Abstract
Rationale:
Current scoring criteria of non-apneic events (ie, hypopnea) require the presence of oxyhemoglobin desaturation and/or arousal. However, other sleep study parameters may help to identify abnormal respiratory events (REs) and assist in making more accurate diagnosis.
Objectives:
To investigate whether non-apneic REs without desaturation or cortical arousal are associated with respiratory and cardiac consequences.
Methods:
Thirteen participants with sleep disturbances (snoring and/or excessive day time sleepiness), were screened using attended in laboratory polysomnography (PSG) while monitoring pressure and airflow via a nasal mask with an attached pneumotach. To separate the contribution of the upper airway resistance (RUA) and total pulmonary resistance (RL), supraglottic and esophageal pressures were measured using Millar pressure catheters. RL and RUA were calculated during baseline and hypopneas. RL was defined as the resistive pressure divided by the maximal flow during inspiration and expiration. Hypopnea was defined 30% decrease in flow with 3% desaturation and/or cortical arousal. REs was defined as 30% decrease in the flow without desaturation and/or cortical arousal. In eight subjects continuous positive airway pressure (CPAP) was titrated to optimal pressure. R-R interval (RRI) was defined as consecutive beat-to-beat intervals on single lead electrocardiograph (ECG) during baseline, RE/hypopnea and on optimal CPAP.
Results:
REs associated with increased expiratory RUA (14.6 ± 11.3 vs. 7.5 ± 4.5 cmH2O L−1 s−1; p < .05), and increased expiratory RL relative to baseline (29.2 ± 14.6 vs. 20.9 ± 11.0 and 23.7 ± 12.1 vs. 14.3 ± 5.6 cmH2O L−1 s−1 during inspiration and expiration, respectively; p < .05). RRI decreased significantly following RE and hypopnea relative to baseline (804.8 ± 33.1 vs. 806.4 ± 36.3 vs. 934.3 ± 45.8 ms; p < .05). Optimal CPAP decreased expiratory RUA (4.0 ± 2.5 vs. 7.5 ± 4.5 cmH2O L−1 s−1; p < .05), decreased inspiratory RL (12.6 ± 14.1 vs. 7.5 ± 4.5 cmH2O L−1 s−1; p < .05), and allowed RRI to return to baseline (p < .05). RRI dips index was an independent predictor of sleep-disordered breathing (SDB) when non-apneic REs were accounted for in symptomatic patients (p < .05).
Conclusions:
Non-apneic REs without cortical arousal or desaturation are associated with significant respiratory and heart rate changes. Optimal CPAP and the reduction of resistive load are associated with the normalization of heart rate indicating potential clinical benefit.
Keywords: hypopnea, inspiratory, expiratory, upper airway resistance, total pulmonary resistance, sleep-disordered breathing.
Statement of Significance
Sleep-disordered breathing (SDB) traditionally include apneic and non-apneic respiratory events (RE) if associated with desaturation or arousals. However, SDB encompasses a spectrum of respiratory abnormalities that could disrupt normal physiology. The objective of this study is to assess the short-term physiologic “consequences” of non-apneic RE without desaturation or cortical arousal before and after continuous positive airway pressure (CPAP) application. This study confirms that non-apneic RE that does not reach current thresholds for the definition of “abnormal events” by criteria based on electroencephalogram (EEG) changes or a fall in oxygen saturation have an impact on heart rate and lung mechanics and improve with CPAP. Accounting for these REs in clinical practice may have significant implications for diagnosis and outcome, which needs to be confirmed in future studies.
INTRODUCTION
Sleep-disordered breathing (SDB) is associated with significant adverse health consequences such as hypertension and cardiovascular disease,1 which may be mitigated with nasal continuous positive airway pressure (CPAP) therapy.2
SDB is characterized by the occurrence of recurrent episodes of apnea and hypopnea, resulting in oxyhemoglobin desaturation and sleep fragmentation. Apneic episodes are identified by the absence of flow, independent of immediate physiologic consequences. In contrast, the qualitative nature of clinical polysomnography (PSG) precludes identification of hypopnea based on ventilatory parameters alone. One of the original definitions of hypopnea included flow reduction associated with subsequent oxyhemoglobin de-saturation ranging from 2–5%.3 Thus, to date all definitions of hypopnea are based on physiologic consequences of decreased alveolar ventilation, namely decreased flow, oxyhemoglobin desaturation, and/or transient cortical arousal.
The criteria for airflow reduction have also been variable across different studies, ranging from perceptible qualitative decrease in oral-nasal flow to 50% drop from baseline. Scoring of hypopnea according to most recent American Academy of Sleep Medicine (AASM) scoring manual requires the presence of physiologic consequences, namely 3% desaturation and/or cortical arousal.4 A common limitation of all definitions of hypopnea is that the magnitude of oxyhemoglobin desaturation depends on individual “host factors” such as body weight and baseline pulmonary function.5 Similarly, the presence of carboxyhemoglobin may shift the oxyhemoglobin dissociation curve to the left and hence dampen the magnitude of oxyhemoglobin desaturation in current smokers.6
The Sleep Heart Health Study investigated the effect of varying the level of hypopnea-related oxyhemoglobin desaturation on the association between SDB and prevalent cardiovascular disease. Specifically, the frequency of hypopneas defined by a threshold of oxyhemoglobin desaturation of 4% or more was associated with cardiovascular disease, while lesser degree of desaturation or cortical arousal was not associated with cardiovascular disease based on self-report.7 However, there are other studies that have examined the immediate cardiovascular response to episodes of decreased flow, independent of the magnitude of desaturation or electroencephalogram (EEG), cortical arousal, and found conflicting results.8,9 While Ayappa et al.8 found less effect on heart rate (HR) increase immediately after non-apneic and mild reduction in flow,9 Guilleminault and colleagues found that non-apneic events were associated more consistelty with HR changes.
