, where the observed number was the number of site-specific cancer cases in the worker cohort, and the expected number was calculated by multiplying the age- and period-specific number of all cancer cases in this worker cohort by the proportion of site-specific cancer relative to all cancers in the general population (22). Ninety-five percent confidence intervals were calculated by using the Vandenbrouke method with Ulm adjustment (22) for standardized mortality ratios, and the use of this method for proportional incidence ratios has been described by van der Gulden and Verbeek (23). All statistical analyses were performed using SAS, version 9.1, software (24).
The study protocol was reviewed and approved by the institutional review boards of the Cancer Prevention Institute of California and the California Health and Human Services Agency Committee for the Protection of Human Subjects.
RESULTS
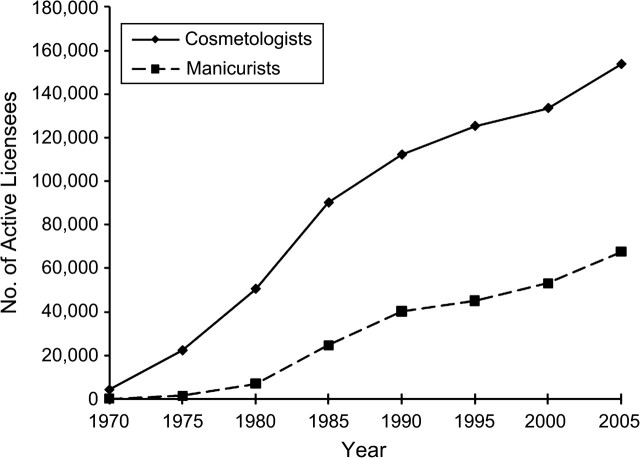
The cohort of female cosmetology workers consisted of 325,228 women licensed in California between 1970 and 2005, with 65.6% licensed as cosmetologists and 34.4% as manicurists. Figure 2 shows a graph of the number of actively licensed cosmetologists and manicurists over time and depicts the growth of the 2 licensee groups from 1970 to 2005. While the cosmetologist licensee group has been steadily growing since 1970, the manicurist licensee group did not really emerge until after 1980, and grew rapidly since then, tripling in size in the 2 most recent decades.
Figure 2.
Number of actively licensed female cosmetologists and manicurists, California, 1970–2005.
Table 1 shows the distribution of licensees by age at entry and year of entry. The majority of these women entered this workforce at a young age; over half of the women were younger than 30 years of age when they became licensed. Most of the licensees (approximately 65%) in this study received their licenses in the 1980s and 1990s.
Table 1.
Distribution of the Number of Female Cosmetologists and Manicurists Initially Licensed in California Between 1970 and 2005, Age at Entry, and Year of Entry
| All Licenseesa |
Cosmetologists |
Manicurists |
||||
| Count | % | Count | % | Count | % | |
| Total | 325,228 | 100 | 213,327 | 100 | 111,901 | 100 |
| Age at entry | ||||||
| <30 years | 203,592 | 62.6 | 143,442 | 67.2 | 60,150 | 53.8 |
| 30–39 years | 78,720 | 24.2 | 46,970 | 22.0 | 31,750 | 28.4 |
| 40–49 years | 33,973 | 10.5 | 18,588 | 8.7 | 15,385 | 13.7 |
| ≥50 years | 8,943 | 2.7 | 4,327 | 2.0 | 4,616 | 4.1 |
| Year of entry | ||||||
| 1970–1979 | 49,440 | 15.2 | 43,872 | 20.6 | 5,568 | 5.0 |
| 1980–1989 | 110,818 | 34.1 | 72,853 | 34.2 | 37,965 | 33.9 |
| 1990–1999 | 99,427 | 30.6 | 58,106 | 27.2 | 41,321 | 36.9 |
| 2000–2005 | 65,543 | 20.2 | 38,496 | 18.0 | 27,047 | 24.2 |
Includes cosmetologist and manicurist licensees.
Table 2 shows the distribution of person-years by age group and time period. The overall distribution by age group shows that less than one-fifth fall in the category of 50 years of age or older, suggesting that this workforce is still fairly young and may not be in the age groups at highest risk for many of the cancer sites of interest. Over 40% of the person-years are distributed in the most recent time period (2000–2005), with manicurists having a slightly larger proportion of person-years in this period relative to cosmetologists.
Table 2.
