Abstract
This scientific commentary refers to ‘Using an engineered glutamate-gated chloride channel to silence sensory neurons and treat neuropathic pain at the source’, by Weir et al. (doi:10.1093/brain/awx201).
This scientific commentary refers to ‘Using an engineered glutamate-gated chloride channel to silence sensory neurons and treat neuropathic pain at the source’, by Weir et al. (doi:10.1093/brain/awx201).
Neuropathic pain syndromes can result from injury to the peripheral or CNS and are characterized by ongoing, often burning pain, dysaesthesias and intense pain that can be provoked by normally innocuous stimulation (Colloca et al., 2017). Mechanical allodynia and cold hypersensitivity predominate. Common peripheral neuropathic pains include post-herpetic neuralgia, diabetic neuropathic pain, phantom limb pain and painful radiculopathy. Central neuropathic pain can also occur post-stroke, after spinal cord injury and in patients with multiple sclerosis. Unlike tissue injury-induced inflammatory pains, neuropathic pains do not respond to non-steroidal anti-inflammatory drugs and are generally poorly responsive to opioids, largely because the high doses required generate intolerable adverse effects, and because of the potential for misuse. As hyperexcitability and ectopic firing of injured peripheral afferents and consequent hyperactivity of CNS circuits are major contributors to neuropathic pain (Haroutounian et al., 2014), anticonvulsant drugs are often used to manage the pain. The mantra in the field, however, is that even the first-line approach to treating neuropathic pain, namely the gabapentinoids, is on average only 30% effective in 30% of patients. In this issue of Brain, Weir and co-workers report preclinical studies of a novel adeno-associated virus (AAV)-based chemogenetic therapy to treat peripheral nerve injury-induced neuropathic pain (Weir et al., 2017).
The rationale for the authors’ approach derived, in part, from the observation that most peripheral neuropathic pains, including phantom limb pain, can be relieved by local anaesthetics that target damaged, hyperexcitable peripheral afferents (Haroutounian et al., 2014; Vaso et al., 2014). Unfortunately, the local anaesthetic action is short-lived and limited by cardiac, motor and CNS side effects. Weir et al. therefore adopted an alternate strategy to silence hyperactive sensory neurons. Their approach followed previous work that examined CNS neurons transfected with a modified, heteromeric invertebrate glutamate-gated chloride channel (GluCl). The modified channel does not respond to the endogenous neurotransmitter, glutamate, but is readily activated by ivermectin (IVM), a well-tolerated broad spectrum anti-parasitic agent. A low dose of IVM acting on the modified GluCl channel can silence mammalian CNS neurons in vivo for days (Lerchner et al., 2007). Weir et al. took advantage of a GluCl channel that had been optimized to increase incorporation of the beta subunit, which, together with a required alpha subunit, forms the functional channel (Frazier et al., 2013). Because AAV-directed channel expression can be targeted to neurons of interest (e.g. those critical to the transmission of the ‘pain’ message), the therapeutic window can be greatly increased, even after systemic IVM administration, compared to traditional systemic analgesic drug approaches.
The authors first completed a comprehensive in vitro characterization of the channel in dissociated dorsal root ganglion (DRG) sensory neurons electroporated with GluCl plasmid DNA. The intrinsic properties of the transfected cells, studied by whole cell patch clamp, did not differ from control neurons; however, a low IVM dose silenced the cells in response to current injection. In parallel studies, using ratiometric Ca2+ imaging, the authors demonstrated that IVM inhibited K+-evoked depolarization of sensory neurons, as well as the responses to the TRP channel agonists capsaicin and menthol, which target sensory neurons directly (Julius, 2013). IVM also inhibited action potential generation in induced pluripotent stem cell-derived human DRG cells expressing the GluCl channel. Interestingly, IVM-induced silencing does not result from hyperpolarization. Because intracellular Cl− levels can be relatively high in sensory neurons compared to CNS neurons (Gilbert et al., 2007), increasing transmembrane Cl− flux via the GluCl channel does not produce the expected hyperpolarization. If anything, there is a slight depolarization. The authors reasoned that the silencing results from shunting of current secondary to an IVM-induced large drop in membrane resistance.
