Supplemental Digital Content is Available in the Text.
Key Words: linkage to HIV care, factors associated with linkage, test-and-treat, linkage strategy, risk factors
Abstract
Introduction:
As countries move toward universal HIV treatment, many individuals fail to link to care after diagnosis of HIV. Efficient and effective linkage strategies are needed.
Methods:
We implemented a patient-centered, multicomponent linkage strategy in the SEARCH “test-and-treat” trial (NCT 01864603) in Kenya and Uganda. After population-based, community-wide HIV testing, eligible participants were (1) introduced to clinic staff after testing, (2) provided a telephone “hot-line” for enquiries, (3) provided an appointment reminder phone call, (4) given transport reimbursement on linkage, and (5) tracked if linkage appointment was missed. We estimated the proportion linked to care within 1 year and evaluated factors associated with linkage at 7, 30, and 365 days after diagnosis.
Results:
Among 71,308 adults tested, 6811 (9.6%) were HIV-infected; of these, 4760 (69.9%) were already in HIV care, and 30.1% were not. Among 2051 not in care, 58% were female, median age was 32 (interquartile range 26–40) years, and median CD4 count was 493 (interquartile range 331–683) cells/µL. Half (49.7%) linked within 1 week, and 73.4% linked within 1 year. Individuals who were younger [15–34 vs. >35 years, adjusted Risk Ratio (aRR) 0.83, 95% confidence interval (CI): 0.74 to 0.94], tested at home vs. community campaign (aRR = 0.87, 95% CI: 0.81 to 0.94), had a high HIV-risk vs. low-risk occupation (aRR = 0.81, 95% CI: 0.75 to 0.88), and were wealthier (aRR 0.90, 95% CI: 0.83 to 0.97) were less likely to link. Linkage did not differ by marital status, stable residence, level of education, or having a phone contact.
Conclusions:
Using a multicomponent linkage strategy, high proportions of people living with HIV but not in care linked rapidly after HIV testing.
INTRODUCTION
The therapeutic and preventive benefits of antiretroviral therapy (ART) highlight the need and the urgency to identify individuals living with HIV, link them to care, and initiate them on ART.1–3 However, many people diagnosed with HIV fail to link to HIV care after diagnosis or link after long delays.4–6 Poor linkage to care is reported across sub-Saharan Africa, the region most heavily burdened by the HIV epidemic,7–9 posing a threat to the 90-90-90 UNAIDS strategy, which aims to ensure 90% of those living with HIV are aware of their status, 90% of those diagnosed are on ART, and 90% of those on ART achieve virological suppression.10,11 Linkage to care may prove an even greater challenge in the context of population-based HIV testing and the current World Health Organization's (WHO) treatment guidelines, under which every person living with HIV is eligible for ART to achieve better patient health outcomes and prevention of new infections.12 The population in greatest need of linkage continues to be those with advanced disease and at highest risk of death; however, in the context of population-wide test and treat, the population in need of linkage may increasingly be recently infected individuals, individuals who previously failed to link, or who have dropped out of care, asymptomatic persons, and individuals who have characteristics, such as mobility, that pose a challenge to both testing and linkage. Population approaches using mobile community-based interventions may further experience difficulties with linkage because testing is conducted outside health facilities. Recently completed and ongoing test-and-treat trials using community-based testing strategies (TasP and PopART trials conducted in South Africa and Zambia) report delayed time to linkage and low rates of linkage to care.11,13 These results highlight the importance of addressing linkage to care where HIV “test-and-treat” is part of the strategy to improve health and control the HIV epidemic.
We sought to evaluate a multicomponent, patient-centered linkage strategy implemented within the context of the SEARCH (Sustainable East Africa Research in Community Health) study, a population-based, community-wide test-and-treat trial in Kenya and Uganda when the ART eligibility criteria for the standard of care was CD <350 or WHO stage 3 or 4 disease.14 The multicomponent linkage strategy consisted of a patient-centered approach tailored to the setting,15 targeting the documented structural, health system, and behavioral barriers that hinder care engagement.16–18 Patient-centered care has been defined as “care that focuses on the patient and the individual's particular health care needs. The goal of patient-centered health care is to empower patients to become active participants in their care”.19 In this analysis, we evaluated linkage to HIV care among individuals newly diagnosed with HIV and those previously diagnosed but not currently in care, and evaluated factors associated with failure to link.
