Abstract
GABAergic dysfunction in hippocampus, a key feature of schizophrenia (SZ), may contribute to cognitive impairment in this disorder. In stratum oriens (SO) of sector CA3/2 of the human hippocampus, a network of genes involved in the regulation of glutamic acid decarboxylase GAD67 has been identified. Several of the genes in this network including epigenetic factors histone deacetylase 1 (HDAC1) and death-associated protein 6 (DAXX), the GABAergic enzyme GAD65 as well as the kainate receptor (KAR) subunits GluR6 and 7 show significant changes in expression in this area in SZ. We have tested whether HDAC1 and DAXX regulate GAD67, GAD65, or GluR in the intact rodent hippocampus. Stereotaxic injections of lentiviral vectors bearing shRNAi sequences for HDAC1 and DAXX were delivered into the SO of CA3/2, followed by laser microdissection of individual transduced GABA neurons. Quantitative PCR (QPCR) analyses demonstrated that inhibition of HDAC1 and DAXX increased expression of GAD67, GAD65, and GluR6 mRNA. Inhibition of DAXX, but not HDAC1 resulted in a significant increase in GluR7 mRNA. Our data support the hypothesis that HDAC1 and DAXX play a central role in coordinating the expression of genes in the GAD67 regulatory pathway in the SO of CA3/2.
Keywords: DAXX, GABA dysfunction, GluR6, GluR7, HDAC1, molecular mechanism
Introduction
Schizophrenia (SZ) is a psychotic disorder associated with abnormalities in cognitive and emotional function. Over the past two decades, several studies have suggested that a decrease in GABAergic neurotransmission is present in key brain regions, including the anterior cingulate cortex, dorsolateral prefrontal region, and hippocampus. It is now believed that the decrease in GABAergic activity (Benes et al. 1991, 1992) is related to a decrease in the expression of the 67-kDa isoform of glutamic acid decarboxylase [GAD67] (Akbarian et al. 1995; Guidotti et al. 2000; Hashimoto et al. 2003; Benes et al. 2007). In the hippocampus of schizophrenics, changes in the GABA system have shown a predilection for sector CA3/2 of the hippocampus, particularly the stratum oriens (SO-CA3/2), a layer in which GABAergic interneurons are the exclusive neuronal cell type (for a review, see Benes 2010). GAD67 and GAD65 mRNAs show highly significant changes in SO-CA3/2 of schizophrenics (Benes et al. 2007), although studies of GAD65 containing terminals do not show an abnormal distribution in hippocampus (Todtenkopf and Benes 1998), prefrontal cortex, or anterior cingulate region of SZs (Benes et al. 2000).
Using laser microdissection (LMD), microarray-based gene expression profiling, and network association analyses (Benes et al. 2007), a functional group of 30 genes has been identified as potentially being involved in the molecular regulation of GAD67 expression in the SO-CA3/2 locus. The individual genes in this regulatory network (GAD67RN) also show abnormal expression patterns and are considered potential candidate genes contributing to circuitry dysfunction in the SO-CA3/2 in SZ (Benes et al. 2007). The genes-of-interest in this network include: 1) the 65-kDa isoform of GAD, which like GAD67, contributes to GABA neuron function primarily through axosomatic synapses on the somata of projection neurons and interneurons; 2) histone deacetylase 1 (HDAC1), an enzyme that forms part of a regulatory complex that represses promoters, 3) death-associated protein 6 (DAXX), a transcription factor that serves as a corepressor of HDAC1 (Ecsedy et al. 2003; Benes et al. 2007) and is involved in the epigenetic regulation of the cell cycle; and 4) the kainate receptor subunits (KARs) GluR5, 6, and 7 that mediate, at least in part, excitatory glutamatergic inputs to GABAergic interneurons in SO-CA3/2 (Gisabella et al. 2012).
