Summary
Background
FTO, a gene recently discovered in genomewide associated studies for type 2 diabetes mellitus (T2D), play an important role in the management of energy homeostasis, nucleic acid demethylation and regulation of body fat mass by lipolysis. The aim of this study was to analyze the association of FTO rs8050136 A>C genetic variant with clinical and biochemical parameters of T2D in the population of West Balkan region (Bosnians and Herzegovinians and Kosovars).
Methods
The study included 638 patients with T2D and prediabetes and 360 healthy controls of both genders, aged from 40 to 65 years. Patients were recruited at the Clinical Centre University of Sarajevo, University Hospital of Clinical Centre in Banja Luka, General Hospital in Tešanj and Health Centre in Prizren. Genotyping of analyzed FTO polymorphism rs8050136 A>C was performed by qPCR allelic discrimination.
Results
Genotype frequencies of the analyzed polymorphism were comparable between patients with T2D, prediabetic patients, and healthy population. Logistic regression analyses didn’t show significant association of FTO rs8050136 A allele with increased risk of T2D. However, risk A allele was significantly associated with higher levels of HbA1c, insulin, HOMA-IR index, diastolic blood pressure, and inflammatory markers (fibrinogen and leukocytes) as well as showed tendency of association with increased values of obesity markers (BMI, waist and hip circumference).
Conclusions
Results of our study showed a significant association of FTO genetic variant rs8050136 A>C with the major markers of insulin resistance, obesity and inflammation, opening new avenues for solving many unclear questions in the pathogenesis of T2D.
Keywords: FTO gene, Type 2 diabetes, obesity, inflammation, gene variant
Kratak sadržaj
Uvod
FTO, gen koji je u cjelogenomskim studijama povezanosti, nedavno otkriven kao kandidatni gen za tip 2 dijabetes (T2D), ima značajnu ulogu u upravljanju energetske homeostaze, demetilaciji nukleinskih kiselina i regulaciji indeksa tjelesne mase (ITM) tako što reguliše proces lipolize. Cilj studije je bio da analiziramo povezanost FTO rs8050136 A>C genetske varijante sa kliničkim i biohemijskim parametrima T2D u populacijama regiona Zapadnog Balkana (Bosancima i Hercegovcima i Kosovarima).
Metode
U studiju je uključeno 638 pacijenata oboljelih od T2D, predijabetesa, i 360 zdravih kontrola, oba spola, starosti od 40 do 65 godina. Pacijenti se regrutovani u Kliničkom centru Univerziteta u Sarajevu, Univerzitetskoj bolnici Kliničkog centra u Banja Luci, Opštoj bolnici u Tešnju i Domu zdravlja u Prizrenu. Genotipizacija FTO genetskog polimorfizma rs8050136 A>C je analizirana korištenjem metode qPCR alelne diskriminacije.
Rezultati
Frekvencije genotipova analiziranog polimorfizma uspoređivane su između pacijenata sa T2D, predijabetesom i zdravih ispitanika. Također, rezultati logistike analize nisu pokazali značajnu povezanost A alela FTO rs8050136 polimorfizma sa povećanim rizikom razvoja T2D. Međutim, rizični A alel je pokazao značajnu asociranost sa višim vrijednostima HbA1c, inzulina, HOMA IR indeksa, diastolnog krvnog pritiska, i inflamatornih markera (fibrinogena i leukocita), kao i tendenciju povezanosti sa povišenim vrijednostima parametara gojaznosti (ITM, opseg struka i opseg kukova).
Zaključak
Rezultati naše studije pokazali su značajnu povezanost FTO genetske varijante rs8050136 A>C sa najbitnijim markerima inzulinske rezistencije, gojaznosti i inflamacije, otvarajući na taj način nove puteve i mogućnosti riješavanja mnogih nejasnih pitanja vezanih za patogenezu T2D.
Ključne reči: FTO gen, tip 2 dijabetes, gojaznost, inflamacija, genetička varijanta
Introduction
Type 2 diabetes (T2D) is a chronic complex disease characterized by hyperglycemia which occurs as a result of reduced insulin secretion, inadequate response of pancreatic cells on progressive development of insulin resistance in peripheral tissues or impaired glucose regulation in the liver (1). Global prevalence of T2D is increasing with average value of 8.7%. Bosnia and Herzegovina belongs to a group of countries with the highest prevalence in Europe of 12.0%. As far as Kosovo is concerned, there are no prevalence data yet available (2). It is well known that the risk of developing T2D is associated with obesity which is now a major global problem, considering that approximately 1.1 billion people worldwide are overweight, while 312 million are obese (3, 4). GWA (genome-wide associated) studies identified around 15 candidate genes responsible for an increase in the visceral depots (which is associated with a number of metabolic disorders such as metabolic syndrome, T2D, and cardiovascular disease) (1). Therefore, it is important to know which genetic loci are associated with obesity in order to better understand their role in pathophysiology of T2D.
One of the major candidate gene associated with obesity is FTO (alpha-ketoglutarate dependent dioxygenase) coding the Fat mass and obesity-associated protein (1). The FTO is a newly identified gene associated with increased risk of T2D (1). FTO was predicted to be a 2-oxyglutarate (2-OG) Fe(II) dependent demethylase. In vitro, recombinant FTO is able to catalyze the Fe(II)- and 2OG-dependent demethylation of 3 methylthymine in single-stranded DNA, as well as 3 methyluracil (3meU), and 6 methyl adenosine (6meA) in single-stranded RNA. This suggests a potential role of FTO in nucleic acid repair or modification (5). However, the exact molecular mechanisms responsible for the effect of FTO on obesity and T2D remain largely unknown. Recent GWA studies revealed that genetic variants in the FTO gene are not associated only with human adiposity and metabolic disorders, but also with cancer, which is as well highly associated with obesity (6, 7, 8, 9).
A large number of studies conducted on different populations has confirmed the impact of the FTO rs8050136 polymorphism on an increased risk of developing T2D (10, 11, 12, 13, 14, 15). Results of several metaanalyses showed a significant association of rs8050136 gene variant with increased T2D and obesity risk (3, 8, 16, 17, 18). Studies performed up to now have shown that FTO gene variant rs8050136 A>C is significantly associated with major markers of obesity (BMI, waist and hip circumference) (13, 18, 19, 20), markers of glucose homeostasis (glucose, HbA1c, insulin) and insulin resistance (HOMA-IR) in different population studies (21, 22, 23). However, studies carried out on the Russian, Mexican Mestizos, Lebanese and Omani population have not confirmed the association of this polymorphism with T2D (24, 25, 26, 27, 28). Also, results of large DiaGene study did not confirmed impact of rs8050136 A>C with increased risk of T2D in Netherland population (29). This is the first study that investigated the impact of FTO candidate gene polymorphism rs8050136 A>C on T2D and its related traits in populations of West Balkan region (Bosnians and Herzegovinians and Kosovars).
