After three intravitreal anti–vascular endothelial growth factor injections, the severity of diabetic retinopathy on color fundus photographs improved, whereas no reperfusion occurred in the nonperfusion areas assessed on ultra–wide-field fluorescein angiography.
Key words: anti-VEGF, diabetic retinopathy, retinal nonperfusion, ultra–wide field, fluorescein angiography
Abstract
Purpose:
To compare the changes in retinal perfusion on ultra–wide-field fluorescein angiography with the changes in diabetic retinopathy lesions observed on ultra–wide-field fundus color photographs after 3 monthly anti–vascular endothelial growth factor injections.
Methods:
Retrospective interventional cohort study analyzing the files of 14 patients with DR (18 eyes). UWF color photos and FA were analyzed at baseline (M0) and 1 month after the third anti-VEGF injection (M3). The main outcomes included the count of the number of red dots (microaneurysms, hemorrhages) and assessment of DR severity score (DRSS); the analysis of non-perfusion areas and disappearance or reappearance of arterioles or venules in the non-perfusion areas on FA.
Results:
Eighteen eyes of 14 diabetic patients, with mean age of 63 ± 5 years, were included. The DRSS score improved by at least one stage in 11/18 (61%) eyes. The mean number of red dots significantly decreased at M3 (n = 80 ± 85) compared with M0 (n = 139 ± 130) (P < 0.0001). No reperfusion of arterioles or venules was observed in or around nonperfusion areas.
Conclusion:
After anti–vascular endothelial growth factor injections, the improvement in the DRSS score based on color fundus photographs can occur without retinal reperfusion on ultra–wide-field fluorescein angiography.
Anti–vascular endothelial growth factor (anti-VEGF) therapy for diabetic macular edema improves the diabetic retinopathy severity scale (DRSS) score assessed on color fundus photographs1 and may reduce DR worsening. Two randomized trials comparing anti-VEGF injections and pan retinal photocoagulation in proliferative DR (PDR) have shown the noninferiority of anti-VEGF over pan retinal photocoagulation to prevent complications of PDR, at least for 1 year to 2 years.2 In some aspects, anti-VEGF seems to be even more effective than pan retinal photocoagulation.3 However, the role of anti-VEGF in retinal perfusion based on fluorescein angiography (FA) remains unclear, and different interpretations have been proposed in DR and retinal vein occlusion.2,4 The discrepancy in assessing the role of anti-VEGF in peripheral retinal perfusion may be due to the difficulty of correctly delineating nonperfusion (NP) areas even on ultra–wide-field (UWF) FA. Nevertheless, the strong correlation between the number of DR lesions and NP, which is well established before any treatment, may no longer be relevant after anti-VEGF therapy.2,4
The aim of this study was to assess this correlation and compare the changes in retinal lesions of DR, that is, microaneurysms and hemorrhages (red dots), observed on UWF fundus photographs with the changes in retinal perfusion seen on UWF FA in eyes with diabetic macular edema at baseline and after 3 monthly anti-VEGF injections.
Patients and Methods
In this retrospective study, the records of consecutive patients with diabetic macular edema, and all inclusion–exclusion criteria, over a 1-year period (January 2016–January 2017) were reviewed. Inclusion criteria were: patients with diabetes (Type 1 or 2) aged 18 years or older, with DR and decreased vision due to diabetic macular edema (with a central macular thickness >310 µm), associated with non-PDR (NPDR) or PDR, treated with 3 monthly anti-VEGF injections (0.5 mg of ranibizumab or 2 mg of aflibercept), and imaged with sufficient quality UWF color fundus photographs and FA before the first injection (M0) and 1 month after the third anti-VEGF injection (M3) as part of the usual procedure to initiate anti-VEGF therapy in diabetic patients. Exclusion criteria were: a history of pan retinal photocoagulation, pars plana vitrectomy, previous intravitreal anti-VEGF therapy, poor image quality due to media opacity, or the association with a nondiabetic retinal disease (including high myopia).