METHODS
Study Setting and Population
The linkage to care intervention was nested in the intervention communities of the SEARCH study (NCT01864603), a community-based cluster randomized trial in rural Kenya and Uganda that evaluated a multidisease, patient-centered approach to reducing HIV incidence and improving community health and productivity. To achieve high uptake of community-wide testing, mobile, multidisease Community Health Campaigns (CHCs) lasting approximately 2 weeks per community were combined with home-based HIV testing (HBT) for those residents who were enumerated in a baseline census but who did not attend the CHC in their community.20 The multidisease screening conducted included hypertension, diabetes, cervical cancer, and HIV screening with treatment of common ailments and immunization for children.20
Identification of the Linkage Cohort
Census-enumerated individuals aged 15 years and older in the 16 SEARCH study intervention communities (10 in Uganda and 6 in Kenya) who tested positive for HIV by rapid HIV antibody tests between June 2013 and June 2014 and were in need of linkage to HIV care were eligible for inclusion in this analysis. Individuals were defined as in need of linkage if they: (1) were newly diagnosed with HIV by rapid HIV antibody tests or (2) self-reported a previous diagnosis of HIV, but stated that they were not currently in HIV care and had no evidence of being in HIV care after review of medical and laboratory records.
Linkage to Care Intervention
Our linkage to care intervention strategy comprised a patient-centered, multicomponent approach with the goal of linking patients to care and starting ART as soon as possible. The accelerated linkage intervention included multiple components, each designed to address a previously identified barrier to linkage. First, at the time of HIV testing, participants were immediately introduced to clinic staff either in person for participants who tested at a health campaign, or using a study-provided phone for participants who tested at home or other location. These personal introductions: (1) established personal rapport between participant and clinic staff; (2) addressed any participant questions about HIV or the care provided in clinic; (3) assured participants of a patient-centered warm friendly environment with flexible clinic hours; and (4) provided patients with a telephone “hot-line.” The “hot-line” consisted of a number that patients could call or text message at any time to ask questions or request support, such as rescheduling clinic appointments without having to attend the clinic in person. Second, patients were provided a 1-time reimbursement for transportation on linkage (2–12.5 US dollars depending on distance from home to clinic). Third, patients received an appointment reminder phone call the day before the clinic appointment. Finally, patients who missed their linkage appointment were called to reschedule their clinic visit; home visits to reschedule clinic visit were performed if the phone call intervention was not successful.
Linkage Outcome Measures
We defined date of linkage to care as date of a first HIV clinic visit made at any health facility within or adjacent to the SEARCH community after the community-based HIV test. “Accelerated linkage” was defined as linking to care within 7 days of HIV diagnosis. At time of the first visit, individuals and their medical records (paper or electronic) were identified at SEARCH trial-affiliated ART clinics by fingerprint-biometric matching and name. At non-SEARCH trial-affiliated clinics, unique identifiers (clinic ID numbers) and demographics were used to identify medical records. Linkage for all participants in this analysis was determined using patient medical records only.
Participant Characteristics
Participant sex, age, marital status, education, occupation, region, stable residence (defined as residing in the community for ≥6 months in the previous year), access to a mobile phone, and self-reported previous HIV testing data were collected at baseline census and at time of HIV testing at CHC or HBT. Socioeconomic status was assessed using an asset score derived from a principal component analysis of the presence of electricity in the home and ownership of clock, radio, television, phone, refrigerator, bicycle, and motorcycle.21 Baseline CD4+ T-cell count and plasma HIV RNA level were measured at time of HIV testing. High HIV-risk occupation was defined as employment in an occupation reported to be associated with higher HIV prevalence in the literature for East Africa22–24 and included fishermen, bar owners, bar workers, tourism personnel, and drivers of trucks, taxis, motorcycles, bikes, or boats. HIV testing location was classified as CHC vs. HBT.
Statistical Analysis
The Kaplan–Meier estimator was used to estimate cumulative linkage over the 365 days after baseline HIV testing at CHC or tracking, censoring at death. We calculated estimates of cumulative linkage by 1, 3, 7, 30, 90, 182, and 365 days after HIV testing.25 To assess factors associated with linkage to care, univariate and multivariate Poisson regression with robust error variance were used to assess predictors of linkage within 7 days, 30 days, and 1 year, treating death before linkage as failure to link. Community of residence was included as a fixed effect. Variables to be included in the adjusted model were selected a priori based on factors identified in literature as predictive of linkage to care and included age, sex, marital status, occupation, education, point of testing, wealth index, baseline CD4+ T cells, previous knowledge of HIV status, and having a phone contact.6,26 Subjects with missing data for asset score measurement were assigned the population mean asset score. Missing baseline laboratory CD4+ T-cell count was treated as its own informative category.
Ethics
The Kenya Medical Research Institute Ethical Review Committee (Kenya), Ugandan National Council on Science and Technology (Uganda), Makerere University School of Medicine Research and Ethics Committee (Uganda), and University of California San Francisco Committee on Human Research (USA) approved the study. All participants provided verbal consent in their preferred language.
