Abstract
Introduction:
Biomarkers of atherosclerosis (pro-inflammatory cytokines and acute phase reactants) are elevated in children with obstructive sleep apnea (OSA). However, their association with cardiovascular endpoints in children are not understood. We hypothesized that biomarkers of atherosclerosis in children with OSA correlate with pulse transit time (PTT), a surrogate measure of vascular stiffness, with some positively influencing and others negatively influencing PTT.
Methods:
Children with OSA and matched controls were recruited to the study. Pro-inflammatory cytokines and acute phase reactants were measured at 6:00 pm and 6:00 am. Polysomnography with beat-to-beat blood pressure was performed. PTT during wakefulness and stage 2 sleep was calculated. Diurnal variation of biomarkers and their associations with PTT was estimated. Factor analysis was used to determine the effect of groups of cytokines on PTT.
Results:
One hundred fifty-five children participated in the study; 90 were healthy controls and 65 had OSA. Children with OSA exhibited a different diurnal variation of biomarkers than healthy controls, with pro-inflammatory cytokines peaking in the morning and acute phase reactants peaking in the afternoon. Structural equation modeling demonstrated that interleukins 6 and 8, tumor necrosis factor-α, and sCD40L had a shortening effect, while serum amyloid A, C-reactive protein, and adiponectin had a prolonging effect on PTT. As a result, there was no difference in PTT between the two groups.
Conclusion:
The differential relationships of acute phase reactants and pro-inflammatory cytokines with PTT suggest that in children with OSA, these mediators may have opposing actions to maintain cardiovascular homeostasis.
Keywords: Pulse transit time, Pediatric obstructive sleep apnea, Acute phase reactants, Inflammation.
Statement of Significance
Atherosclerotic cardiovascular diseases are mediated by several inflammatory pathways which have been described in both adults and children with obstructive sleep apnea (OSA). However, the development of cardiovascular diseases such as heart failure and stroke is limited primarily to adults with OSA. The resilience of children to the development of full blown cardiovascular disease in children with OSA is not fully understood. We have demonstrated that specific groups of inflammatory mediators appear to have opposing effects on vessel stiffness in children and, therefore, maintain cardiovascular homeostasis.
INTRODUCTION
In adults, obstructive sleep apnea (OSA) is associated with cardiovascular diseases such as blood pressure (BP) dysregulation,1 hypertension, heart failure,2 and stroke.3 Similar to adults, children with OSA demonstrate cardiovascular sequelae including altered autonomic function,4,5 diminished baroreflex gain,6,7 decreased BP dipping, and increased BP variability.8 Further, pediatric OSA is associated with left ventricular remodeling and hypertrophy and decreased ventricular function.9 While children with OSA demonstrate many of the risk factors for cardiovascular disease, they rarely develop hypertension, heart failure, or stroke, as observed in the adult population. The difference in phenotypic manifestation of cardiovascular disease between adults and children with OSA could be explained at least in part to the absence of many comorbid conditions in the pediatric age range. Nevertheless, this resilience to the development of full-blown cardiovascular disease in children with OSA occurs despite the presence of the same disease mechanisms associated with atherosclerosis.10–13 Specifically, multiple studies have demonstrated systemic changes in a host of inflammatory mediators, including C-reactive protein (CRP), tumor necrosis factor (TNF)-α, and interleukin (IL)-6, in children with OSA.11,14,15 However, in the absence of cardiovascular disease in children with OSA, the clinical significance and the downstream effect of upregulated systemic inflammation are not clearly understood.
Most of the studies on inflammation in children with OSA have described a relationship between individual inflammatory biomarkers and OSA severity. However, no studies have examined clustering of groups of biomarkers based on their protective or injurious effects on cardiovascular structure or function. As a result, there is a gap in our knowledge concerning how individual inflammatory biomarkers influence cardiovascular endpoints and how they interact together to cause or prevent cardiovascular disease in children with OSA. The objectives of this study are threefold. The first is to examine the relationship between pulse transit time (PTT) measured during wakefulness and sleep to each inflammatory mediator proposed in this study. The second is to determine whether the relationship between inflammatory biomarkers, acute phase reactants, and PTT is modified by sleep status. The third is to examine how biomarkers cluster in different groups based on their positive or negative relationship with PTT.
PTT refers to the time it takes a pulse wave to travel between two arterial sites. The speed at which this arterial pressure wave travels is directly proportional to BP. An increase in vascular tone and stiffness causes the PTT to shorten. Conversely, when vascular tone decreases, PTT increases. It is used as a noninvasive means to estimate changes in vessel tone, BP, and BP variability. PTT is influenced predominantly by vascular tone, but also by the left ventricular pre-ejection period.
We hypothesized that (1) children with OSA have increased pro-inflammatory biomarkers and acute phase reactants which correlate with PTT and that (2) PTT has differential relationships with different groups of biomarkers, some associated with prolongation while others are associated with shortening of PTT. In the present study, we show that, despite elevation of specific inflammatory mediators, no significant difference exists in PTT, a measure of vascular stiffness, in children with OSA compared to healthy controls. Further, specific groups of inflammatory mediators appear to have opposing effects on PTT as a means to maintain cardiovascular homeostasis.
METHODS
Study Participants
Children between 5 and 13 years of age with OSA were recruited to a prospective cohort study at a tertiary academic care center through the pediatric otolaryngology and pulmonary clinics and by advertisements about the study by the clinical trial office. Children with an apnea hypopnea index (AHI) between 1 and 4.9 events/hour (E/hr) were classified as having mild OSA. Children with an AHI ≥ 5 E/hr were classified as having moderate to severe OSA.