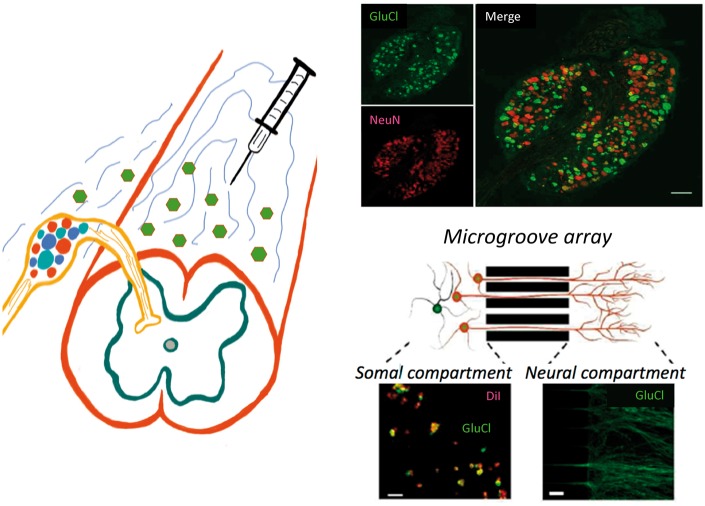
In a particularly elegant demonstration (Fig. 1, bottom right), the authors cultured rat DRG neurons in compartmentalized microfluidic chambers, in which GluCl-expressing DRG neurites grow through microgrooves and can be treated independently of the soma. In this arrangement, treating the terminal field of the neurites with IVM blocked K+-evoked depolarization of the DRG cell bodies. This finding indicates that the GluCl channel synthesized in the cell body is trafficked to the terminals, in both the periphery and spinal cord, and that IVM can silence sensory neuron transmission by binding to GluCl at the level of the axon, as well as the cell body.
Figure 1.
Schematic illustrating intrathecal AAV-mediated delivery of a GluCl channel to DRG cell bodies. The upper panels (right) illustrate selective expression of the GluCl (green) in NeuN positive sensory neurons (red). There was no somatic labelling in the spinal cord. By culturing embryonic rat DRG neurons in a compartmentalized microgroove array, Weir et al. demonstrated neurite transport of GluCl from the cell bodies (somal compartment) to their peripheral terminals (neural compartment). In this arrangement, the terminals could be targeted with a ligand of the GluCl, namely ivermectin, independently of the cell body. Peripheral (axonal) targeting of the ivermectin completely silenced the cell body response to depolarization of the neurites, as it did when applied directly to the cell body. Schematic courtesy of Ms Linda Toschi-Chambers.
Is the approach translatable to the clinic? To test this possibility, the authors transfected sensory neurons by intrathecal injection of an AAV serotype 9 that expressed the alpha subunit and another that expressed the beta subunit (schematic in Fig. 1). By 4 weeks after AAV injection, a fluorescent reporter assay showed that on average 66% of all NeuN positive L4 DRG neurons (red) expressed GluCl (green; Fig. 1, top right). As the GluCl expression was driven by a ubiquitous CAG promoter, it was not selective for nociceptors. However, transgene expression persisted for at least 7 months and the channel was functional. In vitro assessment of DRG neurons from these animals showed that IVM completely prevented the neurons from generating action potentials.
Finally, the authors evaluated IVM-mediated sensory neuron silencing in the sciatic nerve injury (SNI) mouse model of neuropathic pain, in which two of the three branches of the sciatic nerve are transected, isolating the sural nerve. These animals rapidly develop a profound and long lasting mechanical and cold hypersensitivity of the ipsilateral hind paw. Note that in this model, the mechanical input is transmitted to the spinal cord by uninjured fibres, including non-nociceptive, large myelinated Aβ afferents that inappropriately engage dorsal horn pain transmission circuits (Xu et al., 2015). Weir et al. (2017) reported that a low dose of IVM produced a 50% reversal of the mechanical hypersensitivity. This anti-nociceptive activity was maintained for 3 days, only disappearing by 6 days, which is consistent with the slow dissociation of IVM from the channel. Cold hypersensitivity, which is transmitted by TRPM8-expressing sensory neurons (McCoy et al., 2011), was also attenuated. Most importantly, tolerance to IVM did not occur; repeated dosing 3 weeks later also reduced the hypersensitivity. Moreover, the authors found no evidence for motor/sedative side effects, which indicates that the virus did not induce functionally relevant expression of GluCl in muscle afferents (proprioceptors) and did not cross the blood–brain barrier, where it could target motor neurons.
What challenges need to be overcome in order to translate these exciting findings to the clinic? The fact that two AAVs are required to express alpha and beta GluCl subunits adds a complication that perhaps can be mitigated by modifying a single AAV to carry both genes. Unquestionably, however, selective targeting of the relevant DRG sensory neurons is most critical, as this is the basis for the large therapeutic window that the authors demonstrated. Whether a 50% recovery of mechanical threshold will translate into a viable clinical benefit of course, remains to be seen. However, the fact that reducing a 10-point visual analogue score by 2 points is clinically significant makes the present results very encouraging. A potential concern is that baseline mechanical and thermal thresholds increased in the control, uninjured, animals. To what extent that would be acceptable in patients is unclear. If there is a concurrent loss or significant reduction of an otherwise intractable neuropathic pain, then a mild tactile anaesthesia would likely be tolerated. On the other hand, as intrathecal injection leads to bilateral transfection of many DRG neurons, there could be a more widespread and problematic anaesthesia.
