Abstract
Background
The olfactory ensheathing cells (OECs) migrate from the peripheral nervous system to the central nervous system (CNS), a critical process for the development of the olfactory system and axonal extension after injury in neural regeneration. Because of their ability to migrate to the injury site and anti-inflammatory properties, OECs were tested against different neurological pathologies, but were never studied in the context of cancer. Here, we evaluated OEC tropism to gliomas and their potential as a “Trojan horse” to deliver therapeutic transgenes through the nasal pathway, their natural route to CNS.
Methods
OECs were purified from the mouse olfactory bulb and engineered to express a fusion protein between cytosine deaminase and uracil phosphoribosyltransferase (CU), which convert the prodrug 5-fluorocytosine (5-FC) into cytotoxic metabolite 5-fluorouracil, leading to a bystander killing of tumor cells. These cells were injected into the nasal cavity of mice bearing glioblastoma tumors and OEC-mediated gene therapy was monitored by bioluminescence imaging and confirmed with survival and ex vivo histological analysis. All statistical tests were two-sided.
Results
OECs migrated from the nasal pathway to the primary glioma site, tracked infiltrative glioma stemlike cells, and delivered therapeutic transgene, leading to a slower tumor growth and increased mice survival. At day 28, bioluminescence imaging revealed that mice treated with a single injection of OEC-expressing CU and 5-FC had tumor-associated photons (mean [SD]) of 1.08E + 08 [9.7E + 07] vs 4.1E + 08 [2.3E + 08] for control group (P < .001), with a median survival of 41 days vs 34 days, respectively (ratio = 0.8293, 95% confidence interval = 0.4323 to 1.226, P < .001) (n = 9 mice per group).
Conclusions
We show for the first time that autologous transplantation of OECs can target and deliver therapeutic transgenes to brain tumors upon intranasal delivery, the natural route of OECs to the CNS, which could be extended to other types of cancer.
Gliomas are the most common primary tumors that arise in the adult central nervous system (CNS). Glioblastoma (GBM), the most aggressive form, is resistant to current modalities of treatment and has one of the worst 5-year survival rates (<10%) of all human cancers (1). Standard-of-care treatment includes maximal surgical resection followed by radiation and chemotherapy (temozolomide). The neoplastic growth of malignant gliomas is thought to be maintained by a rare population of neural stemlike cells that undergo self-renewal and are resistant to therapy (2). Thus, developing novel therapeutics that target glioma cells and, more importantly, these cancer stemlike cells would be highly beneficial for GBM patients.
The mammalian olfactory epithelium has a unique capacity to replace its olfactory receptor neurons by physiological turnover and following injury throughout life (3,4). The ability to grow axons in the mature CNS milieu has been attributed to the presence of olfactory ensheathing cells (OECs), a glial cell type that closely accompanies the axons as they grow from the olfactory epithelium into the olfactory bulb. OECs are known to migrate from the peripheral nervous system (PNS) to the CNS, a critical process for the development and maintenance of the olfactory system. During neural regeneration, OECs migrate into the injury site and enhance the axon growth because of their permissive environment (5). Recently, OECs were shown to have tropism to astrocytes dissociated from GBM tissue in culture (6). Owing to their strong ability to interact with astrocytes, as well as their immunomodulatory and phagocytic properties, accumulating evidence has led to investigations of the therapeutic potential of OECs in animal models as well as in the clinic for different pathologies, including chronic spinal cord injury and amyotrophic lateral sclerosis (7–10); but OECs were never studied in the context of cancer in vivo. Here, we evaluated the potential of OECs as a Trojan horse to deliver therapeutic transgene to brain tumors through the nasal pathway, their natural route to CNS.
Materials and Methods
Cell Culture of Olfactory Ensheathing Cells
Olfactory ensheathing cells were purified from the olfactory bulbs of C57BL/6 male adult mice following our and others’ previously described protocol (11–15). Briefly, the mice were killed by isoflurane. The olfactory bulbs were dissected and sliced using a Tissue Chopper (Mickle Laboratory, Gomshall UK) in cold Hank’s solution (HBSS, Thermo Fisher Scientific, Waltham, USA). The tissue was dissociated with a TrypLE (Thermo Fisher Scientific) reagent and quenched with Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12; Thermo Fisher Scientific) containing 10% fetal bovine serum (FBS). Cells were then centrifuged at 350×g for 5 min, the supernatant was discarded, and the cell pellet was resuspended in complete DMEM/F-12 supplemented with 10% FBS and 100 U/mL penicillin-streptomycin (Thermo Fisher Scientific), plated onto an uncoated 60-mm dish, and incubated at 37°C in CO2 incubator for 18 hours in preparation for fibroblast removal. Next, floating cells in cell suspension were transferred to a second uncoated dish for astrocyte removal, and incubated using the same conditions for 36 hours. Finally, OECs in the cell suspension were adhered onto a precoated laminin (40 μg/mL, Life Technologies) 60-mm cell dish in DMEM/F-12 complete medium. OECs were maintained in 5% CO2 at 37°C, and the medium was refreshed every three days. Once confluency was reached, OECs were detached using TrypLE and used in the proposed experiments.
OECs were then transduced with a lentivirus vector carrying an expression cassette for the naturally secreted Gaussia luciferase (Gluc) and green fluorescent protein (GFP) separated by an internal ribosomal site (IRES), under the control of cytomegalovirus promoter at a multiplicity of infection of 10 transducing units per cell by adding the virus directly to the cell culture (16). Similarly, OECs were engineered to express a fusion between yeast cytosine deaminase (CD) and uracil phosphoribosyltransferase (UPRT; CU) under the control of cytomegalovirus (CMV) promoter using a previously described lentivirus construct (17). OECs expressing CU are referred to as OCU.
Detailed methods for in vitro luciferase cell proliferation/viability analysis, OEC migration assay, apoptosis detection in cocultured cells, bioluminescence imaging, and immunofluorescence experiments are in the Supplementary Methods (available online).
