Abstract
Aims
Elevation of arginase activity has been linked to vascular dysfunction in diabetes and hypertension by a mechanism involving decreased nitric oxide (NO) bioavailability due to L-arginine depletion. Excessive arginase activity also can drive L-arginine metabolism towards the production of ornithine, polyamines, and proline, promoting proliferation of vascular smooth muscle cells and collagen formation, leading to perivascular fibrosis. We hypothesized that there is a specific involvement of arginase 1 expression within the vascular endothelial cells in this pathology.
Methods and results
To test this proposition, we used models of type 2 diabetes and metabolic syndrome. Studies were performed using wild type (WT), endothelial-specific arginase 1 knockout (EC-A1−/−) and littermate controls(A1con) mice fed high fat-high sucrose (HFHS) or normal diet (ND) for 6 months and isolated vessels exposed to palmitate-high glucose (PA/HG) media. Some WT mice or isolated vessels were treated with an arginase inhibitor, ABH [2-(S)-amino-6-boronohexanoic acid. In WT mice, the HFHS diet promoted increases in body weight, fasting blood glucose, and post-prandial insulin levels along with arterial stiffening and fibrosis, elevated blood pressure, decreased plasma levels of L-arginine, and elevated L-ornithine. The HFHS diet or PA/HG treatment also induced increases in vascular arginase activity along with oxidative stress, reduced vascular NO levels, and impaired endothelial-dependent vasorelaxation. All of these effects except obesity and hypercholesterolemia were prevented or significantly reduced by endothelial-specific deletion of arginase 1 or ABH treatment.
Conclusion
Vascular dysfunctions in diet-induced obesity are prevented by deletion of arginase 1 in vascular endothelial cells or arginase inhibition. These findings indicate that upregulation of arginase 1 expression/activity in vascular endothelial cells has an integral role in diet-induced cardiovascular dysfunction and metabolic syndrome.
Keywords: Obesity, Vascular dysfunction, Arginase, Fibrosis, Diabetes
1. Introduction
Cardiovascular disease (CVD), the leading cause of death worldwide, is linked to many metabolic disorders including obesity, hyperglycemia, and hyperlipidemia.1 Diets high in fat and sugars are well known to promote the development of obesity and type 2 diabetes.2 A major cause of morbidity and mortality in these conditions is vascular dysfunction—impaired vascular endothelium-dependent vasodilation, decreased vascular compliance, and reduced blood flow. Increases in blood glucose, inflammatory cytokines, reactive oxygen species (ROS), and reduced nitric oxide (NO) levels are involved in these vascular pathologies.3,4 A better understanding of the mechanisms leading to obesity-induced CVD is imperative for future therapies to reduce morbidity and mortality.5
Earlier studies of diabetic mice and humans with coronary artery disease have indicated the involvement of arginase 1 in vascular endothelial dysfunction.6–9 Arginase, a urea cycle enzyme, can reciprocally regulate NO production by nitric oxide synthase (NOS) through competition for their common substrate, L-arginine.5,7,10,11 Arginase exists as two isoforms—arginase 1, located in the cytoplasm, is expressed most abundantly in the liver; arginase 2, largely mitochondrial, is the primary isoform in kidney. Both isoforms are found in vascular endothelial and smooth muscle cells and can be upregulated by high glucose and ROS.10,12–14
Arginase catalyzes the hydrolysis of L-arginine to urea and ornithine. Ornithine is metabolized to form polyamines via ornithine decarboxylase and proline via ornithine aminotransferase. Polyamines enhance cell proliferation and proline is essential in collagen synthesis.15,16 While both polyamines and proline are important for wound healing and tissue regeneration,15 they can also promote vascular fibrosis and thickening, resulting in arterial stiffening.10 Arterial stiffening, an important independent risk factor for cardiovascular disease, has been reported in diabetic patients prior to diagnosis of cardiovascular disease.17
The importance of arginase in the regulation of vascular functions has become evident in a variety of conditions characterized by cardiovascular dysfunctions including aging, hypertension, atherosclerosis, vascular injury, inflammation, and erectile dysfunction.4,9–11,18–23 Involvement of elevated arginase in hyperglycemia-induced dysfunction of vascular endothelial and smooth muscle cells has also been established in type 1 diabetic models.6,7,10,22 A recent study in Zucker obese rats reported reduced L-arginine bioavailability, impairment of endothelium-dependent vasorelaxation and elevated blood pressure that could be prevented or reversed by arginase inhibitors or supplemental L-arginine.24 However, the specific cellular mediators are as yet unknown. Here, we used models of high fat-high sucrose (HFHS) diet-induced obesity and metabolic syndrome25 to test the hypothesis that obesity-induced vascular dysfunction and stiffening are mediated by expression of arginase 1 in vascular endothelial cells. The results of these experiments demonstrate for the first time that arginase 1 expression in the vascular endothelium is critically involved in the development of obesity-induced vasculopathies, and further demonstrate that these occur through increases in oxidative stress and decreases in L-arginine bioavailability and NO formation.
2. Methods
2.1 Animal studies
Protocols followed the Guide for the care and use of laboratory animals, Eighth edition (2011) and were approved by the Institutional Animal Care and Use Committee. Male C57BL/6J mice (Jackson Laboratory), wild type (WT) or with an endothelial cell specific knockout of arginase 1 (EC-A1−/−), were used in a model of diet-induced obesity and metabolic syndrome.25 Mice that expressed Cre-recombinase in endothelial cells (Cadherin 5-Cre) (Stock No. 017968) were crossed with mice that carried floxed arginase 1 alleles (loxP sites flanking exons 7 and 8 of the Arg1 gene) (Stock No. 008817) to generate EC-A1−/− knockout mice. Littermates of the EC-A1−/− knockout mice, A1loxp/loxp mice, which have the same phenotype as C57BL/6 J WT mice, were used as knockout controls. We bred the mice according to the JaxLab recommended protocol which results in 25% EC-A1−/− and 25% EC-A1loxp/loxp. In the main text, EC-A1loxp/loxp is referred to as A1con.
Immunohistochemical examination of aortic sections for the presence of A1 in EC-A1−/− mice revealed the absence of arginase 1 in the endothelial cell layer vs. its presence there in control A1con mice. A1 staining is evident in the (see Supplementary material online, Figure S1A). Further, western blot analysis of aortic EC isolated and purified from aortas of A1−/− and A1con mice (∼84% EC) (see Supplementary material online Figure S1B) showed the absence of arginase 1 and presence of arginase 2 in EC-A1−/− cells. Both isoforms were expressed in EC-A1con cells (see Supplementary material online, Figure S1C). Levels of mRNA for arginase 1 were barely detectable in cultures isolated from the in EC-A1−/− mice (see Supplementary material online, Figure S1D). Levels of A2 mRNA in the EC A1−/− cells were similar to the EC-A1con cells. To further examine EC specificity, we determined A1 mRNA levels in aorta with and without intact EC of both genotypes. mRNA levels of A1 in aorta of EC-A1−/− mice were about 60% of that in aorta of A1con mice. However, levels were similar in both genotypes in aorta in which EC were removed by rubbing the luminal surfaces with a wire (see Supplementary material online, Figure S1E). Levels of eNOS were not different between the two groups (data not shown).
Mice were fed a high fat-high sucrose (HFHS) diet (percent of calories: 59% fat, 15% protein, 26% carbohydrate [20% from sucrose], F#1850, BioServe) or a normal diet (percent of calories: 18% fat, 24% protein, 58% carbohydrate [∼5% from sucrose], Harlan) for 6 months. WT mice on normal or HFHS diets were treated with the arginase inhibitor, 2-(S)-amino-6-borono-hexanoic acid (ABH, 10 mg/kg/day in drinking water) for 5 months starting 1 month after beginning diets. ABH is a classical competitive and highly specific inhibitor of arginase 1 and 2 in both humans and rodents.26,27 Body weight and blood/urine glucose levels were measured biweekly. Post-prandial serum insulin levels were measured 2 h after feeding in the week before tissue harvest using an Ultra Sensitive Mouse Insulin ELISA Kit (Crystal Chem USA, Downers Grove, IL, USA). Animals were on a 12-h light/dark cycle and had free access to food and water. Before tissue harvest, mice were overdosed with ketamine/xylazine and exsanguinated.
2.2 Vascular function
Vascular function was measured as described.28 Aorta or first order mesenteric arteries were rapidly excised and placed in cold Krebs buffer (pH 7.4). Perivascular fat was removed and vessels were cut into 2 mm rings. In some experiments, rings were pretreated with M199 media containing 200 μM palmitic acid and 25 mM L-glucose (PA/HG) or M199 control media (CM, no PA, and 5 mM glucose) for 8 or 24 h at 37 °C in culture chambers. Rings were mounted in myograph baths (Danish Myo-Technology) filled with Krebs solution (37 °C) under resting tension of 5.0 mN and bubbled with 95% O2/5% CO2. Isometric force was recorded using a Power Lab system (AD Instruments). After equilibration (1 h), contraction to KCl (8 mmol/L) was assessed. Thereafter, cumulative concentration-response curves to acetylcholine (ACh, endothelium-dependent vasodilator) or sodium nitroprusside (SNP, endothelium-independent vasodilator) were obtained in rings pre-contracted with phenylephrine (PE).