Recent evidence from animal experiments has implicated KARs as mediating the influence of the basolateral amygdala (BLA) on GABA neurons of the hippocampus. Using single cell recording techniques in hippocampal slices taken from rats receiving stereotaxic infusions of picrotoxin in the BLA, selective antagonists of GluR5, 6, and 7 subunits were found to alter the spike frequency, inhibitory postsynaptic currents and inwardly rectifying hyperpolarization-dependent (Ih3) channels in fast-spiking neurons in SO-CA3/2 (Gisabella et al. 2005, 2009, 2012). Changes in the firing pattern of GABA neurons at this locus could contribute to synchronization of gamma oscillations that underlie cortical rhythms that are abnormal in SZ (Colgin and Moser 2010; Uhlhaas and Singer 2010; Rotaru et al. 2012; Benes 2015). In this regard, the GluR5, 6, and 7 subunits of KARs may play discrete roles in modulating the activity of both inhibitory and disinhibitory interneurons within the microcircuitry of SO-CA3/2 (Benes 2015), and could contribute to the characteristics of gamma rhythms generated at this locus (Freund and Buzsaki 1996).
To test the validity of the GAD67RN model, HiB5 postmitotic cell cultures with a GABAergic phenotype have been used to suppress the expression of HDAC1 and DAXX using a viral vector strategy as an initial step in this process (Subburaju et al. 2016). While these in vitro results clearly suggest that these GAD67RN genes regulate GAD67 in hippocampal GABA cells, it was imperative to demonstrate that similar interactions occur in an in vivo system in which HDAC1 and DAXX, as well as KARs, mediate the expression of GAD67 in GABA neurons. Toward this end, the current report presents the results of experiments in which lentiviral vectors have been stereotaxically infused in SO-CA3/2 locus of intact, freely moving rats. LMD of SO-CA3/2 tissue containing transduced neurons as well as microdissected pooled individual labeled GABA neurons within this layer were then used to analyze the expression of GAD67, GAD65, HDAC1, DAXX and the GluR5, 6, and 7 subunits using a high sensitivity methodology.
Materials and Methods
Animal Ethics Statement
Stereotaxic surgery and infusion of lentiviral particles were performed in anesthetized male Sprague-Dawley rats (there is no evidence to suggest sex-specific regulation of GAD67 and GAD65). All procedures were approved by the McLean Hospital IACUC Committee and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Double Immunohistochemistry
Rat brains were perfused with 4% paraformaldehyde (PFA), postfixed overnight, and processed into 40 µm coronal slices. Standard immunofluorescence techniques were used to visualize the colocalization of HDAC1 or DAXX with GAD67 or GAD65. Antibodies employed included monoclonal (anti-GAD67 1:1000 [Sigma], anti-GAD65 1:1000 [Millipore], anti-HDAC1 1:200 [Cell Signaling]) and polyclonal antibodies (rabbit anti-HDAC1 1:200 [Thermoscientific]; rabbit anti-DAXX 1:200 [Santa Cruz]), followed by fluorescence (Alexa)-coupled secondary antibody raised in donkey (1:600; Invitrogen). A Leica fluorescent microscope was used to evaluate fluorescent staining in neuronal and glial cells in the SO of CA3/2 and CA1.
Stereotaxic Infusion of Viral Vectors
Specific gene sequence targets had been selected using the RNAi consortium (TRC-Sigma) Broad Institute algorithm. Three short hairpin RNA constructs that target distinct 21mer sequences within the HDAC1- or DAXX genes had been designed and cloned into the pLKO.1 vector. The U6 promoter drives the shRNA target gene sequences and a turbo green fluorescent protein (tGFP) reporter is under the control of a CMV immediate-early (IE) promoter. All target vector constructs and the respective viral particles were custom made by Sigma-Aldrich. In a preselection process in HiB5 cells, the most efficient shRNA is for HDAC1 and DAXX, respectively (Table 1), as well as optimal conditions for viral transduction were identified (Subburaju et al. 2016).
Table 1.
shRNAi target sequences
Microcannula Preparation
Borosilicate glass pipettes (part # 50613, 1.0 mm, Stoelting Co.) were pulled using a vertical electrode puller to produce glass electrodes with a long, gently tapering shank. Using a microscope stage, the tip of the microcannula was cut with dissection scissors to the desired final tip diameter (~30–50 µm).
