Abstract
There remains debate about whether risk-reducing salpingo-oophorectomy (RRSO), which reduces ovarian cancer risk, also reduces breast cancer risk. We examined the association between RRSO and breast cancer risk using a prospective cohort of 17 917 women unaffected with breast cancer at baseline (7.2% known carriers of BRCA1 or BRCA2 mutations). During a median follow-up of 10.7 years, 1046 women were diagnosed with incident breast cancer. Modeling RRSO as a time-varying exposure, there was no association with breast cancer risk overall (hazard ratio [HR] = 1.04, 95% confidence interval [CI] = 0.87 to 1.24) or by tertiles of predicted absolute risk based on family history (HR = 0.68, 95% CI = 0.32 to 1.47, HR = 0.94, 95% CI = 0.70 to 1.26, and HR = 1.10, 95% CI = 0.88 to 1.39, for lowest, middle, and highest tertile of risk, respectively) or for BRCA1 and BRCA2 mutation carriers when examined separately. There was also no association after accounting for hormone therapy use after RRSO. These findings suggest that RRSO should not be considered efficacious for reducing breast cancer risk.
Several studies have reported evidence for a strong association between risk-reducing salpingo-oophorectomy (RRSO) and reduced breast cancer risk for BRCA1 and BRCA2 mutation carriers (1–4). Heemskerk-Gerritsen et al. (5), however, argued that these risk estimates were biased because of using an inappropriate analysis; they reported no association with breast cancer risk when RRSO was considered as a time-dependent covariate. Time-dependent analyses treat women as unexposed before RRSO and exposed after RRSO. Kotsopoulos et al. (6) confirmed the findings of Heemskerk-Gerritsen et al. (5) using a longer mean follow-up time (5.6 years vs 3.2 years). Both studies had few incident breast cancer cases among women with RRSO (122 and 21 among BRCA1 and BRCA2 mutation carriers, respectively, in Kotsopoulos et al. (6), and 36 and 6 among BRCA1 and BRCA2 carriers, respectively, in Heemskerk-Gerritsen et al. (5)), which limited power.
We examined the association between RRSO and breast cancer risk for women across a wide range of familial and genetic risk using the Prospective Family Study Cohort (7). With our large cohort and long follow-up time, we were also able to investigate whether timing of RRSO mattered and whether use of hormone therapy after RRSO accounted for any lack of evidence for a decreased breast cancer risk.
The Prospective Family Study Cohort includes women from the Breast Cancer Family Registry (BCFR) and the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab) (7). All participants provided written informed consent before enrollment, and study protocols have been approved by institutional review boards at each of the respective institutions. The current analysis used data from all women aged 18 to 79 years who were unaffected with breast cancer at baseline (N = 17 917 women; 7.2% known BRCA1 and BRCA2 mutation carriers). Women were asked at baseline and at follow-up if they ever had one or more ovaries removed. We classified women who reported both ovaries removed at least one year before breast or ovarian cancer diagnosis as being exposed. We used Cox proportional hazard models, with age as the time scale, to estimate hazard ratios (HR) and corresponding 95% confidence intervals (CIs) using a robust variance sandwich estimator. We assessed the proportional hazards assumption by testing the interaction with time. We defined follow-up time in two ways: (1) starting at baseline and (2) starting at age 25 years, given that many of the oophorectomies in this cohort occurred before baseline, with follow-up time ending at first invasive breast cancer diagnosis or censored at risk-reducing bilateral mastectomy, death, last follow-up, or age 80 years, whichever came first.
The analyses were stratified by birth cohort (<1950, 1950–1959, 1960–1969, ≥1970) and adjusted for study center, race/ethnicity, and age at baseline. Screening for germline BRCA1 and BRCA2 mutations by the BCFR and kConFab (8–10) typically involved testing the youngest affected woman in each family and, if identified to be a carrier, testing the other family members for that mutation. Noncarriers were women not known to be carriers and included both tested and untested women. We used the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA) model (11) to estimate absolute 10-year breast cancer risk. Given that many women had RRSO before baseline, we compared the results for follow-up time starting at baseline with those for follow-up time starting at age 25 years. We also compared results based on age at RRSO and stratified based on whether women received hormone therapy after RRSO. All statistical tests based on Cox Proportional Hazard modeling were two sided, and P values less than .05 were considered statistically significant. SAS software version 9.4 (SAS Institute Inc, Cary, NC) was used for all statistical analyses.
