Abstract
Pruritus is one of the common side effects of intrathecal or epidural injection of opioids. The aim of this study was to test the antipruritic effect of acupuncture and its possible mechanism. We used electroacupuncture (EA), toll-like receptor (TLR)2/4 antagonist sparstolonin B (SsnB), and TLR2/4 agonist peptidoglycan (PGN) to precondition female wild-type BALB/c mice, and then prepared a morphine-induced pruritus model. The mRNA and protein expression levels of TLR2, TLR4, MyD88, and NF-κB were detected by RT-PCR and western blotting. The contents of interleukin (IL)-1, IL-6, IL-12, IL-10, and tumor necrosis factor-α in serum were measured by ELISA assays. Flow cytometry was performed to analyze the ratio of M1-phenotype to M2-phenotype macrophages. Our results showed that EA preconditioning improved pruritus; reduced the expressions of TLR2, TLR4, MyD88, and NF-κB both at the mRNA and protein levels (P<0.05); reduced the expression of proinflammatory cytokines IL-1, IL-6, IL-12, and tumor necrosis factor-α; and increased the expression of anti-inflammatory cytokine IL-10 (P<0.05). EA promoted M2-phenotype macrophage differentiation. Moreover, these results showed no significant difference between the SsnB group and the EA+SsnB group (P>0.05), but showed a significant difference between the PGN group and the EA+PGN group (P<0.05). Therefore, we propose that EA may be involved in the remission of pruritus in morphine-induced pruritus model mice through the TLR2/4-MyD88-NF-κB pathway. EA is a potential therapeutic treatment for pruritus.
Keywords: electroacupuncture, inflammatory cytokines, macrophages, pruritus, TLR2/4-MyD88-NF-κB pathway
Introduction
Opioids are used in the management of moderate to severe pain, and pruritus is a common adverse effect. The incidence of pruritus is reported to be 10–50% following intravenous administration of opioids, and 20–100% following neuraxial administration 1. Although not life threatening, pruritus is discomforting and may decrease patient satisfaction. Mu opioid receptor antagonists have been the most consistent in terms of attenuating opioid-induced pruritus, but present problems in dose and administration. And other drugs (e.g. mixed opioid receptor agonist-antagonists, serotonin 5-HT3 receptor antagonists) have also been demonstrated to be useful 2. However, current evidence is still insufficient to clearly mandate prophylactic administration of drugs to prevent opioid-induced pruritus. It has been reported that morphine and other opioids can provoke neuroinflammation by activating the innate immune system, including toll-like receptors (TLRs), but how the compounds turn on TLRs’ signaling remains unclear 3,4.
Acupuncture is an ancient Chinese medical technique that is widely used as a form of complementary and alternative medicine in Asia. Acupuncture has been reported to be a relatively safe and well-tolerated therapy with only minor side effects, such as mild pain and local hematoma 5–7. In clinical settings, reports about acupuncture treatment for pruritus have increased in recent years 8. In a retrospective case series, acupuncture seemed to be effective in alleviating the distressing symptom of itching in patients presenting with neurogenic pruritus 9. Pfab et al. 10 found that acupuncture at Quchi (LI11) and Xuehai (SP10) produced a significant reduction of clinically relevant type I hypersensitivity itch sensation in patients with atopic dermatitis. However, as a traditional Chinese medicine, the underlying mechanism of acupuncture on pruritus is not clear 11.
Sparstolonin B (SsnB), isolated from the Chinese herb Scirpus yagara, is a newly identified polyphenol with structural features of xanthone and isocoumarin 12. Peptidoglycan (PGN) is the main cell wall component of Gram-positive bacteria 13. A number of studies have shown that SsnB and PGN are TLR2/4 antagonist and agonist, respectively 12,14–16. In the current study, we used SsnB to block, and PGN to activate, the TLR2/4-MyD88-NF-κB pathway, to study whether electroacupuncture (EA) plays an antipruritic role through the TLR2/4-MyD88-NF-κB pathway.