2.3 Blood pressure measurements
Systolic blood pressure was measured by the tail-cuff method as described.20 Mice were acclimated to this procedure over 10 days. Final measurements were performed a week before tissue harvest. Ten consecutive readings were recorded and averaged.
2.4 Vascular NO levels
Vascular NO production was determined by using a Sievers NO Analyzer (Boulder, CO) to measure NO-specific chemiluminescence. Levels of nitrite (NO2−), the stable breakdown product of NO, were determined in isolated aorta as previously described.20 Aortic tissues for all groups of mice were incubated in fresh media for 8 hrs at 37 °C. Samples of media were then analyzed for NO content. A portion of each tissue was incubated with the NOS inhibitor L-NAME (0.1 mmol/L) for this same period to determine initial NO levels in these tissues. This value was subtracted from the value obtained without L-NAME addition to provide NO production in that tissue from NOS. Production of NO was normalized to tissue protein content.
In other studies, the NO indicator 4,5-diaminofluorescein diacetate (DAF-2 DA) was used. Cross-sections (10 µm thick) from frozen aorta were incubated in the dark at 37 °C in HEPES buffer containing DAF-2 DA (0.1 μmol/L, 15 min). Sections were incubated with L-NAME (0.1 mmol/L) along with DAF-2 DA as a negative control. Sections were fixed in 4% paraformaldehyde and images were collected by confocal microscopy. Fluorescence intensity was quantified using Zen 2012 software.
2.5 Levels of reactive oxygen species (ROS)
Plasma ROS were assessed by measuring levels of lipid peroxides, as malonaldehyde, spectrophotometrically at 532 nm.29 For assessment of vascular ROS formation, dihydroethidium (DHE) imaging was performed as described.7 Briefly, cross-sections of frozen aorta were incubated in the dark in HEPES buffer containing DHE (0.1 mmol/L) for 30 min and examined by confocal microscopy. Blockade of the DHE signal with superoxide dismutase (SOD, 100 U/ml) or the NOS inhibitor L-NAME (100 μmol/L) was used to demonstrate involvement of superoxide and NOS, respectively. Aortic levels of 3-nitrotyrosine (3-NT), a marker of formation of the potent oxidant peroxynitrite (ONOO−), also were assessed by western blot analysis. Specificity of the antibody was determined by the ability of prior treatment of the membrane (3 h) with Na dithionite and Na borate (both at 100 mmol/L) to block the interaction of 3-NT with the antibody (see Supplementary material online, Figure S4B).
2.6 Vascular stiffness
Aortic stiffness was assessed by measuring pulse wave velocity (PWV) using VEVO 2100 imaging system.28 Aortic pulse waves were assessed at the aortic arch and abdominal aorta proximal to the iliac bifurcation. Pulse travel time was calculated as transit time of each waveform between the sites using R-wave of ECG as a reference point. PWV (m/s) was calculated by dividing the distance by transit time. Each value is an average of 3–5 cardiac cycles.
2.7 Collagen staining
Collagen content was measured as described.28 Aorta were fixed in buffered formalin, embedded in paraffin and tissue sections were stained with Picrosirius Red. Area of collagen staining relative to vessel surface area was quantified using National Institutes of Health ImageJ software.
2.8 Arginase activity assay
Aortic lysates or plasma samples were used for arginase activity assay as described.7 Briefly, 10 mM MnCl2 were added to the samples and heated at 57 °C for 10 min to activate arginase. L-arginine (0.5 mol/L) was added and incubated at 37 °C for 1 h. The reaction was stopped with acid, α-isonitrosopropiophenone was added, the mixture was heated for 45 min (100 °C), and activity was measured by absorbance at 540 nm.
2.9 Plasma amino acid levels
L-arginine, L-ornithine, L-lysine, and L-citrulline levels were assessed for some of our studies using hydrophilic interaction liquid chromatography electrospray tandem mass spectrometry (Shimadzu Scientific Instruments). Systemic arginase activity was assessed as the ratio of plasma ornithine to arginine concentrations. Global arginine bioavailability ratio (GABR) is the ratio of plasma arginine concentration to the sum of concentrations of ornithine and citrulline.30
2.10 Western blot
Lysates from aortic homogenates (20 μg protein) were subjected to electrophoresis on 10% SDS-polyacrylamide gels. Proteins were electro-blotted onto PVDF membranes (Millipore). Membranes were blocked with 5% bovine serum albumin (BSA Fraction V, OmniPur) in TBST (0.2% Tween 20 in 1× Tris-buffered saline) and incubated overnight at 4 °C with primary antibodies [anti-Arginase 1, 1 10, 000 (Kind gift of Dr. Sidney M. Morris Jr, University of Pittsburgh); anti-Arginase 2, 1:250 (Santa Cruz); anti-β-actin, 1:4000, (Sigma-Aldrich); anti-3-nitrotyrosine, 1:1000, (Sigma-Aldrich); and anti-eNOS, 1:1000, (Cell Signalling Tech)]. Membranes were washed, incubated with secondary antibodies conjugated with horseradish peroxidase. Signals were detected using chemiluminescence and analysed using densitometry. Equal loading was determined after stripping the initial gel and re-probing with anti-β-actin or α-tubulin.
2.11 Quantitative reverse transcription-PCR (Q-PCR)
Total RNA was isolated using TRIzol reagent (Invitrogen). Total RNA was reverse transcribed with M-MLV reverse transcriptase (Invitrogen) to generate cDNA. Gene expression was determined by quantitative PCR with SYBR Green Dye Gene Expression Assays or TaqMan Gene Expression Assays (for arginase 1 and arginase 2, Applied Biosystems; for p-eNOS, Life Technologies), which was performed on a StepOne Plus thermocycler (Applied Biosystems). Cycle threshold, determined as the initial increase in fluorescence above background, was determined for each sample. HPRT was used as internal control for normalization.
2.12 Drugs and chemicals
Acetylcholine, phenylephrine, phosphatase cocktail 1 and 2, protease inhibitor, Dihydroethidium (DHE), malondialdehyde bis (phenylimine), trichloroacetic acid, and thiobarbituric acid were from Sigma Aldrich. Serum insulin assay kits came from Crystal Chem. Plasma cholesterol assay kits were from Wako Diagnostics. Arginase inhibitor, 2-(S)-amino-6-boronohexanoic acid (ABH) was a kind gift from Corridor Pharmaceuticals, Inc. (Baltimore, MD). Picrosirius Red Stain Kit was from Polysciences, Inc. DAF-2 (4, 5-Diaminofluorescein) was from Calbiochem. Glycated hemoglobin (A1C-NOW) assay kit came from Bayer Healthcare. ABH is a classical competitive inhibitor for both human arginase 1 and 2 with K(i) values of 0.11 and 0.25 μM, respectively.26 ABH has about 20 times higher affinity to human arginase 1 compared to that of rodents (ABH, Kd = 0.11 μM against rat arginase I; Kd = 5 nM against human arginase 1).27 It has been used in many acute and chronic studies in animals and on human tissues. 28,31,32
2.13 Statistical analysis
Data are presented as mean ± SEM. Statistical differences were determineds using analysis of variance (ANOVA) and Tukey post-test. P values < 0.05 were taken as significant. Analyses used GraphPad Prism, version 4.00 (GraphPAD Software Inc.). Differences among the concentration-response curves were determined using two-way repeated measures ANOVA. All experiments were performed on 4–8 mice/group. For image analysis studies, data values for each mouse were calculated from 2 to 3 sections per mouse.
3. Results
3.1 Metabolic parameters
After 6 months of HFHS feeding WT and A1con mice had significant increases in body weight, fasting blood glucose, glycated hemoglobin, post-prandial serum insulin, plasma cholesterol, and systolic blood pressure compared to ND controls (Table 1). Elevations of body weight, blood glucose, and cholesterol were not altered in mice treated with ABH or those lacking endothelial arginase 1 (EC-A1−/−). These data suggest that arginase activity or EC arginase 1 is not involved in these HFHS-induced changes. Although serum insulin levels were elevated by HFHS in all groups, the rise in insulin was significantly blunted by ABH, indicating a role for arginase activity in insulin resistance. Interestingly, there was also a trend toward a decrease in insulin levels in the EC-A1−/− mice. The HFHS diet-induced increase in systolic blood pressure was blocked by ABH treatment and in EC-A1−/− mice, indicating the involvement of endothelial cell arginase 1 in the blood pressure elevation.
Table 1.