All data were assessed by a chart review, including sex, age, diabetes type and duration, serum glycated hemoglobin (HbA1c) level, visual acuity, intraocular pressure, and previous ophthalmologic treatments.
This study was approved by the Ethics Committee of the French Society of Ophthalmology (IRB 00008855 Société Française d'Ophtalmologie IRB#1) and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained for all patients.
Retinal Image Acquisition
According to current practice, the studied eyes were dilated using tropicamide 1% and phenylephrine 2.5%. Central macular thickness in the central 1-mm diameter circle of the ETDRS thickness map was recorded using the Spectralis device (Heidelberg Eye Explorer, version 1.9.11.0; Heidelberg Engineering, Heidelberg, Germany).
Ultra–wide-field images were obtained using the Optos California v2.14 imaging system (Optos, PLC, Dunfermline, Scotland) equipped with the Optos Vantage review Software v4.0.28. After intravenous administration of fluorescein, UWF FA images were captured in the early (45 seconds), middle (2 minutes), and late (5 minutes) phases of FA.
Image Grading
Images were exported in Tiff format. Fundus photographs and FA images were automatically aligned using i2kRetina software (DualAlign, Clifton Park, NY), and then controlled and cropped to keep only the part common to both images at M0 and M3. Care was taken to analyze the early phase of FA after complete retinal vein filling, that is, about 45 seconds after injection. After alignment, color fundus photographs and FA were divided into 16 identical boxes.
Quantitative Analysis
Color fundus photographs and FA obtained before (M0) and 1 month after three anti-VEGF injections (M3) were presented in a random order, with no indication on the analysis time point, to a retina specialist experienced in DR grading on UWF images, who analyzed the images. Red dots (microaneurysms and retinal hemorrhages), intra retinal microvascular abnormalities (IRMAs), and cotton-wool spots were counted before (M0) and 1 month after three anti-VEGF injections (M3) in each box. This count was performed using ImageJ software (National Institutes of Health, Bethesda, MD). The total number of lesions in the whole retina was used for comparison between M0 and M3.
Qualitative Analysis
To avoid area measurement bias as much as possible, the analysis of NP on FA did not attempt to quantify the areas of NP, but just to track the possible reperfusion of vessels in areas that were not perfused at baseline. Nonperfusion was defined as a fundus area devoid of retinal arterioles, venules, and/or capillaries, with a “pruned” appearance of adjacent vessels. The M0 and M3 FA images of the same patient, divided into 16 boxes, were independently analyzed side-by-side by 2 readers to assess differences in each corresponding box. The possible disappearance or the reappearance of arterioles or venules within or around NP areas between M0 and M3 was noted. Discrepancies between the two readers were adjudicated by common agreement.
The DRSS score was also assessed according to the simplified American Academy of Ophthalmology (AAO) DR grading scale, using a five-stage disease severity, based on the analysis of the seven ETDRS fields.5 An improvement in DR severity was defined as follows, adapted from Bressler et al1: 1) for NPDR: improvement by one or more stages on the AAO DR scale vs. baseline, and 2) for PDR: regression from PDR to no PDR (complete regression of new vessels).
Statistical Analyses
Statistical analyses were performed using GraphPad Prism 5 Software (GraphPad Software, San Diego, CA). Results are expressed as the mean ± SD. Continuous data were analyzed using the Wilcoxon test. P <0.05 was considered significant.
Results
Eighteen eyes of 14 patients with Type 2 diabetes were included (9 men/5 women). The patient mean age was 63 ± 5 years. The mean HbA1c was 8.9 +2.5%, and the mean diabetes duration was 16 ± 8 years. Among the studied eyes, 2 (11%) had mild NPDR, 2 (11%) had moderate NPDR, 11 (61%) had severe NPDR, and 3 (17%) eye had PDR.
