Abstract
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer, with high morbidity and mortality. Racial disparity in HNSCC is observed between African Americans (AAs) and whites, effecting both overall and 5-year survival, with worse prognosis for AAs. In addition to socio-economic status and demographic factors, many epidemiological studies have also identified factors including coexisting human papillomavirus (HPV) infection, primary tumor location, and a variety of somatic mutations that contribute to the prognostic incongruities in HNSCC patients among AAs and whites. Recent research also suggests HPV-induced dysregulation of tumor metabolism and immune microenvironment as the major regulators of HNSCC patient prognosis. Outcomes of several preclinical and clinical studies on targeted therapeutics warrant the need to elucidate the inherent mechanistic and population-based disparities underlying patient responses. This review systematically reports the underlying reasons for inconsistency in disease prognosis and therapy responses among HNSCC patients from different racial populations. The focus of this review is twofold: aside from discussing the causes of racial disparity, we also seek to identify the consequences of such disparity in terms of HPV infection and its associated mutational, metabolic, and immune landscapes. Considering the clinical impact of differential patient outcomes among AA and white populations, understanding the underlying cause of this disparity may pave the way for novel precision therapy for HNSCC.
Head and neck cancer squamous cell carcinoma (HNSCC) is the sixth-most common cancer worldwide, estimated to comprise approximately 3% of all cancers in the United States. In 2018, approximately 51 540 new cases are projected and 10 030 people are expected to die of oral cavity and pharynx cancer in the United States alone (https://seer.cancer.gov/). Despite considerable efforts, the 5-year overall survival (OS) rate of HNSCC patients has not improved substantially in several decades. The median age at diagnosis of the disease is approximately 63 years (1), although initial disease presentation at younger age is on the rise (2). Generally, HNSCC develops in the upper aero-digestive tract of the head and neck, which includes the oral cavity, nasal cavity, larynx, pharynx, and salivary glands. The traditional etiology of HNSCC generally involves tobacco use (either chewing or smoking) and alcohol consumption. Recent epidemiological and laboratory results, however, have implicated human papillomavirus (HPV) as a causative agent for some HNSCC types. HPV generally infects the tonsillar tissue of Waldeyer’s ring. This includes the subsites of the base of the tongue and palatine tonsillar region, both components of the oropharynx. Approximately 70% of oropharyngeal cancers (OPC) in the United States are caused by HPV infection, which generally depicts the younger population as having a very distinct prognosis compared with tobacco-and alcohol-induced OPC (3). HNSCC is endemic in Southeast Asian countries, where tobacco and beetle quid chewing is a cultural norm. In the United States, tobacco remains a main risk factor in cancers of the oral cavity, larynx, and hypopharynx, but a steady downward trend has been noted in tobacco-related HNSCC as a result of proper public health management (4). Although the incidence of tobacco-related HNSCC has been reduced, HPV-induced HNSCC cases have increased considerably in recent years. Currently, about 25% of HNSCCs identified in the United States are thought to arise independent of tobacco use (5). The presence of HPV is thus an independent risk factor for OPC, and, importantly, provides an improved therapeutic response relative to HPV-negative HNSCC (6–9).
Similar to other malignancies, including lung, colon, prostate, and breast, the incidence and mortality of HNSCC varies among different racial and ethnic groups (https://www.cdc.gov/cancer/dcpc/data/race.htm) (10–12). Successful detection and understanding of the risk factors associated with this disparity is critical for control and management of the disease and for overall better patient outcome. The African American (AA) population with HNSCC has a poorer prognostic outcome and higher mortality rate than the whites (13,14). However, there are differences in opinion regarding the cause of differential incidence and mortality rates between the two groups. Lack of adequate health insurance coverage, socio-economic status, and HPV status are considered some of the underlying factors (15). For instance, differences in mortality in the AA and white population, particularly in OPC, may in part be due to greater overall prevalence of HPV in the white population (34%) compared with AAs (4%) (15). Although inconsistencies appear in prognosis and therapy outcomes among racial groups, it is still unclear whether the disparity is based on only genetic and demographic factors, or whether there exists contrasting mutational and biological factors among the races. Accumulating evidence suggests that HPV and its associated immune-metabolic state play a major role in disease prognosis. This review focuses on the cause and consequences for disparity in HNSCC among patient groups and highlights risk factors that would implicate prevention, diagnosis, policy making, and management of the disease.
Impact of Socio-Economic Status on Differential HNSCC Incidence and Outcome
Disproportionate incidence and mortality rates are contributed by differences in access to adequate individual health care coverage and socioeconomic status (SES) (16,17). Disparity in income determines type of insurance coverage availed and thus treatment modalities available, which subsequently affects patient outcomes (14,18,19). In a study from Fox Chase Cancer Center (Philadelphia) examining the differences among Medicaid and uninsured HNSCC patients, 23% of Medicaid and 32% of uninsured patients were less likely to undergo external beam radiotherapy (RT). Similarly, 23% of uninsured patients were less likely to receive oncologic surgery, consequently causing differences in survival and stage of disease at presentation. However, Churilla et al. (20) from the National Cancer Institute’s Surveillance, Epidemiology & End Results (SEER) data (2007–2012) categorically indicated 80.1%, 15%, and 4.9% of the HNSCC patients were Medicaid, non-Medicaid, and uninsured, respectively, suggesting that other unknown risk factors, in addition to insurance coverage and access to health care, are responsible for differences in survival. It is worth mentioning that Medicaid patients were diagnosed with more advanced stage cancer compared with patients enrolled in private health care plans (21,22). It was also observed that most AA patients were lower income and less likely to have insurance coverage, which again resulted in late-stage presentation of the disease (14,23–27). Similar adjustments for stage-at-diagnosis suggests low-income patients have lower rates of survival than high-income patients (28). In a retrospective cohort study, using the state cancer registries of California and Georgia (2002–2006), data predicted that in Medicaid patients, older males and AAs were diagnosed with more advanced stages of the disease and showed high mortality. A larger number of these patients also had succumbed at 12- or 24-month follow-up than whites (29).
Income is also critical in determining individual education (18), and although there is no statistically significant (P = .30) association between low income and education, AA patients were associated with these factors respectively (income, P < .0001, education, P = .002) that may predict the survival outcome of HNSCC patients (18). The univariate and multivariate analyses of patients with high school education or less, income, and advanced age showed a statistically significant independent prediction value with disease-free survival (DFS) of the patients, suggesting its association with the disease. Degree of education can also predict the outcome of patients. For example, patients with a high school education or less had a 44% higher hazard of dying of a cancer-related cause and a 43% higher hazard of recurrence of the disease than higher income patients. With low income and less education, there is a considerable decrease in survival rates of HNSCC patients, primarily due to poor health care access and health behaviors (30). Patients with low income and less education not only have limited access to treatment options but may also have a weak immune system due to malnutrition, thereby affecting their OS (31–33). Although univariate analysis of AA patients demonstrated an association with decreased OS and cancer-specific survival, it was not statistically significant in multivariate analysis, suggesting that socio-economic status and other covariates play a role in disease outcome (18). Moreover, adjustment for socio-economic variables predicted the association between educational status and survival outcomes of HNSCC patients. This suggests that differences in diagnosis and survival outcome among AA and white HNSCC patients may be influenced by SES between the groups.
Variability in Locoregional Origin of HNSCC Tumors
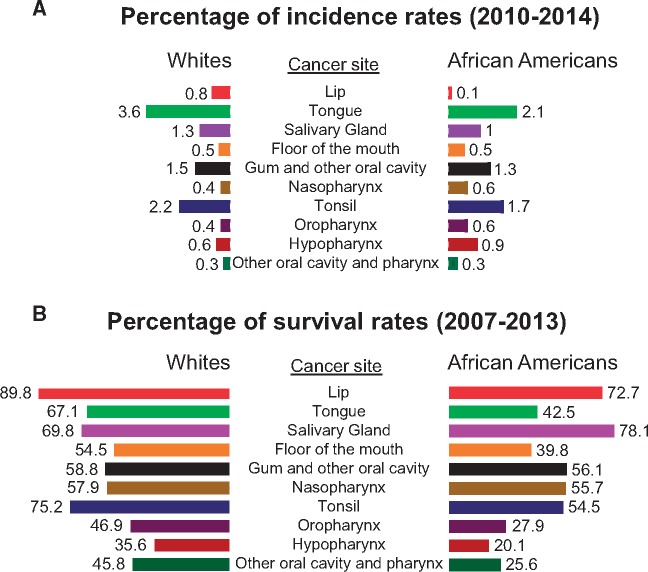
HNSCC originates in squamous cells that line the moist mucosal surfaces of oral and nasal cavity, pharynx, larynx and the salivary gland to a lesser extent. Cancer statistics based on SEER census clearly indicate the difference in cancer incidence according to the site of origin (34). SEER data (17-registry 1973–2007) have shown AAs to harbor approximately 34.3% and 10.1% of all oropharynx and hypopharynx malignancies, respectively. Oral cavity cancer incidence was higher in whites compared with AAs (33.8% vs 23.4%), whereas AAs had a higher incidence of laryngeal primary site malignancies than whites (32.1% vs 26.8%) (34). No clear difference was detected in AA and whites according to SEER incidence rates per 100 000 and age-adjusted (2010–2014) HNSCC patients except for malignancies in the subsites of the lip (0.10% vs 0.80%) and tongue (2.1% vs 3.6%), respectively (Figure 1A). Differences in relative 5-year survival rates (2007–2013) were evident in AAs compared with whites in most of the subsites of HNSCC patients (Figure 1B). However, a retrospective review of 34 182 HNSCC patients from SEER by Zimmerman et al. (35) showed a statistically significant racial survival disparity in AA patients, particularly in sites such as mouth and larynx, and was affected by stage at presentation. Racial survival disparity was also evident in almost all the anatomic sites of HNSCC, with the highest 5-year survival rate of 54.5% in mouth except malignancy in the salivary gland. One of the primary reasons for such difference in survival disparity in AA patients is due to poor tumor recognition in early diagnosis.