Distribution of Person-Years for Female Cosmetologists and Manicurists Initially Licensed in California Between 1970 and 2005 by Age Group and Time Period
| All Licenseesa |
Cosmetologists |
Manicurists |
||||
| Person-Years | % | Person- Years | % | Person-Years | % | |
| Total | 3,837,803.8 | 100 | 2,663,417.1 | 100 | 1,174,386.8 | 100 |
| Age groups | ||||||
| <30 years | 798,682.0 | 20.8 | 540,760.5 | 20.3 | 257,921.5 | 22.0 |
| 30–39 years | 1,293,177.0 | 33.7 | 900,307.1 | 33.8 | 392,870.0 | 33.5 |
| 40–49 years | 1,050,334.9 | 27.4 | 741,649.8 | 27.8 | 308,685.1 | 26.3 |
| ≥50 years | 695,609.9 | 18.1 | 480,699.7 | 18.0 | 214,910.2 | 18.3 |
| Time period | ||||||
| 1988–1994 | 1,201,301.4 | 31.3 | 863,541.8 | 32.4 | 337,759.6 | 28.8 |
| 1995–1999 | 1,088,689.1 | 28.4 | 756,864.7 | 28.4 | 331,824.4 | 28.3 |
| 2000–2005 | 1,547,813.3 | 40.3 | 1,043,010.5 | 39.2 | 504,802.8 | 43.0 |
Includes cosmetologist and manicurist licensees.
We identified 9,044 newly diagnosed invasive cancer cases in this occupational cohort between 1988 and 2005 (Table 3). Approximately two-thirds of the cases were diagnosed at 45 years or older. Non-Hispanic white women comprised over 60% of the cases, followed by Latina and Asian and Pacific Islander women. Most of the cases were initially licensed in the first 2 decades for which licensee information is available (1970s and 1980s).
Table 3.
Characteristics of Female Invasive Cancer Cases Diagnosed Between 1988 and 2005 in California Who Were Licensed as a Cosmetologist or Manicurist in California Between 1970 and 2005
| All Licenseesa |
Cosmetologists |
Manicurists |
||||
| Count | % | Count | % | Count | % | |
| Total | 9,044 | 100 | 6,239 | 100 | 2,805 | 100 |
| Age at diagnosis | ||||||
| <45 years | 2,956 | 32.7 | 2,036 | 32.6 | 920 | 32.8 |
| 45–54 years | 2,955 | 32.7 | 2,118 | 33.9 | 837 | 29.8 |
| ≥55 years | 3,133 | 34.6 | 2,085 | 33.4 | 1,048 | 37.4 |
| Race/ethnicity | ||||||
| Non-Hispanic white | 5,613 | 62.1 | 3,759 | 60.3 | 1,854 | 66.1 |
| Black | 643 | 7.1 | 510 | 8.2 | 133 | 4.7 |
| Latina | 1,442 | 15.9 | 1,187 | 19.0 | 255 | 9.1 |
| Asian/Pacific Islander | 1,233 | 13.6 | 704 | 11.3 | 529 | 18.9 |
| Other or unknown | 113 | 1.2 | 79 | 1.3 | 34 | 1.2 |
| Time of diagnosis | ||||||
| 1988–1994 | 1,923 | 21.3 | 1,304 | 20.9 | 619 | 22.1 |
| 1995–1999 | 2,418 | 26.7 | 1,658 | 26.6 | 760 | 27.1 |
| 2000–2005 | 4,703 | 52.0 | 3,277 | 52.5 | 1,426 | 50.8 |
| Time of initial licensure | ||||||
| 1970–1979 | 3,375 | 37.3 | 2,896 | 46.4 | 479 | 17.1 |
| 1980–1989 | 3,947 | 43.6 | 2,348 | 37.6 | 1,599 | 57.0 |
| 1990–1999 | 1,542 | 17.0 | 896 | 14.4 | 646 | 23.0 |
| 2000–2005 | 180 | 2.0 | 99 | 1.6 | 81 | 2.9 |
| Age at first licensure | ||||||
| <30 years | 3,469 | 38.4 | 2,764 | 44.3 | 705 | 25.1 |
| 30–39 years | 2,729 | 30.2 | 1,864 | 29.9 | 865 | 30.8 |
| 40–49 years | 1,962 | 21.7 | 1,204 | 19.3 | 758 | 27.0 |
| ≥50 years | 884 | 9.8 | 407 | 6.5 | 477 | 17.0 |
Includes cosmetologist and manicurist licensees.