Materials and methods
Animals and reagents
Seventy adult female wild-type BALB/c mice were purchased from the Experimental Animal Center of Sun Yat-sen University. The mice were fed in 12-h light/dark cycles, with a light time of 8:00–20:00. In-vivo experiments were conducted at 9:00–12:00. Both the animal care and study protocol were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and followed the guidelines of the Ethics Committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University. All efforts were made to minimize suffering. SsnB and PGN were purchased from InvivoGen (California, USA). The mice were divided into blank group, model group, EA group, SsnB group, EA+SsnB group, PGN group and EA+PGN group, 10 mice in each group.
Treatment and preparation of the pruritus model
The bilateral acupoints of Quchi (LI11) and Xuehai (SP10) were selected for EA. Ipsilateral Quchi and Xuehai were connected to the same pair of electrodes. Quchi is located in the proximal side of the radial proximal joint of the mouse, and Xuehai is located in the medial hind-limb femur, medial end of the patella to the pubic symphysis junction under the 1/9. The mice were fixed on a mouse frame and treated with an intensity of 2 mA and a frequency of 2/15 Hz dilatational wave for 30 min. The acupuncture needle (7 mm×0.20 mm; Huatuo, Suzhou, China) was inserted into the specific point at a depth of 5 mm vertically. EA preconditioning (i.e. preventive approach before itch provocation) was conducted each day for 5 consecutive days before modeling.
The EA mice were randomly divided into an EA group (intraperitoneal injection of the same volume of normal saline), EA+SsnB group, and EA+PGN group. For SsnB/PGN pretreatments, wild-type BALB/c mice were injected intraperitoneally with 5 mg/kg SsnB or 2 mg/kg PGN, respectively, without EA preconditioning (served as SsnB group and PGN group), or with EA preconditioning (served as EA+SsnB group and EA+PGN group) before modeling. The mice of the blank group were only fixed on the mouse frame for 30 min each day for 5 consecutive days before modeling, but without EA preconditioning or SsnB/PGN pretreatment.
After the last EA intervention, the model group and EA mice were used immediately for modeling. In this study, we used intrathecal morphine injection to induce pruritus 17,18. Each mouse was held firmly by the pelvic girdle in one hand, while a microsyringe (Guangda Co. Ltd, Shanghai, China) was held in the other hand at an angle of about 20° above the vertebral column, and the experimenter inserted it into the tissue to one side of the L5 or L6 spinous process. The needle was then moved carefully forward to the intervertebral space, as the angle of the microsyringe was decreased to about 10°. The tip of the needle was inserted 4 mm into the vertebral column. Five microliters (0.3 nmol) morphine hydrochloride (Northeast Pharmaceutical Group, Shenyang, China) solution was injected for 5 s, and the needle was rotated on withdrawal. The blank group was injected intrathecally with 5 μl of normal saline according to the above procedure.
Behavioral assays
Behavioral assessment was carried out with reference to the literature 15. Each group of mice was placed in the quiet environment of a behavior observation device without human presence, and their activity was recorded within 30 min after modeling using a camera. Using video playback, three persons blinded to the treatment groups independently counted the number of times the mice scratched. Scratching behavior of a mouse within 1 s was recorded as a scratch.
Sampling methods
Mice were anesthetized with halothane and decapitated. The spinal cord tissue was perfused with normal saline. When the liquid became colorless and transparent, it indicated that the spinal cord had been rinsed. The intumescentia lumbalis of the spinal cord was taken out and frozen in liquid nitrogen. After normal saline perfusion and then reperfusion with prechilled 4% paraformaldehyde, the intumescentia lumbalis of the spinal cord was eviscerated and sliced until all tissues were fully fixed (stretching the limbs with a sense of stiffness).