RESULTS
Linkage to Care Cohort
A total of 88,627 household census-enumerated individuals aged 15 years and older were approached for HIV testing in the 16 SEARCH intervention communities. Through mobile community- and HBT, HIV status was determined in 80.5% (n = 71,308) of potentially eligible subjects (Fig. 1). Of these, 6811 individuals had positive HIV rapid antibody tests, corresponding to an HIV prevalence of 9.6%. Of these 6811 HIV-positive cases, 4760 (69.9%) had a medical record or laboratory evidence of engagement in HIV care at time of HIV antibody testing. The remaining 2051 people living with HIV were identified as in need of engagement in care and were thus included in the linkage cohort analysis. Among these, 1624 (79.2%) were newly diagnosed with HIV, and 427 (20.8%) reported a previous HIV diagnosis but were not currently engaged in care.
FIGURE 1.
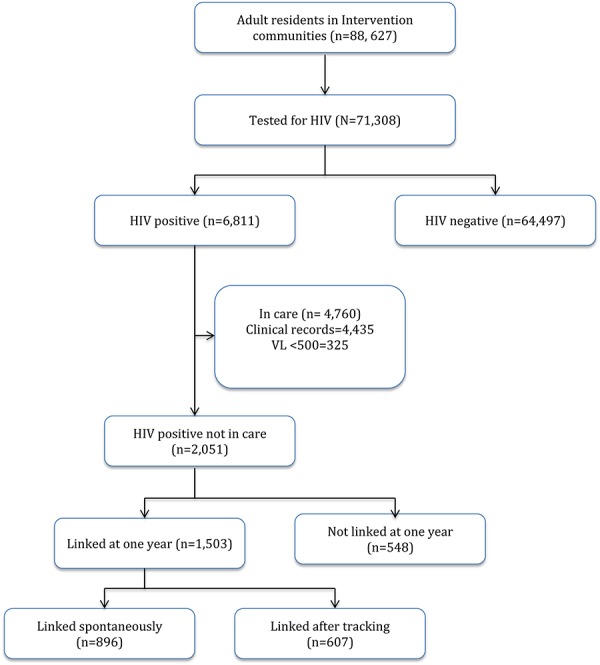
Study profile of residents in 16 SEARCH intervention communities in Kenya and Uganda, July 2013–June 2015. VL, viral load.
Linkage Cohort Characteristics
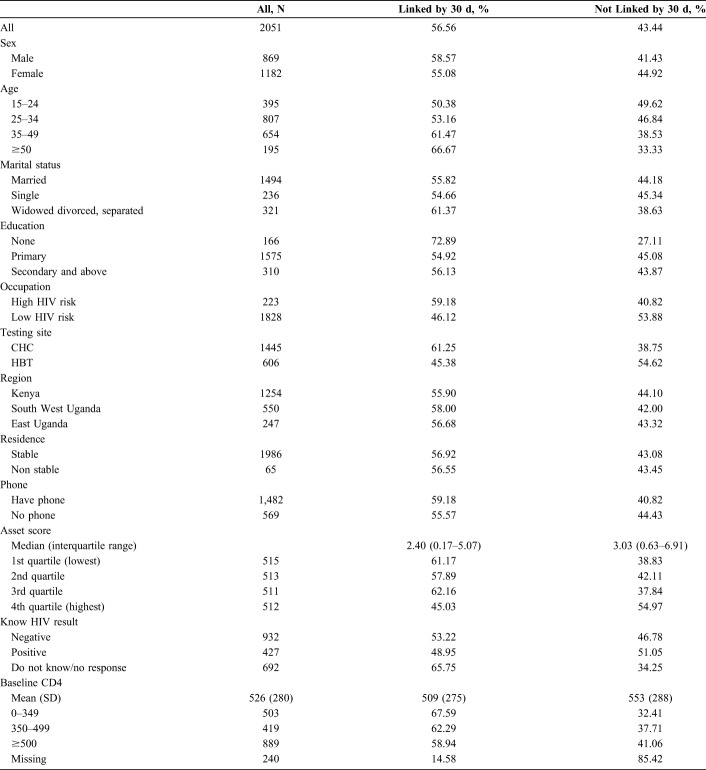
Demographic and clinical characteristics of the linkage cohort are presented in Table 1. The majority (58%) were female, and the median age was 32 years (interquartile range 26–40); 19.3% were younger than 25 years. Geographically, 61.4% resided in Kenya, 27% resided in Southwest Uganda, and 12% resided in East Uganda. The majority (70.5%) tested at a mobile CHC; 29.5% through HBT. Most individuals (72.2%) had access to a mobile phone, and 61.3% had received secondary or tertiary education. At the time of HIV testing, 49% had a CD4 count of ≥500 cells/µL, and 28% had a CD4+ T-cell count below 350 cells/µL. The median plasma HIV RNA level was 34,004 copies/µL.
TABLE 1.