Table 4 shows the rate ratios by cancer site and by license type. Rate ratios for all sites combined were lower than those for the generally female population of all cosmetology workers (rate ratio (RR) = 0.84, 95% confidence interval (CI): 0.83, 0.86), as well as by license type (cosmetologists: RR = 0.84, 95% CI: 0.82, 0.86; manicurists: RR = 0.87, 95% CI: 0.84, 0.90). Elevations in site-specific rate ratios were generally not evident among cosmetology licensees relative to the general population. With few exceptions, rate ratios were similar for the cosmetology and manicurist groups. In comparison, proportional incidence ratio results (shown in Table 5) were closer to the null, with some sites showing slightly higher incidence in cosmetologists, although only the elevations in thyroid cancer among all cosmetologists (proportional incidence ratio = 1.13, 95% CI: 1.04, 1.23) and lung cancer for manicurists (proportional incidence ratio = 1.21, 95% CI: 1.07, 1.36) were statistically significant.
Table 4.
Number of Site-specific Cancer Cases, Age- and Time-adjusted Poisson Rate Ratios, and 95% Confidence Intervals for Female Cosmetology Workers Licensed as Cosmetologists or Manicurists in California, 1988–2005
| Cancer Sites | SEER Codes | All Licenseesa |
Cosmetologists |
Manicurists |
||||||
| No. of Cases | Rate Ratiob,c | 95% CI | No. of Cases | Rate Ratiob,c | 95% CI | No. of Cases | Rate Ratiob,c | 95% CI | ||
| All sites | 20010–37000 | 9,044 | 0.84 | 0.83, 0.86 | 6,239 | 0.84 | 0.82, 0.86 | 2,805 | 0.87 | 0.84, 0.90 |
| Bladder | 29010 | 63 | 0.90 | 0.70, 1.16 | 43 | 0.91 | 0.67, 1.22 | 20 | 0.90 | 0.58, 1.39 |
| Brain | 31010 | 115 | 0.78 | 0.65, 0.94 | 74 | 0.73 | 0.58, 0.91 | 41 | 0.91 | 0.67, 1.24 |
| Breast | 26000 | 3,455 | 0.84 | 0.81, 0.87 | 2,435 | 0.85 | 0.82, 0.89 | 1,020 | 0.81 | 0.76, 0.86 |
| Leukemia | 35011–35043 | 168 | 0.89 | 0.76, 1.04 | 128 | 0.98 | 0.83, 1.17 | 40 | 0.68 | 0.50, 0.93 |
| Lung | 22030 | 777 | 0.87 | 0.81, 0.94 | 496 | 0.81 | 0.74, 0.89 | 281 | 1.00 | 0.89, 1.25 |
| Melanoma of the skin | 25010 | 477 | 0.86 | 0.78, 0.94 | 321 | 0.83 | 0.74, 0.93 | 156 | 0.92 | 0.78, 1.07 |
| Multiple myeloma | 34000 | 83 | 1.05 | 0.85, 1.30 | 59 | 1.09 | 0.84, 1.41 | 24 | 0.97 | 0.65, 1.45 |
| Non-Hodgkin's lymphoma | 33041–33042 | 328 | 0.92 | 0.82, 1.02 | 220 | 0.89 | 0.78, 1.02 | 108 | 0.98 | 0.81, 1.18 |
| Ovary | 27040 | 333 | 0.81 | 0.72, 0.90 | 239 | 0.83 | 0.73, 0.95 | 94 | 0.74 | 0.61, 0.91 |
| Stomach | 21020 | 113 | 0.92 | 0.76, 1.10 | 82 | 0.97 | 0.78, 1.20 | 31 | 0.81 | 0.57, 1.15 |
| Thyroid | 32010 | 503 | 0.99 | 0.91, 1.08 | 350 | 1.00 | 0.90, 1.11 | 153 | 0.98 | 0.84, 1.15 |
| Uterus | 27020–27030 | 459 | 0.75 | 0.68, 0.82 | 308 | 0.73 | 0.65, 0.81 | 151 | 0.80 | 0.68, 0.94 |
Abbreviations: CI, confidence interval; SEER, Surveillance, Epidemiology, and End Results.
Includes both licensed cosmetologist and manicurist licensees.
Rate ratios from Poisson regression models adjusted by 5-year age groups and time periods (1988–1994, 1995–1999, and 2000–2005).