Healthy controls were the study subjects recruited through advertisements by placing brochures at multiple locations at the Children’s hospital and by e-mails sent from the clinical trial office to all hospital employees. The criteria for the enrollment in the control group were the absence of night symptoms of sleep apnea including snoring, a normal physical examination, and a polysomnogram showing an apnea hypopnea index of < 1 per hour of sleep.
Children from both control and OSA groups were excluded from the study if they had chronic or recurrent tonsillitis, chronic pulmonary conditions, cardiac diseases, neuromuscular disorders, developmental delay or genetic syndromes, chronic renal disease, endocrinological disorders, acute or chronic inflammatory conditions, a body mass index z-score (BMI z-score) > 2.5, or were taking medication that influenced the autonomic nervous system. Demographic data and physical examination characteristics, including height and weight and office BP, were recorded. BP was the average of three readings obtained with an automated oscillometric device after a minimum of 5 minutes of rest in the sitting position.
All children underwent overnight polysomnography (PSG), PTT, and beat-to-beat BP measurements. Blood was drawn for biomarker analyses at 6:00 pm and 6:00 am before and after the PSG. An institutional review board (IRB) approval was obtained prior to the initiation of this project. During completion of the procedure, all personnel were blinded except for the scheduling coordinator.
Polysomnography
Overnight, in-laboratory, 16 channel PSG was performed in a pediatric sleep lab using a computerized system (Grass Telefactor, Astro-Med Inc., Westwarwick, RI). The electrocardiogram (EKG) was recorded using the Grass Telefactor System (Grass Telefactor) with standard electrodes. Electroencephalograms, electrooculograms, electromyograms at various locations, nasal/oral airflow, end tidal carbon dioxide (CO2), pulse oximetry waveforms, and video monitoring were performed during the sleep studies. Respiratory cycles were monitored by inductive plethysmography (Somnostar; SensorMedics Corp., Yorba Linda, CA). Simultaneous continuous BP monitoring was performed using finger arterial photoplethysmography (PortaPres; TNO-TPD Biomedical Instrumentation, Amsterdam, The Netherlands). The polysomnograms were reviewed and analyzed by a pediatric sleep physician who was blinded from subject grouping.
Plasma Inflammatory Biomarkers
Seven milliliters of whole blood were collected. A human multiplex cytokine assay (Linco Research Inc., St. Charles, MO) was used to evaluate the samples for serum levels of CRP, serum amyloid A (SAA), monocyte chemoattractant protein (MCP)-1, IL-6 and IL-8, adiponectin, soluble cluster of differentiation (CD)-40 ligand (L), and TNF-α. Cell supernatant was incubated with the antibody-coated bead mixture. Streptavidin-phycoerythrin was added and the sample incubated. The beads were washed and analyzed on a Luminex100 Platform (Luminex Corp., Austin, TX). Cytokine concentrations were calculated from standard curves.
Pulse Transit Time
Custom software (LabVIEW, National Instruments, Inc., United States) was used to identify the R-wave of the EKG and the point of maximum slope of the BP signal for each beat of interest. PTT was reported as the time, in seconds, from the R wave to the point of maximum BP slope. The selection criteria for heart beat analysis consisted of: (1) EKG and BP waveform free from any artifact, (2) heart beat in 30-second epochs which were scored as either awake or stage 2 sleep, (3) absence of any respiratory event, arousal, or stage transition during the 30-second epoch. PTT during wakefulness was derived from a 30-minute recording prior to sleep and from all epochs during the night scored as awake. PTT during stage sleep was derived from all stage 2 sleep throughout the night.
Since the objective of the study was to estimate PTT during periods of sleep that are uninterrupted by respiratory events, arousals, and stage transitions, we limited our measurements to stage 2 sleep.
Statistical Analysis
Descriptive statistics were generated as mean ± SD for continuous variables and the frequency with percentage for categorical variables. A paired t test was used to determine the equality of means between sleep states for the OSA severity levels. Adjusted regression estimates for PTT were determined by fitting univariate linear regression models by sleep state with each cytokine as a covariate. The least square mean estimates for comparing controls versus experimental patients by OSA severity level were obtained from a multivariable regression model with PTT as the response variable, and OSA severity level (three factors: healthy, mild, and severe), sleep state (two factors: awake and nonrapid eye movement [NREM]), BMI z-score, age, and sex as covariates. The results from the confirmatory factor analysis were generated in two phases: (1) factor analysis was used to find the grouping of cytokines, that is, the latent factors, and (2) development of a structural equation model with confirmatory factor analysis to determine the effect of cytokines on PTT. All analyses were performed using SAS (version 9.3, SAS Institute Inc., Cary, NC).
RESULTS
Subjects
One hundred fifty-five children participated in the study; 90 were healthy controls without symptoms or PSG findings consistent with OSA. Sixty-five children with OSA were sub-categorized into a group with mild OSA (n = 23) and a second group with moderate-to-severe OSA (n = 42). Children with moderate-to-severe OSA tended to be more overweight and of higher percentage of African American (Table 1). Although children with OSA did not have significantly higher systolic BPs compared to healthy controls, there was a trend for higher systolic BPs in children with mild and moderate-to-severe OSA.
Table 1.