Thirteen eyes were treated with ranibizumab, and five eyes were treated with aflibercept.
The main characteristics of the studied eyes are shown in Table 1.
Table 1.
Patient Demographic Characteristics

The mean best-corrected visual acuity improved from 0.53 ± 0.28 logMAR (20/63) at baseline to 0.26 ± 0.18 logMAR (20/32) at M3 (P = 0.0002). The mean central macular thickness decreased from 506 ± 194 µm at baseline to 322 ± 114 µm at M3 (P = 0.0002). Changes in the number of DR lesions are shown in Figure 1.
Fig. 1.

Diabetic retinopathy lesions count before (M0) and 1 month after 3 monthly intravitreal anti-VEGF injections (M3). Significant decrease in the red dot count (hemorrhages and microaneurysms) on UWF fundus photographs. ***P < 0.001.
Analysis of Color Fundus Photographs
The DRSS score improved by at least one stage in 11/18 (61%) eyes. The mean number of red dots was significantly decreased at M3 (n = 80 ± 85) compared with M0 (n = 139 ± 130) (P < 0.0001) (Figure 1A and Figure 2, A and B). The number of cotton-wool spots did not change significantly within 3 months (Figure 1D and Figure 2, C and D).
Fig. 2.

Nonproliferative DR before (M0) and 1 month after 3 monthly intravitreal anti-VEGF injections (M3). Decrease in red dots on UWF fundus photographs but no reperfusion on FA (UWF FA). A. Pretreatment (M0) UWF color photograph showing multiple hemorrhages and microaneurysms, both considered as red dots. The yellow line limits the area of the common gradable portion of the image at M0 and 1 month after the third anti-VEGF injection (M3). The grid was used to count red dots in each of the 16 boxes. B. Posttreatment (M3) UWF color photograph showing the significant decrease in red dots. C. Detail of the nasal part of the fundus on UWF color photograph at M0 and (D), the same area at M3 showing that most red dots have vanished. E. The same area on FA at M0 and (F), at M3. Arrows represent some NP area that remained unchanged between M0 and M3. Ellipses show microaneurysms that were no longer visible at M3.
Analysis of UWF FA
At baseline, all eyes showed significant NP areas in the midperiphery and periphery. One month after 3 monthly anti-VEGF injections, the diameter of retinal vessels tended to narrow and fluorescein leakage decreased (Figure 3) due to the restoration of the blood–retinal barrier. The contrast between the barely fluorescent background of the choroid in NP areas and the diffuse hyperfluorescence of the surrounding retinal vascular bed was attenuated. No reperfusion of arterioles or venules was observed in or around NP areas by a side-by-side comparison of fluorescein angiograms, but in 15/18 (83%) studied eyes, a few vessel segments (mean: 6 ± 11 per eye) passing through these areas were occluded at M3 (Figure 4, C–F). New vessels regressed in the three eyes with PDR at baseline (Figure 4, A and B).
Fig. 3.

Nonproliferative DR before and 1 month after 3 monthly intravitreal anti-VEGF injections. Decrease in red dots but no reperfusion on UWF FA. A. Pretreatment (M0) UWF FA: the yellow line limits the area of the common gradable portion of the image at M0 and 1 month after third anti-VEGF injection (M3). The grid was used to count microaneurysms, and intraretinal microvascular anomalies (IRMAs) in each box, and to assess the occlusion or reperfusion of small retinal vessels in NP areas. B. Posttreatment (M3) UWF FA showing no change in retinal perfusion. The white rectangle in (A and B) is analyzed in (C and D). C and D. Magnification of the nasal sector at M0 and M3, respectively. Many microaneurysms regressed (circles). Examples of unchanged NP areas (arrows). E and F. Higher magnification showing the narrowing of retinal vessels (solid arrowhead), and the attenuation, but not the disappearance of an IRMA (empty arrowhead). A dilated capillary network in (E) is attenuated and barely visible in (F).