Total RNA extraction and quantitative RT-PCR
Total RNA was extracted from the spinal cords by using TRIzol reagent (Invitrogen, California, USA). A cDNA Synthesis Kit (Takara, Dalian, China) was used for the synthesis of cDNA according to the manufacturer’s instructions. Quantitative RT-PCR was performed to detect the expression levels of mRNA using SYBR Green and a LightCycler 480 detection system (Roche Diagnostics, Indianapolis, Indiana, USA). GAPDH mRNA levels were used for normalization. The quantitative RT-PCR results were analyzed and expressed as relative mRNA levels on the basis of Ct value, which was then converted to fold change.
Western blot assays
Spinal cord tissue frozen by liquid nitrogen was ground, and then the cells were lysed using Lysis Buffer (P0013; Beyotime, Shanghai, China). The total protein concentration of lysates was measured using a Micro BCA Protein Assay Kit (Pierce, Rockford, Illinois, USA). The samples were separated on 10% SDS-polyacrylamide gels and then electrophoretically transferred to poly-vinylidene difluoride membranes. After incubation in blocking solution (5% non-fat milk) for 1 h, the membranes were incubated with anti-TLR2, anti-MyD88, anti-NF-κB, or GAPDH antibodies (CST, Massachusetts, USA) diluted in blocking solution for 2 h at 37°C. Thereafter, the membranes were washed with 1× TBST solution and treated with horseradish peroxidase-conjugated secondary antibodies for 1 h. Next, the membranes were treated with ECL solution (Millipore, Darmstadt, Germany) according to the manufacturer’s instructions and then quantified using a ChemiDoc image analysis system (Bio-Rad Laboratories, California, USA). The mean gray values of each protein band were measured by Photoshop CS5 software (Adobe, San Jose, California, USA). The relative band intensity is the ratio of the gray value of the protein of interest to that of the corresponding GAPDH.
Detection of inflammatory cytokines by ELISA
Phosphate buffer (0.5 mol/l Na2HPO4 0.5 ml and 0.5 mol/l NaH2PO4 0.5 ml) was added to 100 mg spinal cord tissue and homogenized with a tissue homogenizer in an ice bath. The protein concentration was diluted to 1–5%. After centrifugation, the supernatant was saved. The inflammatory cytokines interleukin (IL)-1β (ab100705; Abcam, Cambridge, USA), IL-6 (ab100713; Abcam), IL-10 (ab108870; Abcam), and tumor necrosis factor (TNF)-α (ab100747; Abcam) were measured using ELISA kits. The procedures were carried out according to the manufacturer’s instructions.
Flow cytometric analysis of macrophages
Macrophage separation and flow cytometric analysis are described in a previous report 19. Fresh spinal cord tissue was ground into a single-cell suspension and filtered through a 45-mm nylon mesh filter. The macrophages were then separated by density gradient centrifugation. Isolated macrophages were collected and washed by PBS, and then incubated with FITC anti-CD86 antibodies (BD Pharmingen; BD Biosciences, San Jose, California, USA) and anti-CD163 antibodies (GeneTex, Irvine, California, USA) for 30 min at room temperature. After being washed by PBS, the cells were analyzed on an FACSCalibur flow cytometer (Accuri C6; BD Biosciences).
Statistical methods
SPSS19.0 statistical analysis software (IBM SPSS, Chicago, Illinois, USA) was utilized for statistical analyses. The significance of the differences was determined by Student’s t-test or one-way analysis of variance. The least significant difference post-hoc test was used where equal variances were assumed, whereas Dunnett’s T3 test was used when equal variances were not assumed. The data are presented as the mean±SD, and P value less than 0.05 indicates that the difference was statistically significant.