Patient-Derived GBM Stemlike Cells and Cell Lines
MGG6, MGG8, and MGG23 primary glioblastoma stemlike cells (GSCs) were derived from surgical specimens obtained from glioblastoma patients undergoing treatment at the Massachusetts General Hospital (Boston, MA), in accordance with the appropriate institutional review board approval, and have been previously characterized (17,18). More details can be found in the Supplementary Methods (available online). GSCs were engineered by a lentivirus vector to express firefly luciferase (Fluc) and mCherry fluorescent protein (mCherry) separated by an IRES, under control of CMV promoter (Fluc-mCherry).
Mouse Studies
All mouse studies were performed in accordance with the Massachusetts General Hospital Subcommittee on Research Animal Care following guidelines set by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Athymic female mice, 8–10 weeks old, were anesthetized with 3% isoflurane under oxygen and stereotactically implanted with 50 000 GBM cells or GSCs (as small spheres) expressing Fluc-mCherry in 2 μL phosphate-buffered saline (PBS) using a 30-gauge Hamilton syringe and the following coordinates: 2.5 mm lateral, 0.5 mm anterior to bregma, and 2.5 mm depth from the skull surface. One week (for therapeutic studies; n = 9 per group) or three weeks (for tropism analysis, n = 3 per experiment) after tumor implantation, mice received 105 OEC-GFP (in 10 μL PBS) via intranasal administration by carefully dropping the cells onto their nostrils, allowing cells to be snorted in drop by drop. One week post-OEC injection, mice were treated with a daily intraperitoneal injection of 5-FC (500 mg/kg) for 7 days. Tumor volume was monitored over time by bioluminescence imaging as detailed in the Supplementary Methods (available online).
Statistical Analysis
GraphPad Prism v6.01 (GraphPad Software, Inc, LaJolla, CA) was used for statistical analysis of all data. Data were expressed as mean (SD). An unpaired two-tailed t test was used for the comparison of two samples. A P value less than .05 was considered statistically significant. One-way (for in-culture work) or two-way (for in vivo work) analysis of variance (ANOVA) followed by the Bonferroni multiple comparison post hoc test was performed to compare differences between groups. All of the mice included in the survival curve were followed to their natural death, and survival was analyzed using Kaplan-Meier curves and log-rank (Mantel-Cox) tests. All statistical tests were two-sided.
Results
Isolation and Characterization of OEC Cultures
We purified the olfactory ensheathing cells from the olfactory bulbs of C57BL/6 male adult mice following previously established protocol (11–15) (summarized in Figure 1A). Costaining of OECs for 2′,3′ cyclic nucleotide 3′-phosphodiesterase, smooth muscle α-actin, glial fibrillary acidic protein, and calcium-binding protein β, specific markers for OEC (13), revealed that our cultures displayed neither unlabeled cells nor cells labeled with only one marker, confirming that our method is highly effective for OEC purification (Figure 1B).
Figure 1.
(spelling of Resuspend; suspension; define ONL). Isolation and characterization of olfactory ensheathing cells (OECs) from the mice olfactory bulb. A) Schematic overview of OEC isolation from the mouse olfactory bulb. B) Phenotypic characterization of OECs by co-immunostaining for 2′,3′ cyclic nucleotide 3′-phosphodiesterase (CNPase), calcium-binding protein β (S100b) (green), smooth muscle α-actin (SMA), and glial fibrillary acidic protein (GFAP) (red). Nuclei were also stained with 6-diamidino-2-phenylindole (DAPI) (blue). Scale bar, 50 µm. ONL = olfactory nerve layer.
OEC Tropism to Brain Tumors via the Nasal Pathway
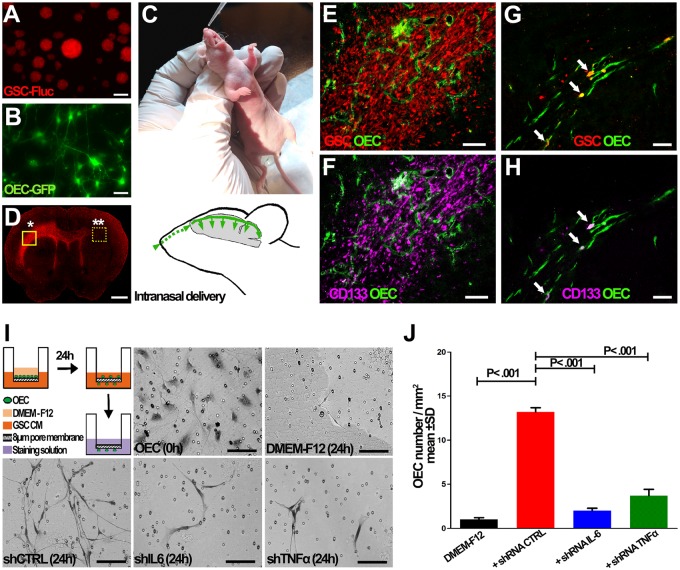
We first evaluated whether OECs can travel their natural route from the olfactory bulb to CNS and target brain tumors. MGG8 patient-derived GSC neurospheres (105 cells) expressing mCherry (Figure 2A) were intracranially injected into the left forebrain. Three weeks after GSC injection, mice received 105 OECs (in 10 µL PBS) expressing GFP via intranasal administration (Figure 2, B and C). One week later, mice were killed and the brains were removed, sectioned, and stained for GFP, as an indirect measure of OEC. OECs migrated from the nasal cavity not only toward the primary tumor site, but also along invasive tumor borders far from their initial site of transplantation (Figure 2, D–H). Costaining with anti-CD133 antibody, a marker for GSCs, revealed that OEC can also target these subpopulations of GBMs, known to invade the brain (Figure 2, E–H). These results were also reproducible in another GBM cell line model (Supplementary Figure 1, available online).
Figure 2.