Body weight, blood glucose, glycated hemoglobin (HbA1C), blood pressure, serum insulin, and plasma cholesterol
| Body weight (g) | Blood glucose (mg/dL) | HbA1C (%) (mmol/mol) | Systolic blood pressure (mmHg) | Insulin after feeding (ng/mL) | Cholesterol (mg/dL) | ||
|---|---|---|---|---|---|---|---|
| WT | ND | 37.7 ± 1.4 | 110.8 ± 3.4 | 4.4 ± 0.2 | 94.3 ± 2.5 | 0.4 ± 0.02 | 115.5 ± 3.9 |
| 24.3 ± 0.9 | |||||||
| HFHS | 51.9 ± 1.9* | 201.4 ± 16.9* | 5.3 ± 0.2* | 120.2 ± 5.7* | 1.6 ± 0.3* | 217.2 ± 23.4* | |
| 34.0 ± 1.0* | |||||||
| ND + | 37.3 ± 1.6 | 107.5 ± 3.1 | 4.4 ± 0.2 | 92.3 ± 1.6 | 0.3 ± 0.05 | 98.2 ± 4.5 | |
| ABH | 24.4 ± 1.2 | ||||||
| HFHS+ | 49.3 ± 1.8* | 195.7 ± 18.4* | 5.1 ± 0.13* | 99.4 ± 1.5 | 1.0 ± 0.2 # | 182.1 ± 18.5* | |
| ABH | 32.2 ± 0.8* | ||||||
| EC-KO | ND A1con | 36.8 ± 1.4 | 106.8 ± 3.9 | 4.5 ± 0.1 | 96.3 ± 2.3 | 0.7 ± 0.2 | 120.1 ± 9.3 |
| 26.0 ± 0.8 | |||||||
| HFHS A1con | 50.7 ± 1.3* | 197.8 ± 10.5* | 5.2 ± 0.1* | 115.9 ± 4.2* | 2.3 ± 0.2* | 190.7 ± 4.6* | |
| 33.6 ± 0.5* | |||||||
| ND | 38.7 ± 1.3 | 102.7 ± 2.4 | 4.5 ± 0.2 | 92.7 ± 1.4 | 0.8 ± 0.4 | 104.7 ± 10.5 | |
| EC-A1−/− | 25.5 ± 1.0 | ||||||
| HFHS | 49.5 ± 1.4* | 199.6 ± 11.3* | 5.2 ± 0.2* | 98.9 ± 3.1 | 1.7 ± 0.3* | 219.5 ± 6.0* | |
| EC-A1−/− | 32.9 ± 1.0* |
Values are means ± S.E.M. *P < 0.05 vs. WT or A1con mice on normal diet (ND), # P < 0.05 vs. WT mice on HFHS diet, n = 4–6. Abbreviations: endothelial cell specific knockout of A1 (EC-A1−/−), high fat high sucrose diet (HFHS), and arginase inhibitor, 2-(S)-amino-6-boronohexanoic acid (ABH).
3.2 Vascular endothelial function
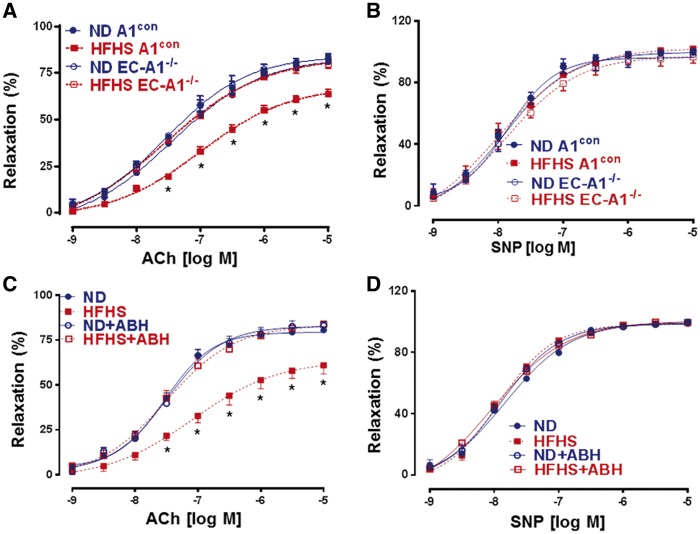
Our previous studies have demonstrated the role of arginase activity in diabetes-induced vascular endothelial dysfunction (VED).6,7,33 To determine the specific role of arginase 1 expression in vascular EC and arginase activity in obesity-induced VED, we analysed acetylcholine-induced vasorelaxation responses in aortas from HFHS and ND-fed mice lacking arginase 1 in EC or treated with ABH. This analysis showed that HFHS feeding caused marked impairment of vasorelaxation responses in aortas from A1con and WT mice as compared with ND mice (Figure 1A and C). Aortas from EC-A1−/− mice were protected from the HFHS diet-induced VED, indicating a central role of endothelial arginase in vascular endothelial dysfunction (VED). Treatment with ABH also preserved normal vasorelaxation responses, confirming the specific role of arginase activity in the VED. Lack of endothelial arginase 1 or its inhibition did not augment vasorelaxation responses in ND aortas. This lack of enhanced function likely relates to sufficient vascular L-arginine availability in normal, non-stress conditions. This observation is in concert with previous reports that arginase knockdown or arginase inhibition under normal physiological, non-stress conditions does not enhance EC-dependent vasorelaxation.34
Figure 1.
EC arginase 1 deletion or arginase inhibition prevents HFHS-induced decreases in endothelium-dependent vasorelaxation to acetylcholine (ACh). (A) Effects of EC arginase 1 deletion or ABH (C) on endothelium-dependent vasorelaxation. Effects on endothelium-independent vasorelaxation to sodium nitroprusside (SNP, B, D). Values are mean ± SEM, n = 6–8. *P < 0.05 vs. WT ND or A1con ND groups.
Endothelium independent relaxation to the NO donor sodium nitroprusside (SNP) was normal in all groups (Figure 1B and D), indicating that smooth muscle function is preserved. These data demonstrate the specific role of EC expression of arginase 1 and arginase activity in HFHS-induced VED.
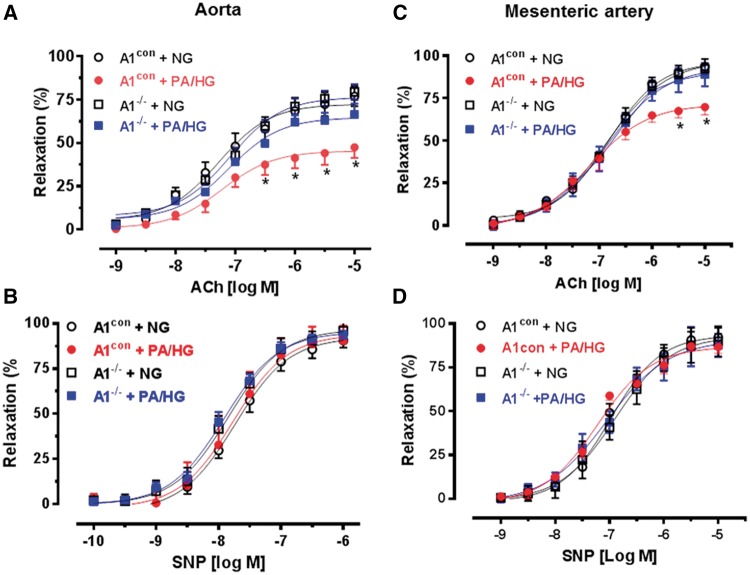
In obesity and metabolic syndrome, the vasculature is exposed to high levels of fatty acids and glucose.35,36 In order to directly test the effects of hyperlipidemia/hyperglycemia on vascular function in the absence of inflammation and other aspects of metabolic disease and to rule out interactions between the effects of diet and age, we mimicked high fat/high glucose conditions ex vivo by exposing vessels freshly isolated from young ND-fed mice (9–11 weeks) to Kreb’s buffer media containing 200 μM palmitic acid and 25 mM L-glucose (PA/HG) or a Kreb’s control media (CM, no PA and 5 mM L-glucose). Aorta from A1con mice exposed to PA/HG (24 h) exhibited significant impairment of endothelial-dependent relaxation compared to aorta maintained in CM. By contrast, vasorelaxation of aortas from EC-A1−/− mice incubated with PA/HG media were not different from aorta of either genotype exposed to CM (Figure 2A). EC-independent vasorelaxation to SNP was not altered by PA/HG exposure (Figure 2B). These response patterns were closely comparable with the in vivo data, indicating that PA/HG treatment mimics the chronic HFHS diet and suggesting that elevated arginase 1 activity is involved in this VED. Studies using vascular resistance vessels (first order mesenteric arteries, MA) from A1con mice also showed impairment in vasorelaxant responses to acetylcholine after exposure to PA/HG (8 r) compared to responses of MA incubated in CM (Figure 2C). Vasorelaxation responses in MA from EC-A1−/− mice exposed to PA/HG were comparable to those in CM control vessels (Figure 2C). Vasorelaxant responses to SNP were not different among the groups (Figure 2D). These data indicate that VED is induced by direct effects of PA/HG exposure in both resistance and conduit vessels and that arginase 1 is involved.
Figure 2.
EC arginase 1 deletion prevents palmitic acid and high glucose (PA/HG)-induced decreases in endothelium-dependent vasorelaxation to acetylcholine (ACh). Effects of EC arginase 1 deletion on endothelium-dependent vasorelaxation in aorta (A) or mesenteric arteries (C) exposed to PA/HG or normal glucose control media (NG). Effects on endothelium-independent vasorelaxation to sodium nitroprusside (SNP, B, D). Values are mean ± SEM, n = 5. *P < 0.05, vs. NG.