Figure 1.
Surveillance, Epidemiology & End Results-based cancer statistics stratified by anatomic sites between African American and white head and neck squamous cell carcinoma (HNSCC) patients. A) Incidence rates per 100 000 (2010–2014) (in percentage). B) Relative 5-year survival rates (2007–2013) (in percentage) in different subsite malignancies of African American and white head and neck squamous cell carcinoma (HNSCC) patients are presented.
Influence of Variable Somatic Mutations on HNSCC Disparity
Tobacco and/or alcohol consumption is associated with the acquisition of somatic mutations (36,37). These mutations lead to defects in metabolic enzymes/pathways of tobacco metabolism that allow accumulation of potent carcinogens to promote tumorigenesis. In AAs, clearance of inhaled tobacco metabolites (nicotine and cotinine) is less effective than in whites (38,39), suggesting the influence of individual genetics. Apart from mutation, it was also proposed that the generation of gene variants is associated with decreased nicotine metabolism in AAs (40–42).
Somatic mutations such as TP53, CDKN2A, PIK3CA, HRAS, FBXW7, and NOTCH1 genes are observed in HNSCC tumors (37,43). However, the acquisition of these mutations may vary based on ethnicity and HPV infection. A retrospective study by Wu et al. (44) included 214 patients with 46% non-Hispanic whites (NHWs), 34% Hispanic whites, and 20% AAs to study the association between somatic mutation and early death (<2 years) in HNSCC. The study indicated EGFR, PIK3CA, and TP53 were the most frequently mutated genes. About 31% Hispanic whites and 18% AA patients potentially acquired at least any three mutations, and the acquisition of at least three gene mutations is associated with high risk of early patient death. Of interest, the acquisition of these mutations also varied among ethnic groups; for example, the prevalence of EGFR, KRAS, HRAS, and TP53 mutations was higher in AAs and Hispanic whites than in NHWs.
Different somatic mutations have been identified in HNC patient samples with various degrees in different anatomical sites of the head and neck. For example, somatic mutations in CDKN2A (17% in larynx, 19% in oral cavity), MET (21% in oropharynx), EGFR (15% in tonsil), KRAS (3% in sinonasal cavity), TP53 (70%–80% oral cavity), BRAF (41% in thyroid), and HRAS (18% in salivary gland) have been detected in different HNC regions. However, the clinical relevance of these mutations in different anatomic regions is not clear (45).
A study using The Cancer Genome Atlas (TCGA) database shows the differences in cancer incidence rates among AAs vs NHWs (−1.83, P = 8.00E-10) and Asians vs NHWs (−3.21, P = 1.00, P = 1.00E-21). Although the study showed that AAs had worse survival compared with the NHW patient population (P = .038) that was suggestive of racial disparity, the study did not find any statistically significant nonsynonymous somatic mutation (driver mutation) burden in different racial subgroups of HNSCC patients (46). In contrast, similar studies conducted on the same data set by Ramakodi et al. (47) defined the presence of different mutational landscapes for AA (n = 13) and NHW patients (n = 57) with laryngeal cancer. The NHW patient population was also seen to harbor a higher mean number of mutations than AA laryngeal cancer counterparts (277.63 vs 151.31). The frequency of nucleotide mutations (C > A, C > G) was also statistically different between AA and NHWs patients. The G > T nucleotide transition, a tobacco-related mutation, is observed in lower proportions in tumors from AA population compared with NHWs. The frequencies of 44 known driver mutations also varied between the AA and NHW HNSCC patient populations (20/44 vs 29/44). However, many NHW patients had mutations in the driver gene, PIK3CA, which is not detected in AA patients, and a greater number of AA patients had mutations in RUNX1T1, KIAA1033, and ZMYM6 compared with NHW patients (47). Therefore, differences in genetic background of AA and NHW HNSCC patients, apart from other nonbiological factors, predispose the two racial groups differentially to tumor-associated mutational burdens. This may advocate for the likelihood of higher laryngeal cancer susceptibility, increased risk of disease development, and poor prognosis in the AA population (48).
HPV Predicts Differential HNSCC Prognosis
HPV is a DNA virus that infects the human keratinocytes of the skin and mucous membranes. Of 200 different strains of HPV viruses, HPV 16, a high-risk subtype, accounts for approximately 90% of all HPV-induced HNSCC (49). HPV infection can spread through sexual contact with an infected person (50). Smoking and alcohol use can further increase the incidence of HPV infection in OPC, primarily due to its local and systematic immunosuppressive action (51–54).
About 99.7% of uterine cervical cancers are caused by HPV DNA (55), with HPV 16 being the most prevalent strain in low-grade and cervical neoplasm. Due to similarities in the morphological features between cervical cancer and HPV-associated lesions in the oral cavity, HPV is hypothesized to play a role in oral and laryngeal cancer (56,57). Multiple studies have indicated HPV as an independent risk factor in OPC (58–64). Although a notable proportion of oropharyngeal and tonsillar cancers are associated with HPV, only 60% of these cancers are mediated by HPV (65). Such differences in risk factor implication between the studies can be due to a wide variation in HPV DNA detection (65).
Epidemiological studies have associated race with differences in cancer incidence and mortality (13,16). Such differences in mortality rates among AAs and whites are driven by disparate diagnosis and prognostic outcome (13,16,23,66–68). Studies from all cancer subsites in HNSCC showed a relatively low 5-year survival in AA compared with white patients (69). One of the primary reasons for high mortality and low response to chemo-radiation therapy (CRT) in AAs is due to low HPV infection rates compared with whites (15,70). The AA patients were also less susceptible to oral, pharynx, nasal, or larynx squamous cell carcinoma, the primary areas of HPV infection (70). The prevalence of HPV 16 infection is, however, higher in oropharyngeal (35.6%) compared with oral (23.5%) and laryngeal (24%) carcinomas (71). This may explain the most prominent 5-year survival difference of AAs and whites in the oropharyngeal locoregion (SEER data) in recently diagnosed cases (1995–2004), which further relates to the surge of HPV-induced HNSCC during this period (72).
Apparently, HPV DNA alone per se does not correlate with the clinical outcome of HNSCC patients (8). During HPV infection, the viral oncoprotein E7 binds to retinoblastoma protein (pRb) causing dysfunction of pRb, which in turn results in the release of the E2F transcription factor 1 from the pRb/E2F transcription factor 1 repression complex. This releases feedback inhibition of p16INK4a expression, resulting in its overexpression (73,74). Thus, HPV DNA along with high levels of p16INK4a is considered a robust prognostic marker. The multi-institutional retrospective cohort analysis of HPV-negative, HPV-inactive, and HPV-active cases demonstrated that only the HPV-active HNSCC group (p16INK4A positive) is associated with OS and DFS. Using this model, it is estimated that 29% of whites harbor an HPV-active strain compared with 0% in AA patients. Apart from differences in active HPV status, the disparity between AA and white patients was clinically significant (P = .01) when the HPV surrogate marker, p16INK4A, was considered (8). Because HPV infection and p16INK4A provide a better outcome in HNSCC patients, the disparity in OS between AA and white HNSCC patients is thought to be propelled by a low prevalence of HPV (15).
Apart from HPV infection itself, differential mutational landscapes and altered metabolic and immunologic profiles driven by viral infection may lead to the variability in prognosis and survival outcome in AA and NHW populations of HNSCC patients. Whole-exome sequencing from 74 tumor-normal pairs identified major driver genes of HNSCC carcinogenesis (NOTCH1, IRF6, and TP53) responsible for squamous differentiation. These genes were observed to harbor mutations; however, the mutation rate in HPV-positive HNSCC patients was one-half that found in HPV-negative cases (75). This observation was consistent with epidemiological differences observed between the two types (75). SES, access to proper health care, differences in sexual lifestyle preferences, host genetics, smoking, and alcohol use are established associated factors for differences in HPV prevalence rates between AAs and whites (70). Several studies have shown whites engage in oral sex more frequently than AAs (76,77), which increases the chances of HPV infection (70). However, it is still unclear whether other differences in behavioral factors (hygiene/eating habits) between the races influence the likelihood of HPV infection (14). It is observed that AAs or low SES children have enlarged tonsil and adenoids (78), which may cause sustained release of inflammatory mediators to process anti-viral components (79). Thus it is postulated that the expression of key molecules required for the viral infection of epithelial cells is genetically driven and may be potentially different between the races.