Person-years adjusted for age-specific out-migration.
Table 5.
Number of Site-specific Cancer Cases, Age- and Time-adjusted Proportional Incidence Ratio, and 95% Confidence Intervals for Female Cosmetology Workers Licensed as a Cosmetologist or Manicurist in California, 1988–2005
| Cancer Sites | SEER Codes | All Licenseesa |
Cosmetologists |
Manicurists |
||||||
| No. of Cases | PIRb,c | 95% CId | No. of Cases | PIRb,c | 95% CId | No. of Cases | PIRb,c | 95% CId | ||
| Bladder | 29010 | 63 | 1.11 | 0.85, 1.40 | 43 | 1.13 | 0.82, 1.49 | 20 | 1.07 | 0.65, 1.59 |
| Brain | 31010 | 115 | 0.92 | 0.76, 1.10 | 74 | 0.86 | 0.68, 1.07 | 41 | 1.06 | 0.76, 1.41 |
| Breast | 26000 | 3,455 | 0.99 | 0.96, 1.02 | 2,435 | 1.00 | 0.96, 1.04 | 1,020 | 0.96 | 0.90, 1.02 |
| Leukemia | 35011–35043 | 168 | 1.05 | 0.90, 1.21 | 128 | 1.17 | 0.98, 1.38 | 40 | 0.79 | 0.57, 1.06 |
| Lung | 22030 | 777 | 1.08 | 1.00, 1.15 | 496 | 1.02 | 0.93, 1.11 | 281 | 1.21 | 1.07, 1.36 |
| Melanoma of the skin | 25010 | 477 | 1.00 | 0.91, 1.09 | 321 | 0.98 | 0.87, 1.09 | 156 | 1.07 | 0.91, 1.24 |
| Multiple myeloma | 34000 | 83 | 1.24 | 0.99, 1.53 | 59 | 1.29 | 0.96, 1.65 | 24 | 1.14 | 0.73, 1.64 |
| Non-Hodgkin's lymphoma | 33041–33042 | 328 | 1.09 | 0.97, 1.21 | 220 | 1.06 | 0.93, 1.21 | 108 | 1.15 | 0.94, 1.37 |
| Ovary | 27040 | 333 | 0.96 | 0.86, 1.07 | 239 | 1.00 | 0.87, 1.13 | 94 | 0.88 | 0.71, 1.06 |
| Stomach | 21020 | 113 | 1.07 | 0.88, 1.28 | 82 | 1.14 | 0.90, 1.40 | 31 | 0.93 | 0.63, 1.29 |
| Thyroid | 32010 | 503 | 1.13 | 1.04, 1.23 | 350 | 1.14 | 1.03, 1.27 | 153 | 1.13 | 0.95, 1.31 |
| Uterus | 27020–27030 | 459 | 0.88 | 0.81, 0.97 | 308 | 0.86 | 0.77, 0.96 | 151 | 0.94 | 0.80, 1.10 |
Abbreviations: CI, confidence interval; PIR, proportional incidence ratio; SEER, Surveillance, Epidemiology, and End Results.
Includes both licensed cosmetologists and manicurists.
PIR values were calculated from Ulm (1990) (22).
PIR adjusted for age (5-year categories) and time period (1988–1994, 1995–1999, and 2000–2005).
Calculated from van der Gulden and Verbeek (23).
DISCUSSION
The overall findings of this study did not show an excess in cancer among female cosmetology licensees compared with the female general population in California. The rate ratio estimates were generally comparable to the proportional incidence ratio estimates.