The objectives of this study were twofold: First, to determine whether respiratory events (REs) that do not meet the hypopnea definition are associated with physiological consequences including an increase in airway resistance and heart rate changes. Second, to determine the effect of continuous positive airway pressure (CPAP) on these physiological consequences. Therefore, we hypothesized that unclassified REs manifesting by decreased flow and no desaturation or cortical arousal would be associated with decreased minute ventilation (VE), increased airway resistance and dynamic fluctuations in heart rate. Results of this study have previously been reported in the form of abstract.10
METHODS
Participants
The experiment protocols were approved by the Human Investigation Committees of Wayne State University School of Medicine and John D. Dingell VA Medical Center. Informed written consents were obtained from all participants who were healthy volunteers recruited from the community in Detroit metropolitan area using approved advertisement. All subjects were screened for SDB including EEG, ventilation, upper airway and esophageal pressure (PES). Subjects were included if they had SDB (ie, apnea-hypopnea index [AHI] ≥ 5 events/h) or sleep related symptoms .
Breathing Circuit
Each participant was connected to the breathing circuit via nasal mask. An appropriate-sized, airtight nasal mask (Respironics, Murrysville, PA) was secured to the face to prevent mask leak. The mask was connected to the Plateau exhalation valve (Respironics, Inc, Pittsburg, PA); via a Pneumotachometer (model 3700A, Hans Rudolph, Kansas City, MO).
Measurements
EEG, electrooculograms, and chin electromeolgrams were recorded using the International 10–20 systems of electrode placement (EEG: C3-A2, and C4-A1; electrooculogram E1-A2). The tidal volume (VT) was obtained from the electronic integration of the flow signal on the pneumotach system. The supraglottic pressure (PSG) was measured using a pressure transducer tipped catheter (Model TC-500 XG, Millar Instruments, Houston, TX) while the tip is positioned in the hypopharynx. This position was obtained by advancing the catheter tip 2 cm behind the tongue.]
In all subjects, PES was measured by a pressure transducer tipped catheter (model TC-500 XG, Millar Instruments, Houston, TX) during sleep throughout the night. The catheter was placed best to reflect pleural pressure approximately in the mid-esophagus. The participant was given 2–4 mL lidocaine (2%) using the syringe through one of the nostrils. After the participant begun to feel the numbing sensation, they were instructed to flex the head (ie, the patient should bend his/her head down, with the chin towards the chest) before and during the procedure. This ensures that the catheter will be more likely to enter the esophagus as opposed to the trachea. The catheter is placed from the angle of the mouth to the tragus of the ear of the same side and then to the sterna angle. The measured length is marked. The catheter then is slowly inserted into the nostril and advanced along the floor of the nostril. The catheter is slowly advanced down the esophagus until placement is achieved at the predetermined taped mark. Using the flash light and the tongue depressor, the catheter position was confirmed behind the tongue at the back of the throat. If found coiled in the mouth, the catheter was pulled out and reinserted. The patient was asked to take several deep breaths. The esophageal catheter position was confirmed by performing Mueller and Valsalva maneuvers while assessing the pressure tracings.
End-tidal CO2 (PETCO2) readings were continuously measured using infrared analyzer (CWE Gemini Gas Analyzer) from tubing placed in the nares via a port in the nasal mask. Arterial O2 saturation (SaO2) was measured by the pulse oximeter (Biox 3700, Ohmeda). Inspired O2 (PIO2) was sampled continuously by an infrared analyzer (model CD-3A, AEI Technologies) via tubing attached to a port on the nasal mask. The signals were displaced on a polygraph recorder (Grass model 15, Astro-Med, West Warwick, RI) and the recording using Powerlab data- acquisition software (AD Instruments, Colorado Springs, CO) for detailed analysis.
CPAP Intervention
To ascertain the effect of CPAP treatment on upper airway and lung mechanics, CPAP was applied and titrated for “optimal pressure” (resolution of inspiratory flow limitation).
Study Design and Data Analysis
Non-apneic REs Analysis
We measured EEG, flow, PSG, and PES simultaneously during wakefulness and sleep. In each hypopnea trial, we compared the three breaths of hypopnea with the three baseline breaths preceding the RE (Figures 1 and 2). Hypopnea was defined 30% decrease in flow with 3% desaturation and/or arousal according to recommended AASM scoring criteria.4 RE was defined as 30% decrease in the flow without desaturation and/or arousal. RE index (REI) was defined as number of Res divided by total sleep time.
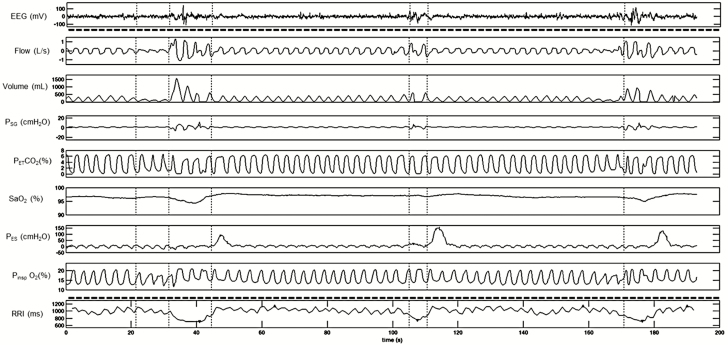
Figure 1.
A representative polygraph record of hypopneas with >3% desaturation and cortical arousals (top part). Note the responses in heart rate and RRI following each hypopnea event (bottom part). RRI tracing was retro-graphed from exported signal that calculates RRI from raw electrocardiograph (ECG) recording. EEG and RRI tracings (top and bottom segments separated by dotted line) were recorded in different systems and time matched with the respiratory tracing. EEG = electroencephalogram; PSG = supraglottic pressure; PETCO2 = end-tidal CO2; SaO2 = oxygen saturation; PES = esophageal pressure; PIO2 = inspired O2; RRI = R-R intervals.
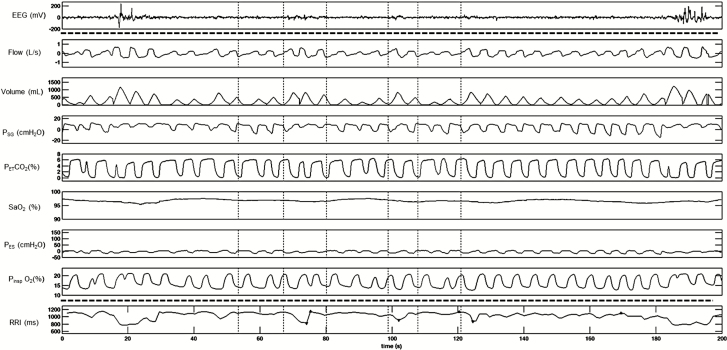
Figure 2.