Baseline Characteristics of a Cohort of 2051 Individuals Newly Diagnosed With HIV (N = 1624) or Previously Diagnosed With HIV But Out of Care (N = 429), Resident in 1 of 16 SEARCH Intervention Communities in Kenya and Uganda, July 2013–June 2015
Linkage to Care
Of the 2051 adults in the linkage cohort, 1503 [73.4%, 95% confidence interval (CI): 71.5% to 75.3%] were linked to care within 1 year of HIV testing. Among those who linked, 60% (n = 896) self-linked to care, with the remaining 40% (n = 607) linking after outreach tracking. Cumulative linkage in days from HIV testing is depicted in Figures 2 and 3. Overall, 42.9% (95% CI: 40.8 to 45.0) individuals linked on the same day of HIV testing, 46.2% (95% CI: 44.1 to 48.4), within 3 days, 49.7% (95% CI: 47.5 to 51.9) within 7 days, 56.6% (95% CI: 47.5 to 58.7) within 30 days, and 61.3% (95% CI: 52.9 to 63.4) within 3 months of testing. Among the 1019 subjects who linked within the “accelerated” linkage period of 7 days, 86% linked on the same day as HIV testing (n = 879), 7% between 0 and 3 days, and 7% between 3 and 7 days. Linkage was higher in Uganda (84%) than Kenya (66%) at 1 year after testing. During the first year after HIV testing, 11 subjects died before linkage—4 from medical causes including 1 with tuberculosis, 1 from traffic accident, and 6 from unknown cause.
FIGURE 2.
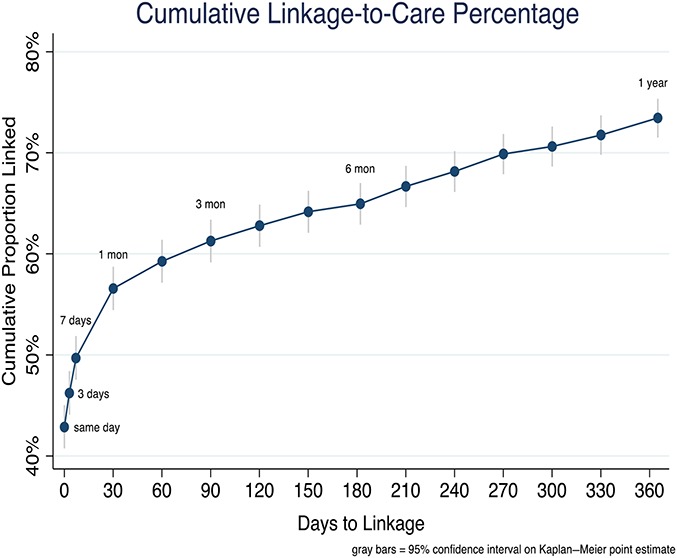
Cumulative linkage to care by time from HIV diagnosis (Kaplan–Meier estimates) in a cohort of 2051 individuals newly diagnosed with HIV (N = 1624) or previously diagnosed with HIV but out of care (N = 429), resident in 1 of 16 SEARCH intervention communities in Kenya and Uganda, July 2013–June 2015. *11 participants died before linkage to care.
FIGURE 3.
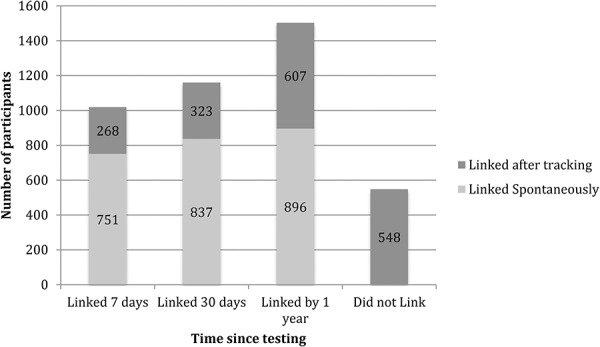
Bar graph of cumulative number linking over time since baseline testing a cohort of 2051 individuals newly diagnosed with HIV (N = 1624) or previously diagnosed with HIV but out of care (N = 429), resident in 1 of 16 SEARCH intervention communities in Kenya and Uganda, July 2013–June 2015.