Olfactory ensheathing cell (OEC) tropism to glioma stem cells (GSCs) in culture and in vivo upon intranasal administration. A) GSCs expressing mCherry were injected into the striatum of nude mice brain. B, C) Three weeks after GSC injection, mice received 105 OECs expressing green fluorescent protein (GFP) (B) via the intranasal cavity (C). D–H) One week after OEC injection, brain sections were analyzed for mCherry showing infiltration of GSCs throughout the brain (D). Brain sections at the GSC injection site (*in D) were costained for GFP (OEC) and CD133; OEC-GFP (green) were colocalized with CD133 (purple) or GSCs (red) (E, F). OEC also migrated to the infiltrative glioma cells and CD133+ GSCs (arrow) at the contralateral side (**in D) (G, H). I, J) Conditioned medium collected from GSCs expressing nontargeted short hairpin RNA (shRNA) control (shCTRL) or against interleukin 6 (shIL-6); tumor necrosis factor alpha (shTNFα) or DMEM-F12 control medium were placed in the lower chambers and OECs in the upper chambers of a Transwell. Twenty-four hours later, lower chambers were analyzed for the presence of OECs by microscopy (I). J) Quantification analysis of results obtained in (I); representative mean (SD), n = 3 independent experiments, P value from two-sided Student t test is shown. Scale bar = 1 mm (D), 150 µm (E, F); 50 µm (A, B; G, H).
Because OECs are known to have tropism to soluble molecules associated with inflammation, we evaluated the migratory potential of OECs to the conditioned medium of GBM cells and GBM stemlike cells in a Transwell assay in culture. Indeed, OECs migrated toward the conditioned medium from GSCs and U87 GBM cells and not control medium (Figure 2, I and J;Supplementary Figure 2, available online). To understand the potential mechanism responsible for this tropism, we explored two major cytokines, the tumor necrosis factor alpha (TNFα) and interleukin 6 (IL-6), previously shown to mediate neural or mesenchymal stem cell tropism to tumors/gliomas (18,19). Indeed, knocking down (using short hairpin RNA [shRNA]) either of these cytokines in GSCs and the U87 GBM line inhibited OEC migration to their conditioned medium (Figure 2, I and J, Supplementary Figures 2 and 3, available online). Quantification analysis revealed a statistically significant increase in OEC migration to conditioned medium from GSCs expressing shCTRL as compared with DMEM-F12 control (13.90 [2.85] OECs per mm2 vs 2.0 [1.00] OEC per mm2, P < .001); however, OEC migration was statistically significantly less to the conditioned medium from GSCs expressing either shTNFα (4.4 [2.16] OECs per mm2, P < .001) or shIL-6 (2.6 [1.06] OECs per mm2, P < .001) (Figure 2, I and J). These results indicate the potential role of these two secreted inflammatory molecules in OEC tropism to glioblastomas.
OEC as a Delivery Vehicle of Therapeutics to Glioma Cells and Stemlike Cells
We evaluated OECs as a vehicle to deliver therapeutic transgenes to brain tumors. We first engineered OECs to express a fusion protein between CD and UPRT under the control of cytomegalovirus promoter (OCU), as a proof-of-concept transgene. The CD converts, on the tumor site, the prodrug 5-FC into the cytotoxic agent 5-fluorouracyl (5-FU), which is further converted to its metabolites 5-fluorodeoxyuridine-5ʹ-monophosphate and 5-fluorouridine-5ʹ-triphosphate intracellularly. These active metabolites are then incorporated into DNA and RNA leading to the inhibition of nucleotide synthesis and cell death. The advantage of this strategy is that the generated 5-FU can be transferred to neighboring nontransduced cells via gap junctions and other mechanisms, leading to a bystander killing of tumor cells. In addition, we chose CU fusion as the transgene because UPRT has been shown to have a synergistic antitumor effect with CD where the 5-FU generated through CD (upon addition of prodrug 5-FC) is further metabolized into 5- fluorodeoxyuridine-5ʹ-monophosphate by UPRT (20–22). Also, CD has been recently evaluated in the first neural stem cell–based gene therapy in humans against malignant gliomas (23).
To assess the potential use of OEC for glioma gene therapy, we used a dual luciferase reporter system to monitor cell viability in both OEC and GSC/GBM cells, simultaneously in the same well (24). OCU or OEC (not expressing transgene as a control) were engineered to express the Gluc (coelenterazine as substrate), which will report for OEC viability. GSCs/GBM cells (three different cultures), on the other hand, were engineered to express Fluc (D-luciferin as substrate). We either cultured each cell type separately or cocultured OECs and GSCs/GBM cells together in the presence or absence of 500 µM 5-FC or 500 µM 5-FU (a positive control). Nine days after treatment, OEC and GSCs/GBM cell viability were assessed by measuring Gluc level in an aliquot of conditioned medium and intracellular Fluc activity, respectively. As expected, in each monoculture, all cells were sensitive to 5-FU, but only OCU were killed by 5-FC, proving the validity of our system (P < .001, Figure 3A). Under the coculture conditions, 5-FC treatment did not cause any cell deaths in either GSCs or OEC (not expressing the transgene; Figure 3, B and C); however, a statistically significant lower number of viable GSCs/GBM cells and OCU (>80%; P < .001) was observed after treatment with 5-FC when cocultured together (Figure 3, B and C). Surprisingly, we observed around a 40% decrease in GSCs/GBM cell proliferation when cocultured with OEC or OCU, even without 5-FC, as compared with the untreated monoculture control, showing that OECs could have an intrinsic antitumor activity on their own. These results, which were reproducible in two different patient-derived GSC cultures and U87 GBM cell line, show that OEC can be used to deliver CU therapeutic transgene to tumor cells, converting prodrug 5-FC to an active agent 5-FU, leading to bystander tumor cell killing.
Figure 3.