Previous cancer studies of cosmetology workers have been primarily restricted to hairdressers, using data from decades earlier and with short follow-up periods (25–35). Those studies typically did not distinguish between hairdressers and manicurists or their specific job tasks (e.g., hair care or nail care services) and were limited to a time period when chemical use may have been different in the cosmetology industry and before the nail care sector began its considerable expansion. Previous literature on this workforce has reported mixed results, partly due to the different methods used to capture occupation and the limited sample sizes. A few studies have reported null results for cancer incidence excess among female hairdressers, after taking into consideration age and race, when such information is available (25, 32, 36). Other studies have shown increased incidence of specific cancer sites, mainly bladder (37), ovarian (28, 34, 38), non-Hodgkin's lymphoma (38, 39), lung (29, 35, 40), and uterine (34). Two of these studies, although much smaller, used a similar methodology, including a roster of hairdressers and beauticians to link to data from cancer registries. A retrospective cohort study in Finland of women who were part of the Finnish Hairdressers Association between 1970 and 1982 found an excess in age-adjusted cancer incidence for all sites combined (standardized incidence ratio (SIR) = 1.64, 95% CI: 1.02, 2.51), as well as for ovarian cancer (SIR = 1.27, 95% CI: 1.11, 1.42) (28). Similarly, a study conducted in Connecticut with data from the state's Cosmetology Licensing Division also found a slight excess in age-adjusted cancer incidence for all sites (SIR = 112, 95% CI: 104, 121) and respiratory-related sites (SIR = 141, 95% CI: 104, 186) in female hairdressers (31). In both of these studies, however, the occupational groups were likely to be more homogenous with respect to racial/ethnic composition, composed mainly of non-Hispanic white women, and thus using the general population as a reference group was more appropriate. By contrast, the workforce in our study is likely to be quite racially/ethnically diverse, as indicated from previous industry estimates (5) and as reflected in the racial/ethnic distribution of our cancer cases.
The majority of cancer cases in our study were licensed in the earlier decades, before the significant growth of the industry and the emergence of the nail care sector. Thus, the observed lack of excess cancer incidence in manicurists may in part be due to insufficient follow-up time for licensees who have entered this workforce in the most recent decades and who may have different occupational exposures. Furthermore, more recent licensees are still fairly young and have not yet entered into age groups at higher risk for many of the cancer sites of interest. Alternatively, the higher proportion of cancer cases among those licensed in the earlier decades may also suggest changes in chemical use over time, whereby those working in the earlier decades may have been exposed to compounds that were more likely to have increased their cancer risk.
The apparent deficits in cancer incidence for several sites in this study could be due to several factors. The healthy worker effect, a bias in which workers usually exhibit a lower rate of illness compared with that of the general population because people who are sick are more likely to be excluded from employment (41), has been shown to influence results for some chronic illnesses, although there is little evidence for a strong healthy worker effect for cancer risk (42, 43). We did not find evidence of a healthy worker effect when we lagged the exposure (time of original licensure).
A second, and more likely, explanation for the observed deficits in our study is that our estimates of person-years at risk (denominator) may have been inflated. There may be 2 reasons for this. First, the licensee file did not contain information on sex, and we therefore applied alternative methods to identify male licensees, whom we excluded from the analysis. Our validation of the names list-assisted method for identifying males yielded a sensitivity of approximately 87% which, while relatively good, suggests that there remained some male licensees that we could not exclude from the denominator. Second, the licensee denominator may be further inflated because we were not able to fully account for out-of-state migration due to the lack of information on residential history. A previous study, however, examined patterns of migration and reported that the annual rates of mobility primarily reflect short-distance migration and that interstate migration tends to comprise less than one-fifth of total migration (44). Furthermore, we adjusted the denominator using published age-specific annual rates of out-of-state migration in California. We reported proportional incidence ratio results, which use case information only and thus do not require calculation of person-years. Although rate ratio and proportional incidence ratio estimates rely on different information, comparisons between the 2 estimates provide a general check on potential errors, especially given the concerns with calculation of person-years. The rate ratio estimates generally did not differ greatly from the proportional incidence ratio estimates, which fact suggests that the denominator may not be subject to large errors.
Recent industry estimates suggest that this is a racially diverse population (5), particularly the nail care sector where there has been a large influx of Vietnamese in the last few decades (45). This is further supported by the racial/ethnic distribution of the cases in our study, where Asian and Pacific Islanders and Latinas comprise approximately one-third of the cases. Unfortunately, the licensee files do not contain information on race/ethnicity. Because cancer incidence is known to differ by racial/ethnic groups, our inability to account for race/ethnicity may have yielded attenuated rate ratio estimates. This is particularly likely to be the case for cancer sites where incidence rates are driven primarily by one racial/ethnic group, such as the notably higher incidence of breast cancer in non-Hispanic white women.
We were able to identify licensees who first became licensed in California as early as 1970; however, the cancer surveillance program did not begin statewide until 1988. There could be a number of workers who were diagnosed with cancer prior to 1988 that would have been missed by our linkage. To explore whether this could potentially affect rate ratio estimates, we conducted analyses on a subset of the cohort who were licensed as of 1988 or later. Although this greatly reduced the sample size and produced much wider confidence intervals, the point estimates did not differ substantially from those in the full analyses, although they tended to be closer to unity.