Test Injections to Determine Coordinates
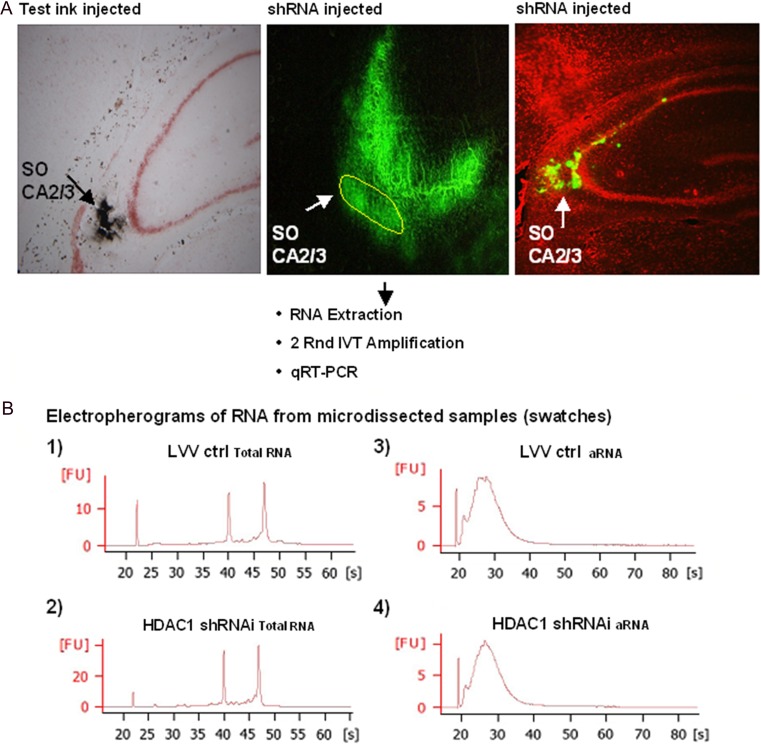
Coordinates for the viral injection into the hippocampal SO of CA3/2 were determined by injections with India ink (Fig. 1A, left) and surrogate viral suspension (0.1 µm latex microspheres).
Figure 1.
Determination of the correct injection site and quality assessment of RNA extracted from swatches. (A) Correct injection site was determined using india ink (left panel; black: india ink, red: Nissl stain) and lentiviral vectors carrying tGFP (middle: unfixed tissue and right: fixed tissue panels; green fluorescence: tGFP-positive transduced neurons; red: fluorescent Nissl stain [NeuroTrace Fluorescent Nissl Stain # N-21482, Molecular Probes]). (B) Assessment of RNA quality in microdissected SO-CA3/2 of tGFP-expressing neurons. Extracted RNA was assessed using an Agilent Bioanalyzer. The sharp peaks at 40 and 47 s depict intact 18s and 28s rRNA, respectively (upper and lower left panels). Panels on the right show typical peaks of amplified RNA (aRNA), ensuring the high quality of the extracted RNA.
Injection of Lentiviral Vectors
Three groups of 8–12 animals were used: 1 control group receiving virus with tGFP only (Fig. 1A, middle and right) and 2 experimental groups receiving virus with tGFP and shRNAi sequences for HDAC1 or DAXX (Table 1). P45 rats (180–210 g) were anesthetized and fixed in a stereotaxic frame, and viral suspension was delivered bilaterally to the SO of CA3/2 using the following coordinates: tooth bar −3.25; bregma −3.4; lateral −4.0; ventral −3.1. Deposits of 0.250 µL (~1.6 × 109 titer) were injected at a rate of 0.125 µL/min. After each injection, the microcannula remained in position for 5 min before withdrawing slowly to avoid back-flow of viral particles.
LMD of Swatches from SO of CA3/2
Five days after the injection, unfixed brains were removed, snap frozen in dry ice-cooled isopentane and cut into 25 µm coronal slices on a cryostat. Using transverse cryostat sections of frozen hippocampus, clusters of tGFP-positive cells were laser microdissected from the SO of CA3/2 as swatches that were roughly rectangular in shape. The labeled cells within the swatches were surrounded by unlabeled non-neuronal cells in the neuropil.