Olfactory ensheathing cells (OECs) as a delivery vehicle of therapeutic transgenes to glioma stem cells (GSCs) or glioblastoma (GBM) cells in culture. A) Different GBM cells or GSCs expressing firefly luciferase (Fluc), OECs expressing Gaussia luciferase (Gluc), or OECs expressing the fusion between cytosine deaminase and uracil phosphorybosyl transferase (OCU) and Gluc were treated with 5-fluorocytosine (5-FC) or 5-fluorouracyl (5-FU) and cell viability was measured 9 days later. B, C) MGG8 and MGG23 GSCs and U87 GBM cell line expressing Fluc were cocultured with OEC-Gluc or OCU-Gluc and treated with or without 5-FC. Nine days post treatment, cell viability was analyzed by microscopic evaluation (B) and by measuring Fluc and Gluc activities, respectively (C). Data in (A) and (C) are shown as mean % cell viability(SD) in which the untreated monoculture cells are set at 100% (n = 8, P value from two-sided Student t test is shown). D) Apoptosis detected in MGG6, MGG8, and MGG23 GSCs cocultured with OCU and treated with or without 5-FC by annexin V/propidium iodide (PI) staining and analyzed by flow cytometry. Representative dot plots (left) for annexin V−/PI− (alive), annexin V+/PI− (apoptotic), annexin V−/PI+ (necrotic), and annexin V+/PI+ (late apoptotic or dead), and the percentage of cells (mean [SD]) in each quadrant (right), representative from n = 3 independent experiments, P value from two-sided Student t test is shown. E) Caspase-3/7 activity (mean [SD]) was detected in MGG6, MGG8, and MGG23 GSCs cocultured with OCU with and without 5-FC (n = 5, P value from two-sided Student t test is shown). Scale bar = 10 µm.
The ability of 5-FC to induce apoptosis in GSCs cocultured with OCU cells was further analyzed by flow cytometry using annexin V/propidium iodide (PI) staining. In three different GSCs cocultured with OCU, the addition of 5-FC induced a statistically significant increase in the percentage of early apoptotic cells (PI−/annexin V+) and/or late apoptotic cells (PI+/annexin V+). For instance, for MGG23, early apoptosis was increased from 16% [0.7%] (MGG23+OCU) to 60.4% [1%] (MGG23+OCU+5-FC; P < .001); for MGG6, late apoptosis was increased from 5% [2.3%] (MGG6+OCU) to 12.3% [2.3%] (MGG6+OCU+5-FC; P < .001); and for MGG8, late apoptosis was increased from 13.6% [2.4%] (MGG8+OCU) to 22.3% [1.4%] (MGG8+OCU+5-FC; P < .001, Figure 3D). OCU on its own (with no 5-FC) also showed some increase in early/late apoptosis in all three GSCs confirming the viability assays above. Similarly, caspase-3/7 activity was also statistically significantly increased (P < .001) in all three GSCs cocultured with OCU when treated with 5-FC (Figure 3E).
OEC as a “Trojan Horse” for Glioma Gene Therapy
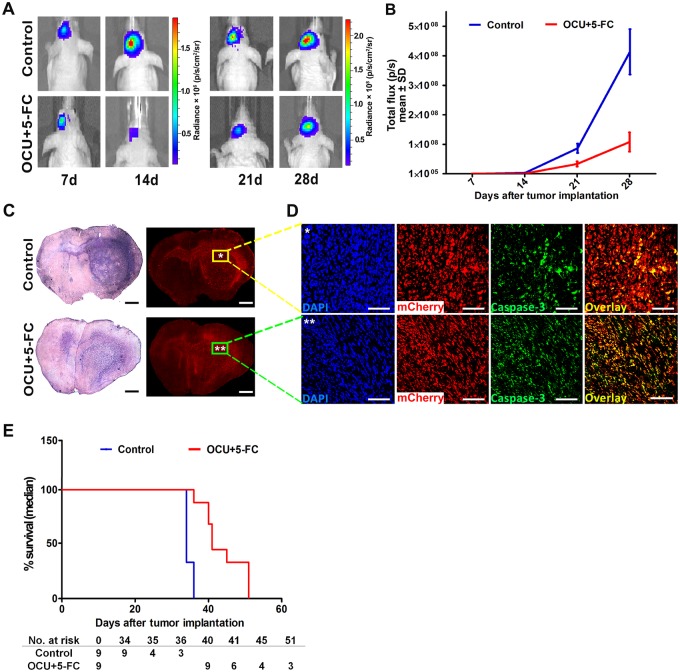
OECs are known to travel along with newly generated olfactory axons from the PNS neurons of the nasal mucosa to their target in the olfactory bulb in CNS. We therefore exploited the natural route of OECs to CNS to deliver CU therapeutic transgene to brain tumors. We injected MGG8 patient-derived GSCs expressing Fluc and mCherry in the striatum of nude mice brains. One week after tumor implantation, mice were divided into two groups (n = 9/group) receiving a single intranasal administration of OCU or PBS (control). One week later, both groups of mice were treated with daily intraperitoneal injection of 5-FC (500 mg/kg) for 7 days. Bioluminescence imaging revealed that mice treated with a single injection of OCU and in combination with 5-FC had a statistically significant reduction of tumor growth compared with the control group treated with 5-FC alone; at day 28, mean (SD) Fluc activity was 1.08E + 08 (9.7E + 07) as compared with 4.1E + 08 (2.3E + 08), respectively (P < .001; Figure 4, A and B). Ex vivo histological analysis for mCherry expression in the tumor as well as hemotoxylin and eosin and cleaved caspase-3 staining confirmed these results and showed that the OCU+5-FC group had a smaller tumor size at the injection site and less invasion/migration throughout the brain, along with increased apoptosis (Figure 4, C and D). Importantly, survival analysis showed that a single injection of OCU and treatment with 5-FC led to a statistically significant increase in the median survival of the mice (41 days for OCU + 5-FC vs 34 days for control, ratio = 0.8293, 95% confidence interval = 0.4323 to 1.226, P < .001, Figure 4E). Taken together, our results indicate that upon intranasal delivery, OCU migrate their natural route toward glioblastomas in a specific manner, and can convert prodrug 5-FC into an active 5-FU drug at the tumor site, leading to an efficient therapeutic bystander effect.