Materials and Methods
Subjects
The study included 998 participants: 638 patients with T2D and prediabetes, and 360 healthy controls of both sexes, aged from 40 up to 65 years. T2D and prediabetes were diagnosed by endocrinologists and diabetologists according to definitions of International Diabetes Federation (IDF) (30). Diabetic patients and healthy subjects were recruited at the Clinic for Endocrinology and Diabetes, University Clinical Centre of Sarajevo, Department of Endocrinology and Internal Medicine, University Hospital of Clinical Centre in Banja Luka, Department of Internal Medicine, General Hospital in Tešanj, and Health Centre in Prizren. Patients treated with insulin and patients with acute and chronic gastrointestinal diseases, chronic kidney disease, endocrine disorders, acute infection and/or inflammation and hormonal therapy were excluded. All patients included in the study were taking heterogeneous therapy (antihypertensive therapy, glucose-lowering drugs, and lipidlowering drugs). Individuals in control group were not taking any medication during the course of the study.
The research was carried out in accordance with ethics principles outlined in the Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects (initiated June 1964, last amended October 2000). The study was approved by Ethics Committee of the International University of Sarajevo and each patient has given a written informed consent.
Anthropometrical and biochemical measurements
Waist circumference, height, weight, systolic and diastolic blood pressure were measured in all participants. BMI was calculated as weight (kg)/(height (m))2. For analysis of all biochemical parameters, IFCC (International Federation of Clinical Chemistry and Laboratory Medicine) standard protocols were used. Serum levels of fasting glucose, triglycerides, total cholesterol, HDL-cholesterol, LDL-cholesterol, HbA1c, fibrinogen and C reactive protein (CRP) were determined by using VITROS auto analyzer 350 Chemistry System (Ortho-Clinical Diagnostics, Rochester, New York, USA). Serum insulin levels were measured by the Abbott AxSYM (Abbott Diagnostics, North Chicago, Illinois, USA) analyzer. HOMA IR index was calculated by using following formula: fasting insulin (mU/L) × fasting glucose (mmol/L)/22.5 (31).
Genetic testing
Blood samples were collected from all participants under fasting conditions from antecubital vein into siliconized tubes with EDTA (BD Vacutainer Systems, Plymouth, UK) and stored at –20 °C. For isolation of genomic DNA, Miller’s protocol and QIAamp DNA Blood Midi Kit(Qiagen, Hilden, Germany) were used (32). Purity and concentration of isolated DNA was determined by UV/VIS spectrophotometer NanoDrop ND-1000. After extraction, DNA samples were stored at –20 °C. Genotyping of FTO gene polymorphism (rs8050136 A>C) was performed by hydrolyzing probes and real-time PCR using TaqMan SNP Genotyping Assays (Applied Biosystems, Foster City, CA), ID C_2031259_10, in cooperation with the Department of Clinical Chemistry, Faculty of Pharmacy, University of Ljubljana (Ljubljana, Slovenia) on LightCycler® 480 Real-Time PCR System (Roche Diagnostics, Switzerland) and Charles University in Prague/University Hospital Hradec Kralove, Czech Republic) on Rotor gene Q machine (Qiagen, Netherlands).
Statistical analysis
Statistical analysis was done using SPSS Statistics v.19.0. Normality evaluation of data distribution was done using Kolmogorov-Smirnov test and Shapiro-Wilk test, respectively (for prediabetes group of patients due its smaller size). Variables that were not normally distributed have been log-transformed. Chi-square (χ2) and Fisher’s exact tests (in the case where frequencies were less or equal to 5) were applied to examine differences in allele frequencies and genotype distributions between healthy controls and patients with T2D or prediabetes, respectively. Logistic regression analysis was used to calculate the odds ratio (OR) with confidence intervals (95% CI) with adjustments to age and gender. Significance of differences of biochemical and anthropometrical measurements according to genotypes of analyzed polymorphisms, sex and age was estimated using ANCOVA test (analysis of covariance) adjusted for sex, age and BMI. A p-value ≤ 0.05 was considered statistically significant.
Results
Clinical and biochemical characteristics of patients with T2D, patients with T2D without treatment (have not use oral hypoglycemics), prediabetic patients and healthy controls are presented in Table I. The most of the measured anthropometric and metabolic parameters were significantly different between the treated T2D patients, T2D patients without treatment, prediabetic patients and healthy controls (Table I).
Table I.
Metabolic and anthropometric characteristics of patients with T2D, patients with T2D without treatment (have not use oral hypoglycemics), prediabetic patients and healthy controls.