A representative polygraph record of respiratory event (RE) without significant desaturation or cortical arousals (top part). Note the responses in heart rate and RRI following each RE (bottom part). RRI tracing was retro-graphed from exported signal that calculates RRI from raw electrocardiograph (ECG) recording. EEG and RRI tracings (top and bottom segments separated by dotted line) were recorded in different systems and time matched with the respiratory tracing. EEG = electroencephalogram; PSG = supraglottic pressure; PETCO2 = end-tidal CO2; SaO2 = oxygen saturation; PES = esophageal pressure; PIO2 = inspired O2; RRI = R-R intervals.
The absolute value of PSG, PES, and flow was measured on a breath-by-breath basis. Upper airway resistance (RUA) was calculated as a numeric representation of the maximal linear part of the pressure-flow loop during inspiratory and expiratory phases. Specifically, RUA was calculated as flow/PSG at the maximal flow. We compared, three baseline breaths preceding each RE to the first three hypopneic breaths and calculated the mean of all identified and scored hypopnea events.
R L was calculated using the standard technique for all analyzed breaths as the resistive pressure divided by the maximal flow during inspiration and expiration, respectively. The lung elastic (recoil) component, measured at points of zero flow, was subtracted from the calculated total pressure gradient to obtain resistive pressure. To estimate the work of breathing during baseline, hypopnea, and RE, we computed the pressure time product for tidal volume (PTP). The PTP was calculated using the following formula as absolute change in PES (cmH2O) multiplied by inspiratory time (seconds) then corrected for the respective mean tidal volume (mL).11
The PSG of every participant was examined for non- apneic REs without significant desaturation was identified. The Electrocardiograph (ECG) signal was assessed for adequacy of analysis and exported to MatLab program to obtain beat-to-beat RRI at the following points: (1) RRI at the beginning of event (BE), (2) RRI at the end of event (EE), (3) nadir RRI following event (NR), (4) RRI at nadir SaO2 (NS), and (5) maximum RRI following nadir (MR). The ECG signal from each participant PSG was exported to a MatLab program developed and validated by our group. The program analyzed each RRI data point and divided it by the average RRI value calculated for the minute in which the RRI data point belongs. If this ratio is less than 90%, the interval index, RRI length, time at which the RRI is found, and the ratios are collected (Supplementary Figure 1E). RRI values for which the ratio is less than 90% are referred to as “dips.” The program analyzed these dips in chronological order and placed them into groups. The program created a group and places all dips in it until it found a pair of dips separated by more than 10 seconds. The number of the dips divided by the total recording time is known as the RRI dips index. The RRI dips were calculated at different thresholds between 90% and 60% drop from preceding baseline. However, the threshold below 90% drop did not correlate with REI or oxygen desaturation index (ODI).
CPAP Intervention Analysis
Eight subjects completed this protocol, and the following parameters were measured: RUA, RL, and RRI change before, during and after each RE. The effect of REs and hypopneas on RUA, RL, and RRI were compared during pre-CPAP and on optimal pressure (when inspiratory flow limitation is eliminated).
Statistical Analysis
A one-way repeated-measures analysis of variance was used to compare each dependent variable (ventilatory parameters, RUA, and RL) during inspiratory and expiratory phases. A paired t test was used to compare the mean values of PTP during baseline and RE breaths. A paired t test was used to compare the mean values of RUA and RL during pre-CPAP and on optimal CPAP. A two-way repeated-measure ANOVA was used to compare RRI between hypopnea and RE during the following periods: baseline, beginning of event (1), end of event (2), nadir RRI following event (3), nadir SaO2 following event (4), maximum RRI following event (5), and on optimal CPAP. To assess determinants of RRI dips index, a Pearson correlation analysis, and linear regression was employed in those who had SDB (n = 10).
RESULTS
We studied 13 volunteers who reported sleep-related symptoms (snoring and/or excessive day-time sleepiness). Detailed demographic characteristics are shown in Table 1. SDB (defined as AHI ≥ 5 events/h) was diagnosed in 10 out of 13 (76.7%) of subjects, and CPAP titration was completed in eight of them. Figure 1 depicts repetitive respiratory and RRI changes between baseline and hypopnea during sleep in a representative subject. Following hypopnea, SaO2 dropped >3% and RRI decreased compared to preceding baseline. Figure 2 illustrates the repetitive respiratory and RRI changes between baseline and RE during sleep in a representative subject. Following RE, RRI decreased significantly compared to preceding baseline without significant change in SaO2.
Table 1.
Subjects Characteristics.
| All participants | CPAP | |
|---|---|---|
| N | 13 | 8 |
| Age (y) | 40.2 ± 12.4 | 45 ± 3.2 |
| BMI (kg/m2) | 28.8 ± 4.1 | 29.1 ± 1.7 |
| NC (cm) | 37.6 ± 5.3 | 38.4 ± 1.5 |
| Gender (M/F) | 8/5 | 5/3 |
| ESSa | 13.1 ± 4.3 | 12.3 ± 1.2 |
| PSQIa | 9.8 ± 10.0 | 9.8 ± 10.9 |
| FSSa | 33.8 ± 18.5 | 40.0 ± 6.6 |
| Cortical arousal index (event/h) | 19.8 ± 14.4 | 21.1 ± 5.0 |
| CAI (event/h) | 0.3 ± 0.7 | 0.2 ± 0.1 |
| ODI (4% desat/h) | 3.0 ± 3.2 | 2.8 ± 1.3 |
| AHI (event/h) | 15.7 ± 13.6 |
13.1 ± 2.4 |
AHI = apnea-hypopnea index; BMI = body mass index; CAI = central apnea index; CPAP = continuous positive airway pressure; ESS = Epworth sleepiness scale; FSS = fatigue severity scale; NC = neck circumference; ODI = oxygen desaturation index (4%); PSQI = Pittsburgh sleep quality index; SD = standard deviation. All data mean ± SD.
a N = 10 for ESS, FSS, and PSQI.