HPV Infection Induced Differential Tumor Metabolism in HNSCC
Altered metabolism is one of the hallmarks of cancer, wherein tumor cells represent a class of metabolically demanding cells (80,81). It is well established that to satisfy energy demand and anabolic building blocks, tumor cells favor aerobic glycolysis over oxidative phosphorylation (OXPHOS), even in with an abundance of oxygen, a finding commonly termed the “Warburg effect.” Like other solid tumors, most studies have identified HNSCC tumors to follow the Warburg effect. It is worth noting that most of these studies were conducted using in vitro monolayer cell cultures, which may not accurately reflect the complete scenario. Recently, studies have started to focus on delineating tumor heterogeneity in terms of cellularity, histology, and metabolic cross-talk. The tumor microenvironment comprises of a wide array of cells, including cancer-associated fibroblasts, immune cells, and proliferating and nonproliferating tumor cells. These cell types have their unique yet symbiotic relationship in the context of metabolism (82). Recent advances in the field indicate that there exists a metabolic compartmentalization inside the tumor that is also closely connected to the differential immune infiltrates. Curry et al. (82) showed in patient samples that the nonproliferating stromal cells are mitochondria poor and have a higher propensity for aerobic glycolysis and lactate release. Nonproliferating tumor cells showed a similar phenotype, with enhanced staining for Monocarboxylase Transporter 4 (MCT4), a plasma membrane transporter for lactate secretion, and a decrease in functional mitochondrial markers such as Cytochrome Oxidase (COX), Translocase of Outer Mitochondrial Membrane 20, and Monocarboxylase Transporter 1 (MCT1), a plasma membrane transporter for lactate uptake. The highly proliferating tumor cells were, however, undergoing extensive OXPHOS and could utilize lactate as a secondary energy source for mitochondrial respiration. The authors also commented that MCT4 expression increases with disease stage and inversely correlates to patient survival. This study also indicated a metabolic symbiosis in concurrence with spatial organization of cells inside the tumor and that the highly proliferating cancer cells along the tumor edge induce the deeply seated nonproliferation cancer cell population to undergo aerobic glycolysis and expel lactate, which in turn is used by the stem cell-like proliferating population. This might explain the correlation between enhanced aggressiveness and MCT4 expression. In another recent study, MCT4 and GLUT1 expression was found to follow an increasing trend in areas closer to hypoxic domains (83). In corroboration with previous studies, MCT4 expression was established as a potential marker for tumor stage and aggressiveness.
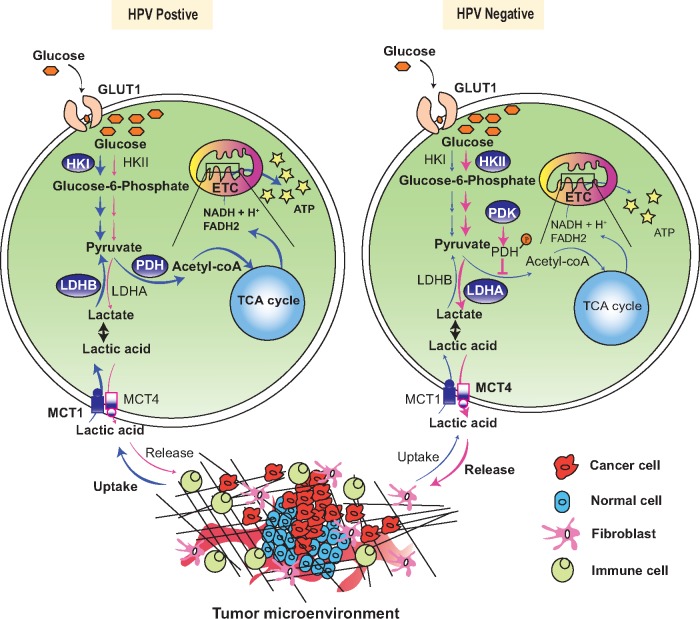
In quest of understanding the reasons behind the differential outcome, recurrence, and response to RT amongst HPV-positive and HPV-negative HNSCC patients, studies have been redirected towards dissecting the metabolic phenotype within these two different tumors. Using cell lines and patient tumors specimens, Krupar et al. (84) found predominance for glucose utilization (GLUT1, plasma membrane glucose transporter) and mitochondrial respiration (LDHB, MCT1, COX5B) in the HPV-positive compared with HPV-negative cases. Depending on the subunit composition of lactate dehydrogenase (LDH), it either converts pyruvate to lactate (LDHA) or lactate to pyruvate (LDHB) (85). Besides, they noticed a differential intra-tumoral distribution of GLUT1 and COX5B in these two classes of tumors; whereas HPV-positive tumors showed centrally localized staining for these markers, HPV-negative tumors had a more peripheral staining along with a higher concentration of lactic acid inside the tumor. Figure 2 explains the altered metabolic pathways in HPV-positive and -negative tumors. This distinct staining pattern correlated with an intra-tumoral abundance of cytotoxic CD8+ T cell infiltration (84).
Figure 2.
Alterations in metabolic pathways in human papillomavirus (HPV)-positive and HPV-negative head and neck squamous cell carcinoma tumors. Glucose enters the tumor cells by Glucose Transporter 1 (GLUT1). Hexokinases I or II (HKI or HKII) convert glucose to glucose-6-phosphate. Pyruvate formed in the subsequent steps of glycolysis is either used for lactate generation in the cytosol or is shuttled inside the mitochondria where it is converted to acetyl-CoA by the multi-enzyme complex pyruvate dehydrogenase (PDH). Acetyl-CoA is then fed into the tricarboxylic acid (TCA) cycle in the mitochondrial matrix. Subsequent steps in the TCA cycle generate high-energy currencies nicotinamide adenine dinucleotide (NADH) and Flavin Adenine Dinucleotide (FADH2), which are harvested in the electron transport chain (ETC) to generate adenosine triphosphate (ATP). HPV-positive tumors: HPV positive tumors are observed to express higher levels of HK I and PDH, thereby ensuring these tumor cells undergo pronounced oxidative phosphorylation (OXPHOS). Besides, higher expression of Monocarboxylate Transporter (MCT1) and Lactate Dehydrogenase B (LDHB) in these tumors helps in the sequestration of lactic acid from the tumor microenvironment and conversion of the same to pyruvate respectively. This pyruvate is utilized in the TCA cycle followed by OXPHOS, leading to higher generation of ATP molecules. HPV-negative tumors: HPV-negative tumors have higher expression of HK II and Pyruvate Dehydrogenase Kinase (PDK). PDK phosphorylates and inactivates PDH, thereby inhibiting the formation of acetyl-CoA. Pyruvate is shuttled towards lactate generation by Lactate Dehydrogenase A (LDHA). A part of the lactate is subsequently expelled to the microenvironment via MCT4. Presence of lactate in the environment results in enhanced tumor aggressiveness (details in text). Blue circles indicate molecules with higher expression and bold arrows indicate more prominent pathways in each of the tumor type.
Although oxidative, radiosensitive, and immunogenic phenotypes of HPV-positive tumors were established in the studies above, contradictory findings exist. The E6 oncoprotein from HPV can activate the mammalian target of rapamycin, which can in turn lead to accumulation of Hypoxia Induced Factor-1α (HIF1α) and its downstream enzymes, including those involved in anaerobic glycolysis leading to enhanced lactate production even in the presence of abundant oxygen (86). Extensive epidemiological evidence exists in support of higher incidences of HPV infection in whites compared with AAs; however, no intricate mechanistic studies have been conducted to elucidate the virus-driven metabolic disparities of HNSCC patients in either of these two racial groups.
One major difference in viral-induced vs tobacco-/alcohol-induced HNSCC tumors is their differential sensitivity to radiation. HPV-negative patients are much more tolerant to radiation and have a higher chance of locoregional recurrence compared with the HPV-positive patients (87). In a recent study by Jung et al. (88), HPV-negative cells were observed to be more glycolytic, whereas HPV-positive cells had better propensity for OXPHOS. Mechanistically, the authors claimed that upon radiation, HIF1α expression is elevated more in the HPV-negative cells leading to elevated expression of its downstream targets like Hexokinase II (HKII), carbonic anhydrase, and Pyruvate Dehydrogenase Kinases (PDKs). On the other hand, HPV-positive patients were able to endure mitochondrial respiration, which could be corroborated by their higher COX/HKII ratio. Interestingly, specific inhibition of PDK led to sensitization of HPV-negative cells to radiation.