Fig. 4.
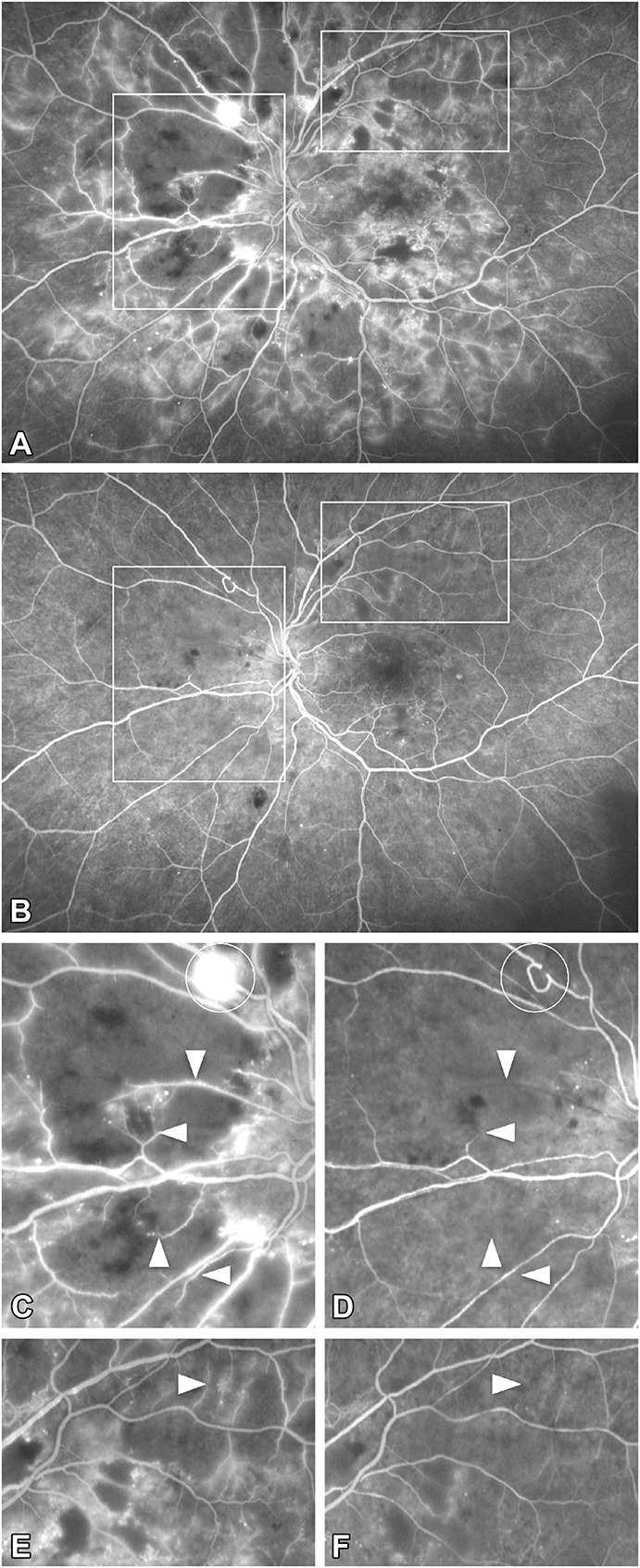
Proliferative DR before and 1 month after 3 monthly intravitreal anti-VEGF injections. Regression of preretinal new vessels but no reperfusion on UWF FA. A. Pretreatment (M0) UWF FA: Diffuse leakage, many areas of capillary NP, and preretinal new vessels in the superonasal quadrant. B. Posttreatment (M3) UWF FA shows the regression of leakage and new vessels but no change in retinal capillary perfusion. The white boxes in (A and B) are analyzed in (C and D). C and D: details corresponding to the nasal box showing the regression of preretinal new vessels (circle) and the occlusion of four segments of small retinal vessels (arrowheads) at between M0 and M3. (D). No capillary reperfusion is seen in these areas. Note that the tone of the choroidal background in the areas of retinal capillary non-perfusion did not change too much. E and F: details corresponding to the supero-temporal box showing the occlusion of one segment of small retinal vessel (arrowhead) at between M0 and M3. (F). Note the regression of capillary leakage and the absence of vessel reperfusion.