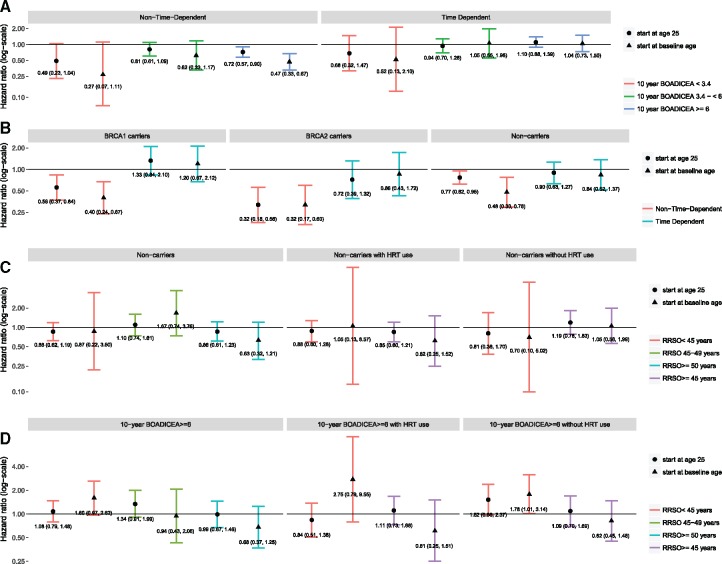
After a median 10.7 years of follow-up from baseline, we observed 1046 incident breast cancer cases (Table 1). Figure 1A summarizes the results for the overall cohort and stratified by tertile of predicted absolute risk. Figure 1B summarizes the results for BRCA1 mutation carriers, BRCA2 mutation carriers, and noncarriers. When we modeled RRSO as a fixed exposure, our risk estimates were similar to other published estimates and suggested a reduced risk from analyses of women in the overall cohort (HR = 0.78, 95%CI = 0.65 to 0.92) as well as in the other risk groups (highest risk tertile of predicted absolute risk, the mutation carriers, and the noncarriers). However, when we fitted RRSO as a time-dependent variable, we observed no statistically significant associations for the overall cohort (HR = 1.04, 95% CI = 0.87 to 1.24) or by tertiles of predicted absolute risk based on family history (HR = 0.68, 95% CI = 0.32 to 1.47, HR = 0.94, 95% CI = 0.70 to 1.26, and HR = 1.10, 95% CI = 0.88 to 1.39, for lowest, middle, and highest tertile of risk, respectively). The results were similar for follow-up time starting at baseline age or at age 25 years. There was also no association after stratification by age at RRSO or by hormone replacement therapy use after RRSO (Figure 1, C and D).
Table 1.
Characteristics of women who were unaffected at baseline, ProF-SC (N = 17 917)
| Descriptive characteristics |
BRCA1 carriers, n (%) |
BRCA2 carriers, n (%) |
Noncarriers*, n (%) |
|||
|---|---|---|---|---|---|---|
| No RRSO | RRSO | No RRSO | RRSO | No RRSO | RRSO | |
| Total cohort unaffected at baseline | 462 (64.5) | 254 (35.5) | 376 (65.6) | 197 (34.4) | 14 731 (88.6) | 1897 (11.4) |
| Incident breast cancer | 69 (14.9) | 47 (18.5) | 61 (16.2) | 19 (9.6) | 748 (5.1) | 102 (5.4) |
| Age at first breast cancer diagnosis, y | ||||||
| ≤35 | 17 (24.6) | 0 (0) | 9 (14.8) | 0 (0) | 19 (2.5) | 0 (0) |
| 36-50 | 39 (56.5) | 22 (46.8) | 24 (39.3) | 7 (36.8) | 225 (30.1) | 11 (10.8) |
| >50 | 13 (18.8) | 25 (53.2) | 28 (45.9) | 12 (63.2) | 504 (67.4) | 91 (89.2) |
| Age at RRSO, y | ||||||
| <30 | — | 4 (1.6) | — | 2 (1.0) | — | 75 (4.0) |
| 30–34 | — | 22 (8.7) | — | 6 (3.1) | — | 126 (6.6) |
| 35–39 | — | 53 (20.9) | — | 27 (13.7) | — | 237 (12.5) |
| 40–44 | — | 62 (24.4) | — | 49 (24.9) | — | 312 (16.5) |
| 45–49 | — | 56 (22.1) | — | 52 (26.4) | — | 438 (23.1) |
| 50–59 | — | 40 (15.8) | — | 47 (23.9) | — | 488 (25.7) |
| ≥60 | — | 17 (6.7) | — | 14 (7.1) | — | 221 (11.7) |
| Hormone replacement therapy use at baseline | ||||||
| Ever | 82 (17.7) | 109 (42.9) | 47 (12.5) | 72 (36.6) | 3134 (21.3) | 1252 (66.0) |
| Never | 373 (80.7) | 144 (56.7) | 324 (86.2) | 124 (62.9) | 11 485 (77.9) | 635 (33.5) |
| Missing | 7 (1.5) | 1 (0.4) | 5 (1.3) | 1 (0.5) | 112 (0.8) | 10 (0.5) |
| Parity at baseline | ||||||
| Nulliparous | 161 (34.9) | 42 (16.5) | 122 (32.5) | 19 (9.6) | 3599 (24.4) | 237 (12.5) |
| Parous | 300 (64.9) | 212 (83.5) | 254 (67.5) | 178 (90.4) | 11 129 (75.6) | 1660 (87.5) |
| Missing | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) | 3 (0.02) | 0 (0) |
| Mean age at first child birth (median), y | 24.0 (24.1) | 24.4 (24.1) | 24.1 (24.1) | 24.5 (24.1 | 24.3 (24.1) | 23.1 (23.0) |
Includes both tested and untested women for mutations in BRCA1 and BRCA2. RRSO = risk-reducing salpingo-oophorectomy.
