Resistance to androgen deprivation therapy remains the major clinical hurdle for treating metastatic prostate cancer. Although next-generation antiandrogens like enzalutamide and abiraterone extend survival (1,2), castration resistant prostate cancer (CRPC) remains a clinical challenge. Recent sequencing efforts have revealed several mechanisms of resistance giving rise to CRPC, largely through androgen receptor gene amplification or mutation, or through alterations that indirectly restore downstream androgen receptor signaling (3,4). In addition, there is evidence that CRPC tumors acquire stem-like properties such as increased expression of stem/progenitor markers (5), a finding that has sparked interest in defining and targeting prostate cancer stem cells, as with other cancers that evolve to drug resistant stages.
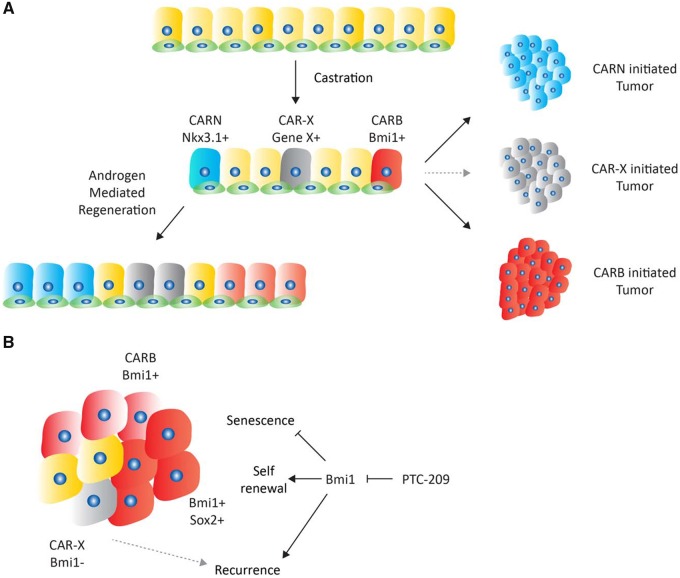
In the current issue of JNCI, Yoo et al. use genetically engineered mouse models to show that Bmi1+/Sox2+ cells within the prostate have cancer stem-like characteristics and that tumors arising from these cells can be targeted with a small molecular inhibitor of BMI1 (6). To place these findings in context, it is important to review the two major epithelial cell types in the normal prostate: luminal cells, which rely on androgen signaling for function and survival, and basal cells, which are androgen independent (7). Although early studies relying on transplantation and cell seeding assays suggested basal cells were prostate stem cells of the prostate (8), more recent work using lineage tracing and organoid culture has revealed the presence of stem cells within the luminal lineage (9–11). During castration, approximately 90% of luminal cells undergo apoptosis, whereas basal cell numbers remain largely unaffected. Upon exogenous testosterone addition, the prostate regenerates to its original size, and normal prostate function is fully restored. Within the 10% remainder of luminal cells, at least two distinct subsets of cells have been identified that have in vivo repopulating capacity: one expressing Nkx3.1 called CARNs (Castration Resistant Nkx3.1 positive cell) (9) and one expressing Bmi1 postcastration called CARBs (12). NXK3.1 encodes a prostate-specific homeobox protein that plays a developmental role in prostate specification (13), whereas BMI1 is member of the polycomb repressor complex 1 and has been implicated in the maintenance of stem cells in several tissues (14). Both CARNs and CARBs can serve as a cell of origin in mouse models of prostate cancer initiated by Pten loss.
The current report by Yoo et al. (6) builds on the earlier CARB study (12) by showing that tumors initiated by Pten loss specifically in these Bmi1+ cells initially have a luminal phenotype with androgen dependency. When treated with castration, luminal tumors regress and remain dormant for approximately 3 months but with evidence of luminal-to-basal lineage switching (Figure 1A). This finding is reminiscent of recent work by several groups implicating lineage plasticity (loss of luminal epithelial features) as a general mechanism of resistance to castration, enzalutamide, or abiraterone. Notably, in these examples, the plasticity is initiated by genomic deletion of TP53, RB1, or PTEN in various combinations (15–17). Interestingly, one common feature in all these examples is the reprogramming factor SOX2, whose increased expression is essential for lineage plasticity (and enzalutamide resistance) initiated by combined TP53/RB1 loss (16).
Figure 1.
Model of prostate cancer stem cell heterogeneity. A) Schematic overview of prostate epithelium showing luminal cells (larger, upper) and basal cells (smaller, lower). After castration, two distinct stem cells have been identified: Nkx3.1-positive CARN cells and Bmi1-positive CARB cells that contribute to androgen mediated regeneration. Moreover, both can serve as a cell of origin for prostate cancer. CAR-X (gray) depicts the possibility of additional luminal progenitor cells distinct from CARNs and CARBs. B) Several distinct cell types are present in CARB-initiated tumors, including Bmi1-positive, Bmi1/Sox2-double positive, and Bmi1-negative cells. Inhibition of Bmi1 with the small molecule PTC-209 leads to an increase in senescent cells and a delay in recurrence. Recurrence is potentially driven by alternate cancer stem cells populations, such as CAR-X. CARN = castration resistant Nkx3.1 positive; CARB = castration resistant Bmi1 positive.