There remain doubts regarding the spatial stratification of metabolic pathways inside the tumor and variabilities in the presence and absence of HPV infection. More comprehensive studies with animal models and patient specimens may bolster findings from cell line models. Stratifying patients on the basis of racial disparity, more specifically their inherent susceptibility to HPV infection and its associated metabolic phenotype, might be beneficial in terms of therapy outcome expectancy and improved patient survival.
HPV Infection Induced Altered Immune Landscape in HNSCC
HNSCC neoplasm development occurs in a very complex and dynamic microenvironment comprising epithelial, endothelial, and immune cells, fibroblasts, and soluble factors such as cytokines, chemokines, and various intermediary metabolites (89). Immune dysfunction together with a tumor-promoting microenvironment has been known to influence tumor development and progression (90). Recent genomic studies using transcriptomic sequencing, data integration, and molecular networking have allowed us to revisit and discover the underlying molecular mechanisms of the progression of various cancers. These genomic studies are not limited to mutational and transcriptomic details that predict prognostic information but also to immune landscapes that will to some extent envisage patient-specific responses to immunotherapy. Comprehensive meta-analysis of TCGA data suggests that HNSCC is one of the most immunologically active tumors, next to lung adenocarcinoma and renal cell carcinoma (91). Single-sample gene set enrichment analysis revealed HNSCC tumors had elevated levels of immune activation measured by granzymes, perforin, and interferon gamma (IFN-γ) signaling enrichment (91). Evidence also suggests that the immune cell composition of the tumor microenvironment is a key determinant of overall HNSCC patient outcomes (92–94).
As mentioned, AAs are often diagnosed with an aggressive advanced HNSCC tumor at presentation, more so than whites, and although the exact mechanism for this fact is still elusive, epidemiological and association studies indicate that the two races differ in their HPV infection rate and mutational landscape. The varied mutational burden and the HPV association may account for the contrasting immune phenotype in the tumor microenvironment, and this may finally lead to a differential prognosis and therapy outcome in this patient population. HPV-positive tumors are commonly observed in the palatine and lingual tonsillar region of head and neck. The palatine tonsils, comprised of nonkeratinized epithelium with incomplete basal lamina, make a fertile ground for immune-mediated infiltration, with cells such as lymphocytes and macrophages on the surface (95). Due to the intensive lymphatic tissue and its proximity to the tumor area, HPV proteins can induce an adaptive immune response directed against viral antigens. HPV-positive HNSCC has a high level of tumor infiltrating lymphocytes, regulatory T cells (Tregs), and PD1+ T cells, all of which are generally associated with a better clinical outcome (84,96–98). One prospective study revealed that RT alone or concurrent CRT had a statistically significant locoregional effect (distant metastasis) in HPV-positive than -negative patients (99). A similar trend of decreased locoregional control and lower OS (35% vs 75% in HPV-positive) is observed in HPV-negative anal cancer, which has frequent TP53 mutations (100). Recent reports suggest that mutant TP53 can fuel pro-inflammatory pathways, ultimately aggravating tumor progression (101,102). It is also known that the tumor suppressor TP53 can regulate immune infiltration by regulating the NFκB pathway (103). This might explain the low immune infiltration in HPV-negative tumors that generally carry mutant TP53.
Given the volume of lymphoid tissue in the oropharyngeal region and the high percentage of HPV infection in oropharyngeal tumors compared with other subsites, numerous studies have shown a strong correlation between the abundance of tumor infiltrating lymphocyte (TIL), HPV status, and improved survival in HNSCC (97,104–107). HPV oncoproteins such as E6/E7 can function as tumor-associated antigens for antigen presentation capability of dendritic cells for the activation of CD8+ cytotoxic T lymphocytes (108). The premise is supported by the clinical observation that the presence of HPV16-specific T cell and E7-specific circulating T lymphocytes in HPV-positive OPC patients (109,110). The presence of an increased number of TILs has been correlated with favorable DFS under resectable conditions and with improved OS (111,112). Moreover, correlation of CD8+ T cell infiltration and improved prognosis has been observed both in HPV-positive (97,104–106,113,114) and HPV-negative OPC (114). In addition, HPV E7-specific CD8+ T cell response was observed in the peripheral blood of HPV-positive patients; this response correlated with survival outcome (115). Overall, the presence of cytotoxic T lymphocyte infiltration and an abundance of IFN-γ have been shown to be associated with favorable outcomes in HPV-positive HNSCC (84,116). The active immunologic landscape in HPV-positive tumors also leads to increased CRT response (117). All these studies suggest that HPV infection activates the immune response and that patients with more tumor immune-infiltrates have better anti-tumor response (118,119). A similar phenotype of higher TILs with better OS has been observed in multiple cancers (120–125). Multicolor surface staining of blood from HNSCC patients suggests the presence of a higher percentage of CD4+CD25+ T cells and Tregs in the peripheral blood of patients compared with healthy controls (126,127). A recent study shows that HPV-specific TILs predominantly produced IFN-γ and IL17, suggesting an HPV-specific T-cell response that ultimately supports favorable OS with small tumor size and low metastasis (128). Further, HPV16-specific CD4 and CD8 T-cells were observed more frequently in the peripheral blood of HPV-positive HNSCC patients (63.6% vs 24.1% in HPV-negative) (109). In support of this notion, in a retrospective analysis of 140 patients, it was shown that HPV‐associated OPC tumors have higher numbers of CD56+ NK cells in the tumor milieu compared with HPV-negative counterparts (91,129). Further, the presence of granzyme B within CD56+ cells on the same patients suggests a higher accumulation of cytotoxic NK cells in HPV‐associated OPC (129) that correlates to improved survival (91,129). Investigation of the tumor-immune profile of HNSCC tissues is crucial to identify responders vs nonresponders for immunotherapy (92–94).
Immune cells, being one of the most functionally plastic cells of the body, can respond to the local environment and undergo variations in their differentiation state, gene expression pattern, and effector functions. Thus, a major part of the current clinical research is focused on understanding tumor metabolism as a determinant of the local immune milieu. Highly proliferating tumor cells and the tumor promoting cells in their neighborhood like cancer-associated fibroblasts and endothelial cells compete for glucose with the immune cells. Glucose deprivation not only restricts the cytotoxicity, motility, and IFN-γ production from the TILs but also abrogates proinflammatory functions of the macrophages (130). Moreover, lactate released from the tumor cells due to enhanced glucose utilization in aerobic glycolysis drives a wide variety of immunosuppressive roles including functional quiescence of the intratumoral CD8 cytotoxic T cells and decreased cytokine release from the dendritic cells. Lactate also serves as a reactive oxygen species (ROS) scavenger. ROS being a critical mediator of CRT efficiency, accumulation of lactate in the tumor microenvironment suppresses CRT response of the patients (131). Hypoxic areas in solid tumors upregulate HIF1α that further enhances tumor cell glucose utilization and lactate release. Thus, oxygen supplementation and treatment with metformin decreased intratumoral hypoxia followed by attenuation of immune evasion (132). A recent study by Krupar et al. (133) bridged the unexplored mechanistic link between intratumoral immunometabolic features in HNSCC patients, their CRT response, and the corresponding survival. In this study, it was observed that high mitochondrial COX5B and low glycolytic GLUT1 correspond to high intratumoral CD8/CD4 ratio, leading to better CRT response and improved short- and long-term survival in HNSCC patients. However, a previous study (84) by the same group showed positive correlation between anti-tumor CD8/CD4 ratio and expression of GLUT1 and COX5B in HPV-positive tumors. This inconsistency in the correlation between GLUT1 prevalence and antitumor CD8/CD4 ratio in the two findings may be explained by the biased patient selection towards a low number of HPV-positive cases in the current study. Further, the authors also observed an improved anti-tumor immune cell chemotaxis towards the HNSCC tumor spheroids upon ROS accumulation following simultaneous administration of radiation and glucose inhibitor. This phenomenon was reversed in the presence of N-acetylcysteine, a known ROS scavenger, thereby indicating that ROS is a critical intermediate between tumor metabolism and the immune microenvironment (133). Thus, an elaborate stratification of HNSCC patients based on the immunometabolic phenotype is a prerequisite to ensure a successful clinical outcome from CRT.
Conclusion and Future Prospectus
The estimated number of HNSCC-related deaths worldwide accounts for more than 0.3 million and 13 000 deaths in the United States. Research in HNSCC disparity has established that differences in racial groups are primarily driven by SES and HPV. As a result of difference in SES among minorities, there is less access to health care, less likelihood of insurance coverage, and a greater probability of a more advanced stage of disease at presentation. HNSCC has long been considered as a homogeneous disease in the context of smoking- and alcohol-induced carcinogenesis, histopathology, locoregional progression, and recurrence after treatment (134). However, anatomical heterogeneity that arises from the oral cavity, pharynx, and larynx also exists (135). Identification of HPV 16 as a risk factor in the oropharynx of head and neck region has added to heterogeneity of the disease. Numerous studies have detected a poorer OS for AAs compared with whites in OPCs, and prognosis depends on low prevalence of HPV among AA patients. To date, the overwhelming evidence in the literature has portrayed the disparity in AA and white HNSCC to be mostly an SES or lifestyle issue; however, detection of variable degrees of somatic mutations in different HNSCC races that potentially drives the prognosis of the disease counters such presumption. Furthermore, variations in predominant somatic mutations and metabolic and immune characteristics associated with HPV infection contribute to the prognosis of the disease in the two racial groups.