3.3 Aortic stiffness and fibrosis
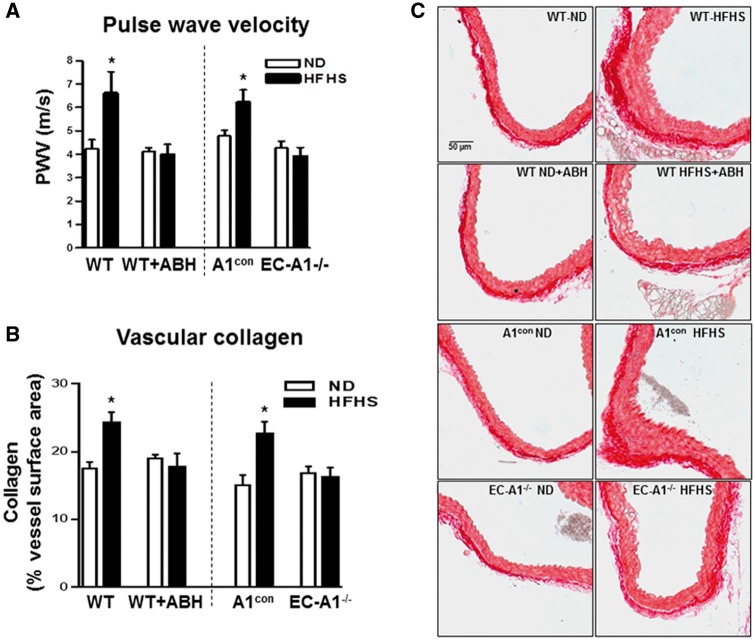
Vascular stiffening seen clinically is directly correlated with increased body weight, fat content, and hyperglycemia.37 Aortic stiffness, assessed as pulse wave velocity (PWV), was significantly increased in A1con and WT mice fed HFHS compared with ND (Figure 3A). HFHS induced aortic stiffening was blocked by arginase 1 deletion in endothelial cells (EC A1−/−) or ABH treatment. Since perivascular fibrosis is involved in arterial stiffening, we examined perivascular collagen deposition in aortic sections. HFHS feeding resulted in significant increases in perivascular collagen levels in both A1con and WT mice as compared to the ND control groups as shown by increased picrosirius red staining (Figure 3B and C). Endothelial arginase 1 deletion or treatment with ABH prevented this increase in collagen fibers. Taken together, these findings demonstrate that endothelial arginase 1 is centrally involved in HFHS diet-induced elevation of aortic fibrosis and stiffness.
Figure 3.
EC arginase 1 deletion or arginase inhibition prevents HFHS-induced aortic stiffening and fibrosis. Effects of arginase 1 deletion or ABH on pulse wave velocity (A) and collagen deposition (B, C). Representative sections stained with Picrosirius Red (C). Values are mean ± SEM, n = 5–6. *P < 0.05 vs. ND.
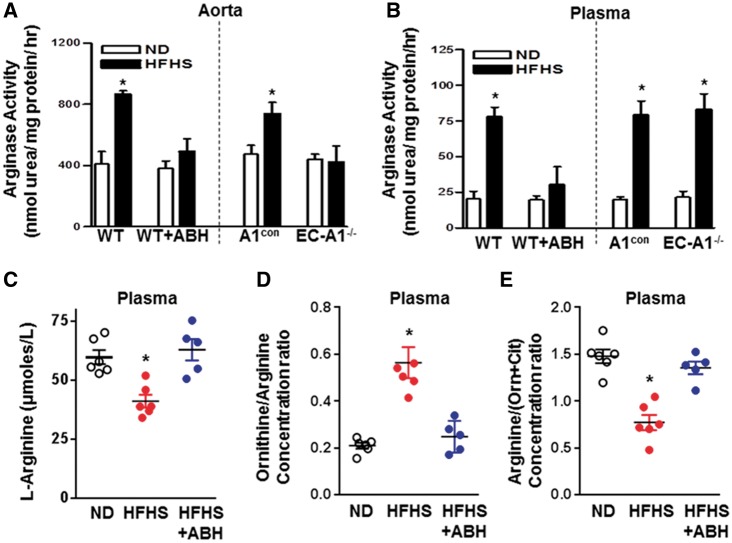
3.4 Arginase activity
We and others have shown the involvement of increased arginase activity in diabetes-induced VED.6,7,9,33 Increases in arginase activity were observed in aortas from HFHS fed A1con and WT mice (Figure 4A). These increases were prevented by EC-A1 deletion or treatment with ABH. This reduction of vascular arginase activity by ABH correlates well with a reduction in arginase 1 expression (Figure 5). This result indicates that endothelial arginase 1 is primarily responsible for HFHS-induced elevation of aortic arginase activity. Aorta from A1con mice exposed to PA/HG (24 h) also exhibited elevated arginase activity compared to levels in aorta of EC-A1−/− mice, which were not different from those of both genotypes exposed to control media (see Supplementary material online, Figure S2A).
Figure 4.
EC arginase 1 deletion or arginase inhibition prevents HFHS-induced increases in arginase activity and decreases in L-arginine bioavailability. Effects of arginase 1 deletion or ABH on aortic (A) and plasma arginase activity (B). Effects of ABH on plasma L-arginine (C), the ratio of plasma L-ornithine to L-arginine (D) (a measure of systemic arginase activity) and ratio of plasma L-arginine to [L-ornithine + L-citrulline] (a measure of L-arginine bioavailability (E). Values are mean ± SEM, n = 5–7. *P < 0.05 vs. ND.
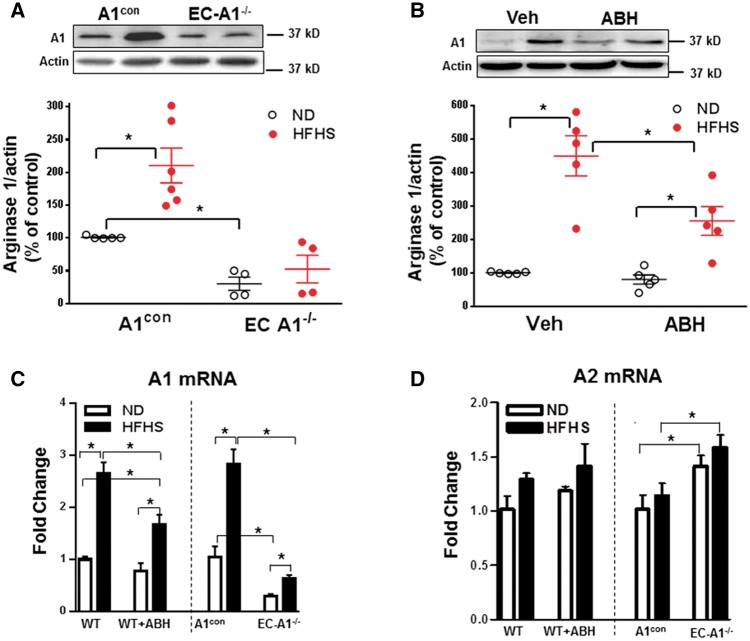
Figure 5.
EC arginase 1 deletion or arginase inhibition prevents HFHS-induced increases in arginase 1 expression in the aorta. Effects of EC arginase 1 deletion (A) and ABH (B) on arginase 1 (A1) protein or arginase 1 (A1) and arginase 2 (A2) mRNA (C, D). Values are mean ± SEM, n = 4–6. *P < 0.05.
To further understand the role of arginase 1 expression in vascular endothelial cells on levels of circulating arginase, we measured plasma arginase activity in EC-A1−/− and WT mice. HFHS feeding caused similar increases in plasma arginase activity in A1con and WT mice (Figure 4B), but unlike the effect of systemic treatment with the arginase inhibitor ABH which prevented this rise, plasma arginase activity in EC-A1−/− mice was elevated comparable to that in A1con and WT mice. This finding suggests that endothelial arginase 1 does not contribute to HFHS-induced elevation of plasma arginase activity.
3.5 L-arginine bioavailability
Bioavailability of L-arginine plays a critical role in endothelial function and, consequently, cardiovascular disease.38 To assess the effects of the HFHS diet feeding on L-arginine bioavailability, we measured plasma levels of L-arginine, L-ornithine, and L-citrulline using hydrophilic interaction liquid chromatography electrospray tandem mass spectrometry. These levels on the normal diet were within the range previously reported for mice.39 This analysis showed that HFHS feeding caused significant reductions in plasma L-arginine concentration as compared with ND mice (Figure 4C), Also, the ratio of plasma L-ornithine to L-arginine was markedly increased, consistent with an increase in systemic arginase activity (Figure 4D). ABH treatment prevented these effects. A measure of systemic L-arginine bioavailability—global arginine bioavailability ratio (GABR)—is an indicator of cardiovascular risks.30 Calculated as plasma levels of arginine divided by the sum of L-ornithine plus L-citrulline levels, GABR has been shown to inversely correlate with increased adverse cardiovascular events.40,41 We observed a marked drop in this ratio in WT HFHS vs. ND fed mice (Figure 4E). This reduction was prevented by ABH treatment. Consistent with the HFHS-induced increases in arginase activity, plasma levels of L-ornithine were elevated by HFHS diet and ABH largely prevented this rise (see Supplementary material online, Figure S2B). There were no differences in plasma levels of L-lysine among the three groups.