Results
Electroacupuncture improved pruritus through the TLR2/4-MyD88-NF-κB pathway
As shown in Fig. 1, compared with the blank group, the number of scratches in the model group was markedly increased (P<0.01). With EA preconditioning only, the number of scratches was significantly reduced (P<0.05). With EA and TLR2 antagonist SsnB preconditioning, EA+SsnB group did not show stronger antipruritic effect than the EA group or the SsnB group (P>0.05). Preconditioning with TLR2 agonist PGN resulted in significantly increased pruritus in the PGN group compared with the model group (P<0.05). Conversely, together with EA and PGN preconditioning, the EA+PGN group had significantly improved pruritus compared with the PGN group (P<0.01).
Fig. 1.
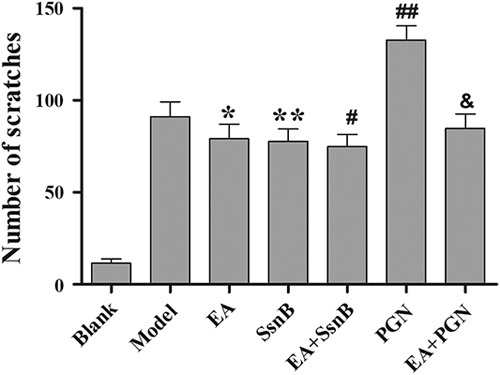
The statistical results of scratch number in each group. Using video playback, three persons blinded to the treatment groups independently counted the number of times the mice scratched. Scratching behavior of a mouse within 1 s was recorded as a scratch. The data are presented as mean±SD (n=10). *P<0.05, **P<0.05 and ##P<0.05 compared with the model group, #P>0.05 compared with the SsnB group. &P<0.01 compared with the PGN group. EA, electroacupuncture; PGN, peptidoglycan; SsnB, sparstolonin B.
Electroacupuncture affected the expressions of TLR2, TLR4, MyD88 and NF-κB
Using qRT-PCR and western blotting (Fig. 2), we found that compared with the blank group, TLR2, TLR4, MyD88 and NF-κB expression was significantly upregulated in the model group (P<0.01), whereas EA preconditioning markedly downregulated their expression levels (P<0.05). Moreover, we also found that the expressions of TLR2, TLR4, MyD88 and NF-κB were significantly downregulated in the SsnB group and the EA+SsnB group compared with the model group (P<0.05), but these results showed no significant difference between the SsnB group and the EA+SsnB group (P>0.05). PGN preconditioning increased TLR2, TLR4, MyD88 and NF-κB expression compared with the model group (P<0.05), whereas EA+PGN treatment reduced TLR2, TLR4, MyD88 and NF-κB expression compared with PGN preconditioning only (P<0.05).
Fig. 2.
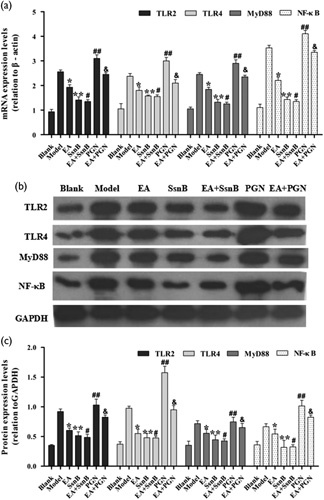
mRNA and protein expression levels of TLR2, MyD88 and NF-κB in each group. (a) Relative mRNA expression levels of TLR2, TLR4, MyD88 and NF-κB by RT-PCR. (b) Western blot detected the expression of TLR2, TLR4, MyD88 and NF-κB. (c) Photoshop CS5 software analysis of the protein expression levels (relation to GAPDH). The data are presented as mean±SD (n=10). Each bar represents the mean of three independent experiments performed in triplicate. *P<0.05, **P<0.05 and ##P<0.05 compared with the model group, #P>0.05 compared with the SsnB group. &P<0.05 compared with the PGN group. EA, electroacupuncture; PGN, peptidoglycan; SsnB, sparstolonin B; TLR, toll-like receptor.