Demographic Characteristics and Sleep Parameters for Children Recruited to the Study.
| Controls (n = 90) | Mild OSA (n = 23) | Moderate-to-severe (n = 42) | |
|---|---|---|---|
| Age, mean ± SD, years | 9.7 ± 2.5 | 9.6 ± 2.5 | 9.0 ± 2.7 |
| Sex | |||
| Male, n (%) | 41 (46%) | 8 (35%) | 18 (43%) |
| Race, n (%)* | |||
| White | 60 (67%) | 12 (52%) | 15 (36%) |
| Black | 20 (22%) | 9 (39%) | 25 (60%) |
| Other | 10 (11%) | 2 (9%) | 2 (5%) |
| BMI, mean ± SD* | 19.4 ± 4.4 | 20.7 ± 4.6 | 23.4 ± 13.5 |
| BMI z-score, mean ± SD, kg/m2 | 0.7 ± 1 | 1.1 ± 1.3 | 1.1 ± 1.2 |
| Total sleep time, min, mean ± SD | 528.4 ± 39.4 | 534.6 ± 38.2 | 522.9 ± 40 |
| Sleep efficiency, % mean ± SD | 80.1 ± 10.9 | 78.9 ± 8.6 | 79.2 ± 11.2 |
| % Time in stage 1, mean ± SD | 3 ± 1.4 | 2.6 ± 0.9 | 3.4 ± 1.8 |
| % Time in stage 2, mean ± SD | 45.4 ± 7.4 | 44.5 ± 6.8 | 45.7 ± 6.8 |
| % Time in stage 3, mean ± SD**,*** | 1.9 ± 0.7 | 1.5 ± 0.4 | 2.1 ± 0.7 |
| % Time in stage 4, mean ± SD | 27.7 ± 6.2 | 27.7 ± 7.0 | 27.8 ± 7.2 |
| % Time in REM, mean ± SD | 29.6 ± 6.4 | 28.7 ± 6.8 | 28.1 ± 7.2 |
| Arousal index, mean ± SD*,*** | 9.8 ± 2.9 | 10.5 ± 2.6 | 17.2 ± 10.1 |
| Obstructive index, mean ± SD*,**,*** | 0.3 ± 0.3 | 3.5 ± 1.1 | 13.8 ± 9.9 |
| Peak end-tidal CO2, mean ± SD | 49.6 ± 2.93 | 50.66 ± 8.61 | 50.92 ± 4.48 |
| Mixed apnea index, mean ± SD | N/A | 0.95 ± 0 | 0.91 ± 0.04 |
| Central apnea index, mean ± SD | 0.93 ± 0.03 | 0.91 ± 0.04 | 0.92 ± 0.03 |
| Obstructive hypopnea index, mean ± SD* | 0.94 ± 0.02 | 0.9 ± 0.04 | 0.87 ± 0.09 |
| Central hypopnea index, mean ± SD | N/A | N/A | N/A |
| SBP, mmHg, mean ± SD | 108.7 ± 14.4 | 113.8 ± 17.5 | 110.3 ± 15 |
| DBP, mmHg, mean ± SD | 60.2 ± 5.6 | 60.8 ± 6.5 | 60.1 ± 4.9 |
| AHI, mean ± SD*,*** | 0.4 ± 0.3 | 3.5 ± 1.1 | 15.2 ± 11.7 |
| % Saturation nadir, mean ± SD* | 93.5 ± 2 | 90.3 ± 3.7 | 86 ± 9.3 |
| % Avg oxygen, REM, mean ± SD | 97.6 ± 1.8 | 97.6 ± 1.3 | 97.5 ± 1.2 |
| % Avg oxygen, NREM, mean ± SD | 97.3 ± 1.3 | 97.4 ± 1.3 | 97.3 ± 1.2 |
| % time with CO2 > 50 mmHg* | 0.5 ± 1.8 | 1.2 ± 3.3 | 1.9 ± 4.4 |
BMI z-score = body mass index z-score; DBP = diastolic blood pressure; n = number; NREM = nonrapid eye movement; OSA = obstructive sleep apnea; SBP = systolic blood pressure. Other race includes Hispanic, Asian, mixed race and those specified as “Other” in the medical record.
*p < .05, Control vs. Severe OSA; **p < .05, Control vs. Mild OSA; ***p < .05, Mild vs. Severe OSA.
Diurnal Variation of Plasma Biomarkers
Healthy Controls
In healthy controls, MCP-1 was higher in the morning compared to the afternoon, while adiponectin, SAA, and TNF-α were higher in the afternoon (Table 2). There was a small but statistically significant difference in CRP between afternoon and morning levels. There was no diurnal variation in the levels of IL-6, IL-8, or sCD40L.
Table 2.
PM vs. AM Cytokine and Acute Phase Reactants Comparisons for Healthy Participants, Children With Mild OSA, and Children With Moderate-to-Severe OSA; Reported as Mean ± SD.