A number of other limitations should be kept in mind when interpreting the findings of this study. This study lacked information on important risk factors that may confound the results, including smoking for smoking-related cancers (e.g., lung, bladder, stomach, and cervical cancer). However, in our recent study of Vietnamese nail salon workers who are thought to comprise a majority of the manicurist workforce, we found that only 3% of female workers smoked cigarettes (46). Reproductive history is also important for hormonally mediated cancer, especially breast and ovarian cancer.
To our knowledge, this is the first comprehensive study that includes all licensed cosmetology workers in California and the first study to examine cancer incidence in manicurists. To date, this is the largest epidemiologic study of cosmetology workers, with over 325,000 cohort members, spanning 3 and a half decades of licensure and nearly 2 decades of cancer data, with over 9,000 invasive cancer cases. Although this initial analysis does not suggest excess cancer risk for this workforce, this work lays the groundwork for future cancer follow-up, which may be particularly important given the significant expansion of the workforce and changes in practice. Future studies should consider the issue of racial/ethnic diversity in this workforce, in addition to the need to extend follow-up time to allow for sufficient time for cancer diagnosis among individuals who entered the cohort in the more recent years.
Acknowledgments
Author affiliations: Cancer Prevention Institute of California, Berkeley, California (Thu Quach, Michael Layefsky, David Nelson, Julie Von Behren, Peggy Reynolds); Asian Health Services, Oakland, California (Phuong An Doan-Billings, Kim Dung Nguyen, Linda Okahara, Alisha Ngoc Tran); and School of Medicine, Stanford University, Stanford, California (Thu Quach, David Nelson, Peggy Reynolds).
This work was supported by the California Breast Cancer Research Program (grant 13BB-3400).
The authors thank Jessica Huynh for her contributions to this study.
Preliminary findings were presented at the American Association for Cancer Research Second Health Disparities Conference, Carefree, Arizona, February 3–6, 2009.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- RR
rate ratio
- SIR
standardized incidence ratio
References
- 1.The European Parliament, the Council of the European Union. Official Journal of the European Union. Norwich, United Kingdom: The Stationery Office, Ltd; 2003. Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products. ( http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:066:0026:0035:en:PDF) [Google Scholar]
- 2.Brown NJ. Health Hazard Manual for Cosmetologists, Hairdressers, Beauticians and Barbers. Albany, NY: Bureau of Occupational Health, New York State Department of Health; 1987. [Google Scholar]
- 3.FDA authority over cosmetics. Silver Spring, MD: Food and Drug Administration; 2005. ( http://www.cfsan.fda.gov/∼dms/cos-206.html). (Accessed July 18, 2008) [Google Scholar]
- 4.Underwood K. Sacramento, CA: California Board of Barbering and Cosmetology; 2007. Letter to: Presidents and Board Members of California Board of Barbering and Cosmetology. [Google Scholar]
- 5.2006 Nail technician demographics. Nails Magazine 2005–2006 Big Book. Torrance, CA: NAILS; 2006. [Google Scholar]
- 6.Roelofs C, Azaroff LS, Holcroft C, et al. Results from a community-based occupational health survey of Vietnamese-American nail salon workers. J Immigr Minor Health. 2008;10(4):353–361. doi: 10.1007/s10903-007-9084-4. [DOI] [PubMed] [Google Scholar]
- 7.Slaga TJ, Klein-Szanto AJ, Triplett LL, et al. Skin tumor-promoting activity of benzoyl peroxide, a widely used free radical-generating compound. Science. 1981;213(4511):1023–1025. doi: 10.1126/science.6791284. [DOI] [PubMed] [Google Scholar]