Discussion
In this work, we showed that 3 monthly injections of anti-VEGF in eyes with NPDR or PDR associated with macular edema significantly reduced the number of red dots such as hemorrhages, microaneurysms, as well as new vessels in the optic disk or elsewhere and macular edema. This evolution is in line with the results of previous randomized studies.6–8 However, despite this improvement in DR severity on color photographs, the retinal perfusion did not improve on FA with no reperfusion of small vessels in capillary NP areas. In other words, DR severity graded on color photographs may improve without concomitant improvement in vessel perfusion. Then, the strong correlation between DR lesions on color photographs and the NP, which is well established before any treatment,2,4 is no longer relevant after anti-VEGF therapy. This may be of importance not only when conducting trials but also when monitoring and evaluating DR in eyes treated with intravitreal injections.
The regression of the signs of DR on color photographs observed after anti-VEGF therapy could be interpreted as an improvement in DR severity in the DRSS score. Indeed, at M3, 11/18 (61%) eyes experienced DR improvement and new vessels, when they were present at baseline, regressed. Such an evolution has also been observed in several recent reports.1,2,9 Unlike these studies, we used UWF color photographs and FA instead of the seven fields of the ETDRS to assess our cases because there is accumulating evidence that UWF can detect more lesions that are missed when using the seven ETDRS fields.2,10,11 However, we also graded DR severity based on the analysis of the seven ETDRS fields for comparison.
We also used FA in addition to color fundus photographs to assess the vascular anomalies of DR. Fluorescein angiography confirmed the presence of retinal NP areas in the periphery or midperiphery in all eyes at baseline and also showed the regression of the new vessels when they were present at baseline. However, even in eyes with improvement in DR severity on color photographs, the retinal perfusion did not improve on FA. We did not observe any reperfusion of small vessels in capillary NP areas, nor an apparent decrease in NP areas despite the regression of red dots (hemorrhages and microaneurysms). This discrepancy between the improvement in the DRSS score on color photographs and the absence of perfusion improvement on FA is a new finding. Indeed, Da Silva and Aiello have shown in a cross-sectional study that hemorrhages and microaneurysms reflect the extent of the NP and ischemia on UWF FA outside the context of injections.2 They then assumed that “the improvement of the signs of DR on color photographs should have had their counterpart on FA after anti-VEGF treatment.” However, this was not the case in our series. Our results are in line with studies, although limited to the macular area, that have shown that the improvement in macular edema after injections does not correspond to capillary reperfusion observed on OCTA.12,13
Anti-VEGF therapy has an antipermeability effect, and is vasoconstrictive and antiangiogenic.14–17 The antipermeability effect could explain the decrease in retinal edema and the resolution of retinal hemorrhages that resorbed progressively and did not recur under treatment. The antiangiogenic effect could explain the complete regression of new vessels reported in more than 20% of cases in various publications.9,18 The vasoconstrictive effect could explain the narrowing of retinal vessels observed in our cases, and perhaps the fact that some small vessels disappeared on FA after 3 months of treatment. We did not assess whether these occluded vessels could reopen after anti-VEGF discontinuation. However, although vasoconstriction and vessel permeability repair prevent the recurrence of new hemorrhages, in our study no reperfusion occurred in the areas where the red dots disappeared. The number of red dots, which is an indirect sign of ongoing capillary closure,2,4 is then no longer a relevant severity criterion of DR after anti-VEGF therapy. The discrepancy between the absence of perfusion improvement observed on FA under anti-VEGF therapy and the improvement in the DRSS score deserves an explanation.