Figure 4.
Olfactory ensheathing cells (OECs) as a Trojan horse for glioma gene therapy via the nasal pathway. A total of 50 000 MGG8 glioma stem cells expressing firefly luciferase (Fluc) and mCherry were implanted in the striatum of athymic mice brain. One week later, mice were randomized into two groups (n = 9/group) receiving either a single intranasal administration of 105 OECs expressing the fusion between cytosine deaminase and uracil phosphorybosyl transferase (OCU) or phosphate-buffered saline (control). One week later, both groups of mice were treated with intraperitoneal injection of 5-fluorocytosine (5-FC, 500 mg/kg) for 7 days. Tumor growth was monitored over time by Fluc imaging and survival was recorded. A) Images of a representative mouse from each group are shown at different time points. B) Tumor-associated bioluminescence signal was quantified (mean total flux [SD] is shown, n = 9, at day 28, P < .001 from two-way ANOVA with a Bonferroni post hoc test). C, D) Hematoxylin and eosin staining and mCherry analysis on brain sections (C), as well as nuclei staining with 6-diamidino-2-phenylindole (DAPI, blue) and cleaved caspase-3 staining (green) colocalized with tumor cells (red, D) at the primary tumor site from a representative mouse from the control (*) and OCU + 5-FC (**) group at day 28. E) Kaplan-Meier survival analysis (median, n = 9, P < .001 from log-rank [Mantel-Cox] test). Scale bar = 1 mm (C) and 100 µm (D).
Discussion
The unique ability of the mammalian olfactory epithelium to continuously replace its olfactory receptor neurons by physiological turnover and following injury throughout life has been attributed to the olfactory ensheathing cells, a glial cell type that closely accompanies the axons as they grow from the olfactory epithelium into the olfactory bulb (3,4). OECs share properties with astrocytes and Schwann cells (25) and are distributed in both the PNS and CNS, which distinguishes them from typical glia (26). OEC migration from PNS to CNS is critical for the development of the olfactory system and axonal extension after injury in neural regeneration. OECs migrate into the injury site and enhance the axon growth because of the permissive OEC environment (27). In stroke, OECs reverses the neurological deficit, protecting the white matter from ischemic injury and improving neurological function (28). OECs combined with olfactory nerve fibroblasts overcome the inhibitory fibrotic scar, increasing the regeneration of the lesioned nigrostriatal dopaminergic neurons in models of Parkinson's disease (29). OECs remyelinate axons after spinal cord lesion (30–33), and induce a less severe host astrocyte response after implantation, downregulating inflammation (34–36). The promising results obtained with OEC transplantation in animal models led to investigating their therapeutic potential in the clinic for different pathologies, including chronic spinal cord injury and amyotrophic lateral sclerosis (7–10), but they were never studied in the context of cancer. Here, we took advantage of these characteristics and, for the first time, evaluated OEC tropism to brain tumors and their potential use as a Trojan horse” to deliver therapeutic transgenes upon intranasal delivery, the OECs natural route to CNS. We showed that OECs navigate the nasal pathway to the primary glioma site and infiltrative GBM stemlike cells and deliver CU therapeutic protein, which could convert the prodrug 5-FC into an active agent 5-FU leading to a strong on-site bystander killing of glioblastomas, even with a single intranasal administration.
Because of their tropism to the tumor site, different stem cell types, including mesenchymal, neural, and embryonic, have been used as a vehicle to deliver therapeutics to gliomas (37). Although these cells have shown some promise, several challenges persist with respect to their isolation and use. For instance, neural stem cells could potentially be harvested from the adult brain, but this process is complicated and time consuming. As an alternative, most studies use stable cell lines of immortalized neural stem cells obtained from embryonic cells, which often makes their use controversial, similar to embryonic stem cells, because of ethical, regulatory, and political concerns. Mesenchymal stem cells are obtained from bone marrow, adipose tissue, peripheral blood, umbilical cord blood, or the placenta; however, as in the case with neural stem cells, local immunosuppression can be observed upon implantation (38). Further, spontaneous tumor formation in long-standing mesenchymal stem cell cultures has been reported, and, after implantation, a small fraction of immortalized neural stem cells continue to proliferate (39–41). Aside from malignant transformation of stem cells, the secretion of growth factors and chemokines, and the direct local immunosuppressive effect of stem cells, may modify the tumor microenvironment such that it promotes tumor growth/metastasis (42–45). OECs, on the other hand, can be easily obtained from the olfactory epithelium, a simple procedure typically done for patients with spinal cord injury allowing autologous transplantation, overcoming both ethical issues and host graft rejection (46,47). Further, the unique properties of OECs, including tissue repair by affecting structural remodeling and support, modulation of the immune system, and clearance of toxic macromolecules, make them an excellent candidate as a delivery vehicle for brain tumor gene therapy. Note that no toxic or tumorigenic potential with OEC transplantation has been reported to date (9,48).
The intranasal pathway has been proposed as a noninvasive alternative route to deliver therapeutics to the brain. This route will bypass the blood-brain barrier, one of the major concerns in delivering any therapeutic to the CNS, and limit systemic side effects. Upon presentation at the nasal cavity, pharmacological agents reach the brain via the olfactory and trigeminal nerves (49,50). Nose-to-brain transport is not exclusively reserved for small molecules and viruses. NSCs and progenitor cells as well as MSCs were shown to target the tumor environment upon intranasal administration in mice bearing brain (and other types) tumors (51–55). In this context, OECs have an advantage over these cells for intranasal delivery, the natural route of OEC from PNS to CNS during olfactory neuron receptor turnover, as well as autologous transplantation, lack of tumorigenicity, restorative potential, and inherent pathotropism toward inflammatory cues.