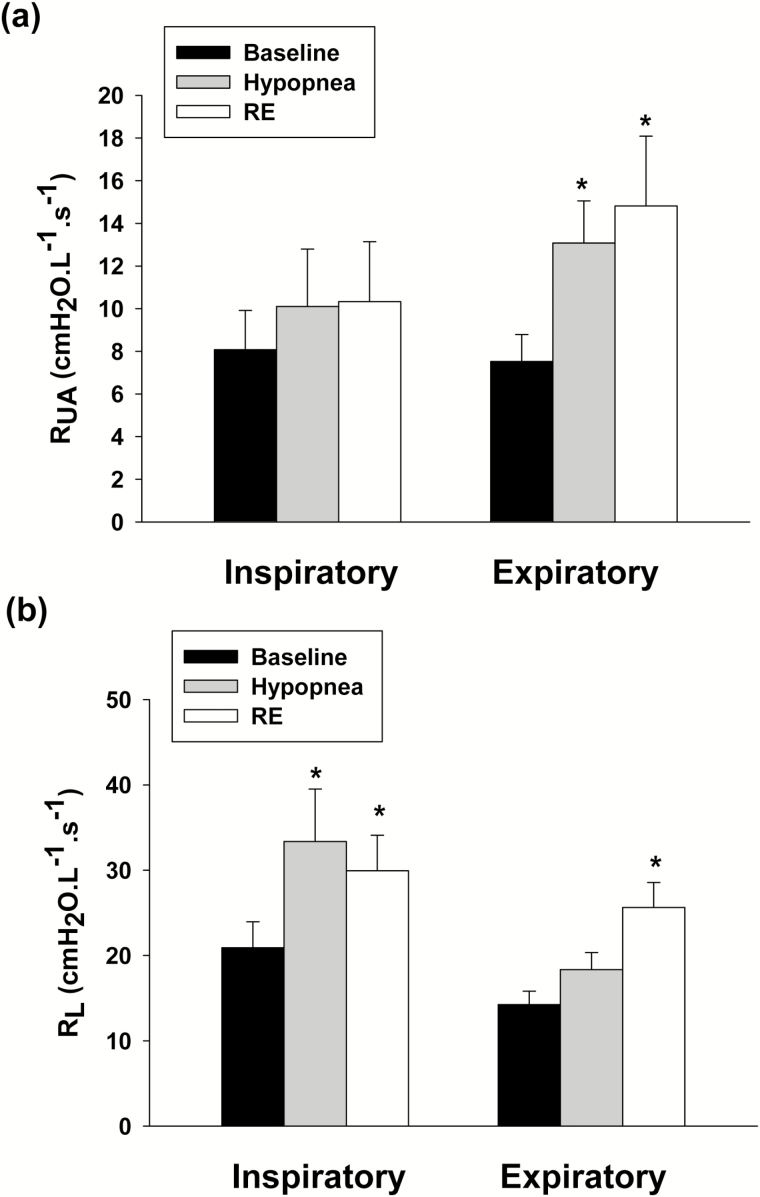
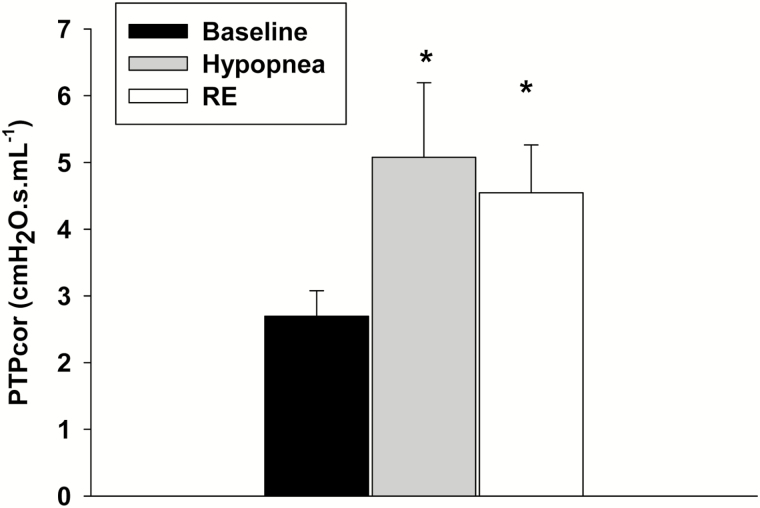
Episodes of decreased inspiratory flow during hypopnea and RE were associated with decreased minute ventilation and tidal volume (VE, VT), and mean inspiratory (VT/TI) flow (p < .05). The alveolar O2 concentration (PAO2) decreased following hypopnea relative to baseline (p < .05), while, no significant change occurred in SaO2 or PETCO2 (Table 2). In addition, episodes of hypopnea and RE were both associated with changes in airway mechanics, manifesting by increased expiratory RUA, compared to the preceding baseline (p < .05) (Figure 3a), and increased inspiratory RL. In contrast, expiratory RL increased significantly (p < .05) during RE only (Figure 3b). Thus, both hypopnea and RE were associated with changes in upper airway mechanics. Furthermore, Figure 4 depicts the estimated change in PTP, a surrogate measure of the work of breathing, between baseline and RE breaths. There was a significant increase in PTP during RE breaths compared to baseline eupneic breathing.
Table 2.