| T2D patients n = 476 | T2D patients without treatment n = 109 | Pre-diabetic patients n=53 | Healthy controls n=360 | p T2D+/T2D- | p T2D+/preD | p T2D+/ctrl | p T2D-/preD | p T2D-/ctrl | p preD/ctrl | |
|---|---|---|---|---|---|---|---|---|---|---|
| Male/ Female ratio | 169 / 277 | 46/63 | 26/27 | 128 / 209 | ||||||
| Age (years) | 55.30 ± 0.478 | 56.89 ± 1.015 | 52.00 ± 1.448 | 48.95 ± 0.477 | 0.362 | 0.851 | <0.001 | 0.299 | <0.001 | 0.004 |
| Fasting glucose (mmol/L) | 9.30 ± 0.190 | 8.76 ± 0.275 | 8.05 ± 1.319 | 5.12 ± 0.503 | 0.686 | <0.001 | <0.001 | 0.003 | <0.001 | <0.001 |
| HbA1c (%) | 7.61 ± 0.082 | 7.78 ± 0.140 | 6.04 ± 0.138 | 5.53 ± 0.055 | 0.510 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Fasting insulin (mU/L) | 15.47 ±0.761 | 17.54 ± 1.871 | 13.92 ±1.289 | 10.21 ± 0.589 | 0.728 | 1.000 | <0.001 | 0.931 | <0.001 | 0.085 |
| + HOMA-IR | 6.38 ± 0.378 | 7.16 ± 1.446 | 4.032 ± 0.428 | 2.41 ± 0.135 | 1.000 | 0.237 | <0.001 | 0.306 | <0.001 | 0.001 |
| BMI (kg/m2) | 31.02 ± 0.343 | 30.32 ± 0.565 | 29.144 ± 0.687 | 26.75 ± 0.389 | 0.830 | 0.701 | <0.001 | 0.974 | <0.001 | 0.070 |
| Waist circumference (cm) | 102.28 ±0.779 | 103.08 ± 1.127 | 101.90 ± 1.184 | 94.35 ± 0.807 | 1.000 | 0.988 | <0.001 | 0.985 | <0.001 | 0.001 |
| Hip circumference (cm) | 109.41 ±0.988 | 108.62 ± 0.975 | 109.02 ± 1.086 | 106.67 ± 0.808 | 0.308 | 0.788 | 0.003 | 0.990 | 0.796 | 0.738 |
| Systolic BP (mm Hg) | 137.25 ±1.378 | 134.43 ± 1.682 | 132.94 ± 2.352 | 122.33 ± 1.093 | 0.077 | 0.108 | <0.001 | 0.971 | <0.001 | 0.004 |
| Diastolic BP (mm Hg) | 88.14 ± 0.521 | 83.75 ± 0.873 | 84.50 ± 1.346 | 79.85 ± 0.498 | 0.001 | 0.088 | <0.001 | 0.982 | 0.003 | 0.014 |
| Triglycerides (mmol/L) | 2.43 ± 0.129 | 2.35 ± 0.139 | 1.95 ± 0.138 | 1.66 ± 0.565 | 0.984 | 0.229 | <0.001 | 0.484 | <0.001 | 0.258 |
| Total cholesterol (mmol/L) | 5.22 ± 0.076 | 5.46 ± 0.126 | 5.11 ± 0.124 | 5.47 ± 0.077 | 0.868 | 0.811 | 0.010 | 0.582 | 0.564 | 0.075 |
| HDL cholesterol (mmol/L) | 1.05 ± 0.016 | 1.16 ± 0.039 | 1.06 ± 0.042 | 1.30 ± 0.027 | 0.324 | 0.961 | <0.001 | 0.428 | <0.001 | <0.001 |
| LDL-cholesterol (mmol/L) | 3.34 ± 0.064 | 3.36 ± 0.120 | 3.22 ± 0.110 | 3.54 ± 0.059 | 0.998 | 0.100 | 0.050 | 0.999 | 0.441 | 0.572 |
| VLDL-cholesterol (mmol/L) | 1.400 ± 0.159 | 0.987 ± 0.063 | 0.755 ± 0.065 | 0.746 ± 0.049 | 0.182 | 0.004 | <0.001 | 0.230 | 0.003 | 0.972 |
| hsCRP (mg/L) | 5.40 ± 0.507 | 5.04 ± 1.110 | 3.60 ± 0.833 | 3.51 ± 0.189 | 0.767 | 0.014 | 0.002 | 0.192 | 0.478 | 0.640 |
| Fibrinogen (g/L) | 3.74 ± 0.092 | 3.92 ± 0.101 | 3.52 ± 0.164 | 3.87 ± 0.407 | 0.791 | 0.743 | 0.258 | 0.361 | 0.031 | 0.999 |
| Leukocytes (109/L) | 7.43 ± 0.125 | 7.92 ± 0.682 | 6.62 ± 0.275 | 6.42 ± 0.109 | 0.999 | 0.230 | <0.001 | 0.277 | <0.001 | 0.648 |
| Sedimentation (mm/h) | 16.62 ± 2.083 | 18.16 ± 2.145 | 8.56 ± 1.621 | 11.61 ± 1.039 | 0.959 | 0.018 | 0.284 | 0.004 | 0.079 | 0.295 |
Values represent mean ± standard error of mean (SEM), p T2D+/T2D -significance between T2D patients and T2D without treatment, p T2D+/preD-significance between T2D patients (treated) and prediabetic patients, p T2D+/ctrl-significance between T2D patients (treated) and healthy controls, p T2D-/preD-significance between T2D without treatment and prediabetic patients, p T2D-/ctrl-significance between T2D without treatment and healthy controls, p preD/ctrl-significance between prediabetic patients and healthy controls. All differences were tested using ANOVA test. BMI, body mass index; BP, blood pressure; HOMA-IR, homeostasis model assessment insulin resistance index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT – γ-glutamyl transferase. HOMA – IR (homeostasis model assessment insulin resistance index) was calculated using the formula: fasting insulin (mU/L) x fasting glucose (mmol/L)/22.5
Allele and genotype frequencies for FTO gene polymorphism rs8050136 for patients with T2D, prediabetic patients and healthy controls are presented in Table II. The allele frequencies for FTO polymorphism – rs8050136 A>C were in Hardy-Weinberg equilibrium in all groups of subjects studied (p>0.05). However, no significant differences in analyzed genotype frequencies were found between T2D, pre-diabetic patients, and healthy controls (Table II). Odds ratios that were adjusted for age and gender did not confirm expected association of FTOrs8050136 with diabetes risk (OR=1.084, 95% CI 0.758–1.551, p=0.659). In the healthy subjects (Table III), FTO gene polymorphism rs8050136 showed a significant association with most important markers of glucose homeostasis, dyslipidemia, inflammation and obesity. Carriers of the risk AA genotype of rs8050136 had higher levels of HbA1c (p = 0.016) and fibrinogen (p=0.013) when compared to the carriers of CC genotype. In addition, carriers of AA genotype had significantly higher levels of diastolic BP (p = 0.020) and leucocytes (p = 0.006), and lower HDL cholesterol levels than carriers of AC genotype (p = 0.040).
Table II.
Genotype and variant allele frequencies for FTO gene polymorphism rs8050136: A>C.
| Polymorphism | Genotype | T2D patients | Mutated allele frequency | Prediabetic patients | Mutated allele frequency | Healthy controls | Mutated allele frequency | p |
|---|---|---|---|---|---|---|---|---|
| rs8050136 | AA | 130 (24.7%) | 0.50 | 17 (41.3%) | 0.46 | 84 (26.0%) | 0.51 | p preD/ctrl = 0.100 |
| AC | 264 (50.1%) | 16 (30.4%) | 150 (46.4%) | p T2D/preD = 0.576 | ||||
| CC | 133 (25.2%) | 13 (28.3%) | 89 (27.6%) | P preD/ctrl = 0.224 | ||||
| Total | 527 | P1 =0.999 | 46 | P2=0.127 | 323 | P3=0.443 | p=0.258 |
p – significance of the 2 test for comparison of genotype frequencies between T2D, prediabetic patients and healthy controls ppreD/ctrl – significance of the 2 test for comparison of genotype frequencies between T2D and prediabetic patients p significance of the 2 test for comparison of genotype frequencies between T2D patients and healthy controls p T2D/preD – significance of the 2 test for comparison of genotype frequencies between prediabetic patients and healthy controls
P1 – value for Hardy-Weinberg equilibrium for T2D patients group
P2 – value for Hardy-Weinberg equilibrium for pre-diabetic patients group
P3 – value for Hardy-Weinberg equilibrium for healthy controls group
Table III.