Aside from their use as a Trojan horse, OECs share several interesting properties that could be an advantage in the context of cancer in general and gliomas in particular. For instance, OECs carry immunomodulatory properties (9,56), are able to phagocytose cellular debris and bacteria (5,57,58), and promote neuroprotection in different CNS disease models (59,60). Our in-culture experiments showed that OECs on their own inhibited GSCs/GBM cells proliferation, potentially having an intrinsic anticancer effect. Recently, the brain-derived neurotrophic factor (BDNF) (61,62), and low-dose curcumin (63), as well as TNFα (64), have been shown to enhance OEC migration to the injury site. Our data revealed that OEC migration decreased upon inhibition of TNFα secretion in glioma cultures, supporting the role of this cytokine in OEC tropism to GBM. It would be interesting to evaluate these OEC properties, together with one of these factors, in our GBM models and their potential use for adjuvant therapy, in combination with radiation and/or temozolomide.
Some limitations of this study should be highlighted, including the choice of the CU transgene, which also kills the OEC upon production of 5-FU at the tumor site. Because our in-culture studies showed that OECs on their own inhibited tumor cell proliferation, it would be worthwhile to use a therapeutic transgene that induces tumor cell killing, but not OEC, which might lead to a more efficient therapy. An example could be soluble TRAIL (tumor necrosis factor apoptosis-inducing ligand), which can be secreted by OEC to bind to death receptors specifically on tumor cells, similar to our published work with neural stem cells (65). Furthermore, the intranasal delivery route could also be limited to the treatment of brain/CNS tumors; however, systemic injection might be more useful for the treatment of other types of cancers.
In conclusion, we show for the first time that OECs can target and deliver therapeutic transgenes to brain tumors upon intranasal administration, the natural route of OEC to CNS. The use of OEC-based gene delivery is not limited to the CU transgene, used here as a proof of concept, but could also be extended to other transgenes and other types of cancer and should not be limited to intranasal administration.
Funding
This work was supported by grants from the National Institutes of Health, the National Institute of Neurological Disorders and Stroke R01NS064983 and P30NS04776 (BAT). We acknowledge the National Council of Technological and Scientific Development (CNPq, Brazil) and the Foundation for Research Support of the State of Rio de Janeiro (FAPERJ, Brazil) for partly supporting Dr Carvalho’s fellowship.
Notes
Affiliations of authors: Experimental Therapeutics and Molecular Imaging Laboratory, Department of Neurology, Neuro-Oncology Division, Massachusetts General Hospital, Boston, MA (LAC, JT, RLF, EIT, MZ, BAT); Neuroscience Program, Harvard Medical School, Boston, MA (LAC, JT, RLF, EIT, MZ, BAT); Laboratory of Neurochemistry, Institute of Biophysics Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (RAdMR).
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. The authors acknowledge the MGH Neuroscience Image Analysis Core (for confocal microscopy) and the MGH Neuroscience Center Vector Core (for producing the viral vector), supported by NIH/NINDS P30NS04776 and a 1S10RR025504 Shared Instrumentation grant for the IVIS imaging system.
Massachusetts General Hospital has filed a provisional patent application for the use of OECs for cancer therapy. The authors declare no conflicts of interest.
LAC, JT, and BAT conceived and designed the study; provided the study material or patents; collected, assembled, analyzed, and interpreted the data; and wrote the manuscript and gave it final approval. RLF, EIT, MZ, and RAMR collected, assembled, analyzed, and interpreted the data. BAT also obtained financial and administrative support for the project.
We would like to thank Dr Hiroaki Wakimoto for providing us the fully characterized patient-derived GBM stem cells used in this study.
Supplementary Material
References
- 1. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 2. Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- 3. Graziadei PP, Graziadei GA.. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979;8(1):1–18. [DOI] [PubMed] [Google Scholar]
- 4. Schwob JE. Neural regeneration and the peripheral olfactory system. Anat Rec. 2002;269(1):33–49. [DOI] [PubMed] [Google Scholar]
- 5. Su Z, Chen J, Qiu Y, et al. Olfactory ensheathing cells: the primary innate immunocytes in the olfactory pathway to engulf apoptotic olfactory nerve debris. Glia. 2013;61(4):490–503. [DOI] [PubMed] [Google Scholar]
- 6. Hashemi M, Fallah A, Aghayan HR, et al. A new approach in gene therapy of glioblastoma multiforme: human olfactory ensheathing cells as a novel carrier for suicide gene delivery. Mol Neurobiol. 2016;53(8):5118–5128. [DOI] [PubMed] [Google Scholar]
- 7. Feron F, Perry C, Cochrane J, et al. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain. 2005;128(Pt 12):2951–2960. [DOI] [PubMed] [Google Scholar]
- 8. Mackay-Sim A, Feron F, Cochrane J, et al. Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial. Brain. 2008;131(pt 9):2376–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tabakow P, Jarmundowicz W, Czapiga B, et al. Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transplant. 2013;22(9):1591–1612. [DOI] [PubMed] [Google Scholar]
- 10. Chen L, Chen D, Xi H, et al. Olfactory ensheathing cell neurorestorotherapy for amyotrophic lateral sclerosis patients: benefits from multiple transplantations. Cell Transplant. 2012;21(suppl 1):65–77. [DOI] [PubMed] [Google Scholar]