Figure 1.
Model comparison for women who were unaffected at baseline, ProF-SC. Adjusted for age at baseline, race/ethnicity, study center, and Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA) score. Time-dependent models count individuals as unexposed before risk-reducing salpingo-oophorectomy (RRSO) and exposed after RRSO; non-time-dependent models treat RRSO as a fixed effect. A) summarizes the results from models treating RRSO as both a fixed and time varying exposure for the overall cohort stratified by tertile of predicted absolute risk of breast cancer as estimated by BOADICEA. B) summarizes results from models treating RRSO as both a fixed and time varying exposure for BRCA1 mutation carriers, BRCA2 mutation carriers, and non-carriers. C) presents time-dependent models for non-carriers stratified by age at RRSO or by age at hormone replacement therapy (HRT) use after RRSO. D) presents time-dependent models for women in the upper tertile of absolute risk of breast cancer as estimated by BOADICEA, stratified by age at RRSO or by age at hormone replacement therapy (HRT) use after RRSO. All panels present models starting follow-up time at at age 25 years or at baseline age.
Our results represent the largest independent prospective replication of the findings by Heemskerk-Gerritsen et al. (5) and suggest that consideration of RRSO, and its timing, should be based solely on reduced risk of ovarian cancer and not breast cancer. We extended the previous analyses by examining these questions using a cohort of women enriched for familial risk but who were highly unlikely to be BRCA1 or BRCA2 mutation carriers. Although it is possible that some noncarriers were mutation carriers, we observed similar associations when we classified women by category of BOADICEA risk, which is highly correlated with BOADICEA’s estimated carrier probability based on family history.
There are several limitations that warrant consideration. We cannot rule out the possibility that RRSO in early adult life, such as before the rapid rise in breast cancer incidence for BRCA1 carriers in their 30 s, could reduce risk (only 10% of our cohort had their RRSO before age 35 years). The rise in breast cancer incidence for BRCA2 carriers occurs in their 40s (12), which might explain a small, although not statistically significant protective association for BRCA2 carriers that was also reported in two previous studies (5,6). In our cohort, only 31.1% of BRCA1 mutation carriers, 17.8% of BRCA2 mutation carriers, and 23.1% of noncarriers had an RRSO before age 40 years. Thus, as in other studies, the power to investigate the role of RRSO at an early age, particularly for BRCA2 mutation carriers, is limited. It remains plausible that RRSO could reduce breast cancer risk, but such a reduction, if it exists, would need to occur much earlier than natural menopause, and the consequences of early RRSO on other aspects of long-term health would need to be considered. Based on the consistency of the recent prospective evidence including our study, we believe that clinical management of BRCA1 and BRCA2 mutation carriers, as well as other high-risk women considering RRSO, should not be based on presumed reduction of breast cancer risk.
Funding
This work was supported by the National Institute of Health USA (grant no. 1RO1CA159868). The ABCFR was supported in Australia by the National Health and Medical Research Council, the New South Wales Cancer Council, the Victorian Health Promotion Foundation, the Victorian Breast Cancer Research Consortium, Cancer Australia, and the National Breast Cancer Foundation. The six sites of the BCFR were supported by grant UM1 CA164920 from the USA National Cancer Institute at NIH.