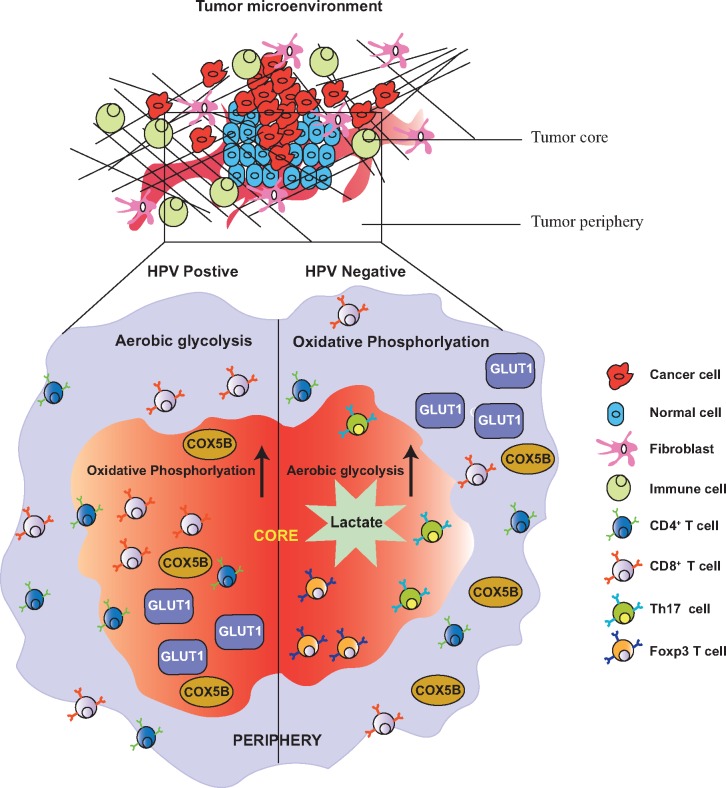
HPV-negative tumors are also speculated to harbor more somatic mutations and are more immunologically cold. The variability in metabolic compartmentalization inside the tumor is partially responsible for such differential immunologic phenotype. HPV-positive tumors are observed to undergo more OXPHOS in the tumor core and higher rates of aerobic glycolysis at the periphery. At the same time, HPV-negative tumors undergo extensive aerobic glycolysis with excessive lactate deposition in the tumor core (84). This may explain the decrease in cytotoxic tumor-infiltrating T cells and the increase in immunosuppressive Tregs inside the tumors of HPV-negative patients, demonstrating that there might exist immune-metabolic crosstalk inside the HPV-positive and negative tumors (Figure 3). Thus, HPV can be one of the major factors in the disparity seen among HNSCC patients of different racial groups, given that the prevalence of viral infection is lower in AAs, and this in turn contributes to poor survival either by itself or via its associated factors (70,136).
Figure 3.
Differential immune-metabolic landscape in the human papillomavirus (HPV)-positive and HPV-negative head and neck squamous cell carcinoma tumors. HPV-positive and negative tumors demonstrate varied spatial organization of the enzymes involved in aerobic glycolysis and oxidative phosphorylation (OXPHOS). The immune phenotype in these two types of tumors also vary grossly. HPV-positive tumors: HPV-positive tumors have enhanced expression of Glucose Transporter 1 (GLUT1) and Cytochrome c Oxidase subunit 5B (COX5B) in the tumor core (red zone), resulting in enhanced mitochondrial OXPHOS in the tumor nest compared with the tumor periphery (blue zone) wherein aerobic glycolysis is more pronounced. This is speculated to propel most of the helper Cluster of Differentiation 4 (CD4)+ and cytotoxic CD8+ T cells towards the tumor interior, resulting in better anti-tumor immune infiltration. HPV-negative tumors: HPV-negative tumors demonstrate higher expression of mitochondrial respiration markers GLUT1 and COX5B in the tumor periphery than that in the tumor core. Thus, HPV-negative tumor interiors undergo more prominent aerobic glycolysis with enhanced deposition of lactate. Higher levels of lactate are implicated in immune suppression. This may partially elucidate the rationale behind lesser anti-tumor CD4+ and CD8+ T cells but pronounced suppressive regulatory (Forkhead Box P3+ Regulatory T cells [FoxP3+Tregs], T-helper cell 17 [Th17]) cells in the HPV-negative tumors.
Not all patients with HPV-related disease do uniformly well. Patients with a long smoking history and HPV-positive tumors have an intermediate risk correlating with pack years (137). Results from trial ECOG 1308, in which patients with HPV-positive disease received induction chemotherapy followed by low-dose primary radiation, showed that those with at least a 10-pack-per-year smoking history saw a drop in 2-year progression-free survival from 80% to 57% (138). This further supports the idea that tobacco augments lethality risk in HPV-positive HNSCC patients.
Defining HPV tumor status is of great clinical importance, given that OS and responsiveness to treatment may vary greatly between the two distinct subtypes of disease. Of interest, despite our current understanding of oropharyngeal disease and viral oncogenesis, the implication and clinical significance of HPV-positive tumors in other nonoropharyngeal head and neck subsites (ie, oral cavity, larynx, hypopharynx, and sinonasal cavity) remains unclear (139). Though the overall prognosis is improved compared with tobacco-related oropharyngeal malignancies, patients with small localized HPV-positive tumors of the tonsil or base of tongue are more likely to have metastatic nodal disease at presentation. There are data to suggest that at least a portion of the survival advantage afforded to patients with HPV-positive disease may be due to demographic factors. Patients with HPV-related disease also tend to be younger and more affluent with fewer medical comorbidities and better access to health care. This OS benefit in HPV-positive disease might be partly due to the decreased risk of developing second primary malignancy (140). These tumors not only show increased radiosensitivity (which can be predicted based on responses to induction CT), but in cases of locoregional treatment failure, these patients also demonstrate improved outcomes following salvage surgery (141). Although current treatment regimens do not take into consideration the HPV tumor status and racial disparity concurrently, several clinical trials are conducted to de-escalate overall RT doses or compliment with other systemic or targeted therapy to decrease patient morbidity without compromising anti-oncologic efficacy. The current therapy regimen and its association with disparity in the outcome of HNSCC patient has been discussed further in the Supplementary Material, available online.
Population-based studies imply the higher propensity of HPV infection in whites, and owing to viral infection, HPV-positive and -negative patients have marked differences in their somatic, immunologic, and metabolic status. Although accumulating evidence supports SES and sexual lifestyle preference as the primary determinant of differential HPV infection and overall prognosis of AA and white patients, it is worth mentioning that the inherent somatic variations and the HPV-driven intratumoral immune-metabolic phenotype of these races are not exclusive phenomenon but interdependent crucial contributors in the disparity. Taking this into consideration, prodigious research is required to stratify the AA and white patient populations on the basis of their HPV status, somatic mutational load, and immune-metabolic characteristics. Further, systematic identification of predisposition and prognostic markers in these two racial groups should be conducted using a global “omics” approach, followed by further validation using tumor samples and cell lines derived from these races. Using such an approach in combination with the association studies holds promise to solve the enigma behind racial disparity in HNSCC patient outcomes and is projected to pave ways for novel, precision-based therapeutic modalities.
Funding
The authors of this manuscript were, in parts, supported by grants from the NIH (PO1 CA217798, UO1 CA185148, UO1 CA200466, P50 CA127297, and P30 CA036727), Nebraska LB595, and UNMC Graduate Studies Fellowship to Koelina Ganguly. SKB is one of the co-founder for Sanguine Diagnostic and Therapeutics Inc. The funders had no role in study design, decision to publish, or preparation of the manuscript.
Notes
Affiliations of authors: Department of Biochemistry and Molecular Biology (SC, KG, SM, RP, SKB, MAM), Department of Otolaryngology/Head and Neck Surgery (ZS, DTJ, MAM), Eppley Institute for Research in Cancer and Allied Diseases (SKB), Fred and Pamela Buffett Cancer Center (SKB), University of Nebraska Medical Center, Omaha, NE.
The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. SKB is one of the co-founder for Sanguine Diagnostic and Therapeutics Inc. Other authors declare no conflict of interest. We thank Dr Adrian E. Koesters, Research Editor at UNMC, for her editorial contribution to the manuscript (supported by UNMC).