Comparison of clinical and biochemical characteristics between different genotypes of FTO gene polymorphism rs8050136: A>C in healthy subjects.
| FTO rs8050136 A>C | ||||||
|---|---|---|---|---|---|---|
| AA (n=79) | AC (n=140) | CC (n=87) | p-value AA/AC | p-value AA/CC | p-value AC/CC | |
| Fasting glucose (mmol/L) | 5.02 ± 0.138 | 5.18 ± 0.055 | 5.15 ± 0.117 | 0.996 | 0.561 | 0.502 |
| HbA1c (%) | 9.37 ± 3.811 | 5.47 ± 0.048 | 5.39 ± 0.062 | 0.119 | 0.016 | 0.246 |
| Fasting insulin (mU/L) | 11.27 ± 2.227 | 11.17 ± 1.105 | 10.443 ± 0.801 | 0.674 | 0.486 | 0.751 |
| + HOMA-IR | 2.212 ± 0.206 | 2.629 ± 0.282 | 2.49 ± 0.217 | 0.744 | 0.618 | 0.842 |
| BMI (kg/m2) | 29.41 ± 2.661 | 26.91 ± 0.589 | 27.02 ± 0.805 | 0.133 | 0.221 | 0.880 |
| Waist circumference (cm) | 98.55 ± 1.638 | 94.07 ± 1.012 | 92.78 ± 2.008 | 0.163 | 0.076 | 0.630 |
| Hip circumference (cm) | 109.25 ± 1.498 | 106.27 ± 0.884 | 106.55 ± 2.574 | 0.414 | 0.635 | 0.194 |
| Systolic BP (mm Hg) | 123.05 ± 2.181 | 120.43 ± 1.803 | 126.75 ± 2.107 | 0.375 | 0.175 | 0.108 |
| Diastolic BP (mm Hg) | 82.65 ± 1.128 | 79.13 ± 0.665 | 80.43 ± 1.112 | 0.020 | 0.362 | 0.179 |
| Triglycerides (mmol/L) | 1.75 ± 0.137 | 1.66 ± 0.082 | 1.64 ± 0.112 | 0.781 | 0.835 | 0.600 |
| Total cholesterol (mmol/L) | 5.37 ± 0.167 | 5.44 ± 0.106 | 5.36 ± 0.157 | 0.521 | 0.679 | 0.850 |
| HDL cholesterol (mmol/L) | 1.21 ± 0.054 | 1.34 ± 0.040 | 1.27 ± 0.051 | 0.040 | 0.169 | 0.585 |
| LDL-cholesterol (mmol/L) | 3.51 ± 0.119 | 3.45 ± 0.088 | 3.48 ± 0.115 | 0.426 | 0.414 | 0.914 |
| VLDL-cholesterol (mmol/L) | 0.735 ± 0.118 | 0.822 ± 0.089 | 0.687 ± 0.066 | 0.152 | 0.576 | 0.365 |
| hsCRP (mg/L) | 3.72 ± 0.433 | 3.39 ± 0.228 | 3.66 ± 0.428 | 0.896 | 0.964 | 0.933 |
| Fibrinogen (g/L) | 5.30 ± 1.584 | 3.81 ± 0.601 | 3.11 ± 0.083 | 0.035 | 0.013 | 0.439 |
| Leukocytes (109/L) | 6.77 ± 0.192 | 6.19 ± 0.170 | 6.44 ± 0.235 | 0.006 | 0.133 | 0.330 |
| Sedimentation (mm/h) | 12.65 ± 2.893 | 11.13 ± 1.320 | 10.06 ± 2.518 | 0.820 | 0.801 | 0.937 |
Values represent mean ± standard error mean (SEM), p-values show the significance of the differences of clinical and biochemical characteristics between the stated genotypes of FTO gene polymorphism rs8050136: A>C. All differences were tested using ANCOVA test (adjusted for age, sex and BMI).
Importantly, tendency of association of AA genotype with higher waist circumference (p = 0.076) was demonstrated when compared to the CC genotype.
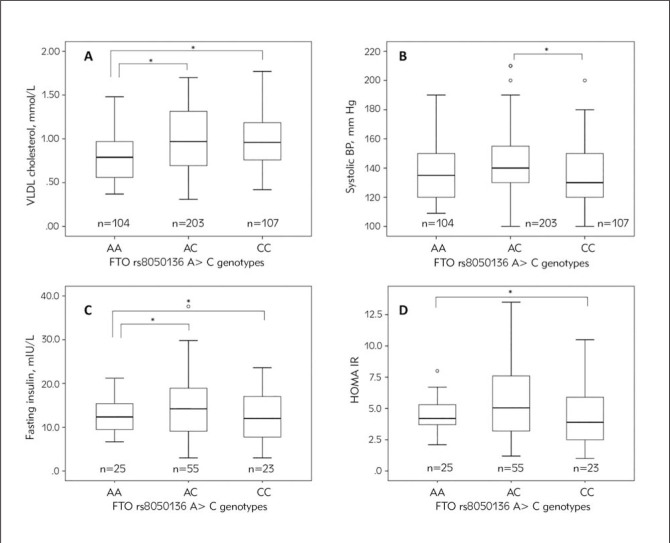
In T2D patients group (Table IV), carriers of AA genotype of rs8050136 had lower VLDL cholesterol levels compared to carriers of AC and CC genotypes (retrospectively p = 0.011; p = 0.021, Figure IA). Carriers of AC genotype had significantly higher systolic BP compared to CC genotype carriers (p=0.049, Figure IB).
Table IV.