This work was supported by grants to kConFab and the kConFab Follow-Up Study from Cancer Australia (grant no. 809195), the Australian National Breast Cancer Foundation (grant no. IF 17 kConFab), the National Health and Medical Research Council (grant nos. 454508, 288704, 145684), the National Institute of Health USA (grant no. 1RO1CA159868), the Queensland Cancer Fund, the Cancer Councils of New South Wales, Victoria, Tasmania and South Australia, and the Cancer Foundation of Western Australia (grant numbers not applicable). KAP is a National Breast Cancer Foundation (Australia) Practitioner Fellow (grant no. PRAC-17–004).
Notes
Affiliations of authors: Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, NY (MBT, XM, NZ, NL); Herbert Irving Comprehensive Cancer Center, Columbia University Medical Center, New York, NY (MBT, WKC); Department of Clinical Genetics, Fox Chase Cancer Center, Philadelphia, PA (MBD); Department of Medical Oncology, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia (KAP); Centre for Epidemiology and Biostatistics, The University of Melbourne, Parkville, Victoria, Australia (KAP, GSD, RJM, RLM, PCW, JLH); Sir Peter MacCallum Department of Oncology, The University of Melbourne, Melbourne, Victoria, Australia (KAP); Cancer Epidemiology and Intelligence Division, Cancer Council Victoria, Melbourne, Victoria, Australia (RJM, RLM, GGG); Departments of Pediatrics and Medicine, Columbia University, New York, NY (WKC); Lunenfeld-Tanenbaum Research Institute, Sinai Health System, Toronto, Ontario, Canada (JAK, GG, ILA); Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Genetic Epidemiology Laboratory, Department of Pathology, The University of Melbourne, Parkville, Victoria, Australia (MCS); Department of Medicine and Huntsman Cancer Institute, University of Utah Health Sciences Center, Salt Lake City, UT (DG, SSB); The Research Department, The Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia (kConFab Investigators); Department of Biostatistics, Mailman School of Public Health, Columbia University, New York, NY (RB); Departments of Molecular Genetics and Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (ILA); Department of Medicine and Stanford Cancer Institute, Stanford University School of Medicine, Stanford, CA (EMJ).
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
The authors have no conflicts of interest to disclose.
The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the BCFR, nor does mention of trade names, commercial products, or organizations imply endorsement by the USA Government or the BCFR.
We thank the entire team of BCFR past and current investigators as well as the kConFab investigators. We also thank Heather Thorne, Eveline Niedermayr, Stephanie Nesci, Lucy Stanhope, Sandra Picken, all the BCFR and kConFab research nurses and staff, the heads and staff of the Family Cancer Clinics, and the many families who contribute to the BCFR and kConFab for their contributions to this resource.
References
- 1. Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304(9):967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Domchek SM, Stopfer JE, Rebbeck TR.. Bilateral risk-reducing oophorectomy in BRCA1 and BRCA2 mutation carriers. J Natl Compr Canc Netw. 2006;4(2):177–182. [DOI] [PubMed] [Google Scholar]
- 3. Eisen A, Lubinski J, Klijn J, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: an international case-control study. J Clin Oncol. 2005;23(30):7491–7496. [DOI] [PubMed] [Google Scholar]
- 4. Kauff ND, Domchek SM, Friebel TM, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. J Clin Oncol. 2008;26(8):1331–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heemskerk-Gerritsen BA, Seynaeve C, van Asperen CJ, et al. Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst. 2015;107(5):djv033. [DOI] [PubMed] [Google Scholar]
- 6. Kotsopoulos J, Huzarski T, Gronwald J, et al. Bilateral oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2017;109(1):djw177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Terry MB, Phillips K-A, Daly MB, et al. Cohort profile: the breast cancer Prospective Family Study Cohort (ProF-SC). Int J Epidemiol. 2016;45(3):683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. John EM, Hopper JL, Beck JC, et al. The Breast Cancer Family Registry: an infrastructure for cooperative multinational, interdisciplinary and translational studies of the genetic epidemiology of breast cancer. Breast Cancer Res. 2004;6(4):R375–R389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andrulis IL, Anton-Culver H, Beck J, et al. Comparison of DNA- and RNA-based methods for detection of truncating BRCA1 mutations. Hum Mutat. 2002;20(1):65–73. [DOI] [PubMed] [Google Scholar]
- 10. Neuhausen SL, Ozcelik H, Southey MC, et al. BRCA1 and BRCA2 mutation carriers in the Breast Cancer Family Registry: an open resource for collaborative research. Breast Cancer Res Treat. 2009;116(2):379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Antoniou AC, Pharoah PP, Smith P, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer. 2004;91(8):1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402–2416. [DOI] [PubMed] [Google Scholar]