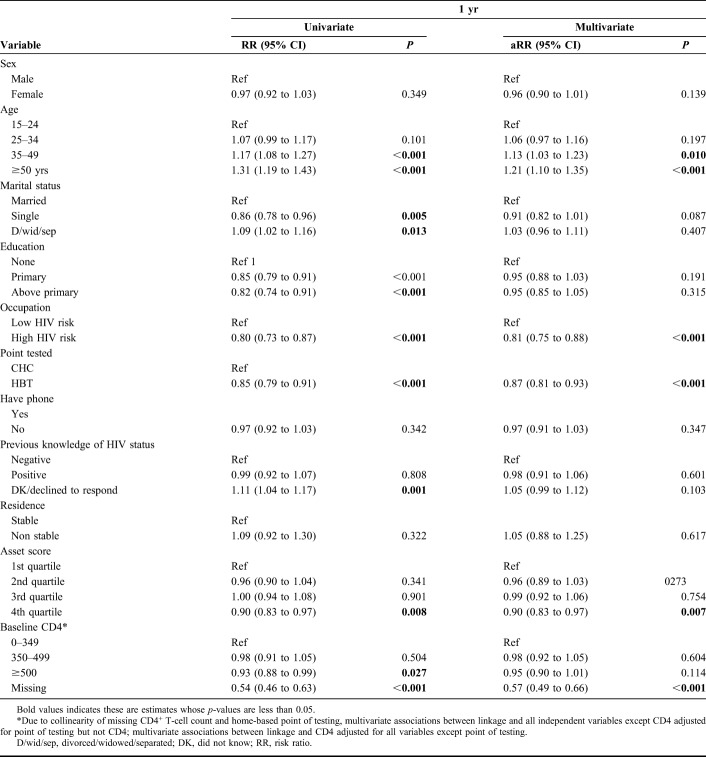
Predictors of Linkage to Care
Univariate and multivariate estimates of the strength of association between sociodemographic and clinical characteristics and linkage to care by 7, 30, and 365 days are shown in Table 2 and Supplemental Table 1, Supplemental Digital Content 1 (http://links.lww.com/QAI/B267). Educational level, having a phone contact, and being a stable community resident were not associated with linkage to care by 7, 30, or 365 days. Age and occupation were associated with linkage to care. There was a 0.7% increase in risk of linkage with each additional year increase in age [adjusted Risk Ratio (aRR) 1.007 95% CI: 1.004 to 1.009]. Compared to people with a low HIV-risk occupation, those in a high-risk occupation were less likely to link to care: aRR 0.74 (95% CI: 0.64 to 0.84) for linkage by 7 days, aRR 0.80 (95% CI: 0.71 to 0.89) for linkage by 30 days, and aRR 0.81 (95% CI: 0.75 to 0.88) for linkage by 365 days. In a multivariate model adjusting for all other variables except point of testing (excluded due to collinearity with baseline CD 4), those with higher CD4+ T-cell count (≥500 cells/µL vs. <350 cells/µL.) were less likely to link within 1 month of testing: aRR 0.84 (95% CI: 0.76 to 0.92) for linkage by 7 days and aRR 0.90 (95% CI: 0.83 to 0.97) for linkage by 30 days. Predictors of linkage did not vary by region, CD4 level, or sex (see Figures 1–3, Supplemental Digital Content, http://links.lww.com/QAI/B267).
TABLE 2.
Factors Associated With Linkage to Care at 1 Year in a Cohort of 2051 Individuals Newly Diagnosed With HIV (N = 1624) or Previously Diagnosed With HIV But Out of Care (N = 429), Resident in 1 of 16 SEARCH Intervention Communities in Kenya and Uganda, July 2013–June 2015
HIV testing characteristics were also associated with linkage to care at each time point. Individuals diagnosed by HBT compared with those tested through CHC were about 25% less likely to link to care within a month of testing: aRR 0.73 (95% CI: 0.65 to 0.82) for linkage by 7 days, aRR 0.78 (95% CI: 0.70 to 0.86) for linkage by 30 days, and aRR 0.87 (95% CI: 0.81 to 0.93) for linkage by 1 year (see Figure 4, Supplemental Digital Content, http://links.lww.com/QAI/B267). Report of previous knowledge of HIV diagnosis was associated with poorer linkage to care at earlier time points but not at 1 year. Compared with those who previously tested HIV negative, individuals who had previously tested positive but were not currently in care were about 20% less likely to link within 7 days after their current HIV test: aRR 0.80 (95% CI: 0.70 to 0.92). On the contrary, those who reported not knowing their HIV status were more likely to link to care within 1 month of testing: aRR 1.17 (95% CI: 1.07 to 1.29) for linkage by 7 days and aRR 1.13 (95% CI: 1.04 to 1.23) for linkage by 30 days. We did not observe effect modification by knowledge of HIV status.
DISCUSSION
After population-based, community-wide HIV testing of more than 70,000 adults in rural Kenya and Uganda, a patient-centered, multicomponent strategy linked half of all HIV-positive persons who were not in care to HIV care within 1 week of testing and by 1 year 3 quarters of all individuals had linked. These linkage rates are substantially higher than rates reported under standard-of-care in Kenya (42% by 4 months) and Uganda (45% by 6 months).8,9 Among other studies including interventions to improve linkage, some have reported higher linkage rates,25–28 but none report rapid linkage rates (within 7 days) as high as observed with this study.