Supplementary Material
References
- 1. Vigneswaran N, Williams MD.. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26(2):123–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu X, Gao XL, Liang XH, Tang YL.. The etiologic spectrum of head and neck squamous cell carcinoma in young patients. Oncotarget. 2016;7(40):66226–66238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berman TA, Schiller JT.. Human papillomavirus in cervical cancer and oropharyngeal cancer: one cause, two diseases. Cancer. 2017;123(12):2219–2229. [DOI] [PubMed] [Google Scholar]
- 4. Chaturvedi AK, Engels EA, Pfeiffer RM.. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607–615. [DOI] [PubMed] [Google Scholar]
- 6. Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–720. [DOI] [PubMed] [Google Scholar]
- 7. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weinberger PM, Merkley MA, Khichi SS, et al. Human papillomavirus‐active head and neck cancer and ethnic health disparities. Laryngoscope. 2010;120(8):1531–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus–positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. [DOI] [PubMed] [Google Scholar]
- 10. Patel MI, Wang A, Kapphahn K, et al. Racial and ethnic variations in lung cancer incidence and mortality: results from the Women’s Health Initiative. J Clin Oncol. 2016;34(4):360.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jackson CS, Oman M, Patel AM, Vega KJ.. Health disparities in colorectal cancer among racial and ethnic minorities in the United States. J Gastrointest Oncol. 2016;7(suppl 1):S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chlebowski RT, Chen Z, Anderson GL, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97(6):439–448. [DOI] [PubMed] [Google Scholar]
- 13. Hoffman HT, Karnell LH, Funk GF, Robinson RA, Menck HR.. The National Cancer Data Base report on cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124(9):951–962. [DOI] [PubMed] [Google Scholar]
- 14. Ragin CC, Langevin SM, Marzouk M, Grandis J, Taioli E.. Determinants of head and neck cancer survival by race. Head Neck. 2011;33(8):1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Settle K, Posner MR, Schumaker LM, et al. Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in black oropharyngeal cancer patients. Cancer Prev Res. 2009;2(9):776–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shavers VL, Harlan LC, Stevens JL.. Racial/ethnic variation in clinical presentation, treatment, and survival among breast cancer patients under age 35. Cancer. 2003;97(1):134–147. [DOI] [PubMed] [Google Scholar]
- 17. Murdock JM, Gluckman JL.. African‐American and white head and neck carcinoma patients in a university medical center setting. Cancer. 2001;91(S1):279–283. [DOI] [PubMed] [Google Scholar]
- 18. Choi SH, Terrell JE, Fowler KE, et al. Socioeconomic and other demographic disparities predicting survival among head and neck cancer patients. PLoS One. 2016;11(3):e0149886.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reeve BB, Cai J, Zhang H, et al. Health‐related quality of life differences between African Americans and non‐Hispanic whites with head and neck cancer. Head Neck. 2013;35(9):1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Churilla T, Egleston B, Dong Y, Lango M, Galloway T.. The impact of health insurance status on the presentation, local management, and outcomes of patients with head and neck cancer in the United States. Int J Radiat Oncol Biol Phys. 2016;94(4):870. [Google Scholar]
- 21. Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY.. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(3):222–231. [DOI] [PubMed] [Google Scholar]
- 22. Chen AY, Schrag NM, Halpern M, Stewart A, Ward EM.. Health insurance and stage at diagnosis of laryngeal cancer: does insurance type predict stage at diagnosis? Arch Otolaryngol Head Neck Surg. 2007;133(8):784–790. [DOI] [PubMed] [Google Scholar]
- 23. Gourin CG, Podolsky RH.. Racial disparities in patients with head and neck squamous cell carcinoma. Laryngoscope. 2006;116(7):1093–1106. [DOI] [PubMed] [Google Scholar]
- 24. Schrank TP, Han Y, Weiss H, Resto VA.. Case‐matching analysis of head and neck squamous cell carcinoma in racial and ethnic minorities in the United States—Possible role for human papillomavirus in survival disparities. Head Neck. 2011;33(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mulligan CR, Meram AD, Proctor CD, Wu H, Zhu K, Marrongi AJ.. Unlimited access to care: effect on racial disparity and prognostic factors in lung cancer. Cancer Epidemiol Prev Biomarkers. 2006;15(1):25–31. [DOI] [PubMed] [Google Scholar]
- 26. Mehta R, Gillan A, Ming Z, et al. Socio-economic deprivation and outcomes following radical nephroureterectomy for clinically localized upper tract transitional cell carcinoma. World J Urol. 2015;33(1):41–49. [DOI] [PubMed] [Google Scholar]
- 27. Richardson MA, Tarsi EJ, Replogle WH, Jefferson GD.. Socioeconomic disparities in Mississippi head and neck cancer patients. Otolaryngol Head Neck Surg. 2013;149(suppl 2):P195. [Google Scholar]
- 28. Byers T, Wolf H, Bauer K, et al. Patterns of care study group: the impact of socioeconomic status on survival after cancer in the United States: findings from the National Program of Cancer Registries Patterns of Care Study. Cancer. 2008;113(3):582–591. [DOI] [PubMed] [Google Scholar]
- 29. Subramanian S, Chen A.. Treatment patterns and survival among low-income medicaid patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2013;139(5):489–495. [DOI] [PubMed] [Google Scholar]
- 30. Steenland K, Henley J, Thun M.. All-cause and cause-specific death rates by educational status for two million people in two American Cancer Society cohorts, 1959–1996. Am J Epidemiol. 2002;156(1):11–21. [DOI] [PubMed] [Google Scholar]
- 31. Duffy SA, Taylor JM, Terrell JE, et al. Interleukin‐6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113(4):750–757. [DOI] [PubMed] [Google Scholar]
- 32. Dowd JB, Palermo TM, Aiello AE.. Family poverty is associated with cytomegalovirus antibody titers in US children. Health Psychol. 2012;31(1):5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI.. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parasher AK, Abramowitz M, Weed D, et al. Ethnicity and clinical outcomes in head and neck cancer: an analysis of the SEER database. J Racial Ethn Health Disparities. 2014;1(4):267–274. [Google Scholar]
- 35. Zimmerman T, Sweeny L, Carroll WR, Day KE, Rosenthal EL.. Analysis and review of outcomes for cutaneous squamous cell carcinoma involving the parotid. Otolaryngol Head Neck Surg. 2013;149(suppl 2):P55–P55. [Google Scholar]
- 36. Agrawal N, Frederick MJ, Pickering CR, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333(6046):1154–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu YF, Chiang SL, Lin CY, et al. Somatic mutations and genetic variants of NOTCH1 in head and neck squamous cell carcinoma occurrence and development. Sci Rep. 2016;6:24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Benowitz NL, Hukkanen J, Jacob P.. Nicotine chemistry, metabolism, kinetics and biomarkers In: Jack E. Henningfield, Edythe D. London, Sakire Pogun, eds, Nicotine Psychopharmacology. Springer, Berlin, Heidelberg: Springer; 2009:29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perez-Stable EJ, Herrera B, Jacob P 3rd, Benowitz NL.. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280(2):152–156. [DOI] [PubMed] [Google Scholar]
- 40. Ho MK, Mwenifumbo JC, Al Koudsi N, et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African‐American light smokers. Clin Pharmacol Ther. 2009;85(6):635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mwenifumbo JC, Al Koudsi N, Ho MK, et al. Novel and established CYP2A6 alleles impair in vivo nicotine metabolism in a population of Black African descent. Hum Mutat. 2008;29(5):679–688. [DOI] [PubMed] [Google Scholar]
- 42. Mwenifumbo JC, Zhou Q, Benowitz NL, Sellers EM, Tyndale RF.. New CYP2A6 gene deletion and conversion variants in a population of Black African descent. Pharmacogenomics. 2010;11(2):189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sun W, Califano JA.. Sequencing the head and neck cancer genome: implications for therapy. Ann N Y Acad Sci. 2014;1333:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu ES, Park JY, Zeitouni JA, et al. Effect of actionable somatic mutations on racial/ethnic disparities in head and neck cancer prognosis. Head Neck. 2016;38(8):1234–1241. [DOI] [PubMed] [Google Scholar]
- 45. Stadler ME, Patel MR, Couch ME, Hayes DN.. Molecular biology of head and neck cancer: risks and pathways. Hematol Oncol Clin. 2008;22(6):1099–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang W, Edwards A, Flemington EK, Zhang K.. Racial disparities in patient survival and tumor mutation burden, and the association between tumor mutation burden and cancer incidence rate. Sci Rep. 2017;7(1):13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ramakodi MP, Kulathinal RJ, Chung Y, Serebriiski I, Liu JC, Ragin CC.. Ancestral-derived effects on the mutational landscape of laryngeal cancer. Genomics. 2016;107(2–3):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cadoni G, Boccia S, Petrelli L, et al. A review of genetic epidemiology of head and neck cancer related to polymorphisms in metabolic genes, cell cycle control and alcohol metabolism. ACTA Otorhinolaryngol Ital. 2012;32(1):1. [PMC free article] [PubMed] [Google Scholar]