Electroacupuncture reduced inflammatory response
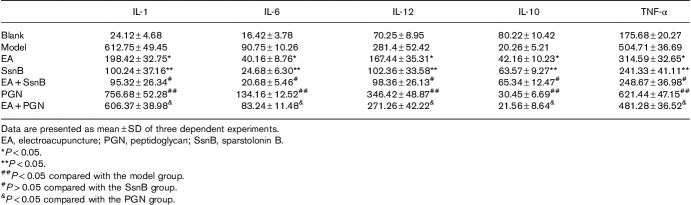
We used ELISA assays to explore the effects of EA on the expression of inflammatory cytokines in serum. ELISA results (Table 1) showed that EA significantly reduced the contents of IL-1, IL-6, IL-12, and TNF-α and increased the concentration of IL-10 compared with the pruritus model mice (P<0.05). Furthermore, we found that the concentrations of IL-1, IL-6, IL-12, and TNF-α were significantly downregulated, and IL-10 upregulated, in the SsnB group and the EA+SsnB group compared with the model group (P<0.01), but these results have no significant difference between the SsnB group and the EA+SsnB group (P>0.05). PGN preconditioning increased the concentrations of IL-1, IL-6, IL-12 and TNF-α, and reduced the concentration of IL-10 compared with the model group (P<0.05). However, EA+PGN treatment significantly reduced the concentrations of IL-1, IL-6, IL-12 and TNF-α, and increased the concentration of IL-10 compared with PGN preconditioning only (P<0.05).
Table 1.
The levels of inflammatory cytokines in each group (n=10)
Electroacupuncture regulated macrophage differentiation
We evaluated the M1-phenotype to M2-phenotype ratio using flow cytometry (Fig. 3). In morphine-induced pruritus model mice, the M1 macrophage ratio (CD86-FITC positive) was 65.2±3.45%, whereas the proportion of M2 macrophages (CD163-PE positive) was only 34.7±3.40%. EA preconditioning markedly downregulated the proportion of M1 macrophages and upregulated the proportion of M2 macrophages compared with the model group (P<0.05). In addition, the ratio of M1/M2 macrophages showed no significant difference between the SsnB group and the EA+SsnB group (P>0.05), but showed significant difference between the PGN group and the EA+PGN group (P<0.05).
Fig. 3.
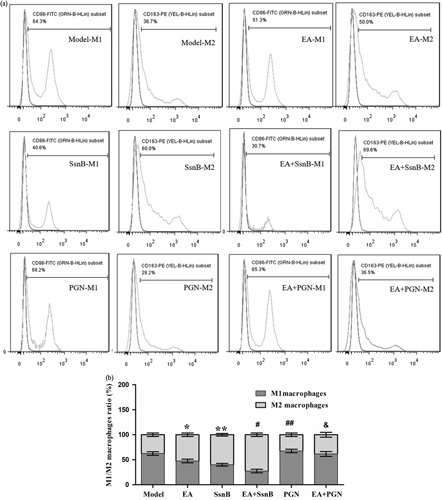
Flow cytometric analysis of M1-phenotype and M2-phenotype. (a) M1-phenotype and M2-phenotype macrophages measured using flow cytometry. (b) The M1-phenotype to M2-phenotype macrophage ratio in each group. CD86 used for M1-phenotype macrophage-specific marker and CD163 used for M2-phenotype macrophage-specific biomarker. The purple line in each image represents the blank group, and the orange line represents the corresponding group. Each bar represents the mean of three independent experiments performed in triplicate. *P<0.05, **P<0.05 and ##P<0.05 compared with the model group, #P>0.05 compared with the SsnB group. &P<0.05 compared with the PGN group. EA, electroacupuncture; PGN, peptidoglycan; SsnB, sparstolonin B.