| Controls | Mild OSA | Moderate-to-severe OSA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PM control n = 90 | AM control n = 78 | Wilcoxon signed ranks p-value | PM mild n = 23 | AM mild n = 20 | Wilcoxon signed ranks p-value | PM severe n = 42 | AM severe n = 33 | Wilcoxon signed ranks p-value | |
| Adiponectin (μg/mL) | 29.7 ± 32.6 | 24.6 ± 13.9 | <.0001 | 23.7 ± 12.8 | 20.8 ± 10.1 | .6777 | 23.1 ± 17.0 | 16.8 ± 8.4 | <.0001 |
| SAA (μg/mL) | 5.2 ± 9.1 | 4.2 ± 6.6 | .0035 | 6.0 ± 9.7 | 4.7 ± 6.3 | .6742 | 11.2 ± 23.6 | 4.6 ± 5.2 | .1966 |
| sCD40L (pg/mL) | 2.5 ± 5.1 | 2.2 ± 2.8 | .5524 | 10.8 ± 18.6 | 8.4 ± 10.4 | .6477 | 6.5 ± 9.7 | 6.5 ± 5.9 | .7121 |
| IL-6 (pg/mL) | 24.6 ± 51.9 | 22 ± 44.2 | .3402 | 76.9 ± 114.9 | 98.2 ± 135 | .0714 | 29.2 ± 62.0 | 39 ± 74.4 | .9543 |
| IL-8 (pg/mL) | 9.8 ± 18.8 | 8.9 ± 14.2 | .3777 | 24.9 ± 31.4 | 30.1 ± 39.5 | .0400 | 11.6 ± 16.7 | 13.5 ± 20.8 | .4943 |
| MCP-1 (pg/mL) | 103.8 ± 50.8 | 108.6 ± 52.4 | .0092 | 100.4 ± 38.0 | 90.9 ± 37 | .9643 | 100.4 ± 43.9 | 88.9 ± 32.1 | .2582 |
| TNF-α (pg/mL) | 10.1 ± 45.4 | 6.2 ± 5.3 | .0044 | 5.9 ± 6.0 | 10.5 ± 11.2 | .0574 | 6.0 ± 4.1 | 6.8 ± 6.7 | .6147 |
| CRP (μg/mL) | 0.7 ± 1.2 | 0.8 ± 2.8 | .0005 | 1.1 ± 1.9 | 0.9 ± 1.7 | .0174 | 2.5 ± 4.5 | 1.2 ± 2.2 | <.0001 |
CRP = C-reactive protein; IL = interleukin; MCP-1 = monocyte chemoattractant protein; OSA = obstructive sleep apnea; TNF-α = tumor necrosis factor.
Children With OSA
In children with OSA, the diurnal variation exhibited a different profile from that of healthy controls, where CRP was higher in the afternoon and IL-8 and TNF-α higher in the morning (Table 2). The levels of IL-6 were also higher in the morning but did not reach statistical significance.
Plasma Cytokines and Acute Phase Reactants in Children With OSA and Healthy Controls
Children with OSA had higher plasma cytokines of IL-6, IL-8, and sCD40L compared to healthy controls (Table 3). These group differences were present in both morning and afternoon levels. CRP was higher in children with OSA during the PM blood draws. These differences between healthy controls and children with OSA varied depending on the severity of OSA.
Table 3.
Plasma Inflammatory Biomarkers for Controls and Children With OSA the Night Before and Morning After PSG.
| LS Means ± SE | |||
|---|---|---|---|
| PM | Control | Mild | Severe |
| Adiponectin (μg /mL) | 28.3 ± 2.0 | 24.4 ± 3.6 | 24.6 ± 2.6 |
| SAA (μg /mL)* | 5.2 ± 0.7 | 5.7 ± 1.5 | 10.3 ± 2.0 |
| sCD40L (pg/mL)*,** | 2.5 ± 0.3 | 11.4 ± 2.9 | 6.3 ± 1.3 |
| IL-6 (pg /mL)**,*** | 24.4 ± 4.0 | 79.1 ± 24.9 | 27.9 ± 6.2 |
| IL-8 (pg /mL)**,*** | 9.8 ± 1.2 | 24.2 ± 5.9 | 11.2 ± 2.0 |
| MCP-1 pg /mL) | 104.1 ± 4.7 | 100.7 ± 8.8 | 100.6 ± 6.6 |
| TNF-α (pg /mL) | 8.2 ± 1.0 | 7.1 ± 1.7 | 6.2 ± 1.1 |
| CRP (μg g/mL)*,*** | 0.6 ± 0.1 | 0.8 ± 0.2 | 2.1 ± 0.3 |
| AM | |||
| Adiponectin (μg /mL)* | 24.4 ± 1.5 | 21.1 ± 2.7 | 17.0 ± 1.6 |
| SAA (μg /mL) | 4.2 ± 0.5 | 3.8 ± 1.0 | 4.1 ± 0.7 |
| sCD40L (pg/mL)*,** | 2.2 ± 0.3 | 7.7 ± 2.0 | 6.3 ± 1.3 |
| IL-6 pg/mL)**,*** | 22.4 ± 3.9 | 89.0 ± 30.8 | 37.5 ± 9.3 |
| IL-8 (pg g/mL)** | 9.0 ± 1.1 | 25.6 ± 6.7 | 12.9 ± 2.5 |
| MCP-1 pg /mL) | 107.0 ± 4.8 | 92.0 ± 8.1 | 90.5 ± 6.2 |
| TNF-α pg /mL) | 6.2 ± 0.6 | 9.9 ± 2.2 | 6.7 ± 1.0 |
| CRP μg /mL)* | 0.7 ± 0.1 | 0.7 ± 0.2 | 1.2 ± 0.2 |
BMI z-score = body mass index z-score; CRP = C-reactive protein; IL = interleukin; LS = least square; MCP-1 = monocyte chemoattractant protein; OSA = obstructive sleep apnea; PSG = polysomnography; SE = standard error; TNF-α = tumor necrosis factor. LS mean ± SE. Variables have been adjusted for gender, BMI z-score and multiple comparisons.
*p < .05, Control vs. Severe OSA; **p < .05, Control vs. Mild OSA; ,***p < .05, Mild vs. Severe OSA.
Afternoon Plasma Cytokines and Acute Phase Reactants
Plasma sCD40L, IL-6, IL-8 and CRP were higher in children with OSA compared to healthy controls. However, adiponectin was higher in the control patients. Children with moderate-to-severe OSA tended to have lower plasma cytokines compared to those in the mild group (Table 3).
Morning Plasma Cytokines and Acute Phase Reactants
The biomarkers that significantly differed between controls and children with OSA included a lower adiponectin in severe OSA and higher sCD40L, IL-6, and IL-8 in mild and moderate-to-severe OSA. There was no difference in TNF-α between the three groups (Table 3).