Comparison of clinical and biochemical characteristics between different genotypes of FTO gene polymorphism rs8050136: A>C in T2D patients treated with hypoglycemics.
| FTO rs8050136 A>C | ||||||
|---|---|---|---|---|---|---|
| AA (n = 104) | AC (n = 203) | CC (n=107) | p-value AA/AC | p-value AA/CC | p-value AC/CC | |
| Fasting glucose (mmol/L) | 8.86 ± 0.361 | 9.50 ± 0.280 | 9.16 ± 0.392 | 0.124 | 0.830 | 0.185 |
| HbA1c (%) | 7.57 ± 0.172 | 7.74 ± 0.124 | 7.53 ± 0.154 | 0.639 | 0.794 | 0.435 |
| Fasting insulin (mU/L) | 16.95 ± 2.283 | 15.29 ± 0.990 | 15.65 ± 1.449 | 0.856 | 0.480 | 0.291 |
| + HOMA-IR | 6.96 ± 1.238 | 6.30 ± 0.434 | 6.65 ± 0.716 | 0.532 | 0.603 | 0.186 |
| BMI (kg/m2) | 30.76 ± 0.727 | 30.98 ± 0.411 | 30.98 ± 0.805 | 0.377 | 0.980 | 0.381 |
| Waist circumference (cm) | 101.09 ± 2.021 | 103.32 ± 0.859 | 101.77 ± 1.814 | 0.346 | 0.268 | 0.731 |
| Hip circumference (cm) | 107.83 ± 2.821 | 110.92 ± 0.945 | 107.23 ± 2.060 | 0.139 | 0.067 | 0.503 |
| Systolic BP (mm Hg) | 134.48 ± 2.878 | 138.50 ± 2.275 | 136.87 ± 1.973 | 0.154 | 0.654 | 0.049 |
| Diastolic BP (mm Hg) | 88.37 ± 1.088 | 88.09 ± 0.763 | 87.16 ± 1.054 | 0.516 | 0.322 | 0.621 |
| Triglycerides (mmol/L) | 2.38 ± 0.259 | 2.31 ± 0.100 | 2.78 ± 0.428 | 0.390 | 0.209 | 0.557 |
| Total cholesterol (mmol/L) | 5.21 ± 0.130 | 5.17 ± 0.118 | 5.29 ± 0,167 | 0.484 | 0.616 | 0.899 |
| HDL-cholesterol (mmol/L) | 1.11 ± 0.036 | 1.03 ± 0.022 | 1.03 ± 0.031 | 0.295 | 0.083 | 0.341 |
| LDL-cholesterol (mmol/L) | 3.29 ± 0.118 | 3.33 ± 0.089 | 3.36 ± 0.155 | 0.841 | 0.740 | 0.855 |
| VLDL-cholesterol (mmol/L) | 0.80 ± 0.064 | 1.51 ± 0.211 | 1.71 ± 0.438 | 0.011 | 0.021 | 0.988 |
| hsCRP (mg/L) | 6.31 ± 1.292 | 4.69 ± 0.557 | 5.64 ± 1.091 | 0.182 | 0.172 | 0.821 |
| Fibrinogen (g/L) | 3.60 ± 0.149 | 3.60 ± 0.114 | 3.76 ± 0.137 | 0.761 | 0.666 | 0.393 |
| Leukocytes (109/L) | 7.12 ± 0.225 | 7.57 ± 0.169 | 7.50 ± 0.311 | 0.132 | 0.407 | 0.609 |
| Sedimentation (mm/h) | 13.38 ± 2.941 | 18.58 ± 4.092 | 16.19 ± 2.940 | 0.543 | 0.178 | 0.356 |
Values represent mean ± standard error mean (SEM), p-values show the significance of the differences of clinical and biochemical characteristics between the stated genotypes of FTO gene polymorphism rs8050136: A>C. All differences were tested using ANCOVA test (adjusted for age, sex and BMI).
Figure 1.
Normalized sigma metric method decision chart for level 1 control.
In T2D patients group without treatment (i.e. without hypoglycemic therapy), FTO rs8050136 was significantly associated with the markers of glucose homeostasis, insulin resistance, and obesity (Table V). Here, carriers of AA and AC genotypes of rs8050136: A>C had significantly higher insulin levels than carriers of CC genotype (retrospectively p=0.016, p=0.044, Figure IC). Carriers of the risk A allele had significantly higher HOMA-IR levels as compared to the carriers of C allele (p=0.018, Figure ID). It is important to mention the tendency of association of AC genotype with higher levels of BMI (p=0.063) as compared to the CC genotype.
Table V.
Comparison of clinical and biochemical characteristics between different genotypes of FTO gene polymorphism rs8050136 in T2D patients without hypoglycemic therapy.
| FTO rs8050136 A>C | ||||||
|---|---|---|---|---|---|---|
| AA (n=25) | AC (n=55) | CC (n = 23) | p-value AA/AC | p-value AA/CC | p-value AC/CC | |
| Fasting glucose (mmol/L) | 9.36 ± 0.658 | 8.27 ± 0.242 | 9.67 ± 0.919 | 0.182 | 0.848 | 0.138 |
| HbA1c (%) | 7.89 ± 0.270 | 7.75 ± 0.177 | 7.95 ± 0.386 | 0.882 | 0.862 | 0.728 |
| Fasting insulin (mU/L) | 26.53 ± 8.243 | 15.95 ± 1.607 | 14.15 ± 2.442 | 0.044 | 0.016 | 0.343 |
| + HOMA-IR | 14.04 ± 7.317 | 5.84 ± 0.604 | 4.76 ± 0.887 | 0.062 | 0.018 | 0.264 |
| BMI (kg/m2) | 30.24 ± 1.297 | 30.64 ± 0.551 | 28.93 ± 0.405 | 0.868 | 0.141 | 0.063 |
| Waist circumference (cm) | 101.52 ± 2.957 | 103.70 ± 1.447 | 105.20 ± 2.211 | 0.311 | 0.217 | 0.649 |
| Hip circumference (cm) | 108.44 ± 2.403 | 109.15 ± 1.365 | 109.46 ± 1.637 | 0.825 | 0.708 | 0.825 |
| Systolic BP (mm Hg) | 139.12 ± 4.085 | 134.81 ± 2.361 | 130.65 ± 3.069 | 0.218 | 0.106 | 0.490 |
| Diastolic BP (mm Hg) | 83.32 ± 1.583 | 83.65 ± 1.356 | 83.91 ± 1.692 | 0.897 | 0.863 | 0.745 |
| Triglycerides (mmol/L) | 2.24 ± 0.294 | 2.08 ± 0.140 | 3.00 ± 0.447 | 0.986 | 0.155 | 0.091 |
| Total cholesterol (mmol/L) | 5.13 ± 0.268 | 5.44 ± 0.161 | 5.84 ± 0.277 | 0.284 | 0.091 | 0.340 |
| HDL-cholesterol (mmol/L) | 1.10 ± 0.067 | 1.16 ± 0.036 | 1.23 ± 0.146 | 0.452 | 0.302 | 0.628 |
| LDL-cholesterol (mmol/L) | 3.04 ± 0.252 | 3.37 ± 0.147 | 3.64 ± 0.266 | 0.218 | 0.103 | 0.456 |
| VLDL-cholesterol (mmol/L) | 0.88 ± 0.115 | 0.89 ± 0.052 | 1.28 ± 0.225 | 0.544 | 0.072 | 0.114 |
| hsCRP (mg/L) | 8.67 ± 4.271 | 4.03 ± 0.515 | 3.73 ± 0.450 | 0.559 | 0.940 | 0.659 |
| Fibrinogen (g/L) | 3.86 ± 0.270 | 4.00 ± 0.127 | 3.73 ± 0.216 | 0.558 | 0.756 | 0.329 |
| Leukocytes (109/L) | 9.87 ± 2.930 | 7.33 ± 0.272 | 7.38 ± 0.307 | 0.527 | 0.696 | 0.883 |
| Sedimentation (mm/h) | 21.27 ± 6.159 | 18.43 ± 3.147 | 14.33 ± 2.404 | 0.504 | 0.523 | 0.893 |
Values represent mean ± standard error mean (SEM), p-values show the significance of the differences of clinical and biochemical characteristics between the stated genotypes of FTO gene polymorphism rs8050136: A>C. All differences were tested using ANCOVA test (adjusted for age, sex and BMI).