- 8.Rudel RA, Attfield KR, Schifano JN, et al. Chemicals causing mammary gland tumors in animals signal new directions for epidemiology, chemicals testing, and risk assessment for breast cancer prevention. Cancer. 2007;109(12 suppl):2635–2666. doi: 10.1002/cncr.22653. [DOI] [PubMed] [Google Scholar]
- 9.Agents Reviewed by the IARC Monographs. Vols. 1–98. France, Lyon: International Agency for Research on Cancer; 2006. [Google Scholar]
- 10.O'Connell JF, Klein-Szanto AJ, DiGiovanni DM, et al. Enhanced malignant progression of mouse skin tumors by the free-radical generator benzoyl peroxide. Cancer Res. 1986;46(6):2863–2865. [PubMed] [Google Scholar]
- 11.Kwapniewski R, Kozaczka S, Hauser R, et al. Occupational exposure to dibutyl phthalate among manicurists. J Occup Environ Med. 2008;50(6):705–711. doi: 10.1097/JOM.0b013e3181651571. [DOI] [PubMed] [Google Scholar]
- 12.Roelofs C, Nguyen H, Azaroff L, et al. Nail salons: health effects and work environment characteristics [abstract] Presented at the American Industrial Hygiene Conference and Exhibition, Chicago, IL, May 17, 2006. [Google Scholar]
- 13.Decker J, Beasley A National Institute for Occupational Safety and Health. Cincinnati, OH: National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Public Health Service, US Department of Health and Human Services; 1992. Tina and Angela's nail salon, Springdale, Ohio: health hazard evaluation report. (Health Hazard Evaluation Report no. 92-128-2241) [Google Scholar]
- 14.Industry Stats: Ethnic Breakdown. Torrance, CA: NAILS; 2001. [Google Scholar]
- 15.Kwong SL, Allen M, Wright WE. Cancer in California, 1988–1992. Sacramento, CA: Cancer Surveillance Section, California Department of Public Health; 2005. [Google Scholar]
- 16.California Department of Finance. Race/Ethnic Population With Age and Sex Detail, 2000–2050. Sacramento, CA: Department of Finance, State of California; 2004. [Google Scholar]
- 17.Demographic Research Unit, California Department of Finance. Current Population Survey, Annual Social and Economic Supplement: California, March 2001–2003. Sacramento, CA: California Department of Finance; 2004. Who moved where and why? [Google Scholar]
- 18.Link Plus. Atlanta, GA: Centers for Disease Control and Prevention; 2008. Record linkage software, version 2. ( http://www.cdc.gov/cancer/npcr/tools/registryplus/lp_features.htm) [Google Scholar]
- 19.Jaro MA. Probabilistic linkage of large public health data files. Stat Med. 1995;14(5–7):491–498. doi: 10.1002/sim.4780140510. [DOI] [PubMed] [Google Scholar]
- 20.Wajda A, Roos LL, Layefsky M, et al. Record linkage strategies: part II. Portable software and deterministic matching. Methods Inf Med. 1991;30(3):210–214. [PubMed] [Google Scholar]
- 21.Jaro MA. Advances in record linkage methodology as applied to matching the 1985 Census of Tampa, Florida. J Am Stat Assoc. 1989;89:414–420. [Google Scholar]
- 22.Ulm K. A simple method to calculate the confidence interval of a standardized mortality ratio (SMR) Am J Epidemiol. 1990;131(2):373–375. doi: 10.1093/oxfordjournals.aje.a115507. [DOI] [PubMed] [Google Scholar]
- 23.van der Gulden JW, Verbeek AL. Re: "A simple method to calculate the confidence interval of a standardized mortality ratio (SMR)" [letter] Am J Epidemiol. 1992;136(9):1170–1172. [PubMed] [Google Scholar]
- 24.SAS Institute, Inc. SAS, version 9.1. Cary, NC: SAS Institute, Inc; 2005. [Google Scholar]
- 25.Gubéran E, Raymond L, Sweetnam PM. Increased risk for male bladder cancer among a cohort of male and female hairdressers from Geneva. Int J Epidemiol. 1985;14(4):549–554. doi: 10.1093/ije/14.4.549. [DOI] [PubMed] [Google Scholar]
- 26.Guidotti S, Wright WE, Peters JM. Multiple myeloma in cosmetologists. Am J Ind Med. 1982;3(2):169–171. doi: 10.1002/ajim.4700030207. [DOI] [PubMed] [Google Scholar]
- 27.Kono S, Tokudome S, Ikeda M, et al. Cancer and other causes of death among female beauticians. J Natl Cancer Inst. 1983;70(3):443–446. [PubMed] [Google Scholar]