As countries move toward universal treatment, a population-based approach that achieves rapid linkage to HIV care for all individuals not currently in care is required to realize the full health and prevention benefits of universal ART. Early data on challenges faced with universal treatment can be found among the first reports of population level HIV test-and-treat studies.11,13 In the PopART study conducted in Zambia and South Africa, 53% of HIV-positive individuals not in care linked and initiated ART by 12 months.13 In the TasP study conducted in South Africa, 29.7% linked by 6 months.11 Barriers identified in these trials included inconvenient clinic hours, overcrowded clinics, health providers' poor attitude, stigma, and shame.29 Our intervention aimed to address some of these structural, behavioral, and health system barriers. A patient-centered approach where, eg, the clinic staff is welcoming and interacting with the client from the time of diagnosis thru ART start, flexible clinical hours, appointment reminders, and provider access to make enquiries through phone may have contributed to the high proportion of patients linking within a week of testing. In addition, we scheduled linkage appointments very soon after diagnosis deliberately to accelerate linkage.
After 1 month, the change in cumulative linkage rate slowed, suggesting that a group of more difficult to engage in care patients was being reached. Our multidisease population-based HIV testing approach achieved high HIV testing coverage across all segments of the population20 and would be expected to result in a population in need of linkage enriched for “hard-to-reach” subgroups such as young adults, men, and people at early stages of disease, as well as providing a new opportunity to link for previously diagnosed individuals currently out of HIV care.30 These hard-to-reach individuals may be less motivated to seek care,31–33 or may face additional barriers to linkage, and may thus require additional interventions for effective linkage. Interestingly, we found that both previously diagnosed individuals not currently in care and those reporting a new diagnosis after an earlier negative HIV test were at higher risk of failing to link than individuals with no previous HIV test, as were individuals with high CD4+ T-cell count. These individuals may be experiencing other barriers to linkage such as protracted phases of grief after their diagnosis, stigma, disclosure challenges, or failure to see the need for care when not feeling ill.26,30,31
Despite the successful implementation of our multiple-component strategy, a quarter of individuals in need of care engagement were not linked to care by 1 year. Our analysis of factors associated with failure to link to care identified some of the most difficult to engage subgroups including young adults. In agreement with other studies,6,26,27,34 being young was associated with a lower likelihood of linking to care at all 3 time points evaluated; other studies have, however, shown conflicting findings with older individuals having lower linkage rates.35 Individuals working in high-HIV-risk occupations such as fishing and transportation were also less likely to link possibly due to the mobile nature of these occupations, rigid work-time schedules, and the high levels of HIV-associated stigma within these subgroups. The young and those in high HIV-risk occupations may also have an elevated risk of transmitting HIV.30 For both their own health and to optimize prevention impacts, innovative approaches are needed to engage them in care.
We also observed lower linkage rates among people identified through HBT compared with individuals diagnosed through the mobile CHCs, those with higher CD4 counts, and those who had been previously diagnosed with HIV but were not in care. This is consistent with findings of other studies.30,36 Home-based testing after community health fairs likely taps into a different, more difficult to engage population and may reach individuals with different health-seeking behaviors, possibly because of higher anticipated HIV stigma.30,37 Being asymptomatic and feeling healthy may be responsible for the lower linkage rates observed among high CD4 participants. Lower linkage among high CD4 patients is a concern in the treat-all era and may present a challenge in using ART for treatment as prevention. This hurdle requires patient empowerment at time of diagnosis through provision of information on benefits of treatment in the early stages of disease even when asymptomatic and use of viral load monitoring to demonstrate to patients the value of treatment. Linkage was also lower in Kenya as compared to Uganda; this may be due to higher stigma and differences in health-seeking behaviors between the 2 countries.38
After adjustment for other risk factors, individuals with the highest household wealth seemed less likely to link, in contrast to findings by other studies.6 This association we speculate may be due to wealthier individuals accessing care at private clinics located distant from the study community due to stigma. Although we confirmed linkage at clinics outside the study communities, we were unable to confirm linkage to care for individuals seen at such clinics.
We did not find a difference in linkage based on sex, education level, or access to a phone. Lack of association with sex confirms findings of other research,35,38 although association has been seen in some settings.34,39–41 Lack of association with formal education level, however, is in contrast to findings by other studies.6 This may be because of the many years of widespread HIV sensitization programs in Uganda and Kenya, which have increased overall knowledge and awareness about HIV to all regardless of educational level.
Our study had several strengths. In contrast to most linkage to care studies that are health facility–based, our population-based study and high HIV testing coverage allows us assess linkage inclusive of difficult to reach patients that may not get tested at a health facility. It is important to note that population-based testing was aimed at determining baseline population HIV prevalence and was also required for determination of the primary endpoint of the main SEARCH study and within this setting; our linkage strategy was aimed at optimizing linkage of those not engaged in care on testing. In addition, this is one of the first studies to examine linkage to care under an implementation of universal HIV treatment and a comprehensive test-and-treat strategy in a rural area. Furthermore, linkage was verified by and based on medical records thus overcoming a social desirability bias that would be introduced by using patient self-report. Our study was also subject to limitations. We were only able to verify linkage in health facilities that we could access within and around our study communities. It is possible that some participants linked to more distant health facilities, and therefore, we are reporting conservative estimates of true linkage rate. Second, we are not able to tell which specific component in our multicomponent strategy made the biggest difference in linkage, but it is important to note that the intervention was designed to be delivered as a package that simultaneously addressed multiple barriers to timely linkage. Finally, participant self-report of knowledge of HIV status may have lead to misclassification as a result of social desirability bias.