In the group of patients with prediabetes results of our study did not show any association of rs8050136: A>C gene variant FTO with analyzed biochemical and anthropometric parameters (data not shown).
Discussion
Obesity is one of today’s biggest global problems (4). Overweight is very important in pathophysiology of T2D and one of major risk factors for developing this complex disease (33). The increasing global prevalence of T2D is tied to rising rates of obesity – which is in part a consequence of social trends toward higher energy intake and reduced energy expenditure (34). However, the mechanisms that underlie individual differences in the predisposition to obesity remain obscure (1). This is a reason why it is important to find out association of different genetic variants of candidate genes of obesity and to find a link with their role in pathophysiology of T2D. For genetic epidemiology of complex diseases, replication studies at various ethnic groups are essential to support the genotype – phenotype linkage to correctly suggest subjects for further (e.g. mechanism uncovering) studies.
Recently, FTO was indicated by GWA study as the candidate gene for development of T2DŠ35). In this study, association of FTO gene polymorphism (rs8050l36) with the traits of T2D was studied for the first time in the West Balkan region population (Bosnians and Herzegovinians and Kosovars), showing associations of genotypes of rs8050136 polymorphism with certain clinical and biochemical parameters of T2D, especially with glucose, insulin and HOMA IR levels, as well as BMI, waist and hips circumference, as indicators of visceral obesity.
Our results demonstrated that no significant differences in analyzed genotype frequencies were found between patients with T2D, prediabetes, and healthy controls. Results of logistic regression analysis did not confirm a significant association of FTO genetic variation (rs8050136: A>C) with diabetes risk, probably due to the low number of subjects in our cohort (OR=1.084, 95% CI 0.758–1.551, p=0.659). Several studies conducted on different populations have confirmed the impact of this genetic variant on the increased risk of developing T2D (10, 11, 12, 13, 14), although there are those made on Russian, Mexican Mestizo, Netherlands, Lebanese and Omani population, which do not confirm this association (24, 25, 26, 27, 28).
Results of our study did not also confirm the previously published significant association of FTO polymorphism rs8050136 A>C with most important markers of obesity. However, risk A allele showed a tendency of association with higher values of BMI, waist circumference and hip circumference in different analyzed groups of our study. Numerous studies have shown association of risk A allele of rs8050136 A>C genetic variant with higher values of obesity biomarkers. In a large study in Indian population, significant association rs8050136 A>C of with higher values of BMI, waist circumference, as well as the relationship with waist-hip ratio has been demonstrated (13). Similar results of association of this genetic variant with BMI were demonstrated in Han Chinese adolescent study (19). Xiao et al. (18) found a significant association of risk A allele of rs805016 gene variant with higher levels of BMI in Uygur population from northwest China. Large meta-analysis which analyzed over 34 000 participants aged from 18 to 100 years, came to finding of greater influence of rs8050136 A>C on elevated BMI in younger patients than in older (20).
Interestingly, our results showed that the risk A allele of rs8050136 A>C polymorphism was significantly associated with decreased HDL cholesterol levels in control subjects. Results of several studies, including large meta-analysis, confirmed the association of rs8050136 A>C with the increased risk of developing metabolic syndrome, which is characterized by higher levels of glucose, waist circumference, triglycerides and total cholesterol, and lower levels of HDL cholesterol (11, 17, 36, 37). The associations of rs8050136 A>C with the markers observed in our study can be explained by the significant effects of metabolic syndrome on the metabolic and clinical parameters including higher values of anthropometric parameters (BMI, waist and hip circumference) and lower HDL cholesterol levels. It is very important to emphasize that most patients within T2D group had developed metabolic syndrome. The results of our study showed a significant association with higher diastolic pressure, which is also one of diagnostic parameters and one of major risk factors of the metabolic syndrome. In T2D patients group, risk A allele showed association with lower values of systolic pressure, which again can be explained by the fact that the most of patients in the study used antihypertensive drugs. Therefore, results obtained for this group of patients need to be interpreted with a special caution.
Interestingly, our results showed a significant association of A allele of rs8050136 A>C with higher HbA1c levels in healthy control group as well as with elevated insulin levels and HOMA IR index in T2D patients without treatment. The presence of the risk A allele appears to lead to the increased levels of HbA1c, insulin, and HOMA IR, pointing out the pronounced insulin resistance in newly diagnosed patients with T2D. Namely, increased insulin levels in the initial stage of developing T2D represent a defence mechanism of the organism against the elevated glucose and HbA1c levels.