- 28.Pukkala E, Nokso-Koivisto P, Roponen P. Changing cancer risk pattern among Finnish hairdressers. Int Arch Occup Environ Health. 1992;64(1):39–42. doi: 10.1007/BF00625949. [DOI] [PubMed] [Google Scholar]
- 29.Skov T, Andersen A, Malker H, et al. Risk for cancer of the urinary bladder among hairdressers in the Nordic countries. Am J Ind Med. 1990;17(2):217–223. doi: 10.1002/ajim.4700170206. [DOI] [PubMed] [Google Scholar]
- 30.Spinelli JJ, Gallagher RP, Band PR, et al. Multiple myeloma, leukemia, and cancer of the ovary in cosmetologists and hairdressers. Am J Ind Med. 1984;6(2):97–102. doi: 10.1002/ajim.4700060204. [DOI] [PubMed] [Google Scholar]
- 31.Teta MJ, Walrath J, Meigs JW, et al. Cancer incidence among cosmetologists. J Natl Cancer Inst. 1984;72(5):1051–1057. [PubMed] [Google Scholar]
- 32.Decoufle P, Stanislawczyc K, Houton L, et al. A Retrospective Survey of Cancer in Relation to Occupation. Cincinnati, OH: National Institute for Occupational Safety and Health; 1977. pp. 77–178. [Google Scholar]
- 33.Osorio AM, Bernstein L, Garabrant DH, et al. Investigation of lung cancer among female cosmetologists. J Occup Med. 1986;28(4):291–295. [PubMed] [Google Scholar]
- 34.Singleton JA, Beaumont JJ. COMS II—California Occupational Mortality 1979–1981, Adjusted for Smoking, Alcohol and Socioeconomic Status. Sacramento, CA: California Department of Health Services; 1989. [Google Scholar]
- 35.Garfinkel J, Selvin S, Brown SM. Brief communication: possible increased risk of lung cancer among beauticians. J Natl Cancer Inst. 1977;58(1):141–143. doi: 10.1093/jnci/58.1.141. [DOI] [PubMed] [Google Scholar]
- 36.Vasama-Neuvonen K, Pukkala E, Paakkulainen H, et al. Ovarian cancer and occupational exposures in Finland. Am J Ind Med. 1999;36(1):83–89. doi: 10.1002/(sici)1097-0274(199907)36:1<83::aid-ajim12>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 37.Dryson E, ‘t Mannetje A, Walls C, et al. Case-control study of high risk occupations for bladder cancer in New Zealand. Int J Cancer. 2008;122(6):1340–1346. doi: 10.1002/ijc.23194. [DOI] [PubMed] [Google Scholar]
- 38.Boffetta P, Andersen A, Lynge E, et al. Employment as hairdresser and risk of ovarian cancer and non-Hodgkin's lymphomas among women. J Occup Med. 1994;36(1):61–65. [PubMed] [Google Scholar]
- 39.Robinson CF, Walker JT. Cancer mortality among women employed in fast-growing U.S. occupations. Am J Ind Med. 1999;36(1):186–192. doi: 10.1002/(sici)1097-0274(199907)36:1<186::aid-ajim26>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 40.Menck HR, Pike MC, Henderson BE, et al. Lung cancer risk among beauticians and other female workers: brief communication. J Natl Cancer Inst. 1977;59(5):1423–1424. [PubMed] [Google Scholar]
- 41.McMichael AJ, Spirtas R, Kupper LL. An epidemiologic study of mortality within a cohort of rubber workers, 1964–72. J Occup Med. 1974;16(7):458–464. [PubMed] [Google Scholar]
- 42.Choi BC. Definition, sources, magnitude, effect modifiers, and strategies of reduction of the healthy worker effect. J Occup Med. 1992;34(10):979–988. [PubMed] [Google Scholar]
- 43.Gridley G, Nyren O, Dosemeci M, et al. Is there a healthy worker effect for cancer incidence among women in Sweden? Am J Ind Med. 1999;36(1):193–199. doi: 10.1002/(sici)1097-0274(199907)36:1<193::aid-ajim27>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 44.Rogerson PA, Han D. The effects of migration on the detection of geographic differences in disease risk. Soc Sci Med. 2002;55(10):1817–1828. doi: 10.1016/s0277-9536(01)00316-1. [DOI] [PubMed] [Google Scholar]
- 45.Federman MN, Harrington DE, Krynski K. Vietnamese manicurists: are immigrants displacing natives or finding new nails to polish? Ind Labor Relat Rev. 2006;59(2):302–318. [Google Scholar]
- 46.Quach T, Nguyen KD, Doan-Billings PA, et al. A preliminary survey of Vietnamese nail salon workers in Alameda County, California. J Community Health. 2008;33(5):336–343. doi: 10.1007/s10900-008-9107-7. [DOI] [PubMed] [Google Scholar]