Pulse Transit Time
Using least square mean estimates from a multivariable regression model adjusted for the effects of BMI z-score, age and gender, PTT was not significantly different between any pair of the three groups during either wakefulness or the NREM sleep states (Table 4). However, there was a significantly longer PTT measured during NREM sleep compared to wakefulness in all three groups.
Table 4.
Differences Between Least Square Mean Estimates for PTT in Healthy Controls, Children With Mild OSA, and Children With Moderate-to-Severe OSA for Awake and NREM Sleep States.
| OSA severity | PTT | |
|---|---|---|
| Awake | NREM | |
| Control* | (N = 322 ± 114) 0.19 ± 0.02 | (N = 322 ± 102) 0.21 ± 0.02 |
| Mild* | (N = 482 ± 482) 0.19 ± 0.03 | (N = 336 ± 155) 0.22 ± 0.03 |
| Severe*,a | (N = 356 ± 95) 0.19 ± 0.02 | (N = 345 ± 76) 0.21 ± 0.03 |
AHI = apnea hypopnea index; NREM = nonrapid eye movement; OSA = obstructive sleep apnea; PTT = pulse transit time. Mild = AHI 1 ≤ x < 5. NREM = Stage N2–N3.
aSevere = Moderate-to-Severe with AHI ≥ 5.
*p values are < .001 for the difference between awake and NREM in PTT.
Relationship Between Plasma Cytokines, Acute Phase Reactants, and PTT
Univariate Analysis
When adjusting for BMI z-score and gender, PM IL-6, IL-8, and sCD40L correlated negatively with PTT measured during wakefulness and during sleep. CRP and SAA correlated positively with PTT measured during wakefulness. AM Il-6 and IL-8 correlated negatively with PTT measured during wakefulness and NREM sleep. CRP correlated positively with PTT measured during wakefulness (Table 5).
Table 5.
Adjusted Regression Coefficient Estimates (With p Values) for the Effects of Each PM and AM Log-Transformed Biomarkers Levels on PTT.
| PM Cytokines | AM Cytokines | |||
|---|---|---|---|---|
| Awake PTT | NREM PTT | Awake PTT | NREM PTT | |
| Adiponectin | −0.0026 (.4) | −0.0027 (.4) | −0.00575 (.1) | −0.0065 (.1) |
| SAA | 0.003 (.02) | 0.00129 (.4) | 0.002181 (.2) | −0.0001 (.9) |
| sCD40L | −0.003 (.03) | −0.0034 (.03) | −0.00189 (.2) | −0.0026 (.1) |
| IL-6 | −0.003 (.009) | −0.00313 (.02) | −0.0036 (.003) | −0.0038 (.009) |
| IL-8 | −0.0042 (.007) | −0.0048 (.005) | −0.0045 (.007) | −0.0048 (.01) |
| MCP-1 | −0.0005 (.9) | −0.00223 (.6) | 0.000853 (.8) | 0.0019 (.7) |
| TNF-α | −0.0025 (.1) | −0.0038 (.049) | −0.00293 (.2) | −0.0027 (.3) |
| CRP | 0.0031 (.02) | 0.00162 (.3) | 0.0036 (.02) | 0.0027 (.1) |
CRP = C-reactive protein; IL = interleukin; MCP-1 = monocyte chemoattractant protein; NREM = nonrapid eye movement; PTT = pulse transit time; TNF-α = tumor necrosis factor. Values were adjusted for gender and body mass index z-score (BMI z-score) and multiple comparisons.
Factor Analysis
As certain cytokines are highly correlated, we adopted a structural equation modeling approach with a confirmatory factor analysis to evaluate the effects of cytokines and cytokine groups on the PTT values. The analyses were conducted separately for the cytokines measured in the afternoon versus the morning.
In this confirmatory factor model with afternoon plasma samples, two factors, group 1 and group 2, were derived. IL-8, IL-6 and TNF-α were clustered into group 1, while SAA, CRP and adiponectin were included in group 2. PM sCD40L and MCP-1 were not found to have any common associations with other specific biomarkers and were, therefore, evaluated individually for their effects on PTT. Group 1 cytokines and sCD40L negatively influenced PTT values measured during wakefulness (Table 6). However, group 2 biomarkers, namely SAA, CRP, and adiponectin, were found to positively influence PTT values. PTT measured during NREM sleep was not influenced by any of the factors.
Table 6.
Effect Estimates From the Structural Equation Models (With p Values) for the PM and AM Cytokines and Cytokine Groups on Vascular Stiffness (PTT) During Awake and NREM Sleep.
| Time of PTT measurement | Estimate | p | Time of PTT measurement | Estimate | p | ||
|---|---|---|---|---|---|---|---|
| PM | AM | ||||||
| Group 1: IL-6, IL-8, TNF-α | PTT during Awake | −0.1046 | .01 | Group 1: IL-6, IL-8, TNF-α, and sCD40L | PTT during Awake | −0.1950 | .002 |
| PTT during NREM | −0.0395 | .1 | PTT during NREM | −0.1401 | .006 | ||
| Group 2: Adiponectin, SAA, CRP | PTT during Awake | 0.1857 | .004 | Group 2: SAA and CRP | PTT during Awake | 0.0407 | .3 |
| PTT during NREM | 0.0701 | .1 | PTT during NREM | 0.0292 | .3 | ||
| sCD40L | PTT during Awake | −0.1206 | .03 | Adiponectin | PTT during Awake | −0.0084 | .8 |
| PTT during NREM | −0.0455 | .1 | PTT during NREM | −0.0060 | .8 | ||
| MCP-1 | PTT during Awake | 0.0140 | .8 | MCP-1 | PTT during Awake | 0.1295 | .04 |
| PTT during NREM | 0.0053 | .8 | PTT during NREM | 0.0930 | .06 | ||
| BMI z-score | PTT during Awake | 0.1174 | .03 | BMI z-score | PTT during Awake | 0.3379 | <.0001 |
CRP = C-reactive protein; BMI z-score = body mass index z-score; IL = interleukin; MCP-1 = monocyte chemoattractant protein; NREM = nonrapid eye movement; PTT = pulse transit time; SAA = serum amyloid A; TNF-α = tumor necrosis factor.