Nonperfusion areas are detected on FA based on variable criteria according to publications. Some authors have not precisely defined the criteria for NP2 or have used categorical grading.19 Others have defined NP as a significant hypofluorescence20 or a hypofluorescent area of at least 1-disk diameter.21 The SCORE study22 has referred to “portions of the fundus devoid of retinal arterioles and/or capillaries, with a “pruned” appearance of adjacent arterioles and a darker appearance of the choroid.” The same approach has been taken in DR by others.23,24 However, although the latter definition includes an objective feature—the loss of vascular branching—it is also based on a more subjective assessment—the background hypofluorescence, although some authors have proposed to apply a minimum threshold to define black and white pixels.25
In our cases, it appeared that the “background hypofluorescence” component was not well adapted to the follow-up of DR under anti-VEGF therapy. Indeed, although the background of NP areas became grayer after anti-VEGF, we never saw the reperfusion of the occluded arterioles and venules in these areas. Of note, this evolution from dark to gray was concomitant with the vasoconstriction of retinal vessels and the resolution of fluorescein leakage, which could have modified the contrast between retinal vessels and the choroidal background, in particular on digital imaging that automatically enhances the gain. We thus considered that the inconstant brightening of the background after anti-VEGF did not correspond to capillary reperfusion in the absence of reperfusion of distal arterioles and venules in the corresponding area.
The decrease in the DRSS score on color fundus photographs under anti-VEGF therapy without improvement in capillary perfusion, which is the leading risk factor for neovascular proliferation, also has clinical implications for the follow-up of these eyes. The absence of reperfusion under anti-VEGF therapy implies that, at least in the short term, in case of treatment discontinuation, the risk of worsening from severe NPDR to PDR persists. Similarly, in the event that a case of PDR returns to the stage of NPDR, the risk of new vessel recurrence is still present. This is a serious issue, as it has been shown in a large series of patients that about 22% of those who received anti-VEGF for DR were lost to follow-up.26 A progression toward PDR has been observed in 2.4% to 9% of cases despite monthly anti-VEGF injections over 36 months,27 and in another study, 78% of patients remained at the PDR stage after regular aflibercept intravitreal injections over 1 year,18 suggesting that there is still a risk of neovascularization in some eyes, and reperfusion cannot be guaranteed after anti-VEGF injections. How to evaluate or monitor these eyes when discontinuation of anti-VEGF injections is planned remains to be determined.
Our study has some limitations. First, it has a retrospective design, and it includes a relatively small number of eyes, but all the cases underwent multimodal imaging with excellent image quality, especially for FA, and careful control of possible biases. Second, it is a short-term study assessing patients after three anti-VEGF injections without a control group (without injection), so that the long-term effect of anti-VEGF cannot be extrapolated. A slowdown of DR worsening with a long-term treatment could not be excluded. We could also assume that a prolonged anti-VEGF treatment could decrease the risk of neovascular proliferation despite NP persistence, but to date, studies seem to suggest the opposite in retinal vein occlusion21 and in DR.3,18,27 Third, we used UWF photographs and FA, which is not yet a validated method for DR severity grading. Indeed, when measuring the NP area, UWF may be subject to distortion errors. Here, we used a method that controlled this bias as much as possible by adequately aligning preintervention and postintervention images and studying robust data such as the perfusion of arterioles and venules, and not only the areas of background darkness. Moreover, the advantage of UWF FA is to display high-quality images in a single shot, not only of the posterior pole but also of the midretinal periphery, which allows precisely locating NP areas for comparisons.2,11,21 Finally, we used the AAO severity scale rather than the ETDRS severity scale, which could be less precise than the latter. However, this is compensated by the fact that UWF imaging improves the diagnosis and classification of DR.10 Nevertheless, we showed here that the DRSS score could improve without any improvement in retinal vessel perfusion while it is assumed to be one of its surrogates. These results should be taken into account in further studies aiming to demonstrate an improvement in peripheral retinal perfusion in DR.