- 49. Mork J, Lie AK, Glattre E, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344(15):1125–1131. [DOI] [PubMed] [Google Scholar]
- 50. Palefsky JM. Human papillomavirus-related disease in men: not just a women’s issue. J Adolesc Health. 2010;46(4):S12–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gillison ML, Alemany L, Snijders PJ, et al. Human papillomavirus and diseases of the upper airway: head and neck cancer and respiratory papillomatosis. Vaccine. 2012;30:F34–F54. [DOI] [PubMed] [Google Scholar]
- 52. Kumar R, Rai AK, Das D, et al. Alcohol and tobacco increases risk of high risk HPV infection in head and neck cancer patients: study from north-east region of India. PLoS One. 2015;10(10):e0140700.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fakhry C, Psyrri A, Chaturvedhi A.. HPV and head and neck cancers: state-of-the-science. Oral Oncol. 2014;50(5):353–355. [DOI] [PubMed] [Google Scholar]
- 54. Hedberg ML, Goh G, Chiosea SI, et al. Genetic landscape of metastatic and recurrent head and neck squamous cell carcinoma. J Clin Invest. 2016;126(1):169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci. 2006;110(5):525–541. [DOI] [PubMed] [Google Scholar]
- 56. Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J.. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12(6):418–424. [DOI] [PubMed] [Google Scholar]
- 57. Syrjänen S, Kellokoski J.. Oral Manifestations of HPV Infections, in Genital Papillomavirus Infections. Springer, Berlin, Heidelberg: Springer; 1990:209–223. [Google Scholar]
- 58. Miller CS, Johnstone BM.. Human papillomavirus as a risk factor for oral squamous cell carcinoma: a meta-analysis, 1982-1997. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91(6):622–635. [DOI] [PubMed] [Google Scholar]
- 59. Wiest T, Schwarz E, Enders C, Flechtenmacher C, Bosch FX.. Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene. 2002;21(10):1510.. [DOI] [PubMed] [Google Scholar]
- 60. Syrjänen S. Human papillomavirus (HPV) in head and neck cancer. J Clin Virol. 2005;32:59–66. [DOI] [PubMed] [Google Scholar]
- 61. Hobbs C, Sterne J, Bailey M, Heyderman RS, Birchall MA, Thomas SJ.. Human papillomavirus and head and neck cancer: a systematic review and meta‐analysis. Clin Otolaryngol. 2006;31(4):259–266. [DOI] [PubMed] [Google Scholar]
- 62. Gillison ML, D'Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16–positive and human papillomavirus type 16–negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407–420. [DOI] [PubMed] [Google Scholar]
- 63. Trottier H, Mahmud SM, Lindsay L, et al. Persistence of an incident human papillomavirus infection and timing of cervical lesions in previously unexposed young women. Cancer Epidemiol Biomarkers Prev. 2009;18(3):854–862. [DOI] [PubMed] [Google Scholar]
- 64. Dayyani F, Etzel CJ, Liu M, Ho CH, Lippman SM, Tsao AS.. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol. 2010;2:15.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Syrjänen S. The role of human papillomavirus infection in head and neck cancers. Ann Oncol. 2010;21(suppl 7):vii243–vii245. [DOI] [PubMed] [Google Scholar]
- 66. Al‐Othman MO, Morris CG, Logan HL, et al. Impact of race on outcome after definitive radiotherapy for squamous cell carcinoma of the head and neck. Cancer. 2003;98(11):2467–2472. [DOI] [PubMed] [Google Scholar]
- 67. Goodwin WJ, Thomas GR, Parker DF, et al. Unequal burden of head and neck cancer in the United States. Head Neck. 2008;30(3):358–371. [DOI] [PubMed] [Google Scholar]
- 68. Molina MA, Cheung MC, Perez EA, et al. African American and poor patients have a dramatically worse prognosis for head and neck cancer. Cancer. 2008;113(10):2797–2806. [DOI] [PubMed] [Google Scholar]
- 69. Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011. CA Cancer J Clin. 2011;61(4):212–236. [DOI] [PubMed] [Google Scholar]
- 70. Jiron J, Sethi S, Ali-Fehmi R, et al. Racial disparities in human papillomavirus (HPV) associated head and neck cancer. Am J Otolaryngol. 2014;35(2):147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kreimer AR, Clifford GM, Boyle P, Franceschi S.. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Prev Biomarkers. 2005;14(2):467–475. [DOI] [PubMed] [Google Scholar]
- 72. Dahlstrom KR, Calzada G, Hanby JD, et al. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center. Cancer. 2013;119(1):81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Khleif SN, DeGregori J, Yee CL, et al. Inhibition of cyclin D-CDK4/CDK6 activity is associated with an E2F-mediated induction of cyclin kinase inhibitor activity. Proc Natl Acad Sci USA. 1996;93(9):4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dyson N, Howley PM, Munger K, Harlow E.. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243(4893):934–937. [DOI] [PubMed] [Google Scholar]
- 75. Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Auslander BA, Biro FM, Succop PA, Short MB, Rosenthal SL.. Racial/ethnic differences in patterns of sexual behavior and STI risk among sexually experienced adolescent girls. J Pediatr Adolesc Gynecol. 2009;22(1):33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Johnson EH, Jackson LA, Hinkle Y, et al. What is the significance of black-white differences in risky sexual behavior? J Natl Med Assoc. 1994;86(10):745–759. [PMC free article] [PubMed] [Google Scholar]
- 78. Boss EF, Smith DF, Ishman SL.. Racial/ethnic and socioeconomic disparities in the diagnosis and treatment of sleep-disordered breathing in children. Int J Pediatr Otorhinolaryngol. 2011;75(3):299–307. [DOI] [PubMed] [Google Scholar]
- 79. Richtsmeier WJ. Human interferon production in tonsil and adenoid tissue cultures. Am J Otolaryngol. 1983;4(5):325–333. [DOI] [PubMed] [Google Scholar]
- 80. Pavlova NN, Thompson CB.. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23(1):27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Warburg O, Wind F, Negelein E.. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Curry JM, Tuluc M, Whitaker-Menezes D, et al. Cancer metabolism, stemness and tumor recurrence: MCT1 and MCT4 are functional biomarkers of metabolic symbiosis in head and neck cancer. Cell Cycle. 2013;12(9):1371–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rademakers SE, Lok J, van der Kogel AJ, Bussink J, Kaanders JH.. Metabolic markers in relation to hypoxia; staining patterns and colocalization of pimonidazole, HIF-1α, CAIX, LDH-5, GLUT-1, MCT1 and MCT4. BMC Cancer. 2011;11:167.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Krupar R, Robold K, Gaag D, et al. Immunologic and metabolic characteristics of HPV-negative and HPV-positive head and neck squamous cell carcinomas are strikingly different. Virchows Arch. 2014;465(3):299–312. [DOI] [PubMed] [Google Scholar]
- 85. Read J, Winter V, Eszes C, Sessions R, Brady R.. Structural basis for altered activity of M‐and H‐isozyme forms of human lactate dehydrogenase. Proteins. 2001;43(2):175–185. [DOI] [PubMed] [Google Scholar]
- 86. Zwerschke W, Mazurek S, Massimi P, Banks L, Eigenbrodt E, Jansen DP.. Modulation of type M2 pyruvate kinase activity by the human papillomavirus type 16 E7 oncoprotein. Proc Natl Acad Sci USA. 1999;96(4):1291–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sethi S, Ali ‐Fehmi R, Franceschi S, et al. Characteristics and survival of head and neck cancer by HPV status: a cancer registry‐based study. Int J Cancer. 2012;131(5):1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jung YS, Najy AJ, Huang W, et al. HPV-associated differential regulation of tumor metabolism in oropharyngeal head and neck cancer. Oncotarget. 2017;8(31):51530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Curry JM, Sprandio J, Cognetti D, et al. Tumor microenvironment in head and neck squamous cell carcinoma. Semin Oncol. 2014;41(2):217–234. [DOI] [PubMed] [Google Scholar]
- 90. Bhardwaj N. Harnessing the immune system to treat cancer. J Clin Invest. 2007;117(5):1130–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mandal R, Şenbabaoğlu Y, Desrichard A, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016;1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Whiteside TL. Immune responses to cancer: are they potential biomarkers of prognosis? Front Oncol. 2013;3:107.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lei Y, Xie Y, Tan YS, et al. Telltale tumor infiltrating lymphocytes (TIL) in oral, head & neck cancer. Oral Oncol. 2016;61:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nguyen N, Bellile E, Thomas D, et al. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2016;38(7):1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Andersen AS, Koldjaer Solling AS, Ovesen T, Ovesen T, Rusan M.. The interplay between HPV and host immunity in head and neck squamous cell carcinoma. Int J Cancer. 2014;134(12):2755–2763. [DOI] [PubMed] [Google Scholar]