In conclusion, timely linkage to care remains critical in treatment and prevention of HIV. Combining a mobile hybrid community-based testing strategy with a novel patient-centered, multicomponent linkage strategy resulted in high linkage rates, with half of all individuals in need of HIV care linked within 1 week of HIV testing and three-quarters linked within a year. Our findings present a feasible and effective linkage approach for adoption by programs in different countries as we move toward universal treatment in similar settings. In addition, our findings draw attention to specific groups that require special attention, namely the young individuals in informal-high-risk occupation sector, home testers, and those with a high CD4+ T-cell count at diagnosis who still pose a challenge and require innovative interventions to further improve linkage. Concerted efforts are thus required to continue to refine and develop linkage strategies to help achieve the maximal benefits of treatment as prevention.
Supplementary Material
ACKNOWLEDGMENTS
The SEARCH project gratefully acknowledges the Ministries of Health of Uganda and Kenya, our research team, collaborators, and especially all communities and participants involved.
Footnotes
Supported by Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health under award number U01AI099959, and in part by the President's Emergency Plan for AIDS Relief.
D.V.H. reports grants from National Institutes of Health, grants from Gates Foundation, and nonfinancial support from Gilead Sciences, during the conduct of the study; outside the submitted work. E.D.C. reports grants from National Institutes of Health, during the conduct of the study. The study was funded by the Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health under award number U01AI099959, and in part by the President's Emergency Plan for AIDS Relief.
The authors have no conflicts of interest to disclose.
J.A., M.L.P., E.D.C., A.V.R., and D.V.H. contributed to the design of the study, conduct of the study, analysis of the data, drafting of the manuscript, and editing/review. L.B.B. contributed to conduct of the study, analysis of data, and editing/review. D.K., M.R.K., E.A.B., and C.R.C. contributed to the conduct of the study and editing/review.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.Danel C, Danel C, Moh R, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808–822. [DOI] [PubMed] [Google Scholar]
- 2.Lundgren JD, Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antinori A, Johnson M, Moreno S, et al. Introduction to late presentation for HIV treatment in Europe. Antivir Ther. 2010;15(suppl 1):1–2. [DOI] [PubMed] [Google Scholar]
- 5.Mocroft A, Lundgren JD, Sabin ML, et al. Risk factors and outcomes for late presentation for HIV-positive persons in europe: results from the collaboration of observational HIV epidemiological research europe study (COHERE). PLoS Med. 2013; 10:e1001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maheu-Giroux M, Tanser F, Boily MC, et al. Determinants of time from HIV infection to linkage-to-care in rural KwaZulu-Natal, South Africa. AIDS. 2017;31:1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kranzer K, Govindasamy D, Ford N, et al. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2012;15:17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wanyenze RK, Hahn JA, Liechty CA, et al. Linkage to HIV care and survival following inpatient HIV counseling and testing. AIDS Behav. 2011;15:751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medley A, Ackers M, Amolloh M, et al. Early uptake of HIV clinical care after testing HIV-positive during home-based testing and counseling in western Kenya. AIDS Behav. 2013;17:224–234. [DOI] [PubMed] [Google Scholar]
- 10.UNAIDS. 90–90–90—An Ambitious Treatment Target to Help End the AIDS Epidemic. Geneva, Switzerland: UNAIDS; 2014. [Google Scholar]
- 11.Iwuji CC, Orne-Gliemann J, Larmarange J, et al. Universal test and treat and the HIV epidemic in rural South Africa: a phase 4, open-label, community cluster randomised trial. Lancet HIV. 2018;5:e116–e125. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organisation. Guidelines on when to Start Antiretroviral Therapy and on Pre-exposure Prophylaxis for HIV. Geneva, Switzerland: WHO; 2015. [PubMed] [Google Scholar]
- 13.Hayes R, Floyd S, Schaap A, et al. A universal testing and treatment intervention to improve HIV control: one-year results from intervention communities in Zambia in the HPTN 071 (PopART) cluster-randomised trial. PLoS Med. 2017;14:e1002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perriat D, Balzer L, Hayes R, et al. Universal test and treat trials consortium (UT3C). Comparative assessment of five trials of universal HIV testing and treatment in sub-Saharan Africa. J Int AIDS Soc. 2018;21:e25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31:143–164. [DOI] [PubMed] [Google Scholar]
- 16.Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS. 2012;26:2059–2067. [DOI] [PubMed] [Google Scholar]