In conclusion, in a series of 18 eyes with NPDR or PDR, examined with UWF color photographs and FA, 3 monthly injections of anti-VEGF improved the DRSS score and macular edema. However, no reperfusion occurred in the NP areas detected at baseline on FA. Thus, after anti-VEGF injections, there is a discrepancy between fundus color photographs and FA interpretation, and the improvement in the DRSS score should be interpreted with caution concerning the risk of neovascularization.
Acknowledgments
Support for English language editing: Sophie Pégorier.
Footnotes
Presented at International Retinal Imaging Society and Macula Society, Los Angeles, CA, February 20 and 24, 2018.
S. Bonnin reports personal fees from Bayer Healthcare, outside the submitted work; B. Dupas reports personal fees from Bayer, personal fees from Novartis, and personal fees from Allergan, outside the submitted work; A. Couturier reports personal fees from Allergan, personal fees from Bayer, and personal fees from Novartis, outside the submitted work. A. Erginay reports personal fees from Allergan, personal fees and nonfinancial support from Bayer, personal fees and nonfinancial support from Optovue, personal fees and nonfinancial support from Novartis, and personal fees from Zeiss, outside the submitted work. A. Gaudric reports personal fees from Novartis, personal fees from Bayer Healthcare, and personal fees from Thrombogenics, outside the submitted work; R. Tadayoni reports personal fees from Novartis, personal fees from Bayer, personal fees from Roche, personal fees from Genentech, personal fees from Allergan, personal fees from Zeiss, and personal fees from Alcon, outside the submitted work. The remaining authors have no financial/conflicting interests to disclose.
All authors attest that they meet the current ICMJE criteria for authorship.
References
- 1.Bressler SB, Liu D, Glassman AR, et al. Change in diabetic retinopathy through 2 years: secondary analysis of a randomized clinical trial comparing aflibercept, bevacizumab, and ranibizumab. JAMA Ophthalmol 2017;135:558–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva PS, Dela Cruz AJ, Ledesmav MG, et al. Diabetic retinopathy severity and peripheral lesions are associated with nonperfusion on ultrawide field angiography. Ophthalmology 2015;122:2465–2472. [DOI] [PubMed] [Google Scholar]
- 3.Bressler SB, Beaulieu WT, Glassman AR, et al. Factors associated with worsening proliferative diabetic retinopathy in eyes treated with panretinal photocoagulation or ranibizumab. Ophthalmology 2017;124:431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimble JA, Brandt BM, McGwin G., Jr Clinical examination accurately locates capillary nonperfusion in diabetic retinopathy. Am J Ophthalmol 2005;139:555–557. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson CP, Ferris FL, III, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677–1682. [DOI] [PubMed] [Google Scholar]
- 6.Boyer DS, Nguyen QD, Brown DM, et al. Outcomes with as-needed ranibizumab after initial monthly therapy: long-term outcomes of the phase III RIDE and RISE trials. Ophthalmology 2015;122:2504–2513 e2. [DOI] [PubMed] [Google Scholar]
- 7.Elman MJ, Bressler NM, Qin H, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2011;118:609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ip MS, Domalpally A, Sun JK, Ehrlich JS. Long-term effects of therapy with ranibizumab on diabetic retinopathy severity and baseline risk factors for worsening retinopathy. Ophthalmology 2015;122:367–374. [DOI] [PubMed] [Google Scholar]
- 9.Writing Committee for the Diabetic Retinopathy Clinical Research Network, Glassman AR, Jampol LM, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA 2015;314:2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghasemi Falavarjani K, Wang K, Khadamy J, Sadda SR. Ultra-wide-field imaging in diabetic retinopathy; an overview. J Curr Ophthalmol 2016;28:57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun JK, Aiello LP. The future of ultrawide field imaging for diabetic retinopathy: pondering the retinal periphery. JAMA Ophthalmol 2016;134:247–248. [DOI] [PubMed] [Google Scholar]