Our findings are consistent with the results of studies which have analyzed association of FTO gene polymorphism, rs8050136 A>C with insulin sensitivity. Result of a study analysing influence of rs8050136 A>C with insulin sensitivity in women with advanced ovarian polycystic syndrome (PCOS), showed a significant association with insulin sensitivity in women without PCOS, indicated a direct effect of rs8050136 A>C on insulin sensitivity, not associated with PCOS (21). A large study of Wang and colleagues confirmed the influence of rs8050136 A>C on the parameters of obesity and glucose homeostasis in various populations in the USA (22). Another study also showed the significant association of the risk A allele with the elevated levels of glucose, C-peptide, and BMI (23). Thus, in our study we confirmed results of the above-mentioned studies of significant association of rs8050136 A>C with important markers of glycaemic control and insulin sensitivity, such as elevated HbA1c, insulin, and HOMA IR levels.
The results of our study also showed significant effects of rs8050136 A>C polymorphism on the increased levels of inflammatory markers (fibrinogen and number of leukocytes). To our knowledge, this is one of the first studies analyzing association of rs8050136 A>C polymorphism in T2D with the inflammatory markers. A recent large meta-analysis examining the impact of candidate genes of metabolic syndrome on the inflammatory processes demonstrated an important association of FTO intron gene variant with the CRP levels (38).
A major limitation of our study is related to the relatively small number of our population cohort, particularly in regards to the patients with prediabetes and patients with newly diagnosed T2D who were not taking any medications. Nevertheless, these categories of patients are very important in terms of analysis of the associations of genetic variants with biomarkers related to the pathophysiology of T2D. Importantly, our results showed a significant association of FTO genetic variant rs8050136 A>C with the major markers of insulin resistance, obesity, T2D, and inflammation, opening new avenues for solving many unclear questions in the pathogenesis of this complex disease. These findings may lead to the new possibilities for prevention, diagnosis, and personalized medical treatment of T2D. Especially, seeing this genetic association through the perspective of obesity-related T2D pathophysiology suggests that obesity prevention and increase in physical activity in the genetically risky subgroups may be a valuable contribution to the T2D prevention.
Acknowledgements
The authors thank all subjects who participated in the study, as well as medical doctors and paramedical staff from the Health Centre of Sarajevo Canton, Clinic for Endocrinology and Diabetes, University Clinical Centre of Sarajevo, University Hospital of Clinical Centre in Banja Luka, General Hospital in Tešanj, and Health Centre in Prizren who assisted in the study.
The work was supported by the research grant for EU-FP7 project preparation by the Council of Ministers of Bosnia and Herzegovina (BH)/Ministry of Civil Affairs BH, awarded to S.S. and by the grant MH CZ – DRO (UHHK, 00179906).
Glossary
List of abbreviations
- T2D
type 2 diabetes
- FTO
alpha-ketoglutarate dependent dioxygenase
- BMI
body mass index
- BP
blood pressure
- HOMA-IR
homeostasis model assessment insulin resistance index
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
- hsCRP
high-sensitivity C-reactive protein
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- GGT
γ-glutamyl transferase
Footnotes
Conflict of interest
Conflict of interest statement: The authors stated that they have no conflicts of interest regarding the publication of this article.
References
- 1.McCarthy MI.. Genomics, type 2 diabetes, and obesity. N Engl J Med. 2010;363:2339–50. doi: 10.1056/NEJMra0906948. [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. IDF Diabetes Atlas. (Sixth) 2013 [Google Scholar]
- 3.Peng S, Zhu Y, Xu F, Ren X, Li XLai M.. FTO gene polymorphisms and obesity risk: a meta-analysis. BMC Med. 2011;9:71. doi: 10.1186/1741-7015-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hossain P, Kawar BEl, Nahas M.. Obesity and diabetes in the developing world – a growing challenge. N Engl J Med. 2007;356:213–5. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 5.Loos RJ, Yeo GS.. The bigger picture of FTO: the first GWAS-identified obesity gene. Nat Rev Endocrinol. 2014;10:51–61. doi: 10.1038/nrendo.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers JC, Elliott P, Zabaneh D, Zhang W, Li Y, Froguel P. et al. Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nat Genet. 2008;40:716–8. doi: 10.1038/ng.156. [DOI] [PubMed] [Google Scholar]
- 7.Meyre D, Delplanque J, Chevre JC, Lecoeur C, Lobbens S, Gallina S. et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet. 2009;41:157–9. doi: 10.1038/ng.301. [DOI] [PubMed] [Google Scholar]
- 8.Lindgren CM, Heid IM, Randall JC, Lamina C, Steinthorsdottir V, Qi L. et al. Genome-wide association scan meta-analysis identifies three Loci influencing adiposity and fat distribution. PLoS Genet. 2009;5:e1000508. doi: 10.1371/journal.pgen.1000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scherag A, Dina C, Hinney A, Vatin V, Scherag S, Vogel CI. et al. Two new Loci for body-weight regulation identified in a joint analysis of genome-wide association studies for early-onset extreme obesity in French and german study groups. PLoS Genet. 2010;6:e1000916. doi: 10.1371/journal.pgen.1000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demirsoy Hİ, Ertural YD, Balci Ş, Çιnkιr Ü, Sezer K, Tamer L, Aras N.. Profiles of circulating miRNAs following metformin treatment in patients with type 2 diabetes. J Med Biochem. 2018;237:502–9. doi: 10.2478/jomb-2018-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotta K, Kitamoto T, Kitamoto A, Mizusawa S, Matsuo T, Nakata Y. et al. Association of variations in the FTO SCG3 and MTMR9 genes with metabolic syndrome in a Japanese population. J Hum Genet. 2011;56:647–51. doi: 10.1038/jhg.2011.74. [DOI] [PubMed] [Google Scholar]
- 12.Rong R, Hanson RL, Ortiz D, Wiedrich C, Kobes S, Knowler WC. et al. Association analysis of variation in/near FTO, CDKAL1, SLC30A8, HHEX, EXT2, IGF2BP2, LOC387761, and CDKN2B with type 2 diabetes and related quantitative traits in Pima Indians. Diabetes. 2009;58:478–88. doi: 10.2337/db08-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chauhan G, Tabassum R, Mahajan A, Dwivedi OP, Mahendran Y, Kaur I. et al. Common variants of FTO and the risk of obesity and type 2 diabetes in Indians. J Hum Genet. 2011;56:720–6. doi: 10.1038/jhg.2011.87. [DOI] [PubMed] [Google Scholar]