Discussion
In long-term practice, acupuncturists have gradually found effective combinations of anti-itching points-Quchi (LI11) and Xuehai (SP10). Wang et al. 20 found that EA at LI11 and SP10 significantly inhibits itching reactions in mice induced by compound 48/80, which might be an effective method for treatment of pruritus. Meng et al. 21 discovered that acupuncture at LI11 and SP10 relieves itching as well as having an antihistamine and antianaphylaxis effect. A further study comparing electrical stimulation of acupoints LI11 and SP10 to cetirizine showed significant itch reduction after verum acupuncture or cetirizine treatment compared with respective placebos and no treatment in atopic dermatitis patients 22. In the present study, we found that EA improved morphine-induced pruritus. This is consistent with the above previous studies suggesting that EA does indeed inhibit pruritus. Specific acupoint selection (LI11 and SP10) and manipulation (electrical stimulation method, needle insertion depth, and time) may play an important role in antipruritic effect. Weak acupoint stimulation might not generate an antipruritic effect with application of intrathecal morphine 23.
The TLR2/4-MyD88-NF-κB pathway is associated with immune response; its activation is key to the development of many diseases, and NF-κB is a pivotal downstream signaling molecule in the TLR2/4-MyD88 pathway 16,24. MyD88, the first identified member of the TLR family, is universally used by all TLRs, except TLR3, and activates the transcription factor NF-κB to induce inflammatory cytokines 25. The subfamily of TLR2 receptors can activate NF-κB and AP-1-regulated genes, including the inflammatory cytokines TNF-α and IL-6 26. The inflammatory cytokines include IL-1, IL-4, IL-5, IL-10, IL-12, IL-13, IL-17, interferon-γ, and TNF-α. In this study, the mRNA and proteins of TLR2, TLR4, MyD88 and NF-κB increased in the morphine-induced pruritus model mice, and EA decreased the expression of all four. Moreover, EA decreased the concentrations of proinflammatory cytokines IL-1, IL-6, IL-12, and TNF-α and increased that of the anti-inflammatory cytokine IL-10. These results showed that EA can regulate the immune response.
Macrophages have functions of damage and repair, and these different effects may be administered by different macrophage subsets: the ‘classic activation’ of proinflammatory (M1) macrophages and ‘alternative activation’ of anti-inflammatory (M2) macrophages 27. Phenotypes associated with M1 and M2 macrophages can be distinguished from the direct effects of microbial stimuli, such as lipopolysaccharide, which induce innate macrophage activation, often through TLRs. Innate stimuli are able to synergize with M1 macrophages to achieve full expression of macrophage effector pathways 28. In the presence of cytokines, the activating macrophages increase their ‘alternatively activated’ M2-phenotype, which promotes matrix remodeling and angiogenesis while suppressing destructive immunity 29. In this study, the M1 macrophages were activated in the morphine-induced pruritus model mice, and EA inhibited M1 macrophage activation. In addition, after EA treatment, the inflammatory cytokine levels decreased, and these effects play a useful role in attenuating pruritus.
A previous study showed that SsnB is a selective TLR2 and TLR4 antagonist that can block TLR2-triggered and TLR4-triggered inflammatory signaling by inhibiting the recruitment of MyD88 to the TIR domains of TLR2 and TLR4 12,14. A number of studies have shown that PGN activates cells through TLR2, which results in the activation of NF-κB 15,16. It is believed that TLR2 agonists mainly induce the production of inflammatory cytokines 30, while, conversely, TLR2 antagonists inhibit the production of inflammatory cytokines. In the present study, compared with the model group, EA, SsnB and the combination of EA and SsnB preconditioning, respectively, improved pruritus; decreased the expression of TLR2, TLR4, MyD88 and NF-κB; decreased the concentrations of proinflammatory cytokines IL-1, IL-6, IL-12, TNF-α; increased the concentration of the anti-inflammatory cytokine IL-10; and inhibited M1 macrophage increase. EA and SsnB did not have mutual enhancement or counteraction with each other but had similar effects on antipruritus and inflammatory response. PGN preconditioning strengthened pruritus. The expressions of TLR2, TLR4, MyD88, NF-κB and related inflammatory response in PGN group had stronger increased effects than those in model group. However, a combination of EA and PGN preconditioning affected the above results markedly compared with PGN preconditioning only. These results indicate that EA exerts its antipruritic effects through the TLR2/4-MyD88-NF-κB pathway.