Ventilatory Parameters.
| Baseline | Hypopnea | RE | |
|---|---|---|---|
| V E (L/min) | 7.2 ± 2.3 | 4.2 ± 0.9* | 4.1 ± 1.2* |
| V T (L) | 0.5 ± 0.2 | 0.3 ± 0.1* | 0.3 ± 0.2* |
| F B (breath/min) | 14.7 ± 3.4 | 15.7 ± 4.8 | 15.5 ± 4.1 |
| T I (s) | 2.0 ± 0.5 | 1.9 ± 0.4 | 1.9 ± 0.3 |
| T E (s) | 2.4 ± 0.7 | 2.2 ± 1.1 | 2.4 ± 1.1 |
| T I/TTOT | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 |
| V T/TI (L/s) | 0.3 ± 0.1 | 0.1 ± 0.0* | 0.1 ± 0.0* |
| SaO2 (%) | 96.6 ± 1.2 | 94.6 ± 7.2 | 96.6 ± 1.5 |
| PAO2 (mmHg) | 97.6 ± 5.9 | 93.2 ± 9.8#+ | 96.7 ± 7.1 |
| PETCO2 (mmHg) | 41.2 ± 3.3 | 41.5 ± 3.4 | 41.8 ± 3.3 |
F B = breathing frequency; PAO2 = alveolar O2; PETCO2 = end-tidal CO2; RE = Respiratory event; SaO2 = Oxygen saturation; SD = standard devaiation; TI = inspiratory time; TE = expiratory time; TI/TTOT = inspiratory time/total breath duration; VE = inspiratory minute ventilation; VT = tidal volume; VT/TI = mean inspiratory flow. Data were presented as mean ± SD. Two-way repeated-measures analysis of variance and Student-Newman-Keuls post hoc test.
*p < .001 vs. baseline; #p < .05 vs. baseline; +p < .05 vs. hypopnea.
Figure 3.
(a) Inspiratory and expiratory upper airway resistance (RUA) measured during the baseline (black bars); hypopnea (gray bars); and respiratory event (RE, white bars). Expiratory RUA increased significantly during hypopnea and RE. (b) Inspiratory and expiratory total pulmonary resistance (RL) measured during the baseline (black bars), hypopnea (gray bars), and RE (white bars). Inspiratory RL increased significantly during hypopnea and RE and expiratory RL increased significantly during RE only. All presented data are mean ± SE; n = 13, *p < .05 vs. baseline RM-ANOVA.
Figure 4.
The corrected pressure time product corrected for tidal volume (PTPcor) during baseline (black bar), hypopnea and (grey bar) and respiratory event (RE, white bar) breaths. All presented data are mean ± SE; n = 10 (there were three missing values in RE), *p < .05 vs. baseline using one-way repeated-measures analysis of variance test.
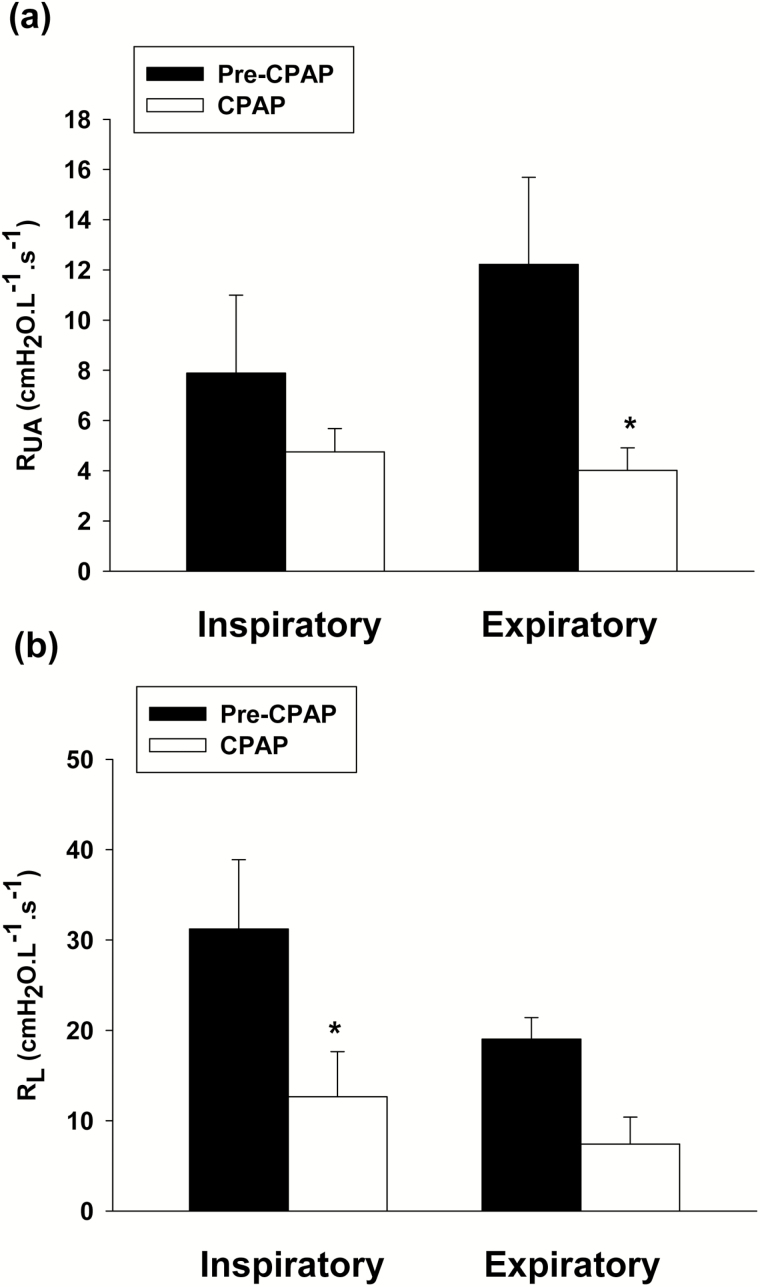
The application of nasal CPAP resulted in a significant decrease in expiratory RUA (Figure 5a; p < .05) and inspiratory RL compared to pre-CPAP baseline (Figure 5b; p < .05). Likewise the application of CPAP resulted in decreased PTP compared to pre-CPAP (all events) but was not statistically significant (Supplementary Figure 2E; p = .11).
Figure 5.
(a) Inspiratory and expiratory upper airway resistance (RUA) measured during the baseline pre-CPAP (black bars) and on optimal CPAP (white bars). Expiratory RUA decreased significantly on optimal CPAP. (b) Inspiratory and expiratory total pulmonary resistance (RL) measured during the baseline pre-CPAP (black bars) and on optimal CPAP (white bars). Inspiratory RL decreased significantly on optimal CPAP. All presented data are mean ± SE; n = 8, *p < .05 vs. pre-CPAP using paired t test.