- 17.Geng EH, Bangsberg DR, Musinguzi N, et al. Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. J Acquir Immune Defic Syndr. 2010;53:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joseph Davey D, Nhavoto JA, Augusto O, et al. Evaluating mobile phone text reminders to improve retention in HIV care for patients on antiretroviral therapy in Mozambique. J Acquir Immune Defic Syndr. 2016;73:e23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reynolds A. Patient-centered care. Radiol Technol. 2009;81:133–147. [PubMed] [Google Scholar]
- 20.Chamie G, Clark TD, Kabami J, et al. A hybrid mobile approach for population-wide HIV testing in rural east Africa: an observational study. Lancet HIV. 2016;3:e111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grosh M, Glewwe P. Designing Household Survey Questionnaires for Developing Countries: Lessons from 15 Years of the Living Standards Measurement Study, Volume 2. Washington, DC: World Bank; 2000. [Google Scholar]
- 22.Allison EH, Seeley JA. HIV and AIDS among fisherfolk: a threat to “responsible fisheries”? Fish Fish. 2004;5:215–234. [Google Scholar]
- 23.Seeley J, Tumwekwase G, Grosskurth H. Fishing for a living but catching HIV: AIDS and changing patterns of the Organization of Work in Fisheries in Uganda. Anthropol Work Rev. 2009;30:66–76. [Google Scholar]
- 24.Lindan CP, Anglemyer A, Hladik W, et al. High-risk motorcycle taxi drivers in the HIV/AIDS era: a respondent-driven sampling survey in Kampala, Uganda. Int J STD AIDS. 2015;26:336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvin S. Statistical Analysis of Epidemiological Data. 3rd ed New York, NY: Oxford University Press; 2004. [Google Scholar]
- 26.Naik R, Doherty T, Jackson D, et al. Linkage to care following a home-based HIV counselling and testing intervention in rural South Africa. J Int AIDS Soc. 2015;18:19843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatcher AM, Turan JM, Leslie HH, et al. Predictors of linkage to care following community-based HIV counseling and testing in rural Kenya. AIDS Behav. 2012;16:1295–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Rooyen H, Barnabas RV, Baeten JM, et al. High HIV testing uptake and linkage to care in a novel program of homebased HIV counseling and testing with facilitated referral in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2013;64:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabapathy K, Mubekapi-Musadaidzwa C, Mulubwa C, et al. Predictors of timely linkage-to-ART within universal test and treat in the HPTN 071 (PopART) trial in Zambia and South Africa: findings from a nested case-control study. J Int AIDS Soc. 2017;20:e25037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bassett IV, Regan S, Luthuli P, et al. Linkage to care following community-based mobile HIV testing compared with clinic-based testing in Umlazi Township, Durban, South Africa. HIV Med. 2014;15:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Govindasamy D, van Schaik N, Kranzer K, et al. Linkage to HIV care from a mobile testing unit in South Africa by different CD4 count strata. J Acquirimmune Deficsyndr. 2011;58:344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. Plos Med. 2011;8:e1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lessells RJ, Mutevedzi PC, Cooke GS, et al. Retention in HIV care for individuals not yet eligible for antiretroviral therapy: rural KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2011;56:e79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Croxford S, Burns F, Copas A, et al. Factors associated with delayed linkage to care following HIV diagnosis in the WHO European Region. HIV Med. 2018;19(suppl 1):40–46. [DOI] [PubMed] [Google Scholar]
- 35.Alaei A, Nautiyal N, Mishkin K, et al. Factors associated with linkage to care for HIV patients in Tajikistan. Int J STD AIDS. 2018;29:1183–1189. [DOI] [PubMed] [Google Scholar]
- 36.Sanga ES, Lerebo W, Mushi AK, et al. Linkage into care among newly diagnosed HIV-positive individuals tested through outreach and facility-based HIV testing models in Mbeya, Tanzania: a prospective mixed-method cohort study. BMJ Open. 2017;7:e013733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and metasynthesis. J Int AIDS Soc. 2013;16(3 suppl 2):18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colombini M, Mutemwa R, Kivunaga J, et al. Experiences of stigma among women living with HIV attending sexual and reproductive health services in Kenya: a qualitative study. BMC Health Serv Res. 2014;14:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meehan SA, Sloot R, Draper HR, et al. Factors associated with linkage to HIV care and TB treatment at community-based HIV testing services in Cape Town, South Africa. PLoS One. 2018;13:e0195208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almirol EA, McNulty MC, Schmitt J, et al. Gender differences in HIV testing, diagnosis, and linkage to care in healthcare settings: identifying African American women with HIV in Chicago. AIDS Patient Care STDS. 2018;32:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parchure R, Kulkarni V, Kulkarni S, et al. Pattern of linkage and retention in HIV care continuum among patients attending referral HIV care clinic in private sector in India. AIDS Care. 2015;27:716–722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.