Quantitative PCR for Swatches
RNA was extracted from microdissected tissue with Trizol reagent (Life Technologies), and purified using the RNeasy Lipid Tissue Mini Kit (Qiagen Inc.). Control and HDAC1 shRNAi samples were amplified for two rounds using a total input of 5 ng. mRNA amplification was performed using MessageAmp II. In amplification round one, T7 oligo (dt) primers were used. The second amplification round was initiated with random hexamer primers. Amplified RNA was tested for quality (Bioanalyser, Agilent Technologies; Fig. 1B) and used to quantify expression of the genes of interest (HDAC1, GAD67, GAD65) using Taqman one-step reverse transcription (RT)-PCR master mix and Taqman gene expression assay according to the manufacturer's instructions.
Optimization of Identifying and Isolating tGFP-Positive Cells in SO of CA2/3 Following Immersion Fixation
Since swatches of tGFP-positive cells with surrounding tissue contain cells that are not transduced, we decided to analyze in a next step only tGFP-positive cells microdissected from the hippocampus. Cryosectioning alone of brains from the tGFP control and HDAC1 groups was not enough to preserve the autofluorescence of tGFP in virus-transduced cells for subsequent LMD of these cells. Therefore, several fixation methods were tried to fix tGFP in cryosections on slides immediately following cutting of the brain. Several different variables were assessed including: fixative type, fixative concentration, temperature of fixative, and immersion time of section in fixative. Fixatives tried included acetone, ethanol, methanol, formaldehyde (37%) vapor, PFA, and glutaraldehyde. Fixation times varied anywhere from 30 s to 24 h, temperatures used were −20°C, 4°C, or RT, and combinations of fixatives were also employed. Thickness of cryosections was also varied as well as the type of slide the sections were mounted on. The only fixative that seemed somewhat effective at preserving tGFP autofluorescence in the brains was 4% PFA. This allowed for visualization of tGFP-positive cells using the fluorescent microscope revealing weak fluorescence that also seemed to leak from cells, was not consistent between brains, and when observed was found only in a small region of the hippocampus (i.e., 3–4 of the 25 µm coronal sections). Also, this fluorescence quickly vanished during storage of the slides.
Visualizing tGFP-Positive Cells and Assessment of RNA Integrity Following Perfusion with Low-Concentration Fixative
While some alternative methods were considered for analyzing tissue from unfixed brains (i.e., rapid immunofluorescence against tGFP using antibodies or LMD of the entire SO) it was decided that perfusing the animals would be the best way to preserve tGFP autofluorescence allowing for identification of virus-transduced cells. To test this, two animals received bilateral injections of tGFP control virus particles into the striatum. Five days after surgery, the animals were perfused with PFA (one animal 1%, one animal 0.1%), the brains removed and cryoprotected in 30% sucrose for 4 days. tGFP-positive cells were observed at the injection site in both animals with no obvious difference in fluorescence intensity between the two concentrations of fixative. However, histogene counterstaining interfered with the GFP fluorescence. An optimized dehydration and drying series was determined for brain sections for subsequent LMD of tGFP-positive cells. This process consisted of passing the slides through RNAse free-graded ethanol solutions (50%, 75%, 95%, and 100% at 4°C, 100% at RT) for 15 s each followed by drying the slide at 37°C for 5 min.
RNA extracted from tissue from the 1% PFA brain was checked for quality using the Agilent 6000 RNA Pico Bioanalyzer Kit. The RNA integrity numbers for two separately processed samples were 5.7 and 7.0, suggesting that the RNA was moderately degraded. In order to shorten the time during which the tissue was exposed to aqueous solutions and potential RNAse activity, it was considered that perfusion of cryoprotectant rather than extensive bath cryoprotection may allow for extraction of better quality RNA. Two animals received bilateral injections of tGFP control virus particles into the striatum. Five days after surgery, animals were perfused with 1% PFA followed by 30% sucrose in 1% PFA. Brains were postfixed/cryoprotected in 30% sucrose in 1% PFA for an additional hour before flash freezing. tGFP-positive cells were visible in these animals. RNA isolated from hippocampal tissue from these animals showed little evidence of degradation when analyzed on the Bioanalyzer. This suggested that this procedure was appropriate for visualizing virus-infected cells in SO of CA2/3 while also allowing for extraction of nondegraded RNA from these cells.