The factor analysis of the morning cytokines showed that IL-8, IL-6, TNF-α, and sCD40Lwere clustered into group 1, while SAA and CRP were included in group 2. Adiponectin, and MCP-1 were not found to have any common associations with other specific cytokines and were therefore, evaluated individually for their effects on the PTT. Group 1 cytokines were found to negatively influence PTT both during wakefulness and during NREM sleep (Table 6).
Collectively, the results of the factor analysis showed that the morning and afternoon levels of IL-8, IL-6, TNF-α, and sCD40L exert a negative effect on PTT while only the afternoon levels of CRP, SAA, and adiponectin were high enough to exert a positive effect on PTT. A differential effect of pro-inflammatory biomarkers and acute phase reactants on PTT exist depending on sleep state (Table 6).
The Effect of BMI on PTT
In the structural model of the influence of AM and PM cytokines on PTT, BMI z-score influenced PTT positively during wakefulness (Table 6).
DISCUSSION
Several new observations highlighting the opposing effects of inflammatory pathways on cardiovascular endpoints have emerged from this study. We report that the acute phase reactants, namely CRP and SAA, had an opposite effect compared to pro-inflammatory cytokines on PTT, a cardiovascular end point which reflects changes in blood vessel stiffness and left ventricular pre-ejection period. Furthermore, we also report that the influence of pro-inflammatory cytokines and acute phase reactants is dependent on the diurnal variation of these biomarkers and on sleep state. These novel observations underscore the clinical significance of the elevated levels of cytokines and acute phase reactants in children with OSA which clearly differ from adults.
In this study, we investigated whether the variation in levels of inflammatory biomarkers from morning to the afternoon in healthy controls is different from that observed in children with OSA. We demonstrated that the diurnal variation indeed differs between the two groups, with children with OSA having higher levels of pro-inflammatory cytokines in the morning compared to the afternoon and higher acute phase reactants in the afternoon. There are several potential explanations to the different profiles of inflammatory biomarkers in children with OSA. One plausible explanation is that the morning rise of pro-inflammatory cytokines stimulates the production of acute phase reactants which, based on their kinetics, peak several hours after the stimulus is applied.16 Another potential explanation is that the profile observed in children with OSA might be the result of desynchronized circadian rhythm controlling the pro-inflammatory cytokines and acute phase reactants, leading to a time shift of their peak levels.
We have also demonstrated that the relationship between PTT and pro-inflammatory cytokines and acute phase reactants observed during wakefulness no longer exists when PTT is measured during NREM sleep. It is possible that the increase in parasympathetic tone during NREM sleep17 becomes the predominant factor influencing vessel tone.
We have observed that children with OSA have higher IL-8, IL-6, and sCD40L compared to healthy controls. Based on published literature, these cytokines are linked mechanistically to the development of atherosclerosis.18–20 However, in our study there was no dose dependent increase of these cytokines with increasing severity of OSA. In fact, children with mild OSA tended to have a significantly higher inflammatory response compared to children with moderate-to-severe OSA and to healthy controls. The higher inflammatory response in children with mild OSA could not be explained by any of the polysomnographic parameters. These findings suggest that the severity of OSA in children, mild or severe, might contribute to the inflammatory response through additional mechanisms other than those related to sleep disruption and abnormal gas exchange.
Two acute phase reactants, namely SAA and CRP, and adiponectin have shown a dose dependent change in their levels after adjustment for obesity and gender. While adiponectin decreased, both SAA and CRP increased in children with OSA compared to controls.
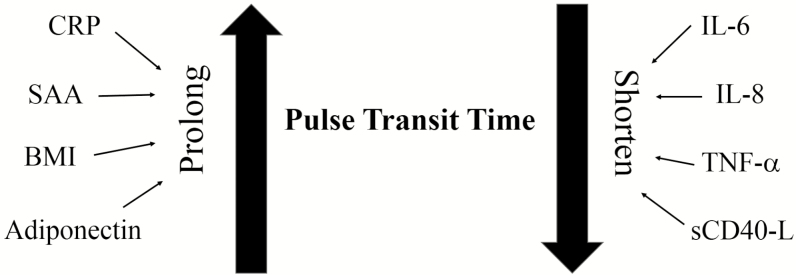
The opposing relationships of the two groups of biomarkers with PTT indicate that IL-8, IL-6, and sCD40L are associated with shortening, while SAA and CRP are associated with prolonging of PTT (Figure 1).
Figure 1.
Representation of groups of biomarkers and body mass index and their effects on pulse transit time (PTT) in children with obstructive sleep apnea (OSA).