3.6 Arginase expression
To further assess isoform-specific roles of vascular arginase, we measured levels of arginase 1 and arginase 2 protein in the isolated aortas. WT and A1con mice fed HFHS showed increased arginase 1 protein (Figure 5A and B), but detection of arginase 2 was faint and no changes were observed (data not shown). Additionally, mRNA levels of arginase 1 rose significantly with HFHS diet in both WT and A1con mice, but arginase 2 mRNA levels were unchanged. These data indicate that arginase 1 is primarily involved in HFHS diet-induced elevation of vascular arginase activities. ABH treatment substantially blocked the HFHS-induced increases in arginase 1 protein and mRNA (Figure 5B and C). This result is most likely due to the action of ABH to reduce production of ROS and activation of RhoA kinase, which are known to elevate arginase 1 expression.13,20,42 HFHS-induced increases in arginase 1 protein and mRNA seen in the A1con mice were largely blocked in the EC-A1−/− mice (Figure 5C). There were no changes in arginase 2 mRNA levels between the WT and WT + ABH groups (Figure 5D), but arginase 2 mRNA levels were slightly higher in EC-A1−/− vs. A1con mice—possibly as a compensation for lack of arginase 1. Control levels of aortic arginase 1 protein and mRNA in EC-A1−/− mice were markedly lower than in A1con mice, indicating a strong contribution of EC to total aortic A1 levels (Figure 5A and C). These data indicate that (i) arginase 1 is the main isoform elevated by HFHS diet, and (ii) endothelium is the predominant source of elevated vascular arginase activity and arginase 1 expression in HFHS mice.
3.7 Vascular nitric oxide production
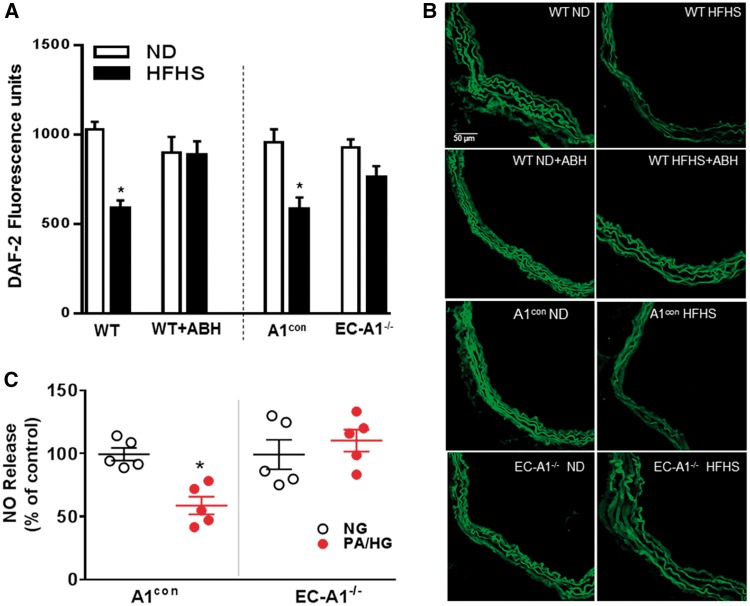
Aortic NO production was assessed by DAF-2 fluorescence imaging and chemiluminescence measurement of nitrite levels in media conditioned by PA/HG-treated vessels (Figure 6). Aortas from both A1con and WT mice on the HFHS diet showed decreased NO levels compared to ND controls (Figure 6A and B). The HFHS diet-induced loss of NO was prevented in EC-A1−/− mice or by treatment with ABH. These findings indicate that vascular arginase is responsible for decreased NO production in HFHS fed mice and that endothelial arginase 1 is involved. Pretreatment of control aorta with the NOS inhibitor L-NAME (0.1 mmol/L) blocked DAF fluorescence (see Supplementary material online, Figure S3A).
Figure 6.
EC arginase 1 deletion or arginase inhibition prevents HFHS or PA/HG -induced decreases in vascular nitric oxide. Effects of EC arginase 1 deletion or ABH on fluorescence for the NO indicator DAF2-DA (A). Representative images of DAF2-DA fluorescence (B). Values are mean ± SEM, n = 5–6. *P < 0.05 vs. ND. Effects of EC arginase 1 deletion on NO production in aorta exposed to PA/HG ex vivo (C). Values are mean ± SEM, n = 5. *P < 0.05 vs. control media (NG).
Chemiluminescence analysis of nitrite levels in PA/HG-treated A1con aorta showed a significant decrease in NO levels compared to control medium (Figure 6C). Levels of NO from EC-A1−/− aorta exposed to CM or PA/HG were not different. These data indicate that plasma factors elevated in high fat diets suppress NO production by an action involving EC-A1.
The other major pathway which metabolizes L-arginine and regulates NO synthesis is eNOS. Analysis of eNOS protein and mRNA expression levels in aorta of the WT HFHS and ND groups revealed no difference between them (see Supplementary material online, Figure S4A).
3.8 Plasma and vascular reactive oxidative species
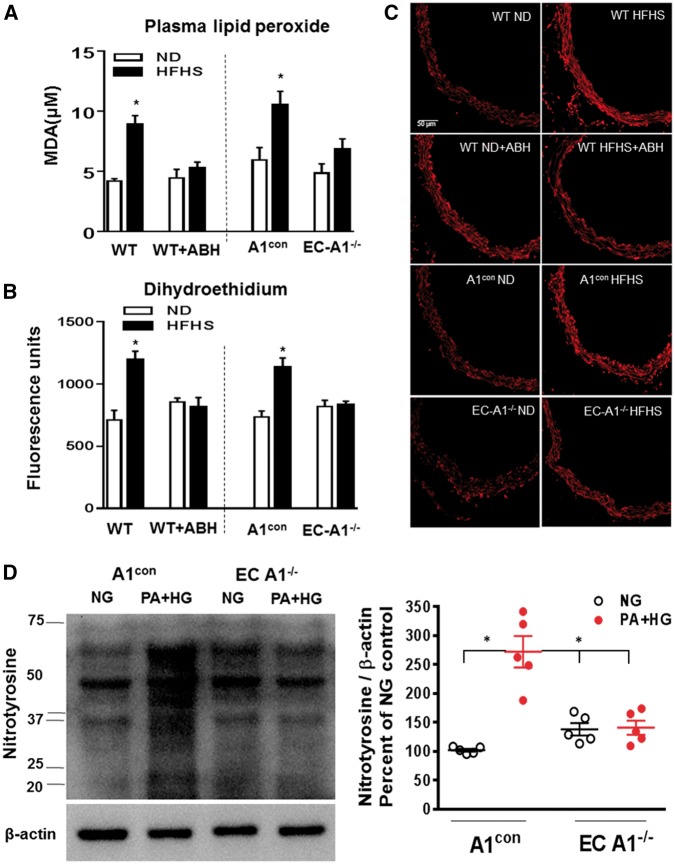
Previous studies have shown that increased oxidative stress is related to increased arginase activity/expression and reduced NO production.7,13,43 In the present study, A1con and WT HFHS mice exhibited increased oxidative stress as demonstrated by increased plasma lipid peroxides (Figure 7A) and vascular reactive oxygen species levels (Figure 7B and C). These HFHS diet-induced increases in oxidative stress were prevented in EC-A1−/− mice or by treatment with ABH, indicating that arginase is involved and that endothelial arginase 1 is largely responsible.
Figure 7.
EC arginase 1 deletion or arginase inhibition prevents HFHS-induced increases in ROS levels. (A) Effects of arginase 1 deletion or ABH on plasma malonaldehyde (MDA) and (B) aortic fluorescence for the ROS indicator dihydroethidium (DHE). (C) Representative images of DHE fluorescence. Values are mean ± SEM, n = 5–6. *P< 0.05 vs. ND. (D) Levels of the peroxynitrite marker—3-nitrotyrosine in aorta from A1con and ECA1−/− mice exposed to normal glucose control media (CM) or palmitic acid and high glucose (PA/HG) for 24 h. Values are mean ± SEM, n = 5. *P< 0.05 vs. NG.
Further, prior exposure of aorta sections to SOD (100 U/ml) or L-NAME (0.1 mmol/L) suppressed the high fat-induced DHE fluorescence, indicating that NOS is a source of ROS and the involvement of superoxide (see Supplementary material online, Figure S3). Other evidence of aortic oxidative stress is the elevated levels of the peroxynitite (ONOO−) biomarker 3-nitrotyrosine. Western blot analysis of A1con aorta exposed to PA/HG (see Figure 2) showed significant increases in the formation of peroxynitrite, compared to vessels exposed to control medium (Figure 7D). Nitrotyrosine levels in EC-A1−/− aorta exposed to normal glucose control media or PA/HG were not different. These data indicate that plasma factors elevated in high fat diets/obesity increase vascular ONOO− formation by an action involving EC-A1.
4. Discussion
Vascular dysfunction is a major cause of morbidity and mortality during type 2 diabetes (T2D), a condition that is reaching epidemic proportions with the increasing incidence of obesity worldwide.44 Here, we demonstrate that arginase 1 expression in vascular endothelial cells plays an integral role in the development of vascular endothelial dysfunction, vascular fibrosis, and stiffening and hypertension resulting from the HFHS diet treatment. Moreover, our studies using vessels from young and healthy mice exposed to palmitate/high glucose media ex vivo show dysfunction similar to that seen after 6 months feeding on a high fat/high sucrose diet. This result demonstrates the specificity of the dysfunction for the diet effects on the vascular endothelium as opposed to inflammation or age-related systemic changes. We further show that the diet-induced vasculopathy involves increases in oxidative stress along with decreases in L-arginine bioavailability, reduced NO formation and increased L-ornithine production.