LMD of Individual tGFP-Positive Cells
Five days after delivery of the lentiviral particles, rats were perfused with 0.1 M phosphate-buffered saline, 1% PFA followed by 30% sucrose in 1% PFA. Brains were removed and snap frozen in powdered dry ice. For LMD, brains were cryo-sectioned at 25 µm. Sections were collected at the level of the anterior commissure (Fig. 2A), single tGFP-positive cells were laser-dissected (Fig. 2B) and the integrity of extracted RNA was assessed by Bioanalyser (Fig. 2C).
Figure 2.
Single cell sampling of microdissected tGFP-positive neurons and evaluation of RNA quantity and quality (Cell-to-Ct). (A) Schema of sampling points in rat hippocampal SO of CA3/2 (from –1.80 mm to –3.80 mm bregma), coronal brain sections adapted from Paxinos and Watson, 1998; red circles: GFP-positive cells. (B) LMD of individual tGFP-positive cells; upper panel, before and after LMD (white arrow: tGFP-positive neuron); lower panel: localization of dissected neurons in SO; (C) evaluation of RNA quality; signals for the mRNA are depicted in red, the other (green) lines represent the zero baseline (parallel to the x axis) and the start and end of the measurement (green dashed vertical lines).
Quantitative PCR for Pooled Individual Cells
Dissected pooled cells were analyzed for expression of HDAC1, GAD67, GAD65, DAXX, and HDAC2 mRNA using the Ambion Cell-to-Ct kit (Life Technologies). Cells were lysed according to the manufacturer's instructions and the RT reaction directly carried out with the lysate without purification of RNA. However, RNA quality was assessed separately. For specific target genes of interest (HDAC1, GAD67, GA65, DAXX, HDAC2, GluR5, 6, 7) and a housekeeping gene (β-2-microglobulin), the respective cDNAs were then preamplified for 10 thermal cycles using pooled diluted TaqMan gene expression assays (Applied Biosystems). Finally, TaqMan quantitative PCR (qPCRs) were performed using 5 µL of the diluted preamplified product. For each sample and gene, three replicates were run in a 96-well plate. Real-time qPCRs were carried out following the manufacturer's protocol. Gene expression values were determined as ΔCt (Ct – Ct B2M) and fold changes between different samples were determined as ΔΔCt (ΔCt vehicle – ΔCt).
Results
GABA Neurons of Rat Hippocampal SO of CA3/2 Express Both HDAC1 and DAXX
To assess whether GABA cells in the SO of CA3/2 express HDAC1 and DAXX, a double immunofluorescence study was performed on coronal sections of rat hippocampus. GAD67 was clearly colocalized with both HDAC1 (Fig. 3A) and DAXX (Fig. 3B) in this specific brain region in rats. HDAC1 and DAXX were also colocalized in the same neurons (Fig. 3C). GAD65 was also colocalized with both HDAC1 and DAXX, and GAD65-positive varicosities were observed in HDAC1- and DAXX-positive neurons (Fig. 3D,E).
Figure 3.
GABA neurons of rat hippocampal SO of CA3/2 express both HDAC1 and DAXX. Coronal brain sections at the level of SO of CA3/2 were incubated with specific antibodies against GAD67 or GAD65 and either HDAC1 or DAXX, or with HDAC1 and DAXX. Fluorescence-coupled secondary antibodies were used to visualize detected proteins. (A) Colocalization of GAD67 and HDAC1; (B) colocalization of GAD67 and DAXX. (C) Colocalization of HDAC1 and DAXX in SO of CA3/2. (D) Colocalization of GAD65 and HDAC1; (E) Colocalization of GAD65 and DAXX. Arrows point to double labeled neurons.
Regulation of GAD67 and GAD65 mRNA after Inhibition of HDAC1 with shRNAi in Swatches
Rats were infused with lentiviral vectors carrying shRNAi for HDAC1 to test whether HDAC1 regulates GAD67 and GAD65 expression in vivo. Swatches of unfixed tissue containing GFP-positive, hippocampal GABA cells were microdissected, the RNA extracted and submitted to 2-round pre-amplification. QRT-PCR showed HDAC1 being downregulated by 90% (Fig. 4A). This coincided with an increase in GAD67 mRNA (2-fold, Fig. 4B) as well as GAD65 mRNA (2.5-fold; Fig. 4C). The results indicate that HDAC1 is instrumental in the regulation of GABA cell function in SO of CA3/2. However, it could not be excluded that cells in the surrounding tissue were contributing to the observed effects. Therefore, an approach was developed to evaluate pooled, individual, tGFP-positive neurons.