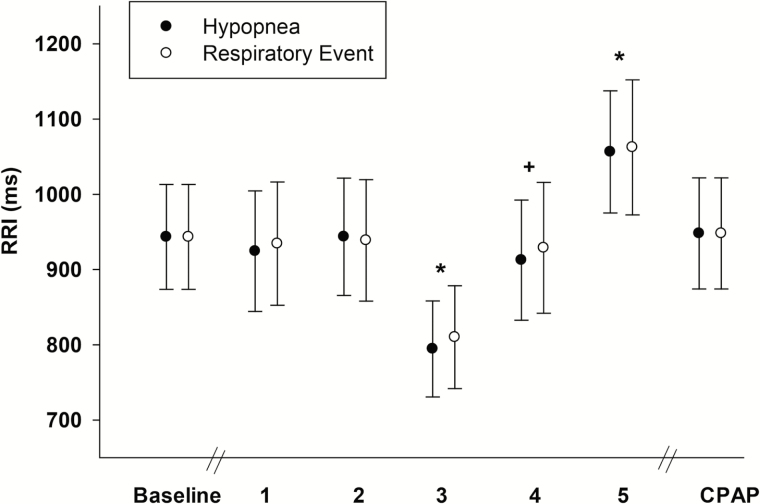
The effect of episodes of decreased flow (hypopnea and REs) on heart rate (RRI) is shown in Figure 6. Both hypopnea and RE affected RRI and led to significant drop in RRI following each event and followed by a significant increase in RRI. Optimal CPAP pressure application returned RRI to baseline (Figure 6, p < .05). Thus, episodes of decreased flow (hypopnea and REs) were associated with similar changes in heart rate.
Figure 6.
The R-R intervals (RRI) following hypopnea (black circles) and respiratory event (RE; white circles) segments during the following periods: baseline, beginning of event (1), end of event (2), nadir RRI following event (3), nadir SaO2 following event (4), maximum RRI following event (5), and on optimal continuous positive airway pressure (CPAP). RRI decreased (3) then increased (5) significantly following both events (hypopnea and RE). All presented data are mean ± SE; n = 6 (ECG of two subjects were not included due to poor signal), *p < .05 vs. baseline, +p < .05 vs. (3) nadir RRI.
To ascertain potential determinants of SDB, we used a multiple linear regression analysis (n = 10). This analysis revealed that the dependent variable: REI can be predicted from the RRI dips index at 90% threshold (r2 = 0.78; p = .007). The predictive power for RRI dips index was 96%. AHI and ODI 4%, however, did not correlate with REI (p = NS) (Table 3).
Table 3.
Results of Multiple Linear Regressions for Determinants of REI During Sleep.
| r 2 | Coefficient | p | |
|---|---|---|---|
| ODI (4%) | .26 | ||
| AHI (events/h) | .44 | ||
| RRI dips index (dips/h) | 0.78 | 0.33 | .007 |
AHI = apnea-hypopnea index (event/h); ODI = oxygen desaturation index (4% desaturation/h); REI = Respiratory event index; RRI dips index = R-R interval dips index at 90% threshold (dips/h).
DISCUSSION
Our study demonstrated several novel and significant findings regarding the effects of SDB on upper airway and total pulmonary mechanics during sleep. (1) Non-apneic REs manifesting by decreased flow—without 3% desaturation or cortical arousal (REs)—were associated with significant physiologic consequences including decreased VT and VE, increased airway resistance and heart rate changes. (2) REs were associated with significant increase in expiratory RUA and significant increase in RL during both inspiration and expiration. (3) REs and hypopneas were associated with similar changes in heart rate (HR increased ~15% from baseline). (4) RRI dips index was an independent predictor of SDB in symptomatic patients. (5) Optimal CPAP decreased expiratory RUA and RL and normalized RRI to baseline.
Physiological Consequences of Unclassified REs During Sleep
This study demonstrated that recurrent reduction of airflow during unclassified REs, without a significant desaturation or cortical arousal, was associated with increased upper and lower airway resistance, leading to a significant drop in minute ventilation and changes in heart rate. These perturbations were similar to the physiologic changes during hypopneas that met the AASM recommended definition. Our study corroborates previous studies which demonstrated a significant correlation between these unclassified events and autonomic arousals using pulse transit time.12 The study by Cracowski et al. was conducted in a similar population of patients and found that 15% of total REs did not fulfill the criteria of hypopneas or respiratory effort-related arousals (RERAs).
In our study we demonstrated that hypopneas and REs displayed similar changes in ventilation and airway mechanics. Increased expiratory RUA may reflect expiratory pharyngeal narrowing during hypopnea and REs.13–15 Our study corroborates previous studies demonstrating expiratory narrowing contributes to upper airway obstruction during sleep. Sanders et al.16 demonstrated in patients with obstructive sleep apnea that both inspiratory and expiratory resistance increase two breaths prior to an obstructive apnea. Likewise, Morrell et al.17 who demonstrated, using fiberoptic imaging that pharyngeal area size decreases progressively at end expiration prior to obstructive apnea. Our study extends these physiological observations to non-apneic events (hypopnea and RE) that are more common type of SDB.
Our study demonstrated that increased inspiratory and expiratory RL at the end of hypopnea and RE. Our finding corroborates previous study, using similar technique, in patients with obstructive sleep apnea which demonstrated progressive and inverse relationship between VT and RL prior to obstructive apnea.18 The study by Martin and colleagues did not assess non-apneic REs, nor did it partition RL into respiratory phases. Furthermore, the increased inspiratory RL in our study may indicate that airway narrowing during non-apneic REs (hypopnea or RE) occurred in the subglottic area of the airway (extra-thoracic). Conversely, increased RL could be secondary to decreased lung volumes during hypopneas and REs. Lower lung volumes would increase RL, and would predispose to more rapid oxygen desaturation leading to classification of an event as a hypopnea.