Shortening of PTT is due in large part to decreased compliance of blood vessels as a result of elevated sympathetic tone and or vessel remodeling.21 In the context of our finding of a negative correlation between PTT and the group of biomarkers IL-6, IL-8, and sCD40L, it is likely that these mediators increase vessel stiffness and, in turn, shorten PTT. The finding that acute phase reactants decrease vessel stiffness contrasts with the knowledge gained from the literature in adults demonstrating a strong association between acute phase reactants and atherosclerotic cardiovascular diseases.22 Our finding raises the question on whether elevation of acute phase reactants in children has the same cardiovascular clinical implications in children compared to adults. It is possible that in children with OSA, acute phase reactants exert anti-inflammatory and/or vasorelaxing effects to maintain cardiovascular homeostasis. This concept could be substantiated by the observations that acute phase reactants, in addition to being pro-inflammatory, also possess an anti-inflammatory effect. CRP causes endothelial-independent vaosrelaxation and hypo-reactivity to phenylephrine mediated by increased nitric oxide (NO) production.23 It also protects against complement mediated cell injury24 and stimulates IL-10, a potent inhibitor of inflammation.25,26 Similarly, SAA induces the production of anti-inflammatory cytokines, including IL-10 and IL-1 receptor antagonist.27 Collectively, these observations suggest that CRP and SAA act as homeostatic regulators of inflammation in children with OSA.
Numerous studies of adults have examined the predictive value of individual cytokines or acute phase reactants to cardiovascular diseases.20 However, it is difficult to discern the true effects of these cytokines by only examining them individually and not considering the more global and physiologically relevant interactions.
Using structural equation modeling, the clinical similarities between cytokines were evaluated to determine which cytokines have similar relationships to PTT. The structural equation modeling provides a different level of understanding of the roles of the different biomarkers than univariate analysis. Specifically, neither TNF-α nor adiponectin correlated with PTT in the univariate analysis. However, in a structural model which examined a group of biomarkers, both were found to have an influence on PTT.
The absence of a difference in PTT between groups is consistent with a previous study that demonstrated no change in pulse wave velocities (PWV), measurements directly related to arterial wall stiffness, in children with OSA compared to control subjects.28 In fact, in this study, changes in vascular tone were only associated with BMI. However, this is inconsistent with data from previous findings.8,29 For example, children with severe SDB have lower diastolic BPs and wider pulse pressures than children with milder forms of the disease.8 Further, OSA was found to be independently associated with changes in flow-mediated dilation and glyceryl trinitrate-mediated dilation of the brachial artery in obese pediatric patients.29 The conflicting results between the different studies could not be explained without a knowledge of the balance between the mediators associated with an increase and those with a decrease in vessel stiffness. Specifically, the effect of an inflammatory milieu on cardiovascular endpoints cannot be examined without an understanding of how pro-inflammatory and anti-inflammatory pathways interact to maintain cardiovascular homeostasis.
We have also demonstrated that BMI has a prolonging effect on PTT, a finding likely explained by the effect of obesity on left ventricular pre-ejection period, a component of PTT. There is evidence that overweight subjects tend to have a prolonged pre-ejection period of the left ventricle.30
Our study has limitations that deserve discussion. PTT was selected as a measure of arterial vascular stiffness, yet we recognize that this measure is influenced by other cardiovascular parameters that are independent from blood vessel stiffness. We have chosen this technique because the noninvasive measurement of pulse wave velocity relies on algorithms validated in adults which limits its use in children. The cross-sectional design of this study limits the observations to a simple association between biomarkers and PTT without establishing a cause and effect relationship. However, the knowledge of the biological actions of the different mediators suggest that the pro-inflammatory mediators increase vessel stiffness while the acute phase reactants have a protective effect. We referred to diurnal variation of the biomarkers as the difference between their levels measured at only two time points. We recognize that the peak and trough of the biomarkers might not have coincided with the time points we have selected.
CONCLUSION
This study provides a new understanding of the inflammatory milieu in children with OSA and how they relate to cardiovascular endpoints. While the same pro-inflammatory cytokines associated with atherosclerosis in adults are elevated in children with OSA, their impact on the cardiovascular endpoints might be dampened by a protective effect of acute phase reactants. Studies which examine the changes in the inflammatory milieu over time are needed to establish risk factors for the development of cardiovascular injury children and adults with systemic inflammation.
FUNDING
The work was funded by National Institutes of Health (NIH) R01HL080670-01, 8 UL 1 TR000077-04 and the American Society of Pediatric Otolaryngology (ASPO) and Centralized Otolaryngology Research Effort (CORE) Basic Research grant.
DISCLOSURE STATEMENT
None declared.