Figure 4.
Regulation of GAD67 and GAD65 after inhibition of HDAC1 with shRNAi in rat hippocampus SO-CA3/2. RNA was extracted from microdissected SO-CA3/2. Control and HDAC1 shRNAi samples were amplified for two rounds using a total RNA input of 5 ng. Amplified RNA was used to quantify gene expression using Taqman qRT-PCR. Statistical significance was evaluated using one-way ANOVA, followed by Student–Newman Keuls post hoc test (*P < 0.05). Data are expressed as means + SEM; n = 3 per group. A P ≤ 0.05 was accepted as statistically significant. (A) HDAC1; (B) GAD67; (C) GAD65.
HDAC1 Silencing Increases GAD67 and GAD65 mRNA in Individual Pooled GABA Cells of SO
The fact that GABA cells are the exclusive neuronal cell type in the SO (Benes and Berretta 2001) makes them an accessible target for microscopic and molecular studies. To assess whether HDAC1 regulates GAD67 and GAD65 expression in individual SO GABA cells in vivo, rats were infused stereotaxically with lentiviral vectors carrying shRNAi for HDAC1.
QRT-PCR showed that HDAC1 was downregulated by 50% (Fig. 5A). This coincided with an increase in GAD67 mRNA (50%, Fig. 5B) as well as GAD65 mRNA (4-fold; Fig. 5C). These findings confirmed previous in vitro data as well as the results from the swatches of unfixed tissue. Similar to the GABAergic HiB5 cells in vitro (Subburaju et al. 2016), HDAC2 mRNA was upregulated by 2-fold (Fig. 5D); this possibly represents a compensatory mechanism on the mRNA level to account for the loss of HDAC1 activity.
Figure 5.
HDAC1 inhibition increases expression of GAD67 and GAD65 in GABA cells of SO. LVV carrying shRNAi for HDAC1 or control vector was infused into hippocampal SO of rats. Individual tGFP-positive cells in SO-CA3/2 were microdissected, pooled for each rat and subjected to Cell-to-Ct qRT-PCR to measure mRNA expression. Data are expressed as fold change compared with tGFP vector control. Statistical significance was evaluated using one-way ANOVA, followed by Student–Newman Keuls post hoc test (*P < 0.05). Data are expressed as means + SEM; n = 4 per group. A P ≤ 0.05 was accepted as statistically significant. (A) HDAC1; (B) GAD67; (C) GAD65; (D) HDAC2.
DAXX Inhibition Results in Increased GAD67 and GAD65 mRNA in Hippocampal SO Cells
To test whether DAXX is involved in the regulation of the GABA phenotype in vivo, rats were transduced with LVV carrying shRNAi against DAXX. Individual tGFP-positive cells were pooled and analyzed by cell-to-ct qPCR. The downregulation of DAXX by about 30% as assessed by qRT-PCR was accompanied by an increase in GAD67 mRNA (about 2-fold); GAD65 was increased by about 3-fold (Fig. 6A–C).
Figure 6.
DAXX inhibition results in increased GAD67 and GAD65 mRNA in hippocampal SO cells. LVV carrying shRNAi for DAXX or control vector was infused into hippocampal SO of rats. tGFP-positive cells were microdissected and subjected to Cell-to Ct qRT-PCR to measure mRNA expression. Data are expressed as fold change compared with tGFP vector control. Statistical significance was evaluated using one-way ANOVA, followed by Student–Newman Keuls post hoc test (*P < 0.05). Data are expressed as means + SEM; n = 4 per group. A P ≤ 0.05 was accepted as statistically significant. (A) DAXX; (B) GAD67; (C) GAD65.
Regulation of Glutamate Receptor Subunits by HDAC1 Inhibition
The GluR subtypes GluR5, 6, and 7 were also identified as differentially regulated in SZ in postmortem hippocampal tissue (Benes et al. 2007). Here, we tested whether an inhibition of HDAC1 influenced the expression of GluR5, 6, or 7. While GluR6 mRNA was increased by about 2-fold (Fig. 7A), GluR7 mRNA expression was not changed as a result of the manipulation of HDAC1 expression (Fig. 7B). GluR5 mRNA expression was too low to be measurable.