- 96. Kong CS, Narasimhan B, Cao H, et al. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2009;74(2):553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Näsman A, Romanitan M, Nordfors C, et al. Tumor infiltrating CD8+ and Foxp3+ lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PLoS One. 2012;7(6):e38711.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Russell S, Angell T, Lechner M, et al. Immune cell infiltration patterns and survival in head and neck squamous cell carcinoma. Head Neck Oncol. 2013;5(3):24.. [PMC free article] [PubMed] [Google Scholar]
- 99. O’Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus–related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31(5):543–550. [DOI] [PubMed] [Google Scholar]
- 100. Meulendijks D, Tomasoa N, Dewit L, et al. HPV-negative squamous cell carcinoma of the anal canal is unresponsive to standard treatment and frequently carries disruptive mutations in TP53. Br J Cancer. 2015;112(8):1358.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Di Minin G, Bellazzo A, Dal Ferro M, et al. Mutant p53 reprograms TNF signaling in cancer cells through interaction with the tumor suppressor DAB2IP. Mol Cell. 2014;56(5):617–629. [DOI] [PubMed] [Google Scholar]
- 102. Cooks T, Harris CC.. p53 mutations and inflammation-associated cancer are linked through TNF signaling. Mol Cell. 2014;56(5):611–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wellenstein MD, de Visser KE.. Cancer-cell-intrinsic mechanisms shaping the tumor immune landscape. Immunity. 2018;48(3):399–416. [DOI] [PubMed] [Google Scholar]
- 104. Oguejiofor K, Hall J, Slater C, et al. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV-positive oropharyngeal squamous carcinoma. Br J Cancer. 2015;113(6):886.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Nordfors C, Grün N, Tertipis N, et al. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur J Cancer. 2013;49(11):2522–2530. [DOI] [PubMed] [Google Scholar]
- 106. Ward M, Thirdborough S, Mellows T, et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br J Cancer. 2014;110(2):489.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wansom D, Light E, Thomas D, et al. Infiltrating lymphocytes and human papillomavirus‐16–associated oropharyngeal cancer. Laryngoscope. 2012;122(1):121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Yin W, Duluc D, Joo H, Oh S.. Dendritic cell targeting vaccine for HPV-associated cancer. Cancer Cell Microenviron. 2016;3(4): e1482.e1482, PMID: 28133621. [PMC free article] [PubMed] [Google Scholar]
- 109. Heusinkveld M, Goedemans R, Briet RJ, et al. Systemic and local human papillomavirus 16-specific T-cell immunity in patients with head and neck cancer. Int J Cancer. 2012;131(2):E74–E85. [DOI] [PubMed] [Google Scholar]
- 110. Albers A, Abe K, Hunt J, et al. Antitumor activity of human papillomavirus type 16 E7–specific T cells against virally infected squamous cell carcinoma of the head and neck. Cancer Res. 2005;65(23):11146–11155. [DOI] [PubMed] [Google Scholar]
- 111. Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29(2):167–178. [DOI] [PubMed] [Google Scholar]
- 112. Watanabe Y, Katou F, Ohtani H, Nakayama T, Yoshie O, Hashimoto K.. Tumor-infiltrating lymphocytes, particularly the balance between CD8+ T cells and CCR4+ regulatory T cells, affect the survival of patients with oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(5):744–752. [DOI] [PubMed] [Google Scholar]
- 113. Badoual C, Hans S, Merillon N, et al. PD-1–expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73(1):128–138. [DOI] [PubMed] [Google Scholar]
- 114. Matlung SE, Wilhelmina van Kempen PM, Bovenschen N, van Baarle D, Willems SM.. Differences in T-cell infiltrates and survival between HPV+ and HPV-oropharyngeal squamous cell carcinoma. Future Sci OA. 2016;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Masterson L, Lechner M, Loewenbein S, et al. CD8+ T cell response to human papillomavirus 16 E7 is able to predict survival outcome in oropharyngeal cancer. Eur J Cancer. 2016;67:141–151. [DOI] [PubMed] [Google Scholar]
- 116. Allen CT, Clavijo PE, Van Waes C, Chen Z.. Anti-tumor immunity in head and neck cancer: understanding the evidence, how tumors escape and immunotherapeutic approaches. Cancers (Basel). 2015;7(4):2397–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Spanos WC, Nowicki P, Lee DW, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus–related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135(11):1137–1146. [DOI] [PubMed] [Google Scholar]
- 118. Stanley M. HPV—immune response to infection and vaccination. Infect Agents Cancer. 2010;5:19.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Best SR, Niparko KJ, Pai SI.. Biology of human papillomavirus infection and immune therapy for HPV-related head and neck cancers. Otolaryngol Clin North Am. 2012;45(4):807–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Clemente CG, Mihm MC Jr., Bufalino R, Zurrida S, Collini P, Cascinelli N.. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77(7):1303–1310. [DOI] [PubMed] [Google Scholar]
- 121. Hu WH, Miyai K, Cajas-Monson LC, Luo L, Liu L, Ramamoorthy SL.. Tumor-infiltrating CD8(+) T lymphocytes associated with clinical outcome in anal squamous cell carcinoma. J Surg Oncol. 2015;112(4):421–426. [DOI] [PubMed] [Google Scholar]
- 122. Rahbar M, Naraghi ZS, Mardanpour M, Mardanpour N.. Tumor-infiltrating CD8+ lymphocytes effect on clinical outcome of muco-cutaneous melanoma. Indian J Dermatol. 2015;60(2):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rathore AS, Kumar S, Konwar R, Makker A, Negi MP, Goel MM.. CD3+, CD4+ & CD8+ tumour infiltrating lymphocytes (TILs) are predictors of favourable survival outcome in infiltrating ductal carcinoma of breast. Indian J Med Res. 2014;140(3):361–369. [PMC free article] [PubMed] [Google Scholar]
- 124. Reynders K, De Ruysscher D.. Tumor infiltrating lymphocytes in lung cancer: a new prognostic parameter. J Thorac Dis. 2016;8(8):E833–E835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Stanton SE, Disis ML.. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Schaefer C, Kim GG, Albers A, Hoermann K, Myers EN, Whiteside TL.. Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer. 2005;92(5):913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Strauss L, Bergmann C, Whiteside TL.. Functional and phenotypic characteristics of CD4+CD25highFoxp3+ Treg clones obtained from peripheral blood of patients with cancer. Int J Cancer. 2007;121(11):2473–2483. [DOI] [PubMed] [Google Scholar]
- 128. Welters MJP, Ma W, Santegoets S, et al. Intratumoral HPV16-Specific T cells constitute a type I-oriented tumor microenvironment to improve survival in HPV16-driven oropharyngeal cancer. Clin Cancer Res. 2018;24(3):634–647. [DOI] [PubMed] [Google Scholar]
- 129. Wagner S, Wittekindt C, Reuschenbach M, et al. CD56‐positive lymphocyte infiltration in relation to human papillomavirus association and prognostic significance in oropharyngeal squamous cell carcinoma. Int J Cancer. 2016;138(9):2263–2273. [DOI] [PubMed] [Google Scholar]
- 130. Chang CH, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Buck MD, Sowell RT, Kaech SM, Pearce EL.. Metabolic instruction of immunity. Cell. 2017;169(4):570–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Scharping NE, Menk AV, Whetstone RD, Zeng X, Delgoffe GM.. Efficacy of PD-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer Immunol Res. 2017;5(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Krupar R, Hautmann MG, Pathak RR, et al. Immunometabolic determinants of chemoradiotherapy response and survival in head and neck squamous cell carcinoma. Am J Pathol. 2018;188(1):72–83. [DOI] [PubMed] [Google Scholar]
- 134. Vokes EE, Agrawal N, Seiwert TY.. HPV-associated head and neck cancer. J Natl Cancer Inst. 2015;107(12):djv344.. [DOI] [PubMed] [Google Scholar]
- 135. Brockstein BE, Vokes EE, Head Neck C, In 2.. Maximizing survival and minimizing toxicity. Nat Rev Clin Oncol. 2011;8(2):72.. [DOI] [PubMed] [Google Scholar]
- 136. Chaturvedi AK, Engels EA, Anderson WF, Gillison ML.. Incidence trends for human papillomavirus-related and-unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–619. [DOI] [PubMed] [Google Scholar]
- 137. Haigentz M Jr., Suarez C, Strojan P, et al. Understanding interactions of smoking on prognosis of HPV-associated oropharyngeal cancers. Adv Ther. 2018;35(3):255–260. [DOI] [PubMed] [Google Scholar]
- 138. Marur S, Li S, Cmelak AJ, et al. E1308: phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx-ECOG-ACRIN cancer research group. J Clin Oncol. 2017;35(5):490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Betiol JC, Sichero L, de Olival Costa HO, et al. Prevalence of human papillomavirus types and variants and p16 INK4a expression in head and neck squamous cells carcinomas in São Paulo, Brazil. Infect Agent Cancer. 2016;11(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Peck BW, Dahlstrom KR, Gan SJ, et al. Low risk of second primary malignancies among never smokers with human papillomavirus–associated index oropharyngeal cancers. Head Neck. 2013;35(6):794–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Cleary C, Leeman J, Higginson D, et al. Biological features of human papillomavirus-related head and neck cancers contributing to improved response. Clin Oncol (R Coll Radiol). 2016;28(7):467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.