Elevated aortic arginase activity was observed after 6 months of HFHS diet. This elevation was associated with increased arginase 1 mRNA and protein while expression of arginase 2 was not altered. Our finding that aortic levels of arginase 1 are upregulated in HFHS diet-induced obesity and hyperglycemia is consistent with our previous findings in models of type 1 diabetes.6,19,45 In the present study, mice lacking arginase 1 in endothelial cells (EC-A1−/−) showed no elevation in vascular arginase activity with HFHS feeding or when exposed to high palmitate/high glucose medium ex vivo (Figure 4A or see Supplementary material online, Figure S2). Vascular expression of arginase 1 (protein and mRNA) in EC-A1−/− mice was only 25–30% of that in mice with intact EC arginase 1 (Figure 5A and C). These data indicate that the endothelium is the dominant source of vascular arginase 1 and of HFHS-induced elevation of arginase activity. Suppression of the elevation of arginase 1 expression by ABH treatment is likely due to the observed reduction of levels of ROS formed by NOS, thus retarding feed-forward actions of ROS on arginase expression/activity.13,20,46 There was no change in aortic arginase 2 protein or mRNA levels in HFHS diet fed mice. Interestingly, a role for arginase 2 has been proposed in a recent study of obesity-associated eNOS dysfunction in C57BL6 mice.47 That study used a 14 week, high fat diet, whereas our study employed a 24 week high fat-high sucrose diet. It would be interesting to determine if differences in diets and duration induce different isoforms of arginase. Arginase 2 has been found to be elevated by oxidized low density lipoprotein and Rho kinase activation in human EC and its expression has been shown to be important in macrophage proinflammatory responses and atherogenesis in mice.48–50
Plasma levels of arginase activity were also elevated by the HFHS diet. The ABH treatment prevented this rise, as would be expected by the presence of the inhibitor in the circulation. However, activity levels in plasma remained high in HFHS EC-A1−/− mice, suggesting that arginase released from tissues other than endothelial cells is responsible for the elevated plasma arginase activity. Plasma arginase activity is known to be elevated in myocardial infarction and liver disease51 and also in obesity-related diabetes.24,52 This could involve the release of arginase from immune cells (monocytes and macrophages),53 which are elevated in inflammatory states of obesity and diabetes.54
Aorta from WT HFHS mice showed impaired endothelium dependent vasodilation which was prevented in mice with selective deletion of EC arginase 1. Treatment with the arginase inhibitor ABH also prevented this vascular endothelial dysfunction confirming the specific involvement of arginase activity. Given that endothelial derived NO is vital for normal vascular relaxation, our findings that EC arginase 1 deletion or inhibition of arginase in the HFHS fed mice maintains good vascular relaxation likely reflects arginase/eNOS competition for their substrate L-arginine. This concept is further supported by our findings that HFHS fed mice had lower circulating levels of L-arginine and higher levels of systemic arginase activity and circulating ornithine. The decrease in L-arginine bioavailability is consistent with our data showing reductions in NO and increases malonaldehyde, superoxide and peroxynitrite formation.
The bioavailability of L-arginine and NO plays a critical role in endothelial function and, consequently, in cardiovascular disease.38 Our analysis of WT HFHS mice showed increases in circulating L-ornithine and decreases in L-arginine and L-citrulline [i.e. global arginine bioavailability ratio (GABR)], a marker inversely correlated with risk of cardiovascular mortality.41 This result shows that these mice had much lower available systemic L-arginine compared to normal diet control mice. ABH treatment normalized GABR, consistent with a reduction in systemic arginase activity. Similar reductions in plasma L-arginine and the GABR have been reported in obese Zucker rats, and also were normalized with arginase inhibitor treatment.24 Endothelial production of NO has been shown to depend on plasma or extracellular levels of L-arginine and its concurrent transport into EC (not intracellular levels).55–57 The intracellular concentrations of L-arginine are well above the Km for arginase.11 The glycocalyx of EC is rich in negatively charged sialic acid residues which can bind cationic molecules like L-arginine and provide a repository of L-arginine on the EC surface. Impaired cellular uptake of L-arginine by the CAT-1 transporter can limit the availability of L-arginine to NOS and may occur either through reduced expression of CAT-157 or via competitors for CAT-1 transport, such as L-ornithine and L-lysine, both of which have affinity similar to L-arginine for the transporter.58 We cannot discount elevated circulating L-ornithine levels, observed in our study, as a factor in the HFHS-induced endothelial dysfunction.
In addition to adverse effects of arginase on endothelial function, elevated production of L-ornithine may contribute to other adverse effects in the vessels of WT HFHS fed mice. L-ornithine can be metabolized into polyamines via ornithine decarboxylase and proline via ornithine aminotransferase. Polyamines play an integral role in cellular mitogenesis, whereas proline is a key precursor for collagen synthesis. Thus, increased arginase expression/activity can potentially stimulate pro-collagen and pro-proliferative pathways in cells of the vascular wall.10,48,59 Indeed, aorta from HFHS fed mice exhibited elevated levels of vascular collagen deposition/fibrosis, and deletion of EC arginase 1 or treatment with ABH blocked this effect. Increased vascular thickening, collagen deposition and fibrosis are major contributors to arterial stiffening,60 an independent cardiovascular risk factor. A previous study using mice fed a similar HFHS diet also observed an increase in arterial stiffness.25 In our study, the increase in arterial stiffness in WT HFHS mice was prevented in mice lacking EC arginase 1 and in mice treated with ABH. These data indicate that HFHS-induced arterial stiffening is mediated by arginase 1 expression in ECs. Our data showing that HFHS diet-induced increases in picrosirius red staining of perivascular collagen were abrogated by EC arginase 1 deletion or ABH treatment suggest a mechanism involving increased collagen production via the ornithine/proline pathway. Further study is needed to evaluate this possibility.
Elevation of blood pressure by the HFHS diet was also prevented by deletion of EC arginase 1 or arginase inhibition. This maintenance of control levels of blood pressure observed in EC-A1−/− mice or ABH treatment was associated with normalization of EC-dependent vasorelaxation. Increased vascular tone/blood pressure may also contribute to vascular stiffening61 and may have been involved in the HFHS-induced elevation of aortic stiffness. However, some anti-hypertensive measures do not reduce arterial stiffness.62,63 On the other hand, those treatments that also reduce inflammation and ROS, both elevated in our HFHS model, have been shown to reduce or prevent arterial stiffening.64,65
Our current study indicates that upregulation of arginase 1 within the vascular endothelium is centrally involved in HFHS diet-induced vasculopathies. We propose that elevated arginase activity could, through reduced L-arginine levels, lead to NOS uncoupling, reduced NO and enhanced superoxide production.66 Superoxide rapidly combines with NO to form the potent oxidant species, peroxynitrite (ONOO−, elevated in our model), which could further uncouple NOS via oxidation of BH4, a key co-factor.67 ONOO− can cause oxidative injury and cell death.68 Moreover, increased superoxide/ONOO− levels elevate arginase activity/expression through a feed-forward mechanism via RhoA/Rho kinase.13,20 Our study shows that HFHS diet markedly increased levels of plasma lipid peroxides and vascular oxidative species and that complete deletion of EC arginase 1 or treatment with ABH abrogates this effect. Moreover, it suggests that limiting arginase activity not only decreases ROS generation but also prevents feed forward upregulation of arginase by ROS. NOX2 and NOX4 which are activated in diabetes and obesity also are generators of ROS and could amplify this cycle.69
Our study did not address molecular mechanisms responsible for the elevation of arginase activity in this diet induced obesity model. Recent studies of obesity suggest that expansion of adipose tissue, vascular rarefaction, hypoxia, and release of inflammatory cytokines and ROS are involved in the vascular pathology (unpublished data).54,70 Hypoxia has been reported to increase arginase 2 expression in bovine and human EC.71,72 Inflammatory cytokines/ROS are largely associated with elevation of arginase 1.9,73,74 However, while the systemic inflammation and metabolic changes associated with HFHS diet feeding are very likely to play a significant role in causing increases in arginase expression/activity and vascular dysfunction, our ex vivo studies indicate that exposure of the endothelium to the high fat/high glucose milieu also has a fundamental role in the dysfunction.
Other important questions remain. (i) The impact of adipose tissue (perivascular or other) and its inflammatory mediators on arginase activity. (ii) Effects of elevated arginase activity and reduced eNOS function on insulin resistance. (iii) Gender differences in the effects of HFHS diet on arginase activity and vascular dysfunctions—we have not yet examined females. (iv) Specific sources of ROS formation in the vascular tissues—while SOD treatment partially blocked the increase in DHE fluorescence in our study, DHE can also be oxidized by other ROS.75 Further studies are required to address these important issues.
In summary, we demonstrate for the first time that high fat/high sucrose diet induces cardiovascular dysfunctions including impaired endothelial-dependent vasorelaxation, arterial fibrosis and stiffening, and increased blood pressure by a mechanism involving the expression of arginase 1 in vascular endothelial cells. Increased arginase expression/activity, oxidative stress and reduced L-arginine, and NO levels contribute to this vascular disease state.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Supplementary Material
Funding
Funding was provided by the National Institute of Health grants: R01 HL070215 (R.W.C.), R01 EY01176 (R.B.C. and R.W.C., and R24 DK-94765 (R.W.C., R.B.C., and R.L.). The work was also supported by the American Diabetes Association grant 1-16-IBS-196 (RL), Veterans Administration Merit Review Award I01BX003221 (RBC) and National Science Foundation for Distinguished Young Scholars of China: 31600937 (LY) .