Figure 7.
Regulation of glutamate receptor subunits by inhibition of HDAC1 or DAXX. LVV carrying shRNAi for HDAC1 or DAXX or control vector was infused into hippocampal SO of rats. tGFP-positive cells were microdissected and subjected to cell-to-ct qRT-PCR to measure mRNA expression. Data are expressed as fold change compared with tGFP vector control. Statistical significance was evaluated using one-way ANOVA, followed by Student–Newman Keuls post hoc test (*P < 0.05). Data are expressed as means + SEM; n = 4 per group. A P ≤ 0.05 was accepted as statistically significant. (A) HDAC1 shRNAi, GluR6; (B) HDAC1 shRNAi, GluR7; (C) DAXX shRNAi, GluR6; (D) DAXX shRNAi, GluR7. NS, not significant.
Influence of DAXX Inhibition on Expression of GluR Subunits in Hippocampus in vivo
The decrease in DAXX mRNA after local injection of lentiviral vectors carrying shRNAi for DAXX resulted in an about 30% increase in GluR6 mRNA expression (Fig. 7C); GluR7 mRNA was increased by about 4-fold (Fig. 7D). Again, GluR5 mRNA expression was too low to be detected.
Discussion
The present study has demonstrated that the epigenetic coregulators, HDAC1 and DAXX, are capable of modulating the expression of GAD67 and GAD65, the key genes involved in the synthesis of GABA in the SO of CA3/2 in vivo. Although HDAC1 is expressed by both neurons and glia at this location, earlier studies have reported that mRNA representing GAD67 or GAD65 is not expressed by glia at this locus. Hence, changes in GAD67 expression induced with specific viral vectors are likely a manifestation of changes taking place in GABAergic interneurons, as the latter are the exclusive neuronal cell type in this layer. The shRNAi-mediated inhibition of HDAC1 or DAXX resulted in a significant increase in GAD67 and GAD65 mRNA expression in SO-CA3/2 tissue; a similar change was observed in pooled individual GABAergic somata harvested at the same site. The pattern detected in our postmortem microarray study of this locus (Benes et al. 2007) demonstrated that an increased expression of HDAC1 or DAXX coincided with a decreased expression of GAD67 and GAD65 mRNA. Consistent with our working hypothesis, the findings described herein show a predictably reversed pattern in response to decreased expression of HDAC1 and DAXX. Taken together, these data expand our understanding of the relationship of these latter genes with GAD67 and GAD65 from one of mere temporal concurrence to one involving functionally interrelated molecular mechanisms. Activity emanating from pyramidal neurons in CA3/2 or other interneurons within this subregion could also contribute to changes in GAD67 and GAD65 regulation in SO-CA3/2. Among the factors that may induce changes in the expression of the GAD67 regulatory network are synaptic inputs from both intrinsic hippocampal and extrinsic amygdalar neuronal systems.
Functionally, the progression of SZ has been linked to a variety of presynaptic and postsynaptic abnormalities in GABA interneurons, resulting in a loss of GABAergic activity in the hippocampus (Benes et al. 1991), as well as the anterior cingulate cortex and dorsolateral prefrontal cortex (Akbarian et al. 1995; Lewis et al. 1999). GABA neuron dysregulation is characterized by decreased high affinity GABA uptake sites (Simpson et al. 1989; Reynolds et al. 1990), a compensatory increase in GABAA receptor binding (Benes et al. 1996), decreased numbers of interneurons (Benes et al. 1998), decreased GAD67 mRNA expression (Akbarian et al. 1995; Guidotti et al. 2000), and decreased GAD65 terminals showing dose-related increases to antipsychotic medication (Todtenkopf and Benes 1998). Our previous studies had demonstrated differential regulation of several genes in SO of sector CA3/2, among them GAD67 and GAD65, which are indispensable for the maintenance of the GABAergic phenotype, as well as a diverse array of epigenetic proteins, transcription factors, signaling cascades, and cell-cycle regulators (Benes et al. 2007, 2008, 2009). Postmortem studies of human brain are subject to many potential confounders, such as premortem agonal changes, deterioration during the postmortem interval, exposure to psychotropic medications, and substance abuse, making it difficult to reliably distinguish changes that are intrinsic to the illness from those that are compensatory or epiphenomenal in nature (Berretta et al. 2014). Moreover, it is not possible to establish whether there are functional relationships among genes and/or their products, as postmortem tissue is typically “snap” frozen and only capable of revealing cross-sectional changes. Accordingly, in assessing the importance of GAD67 and GAD65, we chose two approaches involving viral vectors, one employing neurons cultured in vitro and the other stereotaxic delivery to precise targets within the hippocampus of rats, to evaluate expression differences in our postmortem studies.