To determine the mechanisms of pharyngeal narrowing during non-apneic REs, it is useful to consider the airway as one continuous tube but with different structure and surrounding pressures based on its anatomical location (upper vs. lower airway). We noted that upper airway mechanics were similar in both hypopneas and REs, and manifesting by increased expiratory upper resistance and increased inspiratory RL; the latter may reflect subglottic narrowing of the upper airway in patients with SDB. The difference between hypopneas and REs in expiratory RL is unclear. Overall, both hypopnea and REs appear to share a similar mechanical profile and are hence a similar pathophysiology.
Effect of Non-apneic REs on Heart Rate
Non-apneic REs without desaturation or cortical arousal were associated with recurrent and significant heart rate changes throughout sleep. These respiratory related heart rate changes were characterized on ECG by significant RRI shortening (dips) following each RE independent of desaturation or cortical arousal. In fact the RRI dips were more sensitive in detecting respiratory related consequences than conventional consequences recommended for scoring these events such as desaturation or cortical arousals.4 Heart rate variability during sleep has been linked to SDB and early detection of disease.19 To our knowledge, however, this is the first time the RRI analysis is used as a physiological consequence of non-apneic events in patients with SDB independent of desaturation or cortical arousals. Previous studies suggested that frequency domain analysis of heart rate variability during sleep can be used in patients with suspected sleep apnea syndrome as a low-cost approach to confirm diagnosis and monitor treatment at home using RRI data extracted from pulse oximetry.20 The mechanism of recurrent heart rate changes following non-apneic REs could be explained by autonomic discharges related to these REs during sleep.
Our laboratory has utilized pressure catheters to assess partitioned pressure and resistance changes during inspiratory and expiratory phases during spontaneous sleep.13,15,21 Nevertheless, several considerations may influence the interpretation of the findings, including the inability to directly measure upper airway caliber and obtain multiple levels within the upper airway. Previous studies, however, from our lab showed that upper airway caliber was narrower when RUA was increased during expiratory phase which support the findings in this study.13,15 The application of CPAP decreased PTP compared to pre-CPAP performed on eight subjects during sleep, but was not statistically significant. The lack of statistical significance is likely due to small number of subjects and sample size power in addition to possible role of different CPAP pressure levels in these subjects which should be taken into consideration in future studies.
Clinical Implications
The occurrence of non-apneic REs with decreased ventilation, increased resistive loading, and increased work of breathing followed by significant cardiac responses independent of desaturation or cortical arousals has significant implications regarding the pathogenesis and management of SDB. First, hypopneas and REs demonstrated similar immediate physiologic consequences, including decreased VE and increased PTP; therefore, REs are of potential physiological significance.
The scoring of REs relies on visual pattern recognition. Decreased alveolar ventilation is a common feature in most REs (Table 2). The only exceptions are RERAs. The current AASM Scoring Manual defines RERAs as “a sequence of breaths lasting at least 10 seconds characterized by increasing respiratory effort or by flattening of the inspiratory portion of the nasal pressure (diagnostic study) or positive airway pressure device flow (titration study) waveform leading to arousal from sleep when the sequence of breaths does not meet criteria for an apnea or hypopnea.”22 However, it is difficult to determine if a RERA is associated with decreased VT using clinical PSG signals, since flattening of the flow signal does not necessarily indicate decreased ventilation if inspiratory time is prolonged. Nevertheless, RERAs may meet the more inclusive criteria for hypopnea, if decreased flow and VT occur.
The identification of REs relies on decreased flow, with decreased VT, without accompanying EEG arousal. Whether a discrete event is scored as hypopnea, RERA or RE may depend on the underlying etiology, and the magnitude of immediate physiologic response, namely oxygenation, respiratory effort or EEG arousal. We posit that the underlying etiology (central vs. occlusive) may influence the morphologic manifestations of an abnormal RE. For example, decreased ventilatory motor output (central etiology) may result in hypopnea or RE depending on the presence or absence of significant oxyhemoglobin desaturation. In contrast, upper airway narrowing may manifest as RERA or hypopnea, depending on the degree of decreased VT, desaturation or the occurrence of EEG arousal. Therefore, abnormal REs may span a spectrum of events starting with apnea, hypopnea, followed by RERAs and REs. Accordingly, RERAs and REs represent potentially significant events, albeit challenging to be identified reliably.22 Thus, identification of SDB that includes apnea, hypopnea, RERAs, and REs may capture the full continuum of SDB in symptomatic individuals. This notion is supported by the salutary effect of nasal CPAP, which eliminated all these physiological changes suggest that clinical outcome can be modified using optimal CPAP in symptomatic patients who are currently left untreated based on current AHI definition.4
The association between REI and the RRI dips index independent of AHI and ODI explains the potential role of more sensitive physiological consequences than ODI or cortical arousals in predicting SDB diagnosis and outcome earlier (Table 3). SDB exerts several deleterious effects on the cardiovascular system either through hypoxia, sympathetic hyperactivity, repetitive intrathoracic pressure changes or humoral, neurohumoral, and endothelial changes.23 However, recent randomized clinical trial,24 demonstrated that in patients with SDB abolishing intermittent hypoxia by using supplemental oxygen, was not associated with a reduction in blood pressure despite greater adherence to therapy when compare to CPAP. A major physiological result of repetitive nocturnal respiratory events is intermittent sympathetic stimulation.25 These physiological consequences during sleep may affect cardiovascular function during the day leading to increased incidence of cardiovascular disease such hypertension,1 coronary heart disease and stroke.26 Sympathetic stimulation can also contribute to insulin resistance and modulate leptin expression, and facilitate the development of obesity, and hypertension.26
In summary, we have shown the occurrence of non-apneic REs during sleep is associated with increased expiratory pharyngeal resistance and RL leading to recurrent heart rate changes independent of desaturation or cortical arousal. The REI correlated with RRI dips index and was an independent predictor of SDB in symptomatic patients.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at SLEEP online.
FUNDING
This work was supported by Career Development Award # 1IK2CX000547 (to AS) and Merit Review Awards # 1I01CX001040 and # 1I01RX002116 (to MSB and AS) from the Clinical Science Research and Development Service from the Rehabilitation Research and Development service from the (US) Department of Veterans Affairs. The authors are also supported by DMC Foundation (to AS), Cardiovascular Research Institute (CVRI) (to AS), and NIH 1R01HL130552 (to MSB and AS).