Limitations
Our study has several limitations. First, we did not use gene knockout mice to confirm the specific role of TLR2 or TLR4 related to the mechanisms of acupuncture-related antipruritic effect. Second, besides TLR2/4-MyD88-NF-κB pathway, there are many other mediators and neuronal pathways in the relieving process of itch, the potential effects of which we cannot exclude. Third, there was no acupuncture control in this study. Therefore, the changes in this research might not be induced by acupuncture or acupoint specificity but rather by mechanical and electrical stimulation of acupuncture. Hence, further observations with stricter control of these relevant variables are inevitable to undrape the antipruritic mechanism of acupuncture.
Conclusion
EA was involved in the remission of pruritus in morphine-induced pruritus model mice through the TLR2/4-MyD88-NF-κB pathway, and EA may be a potential therapeutic treatment for pruritus. Further research is needed to assess the effect of acupuncture on morphine-induced pruritus in human patients and to explore other related molecular events.
Acknowledgements
Yu Shan Ye and Ai Zhen Pan had the same contribution to the paper. Yan Zhen, Meng Ru Kang and Bin Zhang participated in the research. Wei Min Yi wrote the final paper.
This research was supported by grants from the National Natural Science Foundation of China (no: 81503651).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Horta ML, Ramos L, Goncalves ZR. The inhibition of epidural morphine-induced pruritus by epidural droperidol. Anesth Analg 2000; 90:638–641. [DOI] [PubMed] [Google Scholar]
- 2.Ganesh A, Maxwell LG. Pathophysiology and management of opioid-induced pruritus. Drugs 2007; 67:2323–2333. [DOI] [PubMed] [Google Scholar]
- 3.Shah M, Choi S. Toll-like receptor-dependent negative effects of opioids: a battle between analgesia and hyperalgesia. Front Immunol 2017; 8:642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talkington MW, McCaffrey P. Morphine mimics endotoxin action at innate immune receptor TLR4. Available at: https://www.painresearchforum.org/node/15713 [27 April 2012].
- 5.Sniezek DP, Siddiqui IJ. Acupuncture for treating anxiety and depression in women: a clinical systematic review. Med Acupunct 2013; 25:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi WM, Chen Q, Liu CH, Hou JY, Chen LD, Wu WK. Acupuncture for preventing complications after radical hysterectomy: a randomized controlled clinical trial. Evid Based Complement Alternat Med 2014; 25:802134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He X, Zhu Y, Li C, Park K, Mohamed AZ, Wu H, et al. Acupuncture-induced changes in functional connectivity of the primary somatosensory cortex varied with pathological stages of Bell’s palsy. Neuroreport 2014; 25:1162–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu C, Zhang P, Lv ZT, Li JJ, Li HP, Wu CH, et al. Efficacy of acupuncture in itch: a systematic review and meta-analysis of clinical randomized controlled trials. Evid Based Complement Alternat Med 2015; 2015:208690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stellon A. Neurogenic pruritus: an unrecognised problem? A retrospective case series of treatment by acupuncture. Acupunct Med 2002; 20:186–190. [DOI] [PubMed] [Google Scholar]
- 10.Pfab F, Huss-Marp J, Gatti A, Fuqin J, Athanasiadis GI, Irnich D, et al. Influence of acupuncture on type I hypersensitivity itch and the wheal and flare response in adults with atopic eczema – a blinded, randomized, placebo-controlled, crossover trial. Allergy 2010; 65:903–910. [DOI] [PubMed] [Google Scholar]
- 11.Han JB, Kim CW, Sun B, Kim SK, Lee MG, Park DS, et al. The antipruritic effect of acupuncture on serotonin-evoked itch in rats. Acupunct Electrother Res 2008; 33:145–156. [DOI] [PubMed] [Google Scholar]