REFERENCES
- 1. Ren R, Li Y, Zhang J, et al. Obstructive sleep apnea with objective daytime sleepiness is associated with hypertension. Hypertension. 2016; 68(5): 1264–1270. [DOI] [PubMed] [Google Scholar]
- 2. Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010; 122(4): 352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005; 353(19): 2034–2041. [DOI] [PubMed] [Google Scholar]
- 4. O’Brien LM, Gozal D. Autonomic dysfunction in children with sleep-disordered breathing. Sleep. 2005; 28(6): 747–752. [DOI] [PubMed] [Google Scholar]
- 5. Walter LM, Nixon GM, Davey MJ, Anderson V, Walker AM, Horne RS. Autonomic dysfunction in children with sleep disordered breathing. Sleep Breath. 2013; 17(2): 605–613. [DOI] [PubMed] [Google Scholar]
- 6. Crisalli JA, McConnell K, Vandyke RD, et al. Baroreflex sensitivity after adenotonsillectomy in children with obstructive sleep apnea during wakefulness and sleep. Sleep. 2012; 35(10): 1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McConnell K, Somers VK, Kimball T, et al. Baroreflex gain in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2009; 180(1): 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amin RS, Carroll JL, Jeffries JL, et al. Twenty-four-hour ambulatory blood pressure in children with sleep-disordered breathing. Am J Respir Crit Care Med. 2004; 169(8): 950–956. [DOI] [PubMed] [Google Scholar]
- 9. Amin RS, Kimball TR, Bean JA, et al. Left ventricular hypertrophy and abnormal ventricular geometry in children and adolescents with obstructive sleep apnea. Am J Respir Crit Care Med. 2002; 165(10): 1395–1399. [DOI] [PubMed] [Google Scholar]
- 10. Li AM, Hung E, Tsang T, et al. Induced sputum inflammatory measures correlate with disease severity in children with obstructive sleep apnoea. Thorax. 2007; 62(1): 75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tam CS, Wong M, McBain R, Bailey S, Waters KA. Inflammatory measures in children with obstructive sleep apnoea. J Paediatr Child Health. 2006; 42(5): 277–282. [DOI] [PubMed] [Google Scholar]
- 12. Biltagi MA, Maguid MA, Ghafar MA, Farid E. Correlation of 8-isoprostane, interleukin-6 and cardiac functions with clinical score in childhood obstructive sleep apnoea. Acta Paediatr. 2008; 97(10): 1397–1405. [DOI] [PubMed] [Google Scholar]
- 13. Gozal D, Crabtree VM, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med. 2007; 176(2): 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tauman R, O’Brien LM, Gozal D. Hypoxemia and obesity modulate plasma C-reactive protein and interleukin-6 levels in sleep-disordered breathing. Sleep Breath. 2007; 11(2): 77–84. [DOI] [PubMed] [Google Scholar]
- 15. De Luca Canto G, Pachêco-Pereira C, Aydinoz S, Major PW, Flores-Mir C, Gozal D. Biomarkers associated with obstructive sleep apnea and morbidities: a scoping review. Sleep Med. 2015; 16(3): 347–357. [DOI] [PubMed] [Google Scholar]
- 16. Jain S, Gautam V, Naseem S. Acute-phase proteins: as diagnostic tool. J Pharm Bioallied Sci. 2011; 3(1): 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trinder J, Kleiman J, Carrington M, et al. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001; 10(4): 253–264. [DOI] [PubMed] [Google Scholar]
- 18. Cavusoglu E, Marmur JD, Yanamadala S, et al. Elevated baseline plasma IL-8 levels are an independent predictor of long-term all-cause mortality in patients with acute coronary syndrome. Atherosclerosis. 2015; 242(2): 589–594. [DOI] [PubMed] [Google Scholar]
- 19. Zhao W, Zhang F, Li Z, Yu H, Li Z, Gao W. Soluble CD40 ligand is associated with angiographic severity of coronary artery disease in patients with acute coronary syndrome. Chin Med J (Engl). 2014; 127(12): 2218–2221. [PubMed] [Google Scholar]
- 20. Yuan M, Fu H, Ren L, Wang H, Guo W. Soluble CD40 ligand promotes macrophage foam cell formation in the etiology of atherosclerosis. Cardiology. 2015; 131(1): 1–12. [DOI] [PubMed] [Google Scholar]
- 21. Vlahandonis A, Biggs SN, Nixon GM, Davey MJ, Walter LM, Horne RS. Pulse transit time as a surrogate measure of changes in systolic arterial pressure in children during sleep. J Sleep Res. 2014; 23(4): 406–413. [DOI] [PubMed] [Google Scholar]
- 22. Koenig W, Rosenson RS. Acute-phase reactants and coronary heart disease. Semin Vasc Med. 2002; 2(4): 417–428. [DOI] [PubMed] [Google Scholar]
- 23. Clapp BR, Hirschfield GM, Storry C, et al. Inflammation and endothelial function: direct vascular effects of human C-reactive protein on nitric oxide bioavailability. Circulation. 2005; 111(12): 1530–1536. [DOI] [PubMed] [Google Scholar]
- 24. Li SH, Szmitko PE, Weisel RD, et al. C-reactive protein upregulates complement-inhibitory factors in endothelial cells. Circulation. 2004; 109(7): 833–836. [DOI] [PubMed] [Google Scholar]
- 25. Mold C, Rodriguez W, Rodic-Polic B, Du Clos TW. C-reactive protein mediates protection from lipopolysaccharide through interactions with Fc gamma R. J Immunol. 2002; 169(12): 7019–7025. [DOI] [PubMed] [Google Scholar]
- 26. Singh U, Devaraj S, Dasu MR, Ciobanu D, Reusch J, Jialal I. C-reactive protein decreases interleukin-10 secretion in activated human monocyte-derived macrophages via inhibition of cyclic AMP production. Arterioscler Thromb Vasc Biol. 2006; 26(11): 2469–2475. [DOI] [PubMed] [Google Scholar]
- 27. Sun L, Zhou H, Zhu Z, et al. Ex vivo and in vitro effect of serum amyloid a in the induction of macrophage M2 markers and efferocytosis of apoptotic neutrophils. J Immunol. 2015; 194(10): 4891–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koren D, Chirinos JA, Katz LE, et al. Interrelationships between obesity, obstructive sleep apnea syndrome and cardiovascular risk in obese adolescents. Int J Obes (Lond). 2015; 39(7): 1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dubern B, Aggoun Y, Boulé M, Fauroux B, Bonnet D, Tounian P. Arterial alterations in severely obese children with obstructive sleep apnoea. Int J Pediatr Obes. 2010; 5(3): 230–236. [DOI] [PubMed] [Google Scholar]
- 30. Romano M, Carella G, Cotecchia MR, et al. Abnormal systolic time intervals in obesity and their relationship with the amount of overweight. Am Heart J. 1986; 112(2): 356–360. [DOI] [PubMed] [Google Scholar]