References
- 1. Galassi A, Reynolds K, He J.. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med 2006;119:812–819. [DOI] [PubMed] [Google Scholar]
- 2. Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffe-Scrive M.. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism 1995;44:645–651. [DOI] [PubMed] [Google Scholar]
- 3. Kim TN, Kim S, Yang SJ, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM.. Vascular inflammation in patients with impaired glucose tolerance and type 2 diabetes: analysis with 18F-fluorodeoxyglucose positron emission tomography. Circ Cardiovasc Imaging 2010;3:142–148. [DOI] [PubMed] [Google Scholar]
- 4. Qin Z, Hou X, Weisbrod RM, Seta F, Cohen RA, Tong X.. Nox2 mediates high fat high sucrose diet-induced nitric oxide dysfunction and inflammation in aortic smooth muscle cells. J Mol Cell Cardiol 2014;72:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jung C, Gonon AT, Sjoquist PO, Lundberg JO, Pernow J.. Arginase inhibition mediates cardioprotection during ischaemia-reperfusion. Cardiovasc Res 2010;85:147–154. [DOI] [PubMed] [Google Scholar]
- 6. Romero MJ, Iddings JA, Platt DH, Ali MI, Cederbaum SD, Stepp DW, Caldwell RB, Caldwell RW.. Diabetes-induced vascular dysfunction involves arginase I. Am J Physiol Heart Circ Physiol 2012;302:H159–H166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW.. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res 2008;102:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beleznai T, Feher A, Spielvogel D, Lansman SL, Bagi Z.. Arginase 1 contributes to diminished coronary arteriolar dilation in patients with diabetes. Am J Physiol Heart Circ Physiol 2011;300:H777–H783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shemyakin A, Kovamees O, Rafnsson A, Bohm F, Svenarud P, Settergren M, Jung C, Pernow J.. Arginase inhibition improves endothelial function in patients with coronary artery disease and type 2 diabetes mellitus. Circulation 2012;126:2943–2950. [DOI] [PubMed] [Google Scholar]
- 10. Peyton KJ, Ensenat D, Azam MA, Keswani AN, Kannan S, Liu XM, Wang H, Tulis DA, Durante W.. Arginase promotes neointima formation in rat injured carotid arteries. Arterioscler Thromb Vasc Biol 2009;29:488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caldwell RB, Toque HA, Narayanan SP, Caldwell RW.. Arginase: an old enzyme with new tricks. Trends Pharmacol Sci 2015;36:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pandey D, Bhunia A, Oh YJ, Chang F, Bergman Y, Kim JH, Serbo J, Boronina TN, Cole RN, Van Eyk J, Remaley AT, Berkowitz DE, Romer LH.. OxLDL triggers retrograde translocation of arginase2 in aortic endothelial cells via ROCK and mitochondrial processing peptidase. Circ Res 2014;115:450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chandra S, Romero MJ, Shatanawi A, Alkilany AM, Caldwell RB, Caldwell RW.. Oxidative species increase arginase activity in endothelial cells through the RhoA/Rho kinase pathway. Br J Pharmacol 2012;165:506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morris SM., Jr. Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharmacol 2009;157:922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lange PS, Langley B, Lu P, Ratan RR.. Novel roles for arginase in cell survival, regeneration, and translation in the central nervous system. J Nutr 2004;134:2812S–2817S. [DOI] [PubMed] [Google Scholar]
- 16. Li H, Meininger CJ, Hawker JR Jr, Haynes TE, Kepka-Lenhart D, Mistry SK, Morris SM Jr, Wu G.. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am J Physiol Endocrinol Metab 2001;280:E75–E82. [DOI] [PubMed] [Google Scholar]
- 17. Llaurado G, Ceperuelo-Mallafre V, Vilardell C, Simo R, Freixenet N, Vendrell J, Gonzalez-Clemente JM.. Arterial stiffness is increased in patients with type 1 diabetes without cardiovascular disease: a potential role of low-grade inflammation. Diabetes Care 2012;35:1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, Hare JM.. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation 2003;108:2000–2006. [DOI] [PubMed] [Google Scholar]
- 19. Toque HA, Nunes KP, Yao L, Xu Z, Kondrikov D, Su Y, Webb RC, Caldwell RB, Caldwell RW, Emanueli C.. Akita spontaneously type 1 diabetic mice exhibit elevated vascular arginase and impaired vascular endothelial and nitrergic function. PLoS One 2013;8:e72277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shatanawi A, Romero MJ, Iddings JA, Chandra S, Umapathy NS, Verin AD, Caldwell RB, Caldwell RW.. Angiotensin II-induced vascular endothelial dysfunction through RhoA/Rho Kinase/p38 mitogen-activated protein kinase/arginase pathway. Am J Physiol Cell Physiol 2011;300:1181–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pernow J, Jung C.. Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal?. Cardiovasc Res 2013;98:334–343. [DOI] [PubMed] [Google Scholar]
- 22. Wang XP, Chen YG, Qin WD, Zhang W, Wei SJ, Wang J, Liu FQ, Gong L, An FS, Zhang Y, Chen ZY, Zhang MX.. Arginase I attenuates inflammatory cytokine secretion induced by lipopolysaccharide in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 2011;31:1853–1860. [DOI] [PubMed] [Google Scholar]
- 23. Zhang W, Baban B, Rojas M, Tofigh S, Virmani SK, Patel C, Behzadian MA, Romero MJ, Caldwell RW, Caldwell RB.. Arginase activity mediates retinal inflammation in endotoxin-induced uveitis. Am J Pathol 2009;175:891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson FK, Peyton KJ, Liu XM, Azam MA, Shebib AR, Johnson RA, Durante W.. Arginase promotes endothelial dysfunction and hypertension in obese rats. Obesity (Silver Spring) 2015;23:383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, Seta F.. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension 2013;62:1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steppan J, Nyhan D, Berkowitz DE.. Development of novel arginase inhibitors for therapy of endothelial dysfunction. Front Immunol 2013;4:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Di Costanzo L, Sabio G, Mora A, Rodriguez PC, Ochoa AC, Centeno F, Christianson DW.. Crystal structure of human arginase I at 1.29-A resolution and exploration of inhibition in the immune response. Proc Natl Acad Sci U S A 2005;102:13058–13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bhatta A, Yao L, Toque HA, Shatanawi A, Xu Z, Caldwell RB, Caldwell RW.. Angiotensin II-induced arterial thickening, fibrosis and stiffening involves elevated arginase function. PLoS One 2015;10:e0121727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mihara M, Uchiyama M.. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 1978;86:271–278. [DOI] [PubMed] [Google Scholar]
- 30. Tang WH, Wang Z, Cho L, Brennan DM, Hazen SL.. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J Am Coll Cardiol 2009;53:2061–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pera T, Zuidhof AB, Smit M, Menzen MH, Klein T, Flik G, Zaagsma J, Meurs H, Maarsingh H.. Arginase inhibition prevents inflammation and remodeling in a guinea pig model of chronic obstructive pulmonary disease. J Pharmacol Exp Ther 2014;349:229–238. [DOI] [PubMed] [Google Scholar]
- 32. George TJ, Arnaoutakis GJ, Beaty CA, Jandu SK, Santhanam L, Berkowitz DE, Shah ASA.. Physiologic and biochemical profile of clinically rejected lungs on a normothermic ex vivo lung perfusion platform. J Surg Res 2013;183:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Elms SC, Toque HA, Rojas M, Xu Z, Caldwell RW, Caldwell RB.. The role of arginase I in diabetes-induced retinal vascular dysfunction in mouse and rat models of diabetes. Diabetologia 2013;56:654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. White AR, Ryoo S, Li DC, Champion HC, Steppan J, Wang DM, Nyhan D, Shoukas AA, Hare JM, Berkowitz DE.. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension 2006;47:245–251. [DOI] [PubMed] [Google Scholar]
- 35. Zhao L, Guo X, Wang O, Zhang H, Wang Y, Zhou F, Liu J, Ji B.. Fructose and glucose combined with free fatty acids induce metabolic disorders in HepG2 cell: a new model to study the impacts of high-fructose/sucrose and high-fat diets in vitro. Mol Nutr Food Res 2016;60:909–921. [DOI] [PubMed] [Google Scholar]
- 36. Mika A, Sledzinski T.. Alterations of specific lipid groups in serum of obese humans: a review. Obes Rev 2017;18:247–272. [DOI] [PubMed] [Google Scholar]
- 37. Sutton-Tyrrell K, Newman A, Simonsick EM, Havlik R, Pahor M, Lakatta E, Spurgeon H, Vaitkevicius P.. Aortic stiffness is associated with visceral adiposity in older adults enrolled in the study of health, aging, and body composition. Hypertension 2001;38:429–433. [DOI] [PubMed] [Google Scholar]
- 38. Davignon J, Ganz P.. Role of endothelial dysfunction in atherosclerosis. Circulation 2004;109:III27–III32. [DOI] [PubMed] [Google Scholar]