DISCLOSURE STATEMENT
The authors (AS and SM and MSB) declare a pending patent application 9/16/16-Serial number 62/395,634 for the Detection of Sleep Disordered Breathing Using Cardiac Autonomic Responses.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank all the subjects in this study. Conception and design: AS and MB; analysis and interpretation: AS, SP, SM, AMH, and MSB; drafting the manuscript for important intellectual content: AS and MSB.
REFERENCES
- 1. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000; 342: 1378–1384. [DOI] [PubMed] [Google Scholar]
- 2. Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005; 365: 1046–1053. [DOI] [PubMed] [Google Scholar]
- 3. Gould GA, Whyte KF, Rhind GB, et al. The sleep hypopnea syndrome. Am Rev Respir Dis. 1988; 137: 895–898. [DOI] [PubMed] [Google Scholar]
- 4. Berry R, Brooks R, Gamaldo C, Harding S, Marcus C, Vaughn B. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.0. Darien, IL: American Academy of Sleep Medicine; 2012: 47. [Google Scholar]
- 5. Peppard PE, Ward NR, Morrell MJ. The impact of obesity on oxygen de-saturation during sleep-disordered breathing. Am J Respir Crit Care Med. 2009; 180(8): 788–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kambam JR, Chen LH, Hyman SA. Effect of short-term smoking halt on carboxyhemoglobin levels and P50 values. Anesth Analg. 1986; 65(11): 1186–1188. [PubMed] [Google Scholar]
- 7. Punjabi NM, Newman AB, Young TB, Resnick HE, Sanders MH. Sleep-disordered breathing and cardiovascular disease. Am J Respir Crit Care Med. 2008; 177: 1150–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ayappa I, Rapaport BS, Norman RG, Rapoport DM. Immediate consequences of respiratory events in sleep disordered breathing. Sleep Med. 2005; 6: 123–130. [DOI] [PubMed] [Google Scholar]
- 9. Guilleminault C, Poyares D, Rosa A, Huang YS. Heart rate variability, sympathetic and vagal balance and EEG arousals in upper airway resistance and mild obstructive sleep apnea syndromes. Sleep Med. 2005; 6(5): 451–457. [DOI] [PubMed] [Google Scholar]
- 10. Pranathiageswaran S, Sankari A, Badr M, Mina N. Oxyhemoglobin de-saturation may underestimate the severity of sleep-disordered breathing in non obese individuals. Am J Respir Crit Care Med. 2014; 189: A5609. [Google Scholar]
- 11. Sassoon CS, Light RW, Lodia R, Sieck GC, Mahutte CK. Pressure-time product during continuous positive airway pressure, pressure support ventilation, and T-piece during weaning from mechanical ventilation. Am Rev Respir Dis. 1991; 143: 469–475. [DOI] [PubMed] [Google Scholar]
- 12. Cracowski C, PÉPin J-L, Wuyam B, LÉVy P. Characterization of obstructive nonapneic respiratory events in moderate sleep apnea syndrome. Am J Respir Crit Care Med. 2001; 164(6): 944–948. [DOI] [PubMed] [Google Scholar]
- 13. Sankri-Tarbichi AG, Richardson NN, Chowdhuri S, Rowley JA, Badr MS. Hypocapnia is associated with increased upper airway expiratory resistance during sleep. Respir Physiol Neurobiol. 2011; 177(2): 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sankri-Tarbichi AG, Rowley JA, Badr MS. Inhibition of ventilatory motor output increases expiratory retro palatal compliance during sleep. Respir Physiol Neurobiol. 2011; 176(3): 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sankri-Tarbichi AG, Rowley JA, Badr MS. Expiratory pharyngeal narrowing during central hypocapnic hypopnea. Am J Respir Crit Care Med. 2009; 179(4): 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sanders MH, Moore SE. Inspiratory and expiratory partitioning of airway resistance during sleep in patients with sleep apnea. Am Rev Respir Dis. 1983; 127(5): 554–558. [DOI] [PubMed] [Google Scholar]
- 17. Morrell MJ, Arabi Y, Zahn B, Badr MS. Progressive retropalatal narrowing preceding obstructive apnea. Am J Respir Crit Care Med. 1998; 158(6): 1974–1981. [DOI] [PubMed] [Google Scholar]
- 18. Martin R, Pennock B, Orr W, Sanders M, Rogers R. Respiratory mechanics and timing during sleep in occlusive sleep apnea. J Appl Physiol Respir Environ Exerc Physiol. 1980; 48(3): 432–437. [DOI] [PubMed] [Google Scholar]
- 19. Roche F, Gaspoz J-M, Minini P, et al. Screening of obstructive sleep apnea syndrome by heart rate variability analysis. Circulation. 1999; 100: 1411–1415. [DOI] [PubMed] [Google Scholar]
- 20. Shiomi T, Guilleminault C, Sasanabe R, Hirota I, Maekawa M, Kobayashi T. Augmented very low frequency component of heart rate variability during obstructive sleep apnea. Sleep. 1996; 19: 370–377. [DOI] [PubMed] [Google Scholar]
- 21. Badr MS, Skatrud JB, Dempsey JA. Effect of chemoreceptor stimulation and inhibition on total pulmonary resistance in humans during NREM sleep. J Appl Physiol (1985). 1994; 76(4): 1682–1692. [DOI] [PubMed] [Google Scholar]
- 22. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med. 2012; 8(5): 597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Quan SF, Gersh BJ. Cardiovascular consequences of sleep-disordered breathing: Past, present and future report of a workshop from the national center on sleep disorders research and the national heart, lung, and blood institute. Circulation. 2004; 109(8): 951–957. [DOI] [PubMed] [Google Scholar]
- 24. Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014; 370: 2276–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995; 96: 1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Phillips BG, Somers VK. Sleep disordered breathing and risk factors for cardiovascular disease. Curr Opin Pulm Med. 2002; 8: 516–520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.