- 14.Votsi C, Toufexis C, Michailidou K, Antoniades A, Skordis N, Karaolis M. et al. Type 2 Diabetes Susceptibility in the Greek-Cypriot Population: Replication of Associations with TCF7L2, FTO, HHEX, SLC30A8 and IGF2BP2 Polymorphisms. Genes (Basel) 2017:8. doi: 10.3390/genes8010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao S, Zeng X, Fan Y, Su Y, Ma Q, Zhu J. et al. Gene Polymorphism Association with Type 2 Diabetes and Related Gene-Gene and Gene-Environment Interactions in a Uyghur Population. Med Sci Monit. 2016;22:474–87. doi: 10.12659/MSM.895347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang YC, Liu PH, Yu YH, Kuo SS, Chang TJ, Jiang YD. et al. Validation of type 2 diabetes risk variants identified by genome-wide association studies in Han Chinese population: a replication study and meta-analysis. PLoS One. 2014;9:e95045. doi: 10.1371/journal.pone.0095045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Dong S, Xu H, Qian J, Yang J.. Genetic variants in FTO associated with metabolic syndrome: a meta- and gene-based analysis. Mol Biol Rep. 2012;39:5691–8. doi: 10.1007/s11033-011-1377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao S, Zeng X, Quan L, Zhu J.. Correlation between polymorphism of FTO gene and type 2 diabetes mellitus in Uygur people from northwest China. Int J Clin Exp Med. 2015;8:9744–50. [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J, Xu J, Zhang Z, Ren J, Li Y, Wang J. et al. Association of FTO polymorphisms with obesity and metabolic parameters in Han Chinese adolescents. PLoS One. 2014;9:e98984. doi: 10.1371/journal.pone.0098984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graff M, Gordon-Larsen P, Lim U, Fowke JH, Love SA, Fesinmeyer M. et al. The influence of obesity-related single nucleotide polymorphisms on BMI across the life course: the PAGE study. Diabetes. 2013;62:1763–7. doi: 10.2337/db12-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatziagelaki E, Wagner R, Kantartzis K, Heni M, Linder K, Ketterer C. et al. Insulin resistant phenotype of polycystic ovary syndrome does not seem to be caused by variation in FTO. Horm Metab Res. 2012;44:810–3. doi: 10.1055/s-0032-1321817. [DOI] [PubMed] [Google Scholar]
- 22.Wing MR, Ziegler JM, Langefeld CD, Roh BH, Palmer ND, Mayer-Davis EJ. et al. Analysis of FTO gene variants with obesity and glucose homeostasis measures in the multiethnic Insulin Resistance Atherosclerosis Study cohort. Int J Obes (Lond) 2011;35:1173–82. doi: 10.1038/ijo.2010.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen J, Ronn T, Olsson A, Yang Z, Lu B, Du Y. et al. Investigation of type 2 diabetes risk alleles support CDKN2A/B, CDKAL1, and TCF7L2 as susceptibility genes in a Han Chinese cohort. PLoS One. 2010;5:e9153. doi: 10.1371/journal.pone.0009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almawi WY, Nemr R, Keleshian SH, Echtay A, Saldanha FL, Al-Doseri FA. et al. A replication study of 19 GWAS-validated type 2 diabetes at-risk variants in the Lebanese population. Diabetes Res Clin Pract. 2013;102:117–22. doi: 10.1016/j.diabres.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Al-Sinani S, Woodhouse N, Al-Mamari A, Al-Shafie O, Al-Shafaee M, Al-Yahyaee S. et al. Association of gene variants with susceptibility to type 2 diabetes among Omanis. World J Diabetes. 2015;6:358–66. doi: 10.4239/wjd.v6.i2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikitin AG, Potapov VY, Brovkina OI, Koksharova EO, Khodyrev DS, Philippov YI. et al. Association of polymorphic markers of genes FTO KCNJ11, CDKAL1, SLC30A8, and CDKN2B with type 2 diabetes mellitus in the Russian population. PeerJ. 2017;5:e3414. doi: 10.7717/peerj.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gamboa-Melendez MA, Huerta-Chagoya A, Moreno-Macias H, Vazquez-Cardenas P, Ordonez-Sanchez ML, Rodriguez-Guillen R. et al. Contribution of common genetic variation to the risk of type 2 diabetes in the Mexican Mestizo population. Diabetes. 2012;61:3314–21. doi: 10.2337/db11-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Chapa EG, Leal-Ugarte E, Peralta-Leal V, Duran-Gonzalez J, Meza-Espinoza JP.. Genetic Epidemiology of Type 2 Diabetes in Mexican Mestizos. Biomed Res Int. 2017;2017:3937893. doi: 10.1155/2017/3937893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Herpt TTW, Lemmers RFH, van Hoek M, Langendonk JG, Erdtsieck RJ, Bravenboer B. et al. Introduction of the DiaGene study: clinical characteristics, pathophysiology and determinants of vascular complications of type 2 diabetes. Diabetol Metab Syndr. 2017;9:47. doi: 10.1186/s13098-017-0245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.International Diabetes federation (IDF) Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. 2006 [Google Scholar]
- 31.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC.. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 32.Miller SA, Dykes DD, Polesky HF.. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puhalo Sladoje D, Kisić B, Mirić D.. Lipid concentration in obese subjects with metabolic. J Med Biochem. 2017;36:366–74. doi: 10.1515/jomb-2017-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baldane S, Kendir CI, Kirac OC, Ipekci S, Tekin G, Unlu A, Kebapcilar L.. Healthy subjects and newly diagnosed type 2 diabetic patients. J Med Biochem. 2018;37:373–8. doi: 10.1515/jomb-2017-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL. et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–5. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheung CY, Tso AW, Cheung BM, Xu A, Ong KL, Law LS. et al. Genetic variants associated with persistent central obesity and the metabolic syndrome in a 12-year longitudinal study. Eur J Endocrinol. 2011;164:381–8. doi: 10.1530/EJE-10-0902. [DOI] [PubMed] [Google Scholar]
- 37.Elouej S, Nagara M, Attaoua R, Sallem OK, Rejeb I, Hsouna S. et al. Association of genetic variants in the FTO gene with metabolic syndrome: A case-control study in the Tunisian population. J Diabetes Complications. 2016;30:206–11. doi: 10.1016/j.jdiacomp.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 38.Kraja AT, Chasman DI, North KE, Reiner AP, Yanek LR, Kilpelainen TO. et al. Pleiotropic genes for metabolic syndrome and inflammation. Mol Genet Metab. 2014;112:317–38. doi: 10.1016/j.ymgme.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]