- 12.Karst SG, Deak GG, Gerendas BS, et al. Association of changes in macular perfusion with ranibizumab treatment for diabetic macular edema: a subanalysis of the RESTORE (extension) study. JAMA Ophthalmol 2018;136:315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mané V, Dupas B, Gaudric A, et al. Correlation between cystoid spaces in chronic diabetic macular edema and capillary nonperfusion detected by optical coherence tomography angiography. Retina 2016;36:S102–S110. [DOI] [PubMed] [Google Scholar]
- 14.Aiello LP, Pierce EA, Foley ED, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA 1995;92:10457–10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonnin P, Pournaras JA, Makowiecka K, et al. Ultrasound assessment of ocular vascular effects of repeated intravitreal injections of ranibizumab for wet age-related macular degeneration. Acta Ophthalmol 2014;92:e382–e387. [DOI] [PubMed] [Google Scholar]
- 16.Papadopoulou DN, Mendrinos E, Mangioris G, et al. Intravitreal ranibizumab may induce retinal arteriolar vasoconstriction in patients with neovascular age-related macular degeneration. Ophthalmology 2009;16:1755–1761. [DOI] [PubMed] [Google Scholar]
- 17.Sacu S, Pemp B, Weigert G, et al. Response of retinal vessels and retrobulbar hemodynamics to intravitreal anti-VEGF treatment in eyes with branch retinal vein occlusion. Invest Ophthalmol Vis Sci 2011;52:3046–3050. [DOI] [PubMed] [Google Scholar]
- 18.Sivaprasad S, Prevost AT, Bainbridge J, et al. Clinical efficacy and mechanistic evaluation of aflibercept for proliferative diabetic retinopathy (acronym CLARITY): a multicentre phase IIb randomised active-controlled clinical trial. BMJ Open 2015;5:e008405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mir TA, Kherani S, Hafiz G, et al. Changes in retinal nonperfusion associated with suppression of vascular endothelial growth factor in retinal vein occlusion. Ophthalmology 2016;123:625–634 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JH, Jung HG, Chung HJ, et al. Simplified correction of ischemic index in diabetic retinopathy evaluated by ultra-widefield fluorescein angiography. Kor J Ophthalmol 2015;29:168–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wessel MM, Aaker GD, Parlitsis G, et al. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina 2012;32:785–791. [DOI] [PubMed] [Google Scholar]
- 22.Chan CK, Ip MS, Vanveldhuisen PC, et al. SCORE study report #11 incidences of neovascular events in eyes with retinal vein occlusion. Ophthalmology 2011;118:1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan W, Wang K, Ghasemi Falavarjani K, et al. Distribution of nonperfusion area on ultra-widefield fluorescein angiography in eyes with diabetic macular edema: DAVE study. Am J Ophthalmol 2017;180:110–116. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson L, Vazquez-Alfageme C, Ramu J, et al. Validation of concentric rings method as a topographic measure of retinal nonperfusion in ultra-widefield fluorescein angiography. Am J Ophthalmol 2015;160:1217–1225 e2. [DOI] [PubMed] [Google Scholar]
- 25.Levin AM, Rusu I, Orlin A, et al. Retinal reperfusion in diabetic retinopathy following treatment with anti-VEGF intravitreal injections. Clin Ophthalmol 2017;11:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obeid A, Gao X, Ali FS, et al. Loss to follow-up in patients with proliferative diabetic retinopathy after panretinal photocoagulation or intravitreal anti-VEGF injections. Ophthalmology 2018;125:1386–1392. [DOI] [PubMed] [Google Scholar]
- 27.Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials. Ophthalmology 2013;120:2013–2022. [DOI] [PubMed] [Google Scholar]