This concern, of course, can be obviated either by directly injecting DRG neurons or by administering the IVM topically, in the zone of hypersensitivity. Ongoing pain that is driven from hyperexcitable, injured afferents trapped in a neuroma could conceivably be treated locally as well, but direct DRG targeting is more likely. Even more selective pain control will depend on ongoing research to identify and incorporate promoters that drive GluCl expression, ideally long-term, into critical subsets of sensory neurons. Whether this will require targeting unmyelinated nociceptors, to prevent the ongoing (ectopic) activity of injured afferents (Haroutounian et al., 2014; Vaso et al., 2014), and/or the Aβ myelinated afferents that carry the mechanical allodynia that is readily evoked in these patients, remains to be seen.
Glossary
Chemogenetics: A molecular approach to regulating neuronal function. Specific populations of neurons are administered a virus that expresses a channel or receptor that is not engaged by an endogenous molecule (e.g. a neurotransmitter). Rather, the channel/receptor is mutated so that it is selectively engaged by a drug that only interacts with that novel channel/receptor.
Ectopic firing: Peripheral axons normally generate action potentials after a stimulus is applied to nerve terminals (in skin, muscle, etc.). However, injured peripheral sensory neurons may display ectopic firing, characterized by spontaneous activity, hyperexcitability and often mechanical sensitivity at the site of damage. This abnormal activity is a critical contributor to many neuropathic pain conditions.
Ivermectin sensitive glutamate-chloride channel (GluCl): In contrast to vertebrates, which express glycine-activated chloride channels, invertebrates express a GluCl channel. The chemogenetic approach used in the present study involves expression of a mutant version of the GluCl channel that is not responsive to endogenous glutamate, but can be activated by ivermectin, a broad spectrum anti-parasitic agent. Ivermectin binding to the mutant GluCl channel increases Cl− conductance and inhibits neuronal activity.
What is exciting, of course, is that these are all hurdles that can be overcome. Unlike ablative procedures that try to silence aberrant firing of afferents, the present approach is both amenable to dosing and reversible. And, of course, with a chemogenetic approach to silencing sensory neurons, there would be no fear of generating a deafferentation pain/anaesthesia dolorosa. Clearly, the possibility of long-term, controllable silencing of the sensory neurons that drive many neuropathic pain conditions offers tremendous hope that a new era in the management of neuropathic pain can be envisioned: an era of effective pain control, repeated dosing without tolerance, limited adverse effects, and less misuse potential. These are the ideal properties of the non-opioid pain management regime that clinicians seek. Only time will tell how translatable these findings will be, but Weir et al. (2017) have definitely paved an exciting path forward.
References
- Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, et al. Neuropathic pain. Nat Rev Dis Primers 2017; 3: 17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier SJ, Cohen BN, Lester HA. An engineered glutamate-gated chloride (GluCl) channel for sensitive, consistent neuronal silencing by ivermectin. J Biol Chem 2013; 288: 21029–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D, Franjic-Wurtz C, Funk K, Gensch T, Frings S, Mohrlen F. Differential maturation of chloride homeostasis in primary afferent neurons of the somatosensory system. Int J Dev Neurosci 2007; 25: 479–89. [DOI] [PubMed] [Google Scholar]
- Haroutounian S, Nikolajsen L, Bendtsen TF, Finnerup NB, Kristensen AD, Hasselstrom JB, et al. Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy. Pain 2014; 155: 1272–9. [DOI] [PubMed] [Google Scholar]
- Julius D. TRP channels and pain. Annu Rev Cell Dev Biol 2013; 29: 355–84. [DOI] [PubMed] [Google Scholar]
- Lerchner W, Xiao C, Nashmi R, Slimko EM, van Trigt L, Lester HA, et al. Reversible silencing of neuronal excitability in behaving mice by a genetically targeted, ivermectin-gated Cl- channel. Neuron 2007; 54: 35–49. [DOI] [PubMed] [Google Scholar]
- McCoy DD, Knowlton WM, McKemy DD. Scraping through the ice: uncovering the role of TRPM8 in cold transduction. Am J Physiol Regul Integr Comp Physiol 2011; 300: R1278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaso A, Adahan HM, Gjika A, Zahaj S, Zhurda T, Vyshka G, et al. Peripheral nervous system origin of phantom limb pain. Pain 2014; 155: 1384–91. [DOI] [PubMed] [Google Scholar]
- Weir G, Middleton S, Clark A, Daniel T, Khovanov N, McMahon S, et al. Using an engineered GluCl channel to silence sensory neurons and treat neuropathic pain at the source. Brain 2017; 140: 2570–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZZ, Kim YH, Bang S, Zhang Y, Berta T, Wang F, et al. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat Med 2015; 21: 1326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]