- 11. Carvalho LA, Nobrega AF, Soares ID, et al. The mannose receptor is expressed by olfactory ensheathing cells in the rat olfactory bulb. J Neurosci Res. 2013;91(12):1572–1580. [DOI] [PubMed] [Google Scholar]
- 12. Carvalho LA, Vitorino LC, Guimaraes RP, et al. Selective stimulatory action of olfactory ensheathing glia-conditioned medium on oligodendroglial differentiation, with additional reference to signaling mechanisms. Biochem Biophys Res Commun. 2014;449(3):338–343. [DOI] [PubMed] [Google Scholar]
- 13. Macedo-Ramos H, Campos FS, Carvalho LA, et al. Olfactory ensheathing cells as putative host cells for Streptococcus pneumoniae: evidence of bacterial invasion via mannose receptor-mediated endocytosis. Neurosci Res. 2011;69(4):308–313. [DOI] [PubMed] [Google Scholar]
- 14. Goulart CO, Angelo Durco DF, de Carvalho LA, et al. Olfactory ensheathing glia cell therapy and tubular conduit enhance nerve regeneration after mouse sciatic nerve transection. Brain Res. 2016;1650:243–251. [DOI] [PubMed] [Google Scholar]
- 15. Nash HH, Borke RC, Anders JJ.. Ensheathing cells and methylprednisolone promote axonal regeneration and functional recovery in the lesioned adult rat spinal cord. J Neurosci. 2002;22(16):7111–7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wurdinger T, Badr C, Pike L, et al. A secreted luciferase for ex vivo monitoring of in vivo processes. Nat Methods. 2008;5(2):171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Badr CE, Niers JM, Morse D, et al. Suicidal gene therapy in an NF-kappaB-controlled tumor environment as monitored by a secreted blood reporter. Gene Ther. 2011;18(5):445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Egea V, von Baumgarten L, Schichor C, et al. TNF-alpha respecifies human mesenchymal stem cells to a neural fate and promotes migration toward experimental glioma. Cell Death Differ. 2011;18(5):853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao D, Najbauer J, Annala AJ, et al. Human neural stem cell tropism to metastatic breast cancer. Stem Cells. 2012;30(2):314–325. [DOI] [PubMed] [Google Scholar]
- 20. Andersen PS, Smith JM, Mygind B.. Characterization of the upp gene encoding uracil phosphoribosyltransferase of Escherichia coli K12. Eur J Biochem. 1992;204(1):51–56. [DOI] [PubMed] [Google Scholar]
- 21. Adachi Y, Tamiya T, Ichikawa T, et al. Experimental gene therapy for brain tumors using adenovirus-mediated transfer of cytosine deaminase gene and uracil phosphoribosyltransferase gene with 5-fluorocytosine. Hum Gene Ther. 2000;11(1):77–89. [DOI] [PubMed] [Google Scholar]
- 22. Tanaka T, Duflot-Dancer A, Tiraby M, et al. Bystander effect from cytosine deaminase and uracil phosphoribosyl transferase genes in vitro: a partial contribution of gap junctions. Cancer Lett. 2009;282(1):43–47. [DOI] [PubMed] [Google Scholar]
- 23. Portnow J, Synold TW, Badie B, et al. Neural stem cell-based anticancer gene therapy: a first-in-human study in recurrent high-grade glioma patients. Clin Cancer Res. 2017;23(12):2951–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bovenberg MS, Degeling MH, Hejazi S, et al. Multiplex blood reporters for simultaneous monitoring of cellular processes. Anal Chem. 2013;85(21):10205–10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gudino-Cabrera G, Nieto-Sampedro M.. Schwann-like macroglia in adult rat brain. Glia. 2000;30(1):49–63. [DOI] [PubMed] [Google Scholar]
- 26. Ramon-Cueto A, Avila J.. Olfactory ensheathing glia: properties and function. Brain Res Bull. 1998;46(3):175–187. [DOI] [PubMed] [Google Scholar]
- 27. Chehrehasa F, Windus LC, Ekberg JA, et al. Olfactory glia enhance neonatal axon regeneration. Mol Cell Neurosci. 2010;45(3):277–288. [DOI] [PubMed] [Google Scholar]
- 28. Shi X, Kang Y, Hu Q, et al. A long-term observation of olfactory ensheathing cells transplantation to repair white matter and functional recovery in a focal ischemia model in rat. Brain Res. 2010;1317:257–267. [DOI] [PubMed] [Google Scholar]
- 29. Teng X, Nagata I, Li HP, et al. Regeneration of nigrostriatal dopaminergic axons after transplantation of olfactory ensheathing cells and fibroblasts prevents fibrotic scar formation at the lesion site. J Neurosci Res. 2008;86(14):3140–3150. [DOI] [PubMed] [Google Scholar]
- 30. Franklin RJ, Gilson JM, Franceschini IA, et al. Schwann cell-like myelination following transplantation of an olfactory bulb-ensheathing cell line into areas of demyelination in the adult CNS. Glia. 1996;17(3):217–224. [DOI] [PubMed] [Google Scholar]
- 31. Imaizumi T, Lankford KL, Kocsis JD.. Transplantation of olfactory ensheathing cells or Schwann cells restores rapid and secure conduction across the transected spinal cord. Brain Res. 2000;854(1-2):70–78. [DOI] [PubMed] [Google Scholar]
- 32. Imaizumi T, Lankford KL, Waxman SG, et al. Transplanted olfactory ensheathing cells remyelinate and enhance axonal conduction in the demyelinated dorsal columns of the rat spinal cord. J Neurosci. 1998;18(16):6176–6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Plant GW, Currier PF, Cuervo EP, et al. Purified adult ensheathing glia fail to myelinate axons under culture conditions that enable Schwann cells to form myelin. J Neurosci. 2002;22(14):6083–6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leaver SG, Harvey AR, Plant GW.. Adult olfactory ensheathing glia promote the long-distance growth of adult retinal ganglion cell neurites in vitro. Glia. 2006;53(5):467–476. [DOI] [PubMed] [Google Scholar]
- 35. Runyan SA, Phelps PE.. Mouse olfactory ensheathing glia enhance axon outgrowth on a myelin substrate in vitro. Exp Neurol. 2009;216(1):95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sonigra RJ, Brighton PC, Jacoby J, et al. Adult rat olfactory nerve ensheathing cells are effective promoters of adult central nervous system neurite outgrowth in coculture. Glia. 1999;25(3):256–269. [DOI] [PubMed] [Google Scholar]