- 39. Erdely A, Kepka-Lenhart D, Salmen-Muniz R, Chapman R, Hulderman T, Kashon M, Simeonova PP, Morris SM, Deli MA Jr.. Arginase activities and global arginine bioavailability in wild-type and ApoE-deficient mice: responses to high fat and high cholesterol diets. PLoS One 2010;5:e15253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tripolt NJ, Meinitzer A, Eder M, Wascher TC, Pieber TR, Sourij H.. Multifactorial risk factor intervention in patients with type 2 diabetes improves arginine bioavailability ratios. Diabet Med 2012;29:e365–e368. [DOI] [PubMed] [Google Scholar]
- 41. Sourij H, Meinitzer A, Pilz S, Grammer TB, Winkelmann BR, Boehm BO, Marz W.. Arginine bioavailability ratios are associated with cardiovascular mortality in patients referred to coronary angiography. Atherosclerosis 2011;218:220–225. [DOI] [PubMed] [Google Scholar]
- 42. Horowitz S, Binion DG, Nelson VM, Kanaa Y, Javadi P, Lazarova Z, Andrekopoulos C, Kalyanaraman B, Otterson MF, Rafiee P.. Increased arginase activity and endothelial dysfunction in human inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 2007;292:G1323–G1336. [DOI] [PubMed] [Google Scholar]
- 43. Tawfik HE, El-Remessy AB, Matragoon S, Ma G, Caldwell RB, Caldwell RW.. Simvastatin improves diabetes-induced coronary endothelial dysfunction. J Pharmacol Exp Ther 2006;319:386–395. [DOI] [PubMed] [Google Scholar]
- 44. Coutinho T, Goel K, Correa de Sa D, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, Torp-Pedersen C, Cottin Y, Lorgis L, Lee SH, Kim YJ, Thomas R, Roger VL, Somers VK, Lopez-Jimenez F.. Central obesity and survival in subjects with coronary artery disease: a systematic review of the literature and collaborative analysis with individual subject data. J Am Coll Cardiol 2011;57:1877–1886. [DOI] [PubMed] [Google Scholar]
- 45. Yao L, Chandra S, Toque HA, Bhatta A, Rojas M, Caldwell RB, Caldwell RW.. Prevention of diabetes-induced arginase activation and vascular dysfunction by Rho kinase (ROCK) knockout. Cardiovasc Res 2013;97:509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sankaralingam S, Xu H, Davidge ST.. Arginase contributes to endothelial cell oxidative stress in response to plasma from women with preeclampsia. Cardiovasc Res 2010;85:194–203. [DOI] [PubMed] [Google Scholar]
- 47. Yu Y, Rajapakse AG, Montani JP, Yang Z, Ming XF.. p38 mitogen-activated protein kinase is involved in arginase-II-mediated eNOS-uncoupling in obesity. Cardiovasc Diabetol 2014;13:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, Lim HK, Sohi J, Santhanam L, Soucy K, Tuday E, Baraban E, Ilies M, Gerstenblith G, Nyhan D, Shoukas A, Christianson DW, Alp NJ, Champion HC, Huso D, Berkowitz DE.. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circulation Research 2008;102:923–932. [DOI] [PubMed] [Google Scholar]
- 49. Ming XF, Barandier C, Viswambharan H, Kwak BR, Mach F, Mazzolai L, Hayoz D, Ruffieux J, Rusconi S, Montani JP, Yang Z.. Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway: implications for atherosclerotic endothelial dysfunction. Circulation 2004;110:3708–3714. [DOI] [PubMed] [Google Scholar]
- 50. Ming XF, Rajapakse AG, Yepuri G, Xiong Y, Carvas JM, Ruffieux J, Scerri I, Wu Z, Popp K, Li J, Sartori C, Scherrer U, Kwak BR, Montani JP, Yang Z.. Arginase II promotes macrophage inflammatory responses through mitochondrial reactive oxygen species, contributing to insulin resistance and atherogenesis. J Am Heart Assoc 2012;1:e000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Morris SM., Jr. Arginases and arginine deficiency syndromes. Curr Opin Clin Nutr Metab Care 2012;15:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jung C, Figulla HR, Lichtenauer M, Franz M, Pernow J.. Increased levels of circulating arginase I in overweight compared to normal weight adolescents. Pediatr Diabetes 2014;15:51–56. [DOI] [PubMed] [Google Scholar]
- 53. Xu XD, Hu J, Wang M, Peng F, Tian R, Guo XJ, Xie Y, Qin RY.. Circulating myeloid-derived suppressor cells in patients with pancreatic cancer. Hepatobiliary Pancreat Dis Int 2016;15:99–105. [DOI] [PubMed] [Google Scholar]
- 54. Hu H, Moon J, Chung JH, Kim OY, Yu R, Shin MJ.. Arginase inhibition ameliorates adipose tissue inflammation in mice with diet-induced obesity. Biochem Biophys Res Commun 2015;464:840–847. [DOI] [PubMed] [Google Scholar]
- 55. Shin S, Mohan S, Fung HL.. Intracellular L-arginine concentration does not determine NO production in endothelial cells: implications on the “L-arginine paradox”. Biochem Biophys Res Commun 2011;414:660–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zani BG, Bohlen HG.. Transport of extracellular l-arginine via cationic amino acid transporter is required during in vivo endothelial nitric oxide production. Am J Physiol Heart Circ Physiol 2005;289:H1381–13H1390. [DOI] [PubMed] [Google Scholar]
- 57. Martens CR, Kuczmarski JM, Lennon-Edwards S, Edwards DG.. Impaired L-arginine uptake but not arginase contributes to endothelial dysfunction in rats with chronic kidney disease. J Cardiovasc Pharmacol 2014;63:40–48. [DOI] [PubMed] [Google Scholar]
- 58. Hatzoglou M, Fernandez J, Yaman I, Closs E.. Regulation of cationic amino acid transport: the story of the CAT-1 transporter. Annu Rev Nutr 2004;24:377–399. [DOI] [PubMed] [Google Scholar]
- 59. Bagnost T, Ma L, da Silva RF, Rezakhaniha R, Houdayer C, Stergiopulos N, Andre C, Guillaume Y, Berthelot A, Demougeot C.. Cardiovascular effects of arginase inhibition in spontaneously hypertensive rats with fully developed hypertension. Cardiovasc Res 2010;87:569–577. [DOI] [PubMed] [Google Scholar]
- 60. Benetos A, Safar ME.. Aortic collagen, aortic stiffness, and AT1 receptors in experimental and human hypertension. Can J Physiol Pharmacol 1996;74:862–866. [PubMed] [Google Scholar]
- 61. Greenwald SE. Ageing of the conduit arteries. J Pathol 2007;211:157–172. [DOI] [PubMed] [Google Scholar]
- 62. Takami T, Shigemasa M.. Efficacy of various antihypertensive agents as evaluated by indices of vascular stiffness in elderly hypertensive patients. Hypertens Res 2003;26:609–614. [DOI] [PubMed] [Google Scholar]
- 63. Munakata M, Nagasaki A, Nunokawa T, Sakuma T, Kato H, Yoshinaga K, Toyota T.. Effects of valsartan and nifedipine coat-core on systemic arterial stiffness in hypertensive patients. Am J Hypertens 2004;17:1050–1055. [DOI] [PubMed] [Google Scholar]
- 64. Maki-Petaja KM, Hall FC, Booth AD, Wallace SM, Yasmin , Bearcroft PW, Harish S, Furlong A, McEniery CM, Brown J, Wilkinson IB.. Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy. Circulation 2006;114:1185–1192. [DOI] [PubMed] [Google Scholar]
- 65. Majeed B, Tawinwung S, Eberson LS, Secomb TW, Larmonier N, Larson DF.. Interleukin-2/anti-interleukin-2 immune complex expands regulatory T cells and reduces angiotensin II-induced aortic stiffening. Int J Hypertens 2014;2014:126365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA Jr.. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A 1998;95:9220–9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Katusic ZS, D’uscio LV, Nath KA.. Vascular protection by tetrahydrobiopterin: progress and therapeutic prospects. Trends Pharmacol Sci 2009;30:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pacher P, Beckman JS, Liaudet L.. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007;87:315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Han CY. Roles of reactive oxygen species on insulin resistance in adipose tissue. Diabetes Metab J 2016;40:272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nakamura K, Fuster JJ, Walsh K.. Adipokines: a link between obesity and cardiovascular disease. J Cardiol 2014;63:250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang L, Bhatta A, Toque HA, Rojas M, Yao L, Xu Z, Patel C, Caldwell RB, Caldwell RW.. Arginase inhibition enhances angiogenesis in endothelial cells exposed to hypoxia. Microvasc Res 2015;98:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Krotova K, Patel JM, Block ER, Zharikov S.. Hypoxic upregulation of arginase II in human lung endothelial cells. Am J Physiol Cell Physiol 2010;299:C1541–C1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang C, Wu J, Xu X, Potter BJ, Gao X.. Direct relationship between levels of TNF-alpha expression and endothelial dysfunction in reperfusion injury. Basic Res Cardiol 2010;105:453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhu M, Goetsch SC, Wang Z, Luo R, Hill JA, Schneider J, Morris SM Jr, Liu ZP.. FoxO4 promotes early inflammatory response upon myocardial infarction via endothelial Arg1. Circ Res 2015;117:967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B.. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci U S A 2005;102:5727–5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.