- 12.Liang Q, Wu Q, Jiang J, Duan J, Wang C, Smith MD, et al. Characterization of sparstolonin B, a Chinese herb-derived compound, as a selective Toll-like receptor antagonist with potent anti-inflammatory properties. J Biol Chem 2011; 286:26470–26479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arbeloa A, Hugonnet JE, Sentilhes AC, Josseaume N, Dubost L, Monsempes C, et al. Synthesis of mosaic peptidoglycan cross-bridges by hybrid peptidoglycan assembly pathways in gram-positive bacteria. J Biol Chem 2004; 279:41546–41556. [DOI] [PubMed] [Google Scholar]
- 14.Liang Q, Dong S, Lei L, Liu J, Zhang J, Li J, et al. Protective effects of Sparstolonin B, a selective TLR2 and TLR4 antagonist, on mouse endotoxin shock. Cytokine 2015; 75:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dziarski R, Wang Q, Miyake K, Kirschning CJ, Gupta D. MD-2 enables Toll-like receptor 2 (TLR2)-mediated responses to lipopolysaccharide and enhances TLR2-mediated responses to Gram-positive and Gram-negative bacteria and their cell wall components. J Immunol 2001; 166:1938–1944. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Dziarski R, Kirschning CJ, Muzio M, Gupta D. Micrococci and peptidoglycan activate TLR2→MyD88→IRAK→TRAF→NIK→IKK→NF-κB, signal transduction pathway that induces transcription of interleukin-8. Infect Immun 2001; 69:2270–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu XY, Liu ZC, Sun YG, Ross M, Kim S, Tsai FF, et al. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell 2011; 147:447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol 1980; 67:313–316. [DOI] [PubMed] [Google Scholar]
- 19.Baiyila B, He B, He G, Long T. Anti-inflammatory effect of Mongolian drug Naru-3 on traumatic spinal cord injury and its mechanism of action. J Int Med Res 2018; 46:2346–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Tang ZX, Bin XU. Effects of electroacupuncture at Quchi (LI 11) and Xuehai (SP 10) on compound 48/80-induced pruritus model of mice. China J Tradit Chin Med Pharm 2014; 29:117–120. [Google Scholar]
- 21.Meng H, Shi YJ. Effect of Acupuncture at Quchi(LI11) and Xuehai(SP10) on Mice Model with Itching. Chin J Inform Tradit Chin Med 2011; 18:40–41. [Google Scholar]
- 22.Pfab F, Kirchner MT, Huss-Marp J, Schuster T, Schalock PC, Fuqin J, et al. Acupuncture compared with oral antihistamine for type I hypersensitivity itch and skin response with atopic dermatitis: a patient- and examiner-blinded, randomized, placebo-controlled, crossover trial. Allergy 2012; 67:566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazda Y, Kikuchi T, Yoshimatsu A, Kato A, Nagashima S, Terui K. Acupuncture for reducing pruritus induced by intrathecal morphine at elective cesarean delivery: a placebo-controlled, randomized, double-blinded trial. Int J Obstet Anesth 2018; 36:66–76. [DOI] [PubMed] [Google Scholar]
- 24.Segovia J, Sabbah A, Mgbemena V, Tsai SY, Chang TH, Berton MT, et al. TLR2/MyD88/NF-κB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS One 2012; 7:e29695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006; 124:783–801. [DOI] [PubMed] [Google Scholar]
- 26.Toshchakov V, Jones BW, Perera PY, Thomas K, Cody MJ, Zhang S, et al. TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat Immunol 2002; 3:392–398. [DOI] [PubMed] [Google Scholar]
- 27.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 2009; 29:13435–13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 2010; 32:593–604. [DOI] [PubMed] [Google Scholar]
- 29.Popovich PG, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci 2008; 9:481–493. [DOI] [PubMed] [Google Scholar]
- 30.Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol 2009; 10:1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]