- 37. Bovenberg MS, Degeling MH, Tannous BA.. Advances in stem cell therapy against gliomas. Trends Mol Med. 2013;19(5):281–291. [DOI] [PubMed] [Google Scholar]
- 38. Jones BJ, McTaggart SJ.. Immunosuppression by mesenchymal stromal cells: from culture to clinic. Exp Hematol. 2008;36(6):733–741. [DOI] [PubMed] [Google Scholar]
- 39. Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97(23):12846–12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Amariglio N, Hirshberg A, Scheithauer BW, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6(2):e1000029.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu J, Zhang Y, Bai L, et al. Rat bone marrow mesenchymal stem cells undergo malignant transformation via indirect co-cultured with tumour cells. Cell Biochem Funct. 2012;30(8):650–656. [DOI] [PubMed] [Google Scholar]
- 42. Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102(10):3837–3844. [DOI] [PubMed] [Google Scholar]
- 43. Houghton J, Stoicov C, Nomura S, et al. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306(5701):1568–1571. [DOI] [PubMed] [Google Scholar]
- 44. Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. [DOI] [PubMed] [Google Scholar]
- 45. Spaeth EL, Dembinski JL, Sasser AK, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009;4(4):e4992.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mackay-Sim A, St John JA.. Olfactory ensheathing cells from the nose: clinical application in human spinal cord injuries. Exp Neurol. 2011;229(1):174–180. [DOI] [PubMed] [Google Scholar]
- 47. Tabakow P, Raisman G, Fortuna W, et al. Functional regeneration of supraspinal connections in a patient with transected spinal cord following transplantation of bulbar olfactory ensheathing cells with peripheral nerve bridging. Cell Transplant. 2014;23(12):1631–1655. [DOI] [PubMed] [Google Scholar]
- 48. Rao Y, Zhu W, Guo Y, et al. Long-term outcome of olfactory ensheathing cell transplantation in six patients with chronic complete spinal cord injury. Cell Transplant. 2013;22(suppl 1):S21–S25. [DOI] [PubMed] [Google Scholar]
- 49. Kozlovskaya L, Abou-Kaoud M, Stepensky D.. Quantitative analysis of drug delivery to the brain via nasal route. J Control Release. 2014;189:133–140. [DOI] [PubMed] [Google Scholar]
- 50. Kapoor M, Cloyd JC, Siegel RA.. A review of intranasal formulations for the treatment of seizure emergencies. J Control Release. 2016;237:147–159. [DOI] [PubMed] [Google Scholar]
- 51. Oppliger B, Joerger-Messerli M, Mueller M, et al. Intranasal delivery of umbilical cord-derived mesenchymal stem cells preserves myelination in perinatal brain damage. Stem Cells Dev. 2016;25(16):1234–1242. [DOI] [PubMed] [Google Scholar]
- 52. Lee ES, Im HJ, Kim HS, et al. In vivo brain delivery of v-myc overproduced human neural stem cells via the intranasal pathway: tumor characteristics in the lung of a nude mouse. Mol Imaging. 2015;14(1):doi:10.2310/7290.2014.00042. [DOI] [PubMed] [Google Scholar]
- 53. Danielyan L, Beer-Hammer S, Stolzing A, et al. Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer’s and Parkinson’s disease. Cell Transplant. 2014;23(1_suppl):123–139. [DOI] [PubMed] [Google Scholar]
- 54. Balyasnikova IV, Prasol MS, Ferguson SD, et al. Intranasal delivery of mesenchymal stem cells significantly extends survival of irradiated mice with experimental brain tumors. Mol Ther. 2014;22(1):140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jiang Y, Zhu J, Xu G, et al. Intranasal delivery of stem cells to the brain. Expert Opin Drug Deliv. 2011;8(5):623–632. [DOI] [PubMed] [Google Scholar]
- 56. Harris JA, West AK, Chuah MI.. Olfactory ensheathing cells: nitric oxide production and innate immunity. Glia. 2009;57(16):1848–1857. [DOI] [PubMed] [Google Scholar]
- 57. Wewetzer K, Kern N, Ebel C, et al. Phagocytosis of O4+ axonal fragments in vitro by p75- neonatal rat olfactory ensheathing cells. Glia. 2005;49(4):577–587. [DOI] [PubMed] [Google Scholar]
- 58. Panni P, Ferguson IA, Beacham I, et al. Phagocytosis of bacteria by olfactory ensheathing cells and Schwann cells. Neurosci Lett. 2013;539:65–70. [DOI] [PubMed] [Google Scholar]
- 59. Pellitteri R, Cova L, Zaccheo D, et al. Phenotypic modulation and neuroprotective effects of olfactory ensheathing cells: a promising tool for cell therapy. Stem Cell Rev Rep. 2016;12(2):224–234. [DOI] [PubMed] [Google Scholar]
- 60. Guerout N, Paviot A, Bon-Mardion N, et al. Co-transplantation of olfactory ensheathing cells from mucosa and bulb origin enhances functional recovery after peripheral nerve lesion. PLoS One. 2011;6(8):e22816.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang Y, Teng HL, Gao Y, et al. Brain-derived neurotrophic factor promotes the migration of olfactory ensheathing cells through TRPC channels. Glia. 2016; 64:2154–2165. [DOI] [PubMed] [Google Scholar]
- 62. Cao L, Su Z, Zhou Q, et al. Glial cell line-derived neurotrophic factor promotes olfactory ensheathing cells migration. Glia. 2006;54(6):536–544. [DOI] [PubMed] [Google Scholar]
- 63. Tello Velasquez J, Watts ME, Todorovic M, et al. Low-dose curcumin stimulates proliferation, migration and phagocytic activity of olfactory ensheathing cells. PLoS One. 2014;9(10):e111787.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lankford KL, Sasaki M, Radtke C, et al. Olfactory ensheathing cells exhibit unique migratory, phagocytic, and myelinating properties in the X-irradiated spinal cord not shared by Schwann cells. Glia. 2008;56(15):1664–1678. [DOI] [PubMed] [Google Scholar]
- 65. Teng J, Hejazi S, Badr CE, et al. Systemic anticancer neural stem cells in combination with a cardiac glycoside for glioblastoma therapy. Stem Cells. 2014;32(8):2021–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.