After demonstrating that prostate tumors can evolve from CARBs in intact mice in the Pten model, Yoo et al. (6) used a retracing strategy with the R26R-confetti allele to show that Bmi1-expressing CARBs within the tumor were responsible for driving castration resistance. They next asked if these tumors could be targeted using the Bmi1 small molecule inhibitor PTC-209 (18). Inhibition of Bmi1 resulted in decreased expression of Sox2 and increased cellular senescence, consistent with well-known regulation of the INK4a/ARF locus by Bmi1. PTC-209 treatment also resulted in a statistically significant delay in tumor recurrence after castration, suggesting that targeting prostate cancer stem cells could be an effective strategy to prevent resistance to hormone therapy.
While provocative, clinical testing of BMI1 inhibitors in prostate cancer requires further consideration. First, PTC-209 is an early stage compound with limited potency and poor pharmacokinetic properties that make it a poor candidate for clinical development; however, a newer BMI1 inhibitor PTC596 may be more promising (19). In addition, BMI1 is broadly expressed and implicated in self-renewal and stem cell maintenance in many tissues, raising potential concerns about toxicity if continuous BMI1 inhibition is required to prevent hormone therapy resistance. Assuming these hurdles can be overcome, there is the additional question of whether BMI1 inhibition alone would be sufficient to target all the relevant prostate cancer stem cells. Specifically, CARBs and CARNs define two distinct populations of luminal stem cells, and it is possible that CARNs or other BMI1-negative stem cell populations could emerge (20,21), particularly in light of growing evidence for lineage plasticity (Figure 1B). This concern would be particularly relevant in tumors with TP53 or RB1 loss, which account for a substantial fraction of CRPC cancers. On an optimistic note, technologies for single cell analysis of whole transcriptomes (single cell RNA-seq) offer the promise of bringing increasing clarity to the complex and heterogeneous populations of cancer-initiating cells in prostate cancer as well as other tumor types, and will be an important tool to track the evolution of tumors treated with stem cell therapy.
Funding
WRK is funded by the Dutch Cancer Foundation (KWF BUIT-2015–7545) and a Prostate Cancer Foundation Young Investigator Award (17YOUN10). C.L.S. is an investigator of the HHMI and is supported by National Institutes of Health grants CA155169, CA193837, CA224079, CA092629, CA160001, and CA008748 and the Starr Cancer Consortium grant I10–0062.
Notes
Affiliations of authors: Human Oncology and Pathogenesis Program, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, New York 10065, USA. (WRK, CLS); 2Howard Hughes Medical Institute, Chevy Chase, Maryland 20185, USA (CLS).
The funders had no role in the writing of this editorial or the decision to submit it for publication. The authors have no conflicts of interest to disclose.
References
- 1. Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Watson PA, Arora VK, Sawyers CL.. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15(12):701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang D, Park D, Zhong Y, et al. Stem cell and neurogenic gene-expression profiles link prostate basal cells to aggressive prostate cancer. Nat Commun. 2016;7:10798.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoo YA, Vatapalli R, Lysy B, et al. The role of castration-resistant Bmi1+Sox2+ cells in driving recurrence in prostate cancer. J Natl Cancer Inst. 2019;111(3):djy142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shen MM, Abate-Shen C.. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 2010;24(18):1967–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldstein AS, Lawson DA, Cheng D, et al. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc Natl Acad Sci USA 2008;105(52):20882–20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang X, Kruithof-de Julio M, Economides KD, et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461(7263):495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karthaus WR, Iaquinta PJ, Drost J, et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell. 2014;159(1):163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi N, Zhang B, Zhang L, et al. Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell. 2012;21(2):253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoo YA, Roh M, Naseem AF, et al. Bmi1 marks distinct castration-resistant luminal progenitor cells competent for prostate regeneration and tumour initiation. Nat Commun. 2016;7:12943.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shen MM, Abate-Shen C.. Roles of the Nkx3.1 homeobox gene in prostate organogenesis and carcinogenesis. Dev Dyn. 2003;228(4):767–778. [DOI] [PubMed] [Google Scholar]
- 14. Park IK, Qian D, Kiel M, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423(6937):302–305. [DOI] [PubMed] [Google Scholar]
- 15. Zou M, Toivanen R, Mitrofanova A, et al. Transdifferentiation as a mechanism of treatment resistance in a mouse model of castration-resistant prostate cancer. Cancer Discov. 2017;7(7):736–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mu P, Zhang Z, Benelli M, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science. 2017;355(6320):84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ku SY, Rosario S, Wang Y, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science. 2017;355(6320):78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kreso A, van Galen P, Pedley NM, et al. Self-renewal as a therapeutic target in human colorectal cancer. Nat Med. 2014;20(1):29–36. [DOI] [PubMed] [Google Scholar]
- 19. Nishida Y, Maeda A, Kim MJ, et al. The novel BMI-1 inhibitor PTC596 downregulates MCL-1 and induces p53-independent mitochondrial apoptosis in acute myeloid leukemia progenitor cells. Blood Cancer J. 2017;7(2):e527.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Agarwal S, Hynes PG, Tillman HS, et al. Identification of different classes of luminal progenitor cells within prostate tumors. Cell Rep. 2015;13(10):2147–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang D, Jeter C, Gong S, et al. Histone 2B-GFP label-retaining prostate luminal cells possess progenitor cell properties and are intrinsically resistant to castration. Stem Cell Reports. 2018;10(1):228–242. [DOI] [PMC free article] [PubMed] [Google Scholar]