For our in vitro model, we chose a hippocampal progenitor cell line (HiB5) (Renfranz et al. 1991) experimentally prompted to differentiate into neurons with a GABAergic phenotype (Subburaju and Benes 2012). In the cultured HiB5-derived GABAergic neurons, inhibition of HDAC1 or DAXX mRNA and protein expression with lentivirus-based shRNAi resulted in a significant upregulation of GAD67 mRNA and protein expression. HDAC1 and DAXX did not regulate each other's expression.
In vitro models are limited by the lack of presynaptic and postsynaptic contacts with other neurons, which might influence regulatory processes in situ under normal and abnormal physiological conditions. To overcome this limitation, we employed direct stereotaxic injection of lentivirus-based shRNAi specifically into the SO of CA3/2, followed by LMD to obtain discrete samples of tissue from tGFP-labeled specific layers (swatches) or individual tGFP-positive neurons. As with the HiB5-derived GABA neurons, swatches of SO containing lentivirus-transduced GABA neurons showed an inhibition of HDAC1 expression that was associated with a significant upregulation of GAD67 and GAD65 .
It was important to consider whether non-neuronal cells surrounding the transduced GABA neurons might be contributing to the findings. Towards this end, the expression of HDAC1, DAXX, GAD67, and GAD65 was analyzed in pooled individual GABA neurons transduced with shRNAi for HDAC1 or DAXX.
Consistent with our previous data in vitro and with the results from swatches of hippocampal tissue, HDAC1 inhibition in vivo resulted in an increase in GAD67 and GAD65 mRNA in pooled individual neurons. Interestingly, we could also demonstrate upregulation of HDAC2 mRNA in response to HDAC1 inhibition, a counter regulatory phenomenon that had also been observed in differentiated HiB5 in vitro (Subburaju et al. 2016). However, since our in vitro data clearly showed that HDAC2 protein is not increased, it seems doubtful whether this counter-regulation is functionally effective. Suppressed expression of DAXX mRNA was also associated with an increased expression of GAD67 and GAD65 mRNA, suggesting that DAXX and HDAC1 may be acting in parallel in the regulation of GAD67 and GAD65 in vivo (Amin et al. 2001; Subburaju et al. 2016).
Previous experimental evidence had already suggested that epigenetic mechanisms contribute to the pathogenesis of SZ. Reduction of GAD67 mRNA and protein in postmortem hippocampal tissue from SZ patients coincides with the hypermethylation of histone H3K27, a marker for transcriptional repression, and the hypomethylation of H3K4, an indicator of transcriptional activation, in the promoter region of the GAD67 gene (Huang and Akbarian 2007; Huang et al. 2007; Kouzarides 2007). Epigenetic regulators including DNA methyl transferase 1 (DNMT1), expressed in GABA interneurons of the adult brain, and HDAC1, a DNMT1 binding partner, are upregulated in the brains of SZ patients (Veldic et al. 2004; Benes et al. 2007; Ruzicka et al. 2007). In mice, overexpression of HDAC1 is associated with a dysfunction of the prefrontal cortex and a specific impairment of contextual fear memory in the hippocampus (Bahari-Javan et al. 2012; Jakovcevski et al. 2013). Consistent with these studies, HDAC1 in our in vivo and in vitro studies appears to exert a regulatory influence on GAD67 expression. This current study demonstrates for the first time, however, that DAXX takes part in regulation of GAD67 and GAD65 in vivo, possibly through complex formation with its corepressor HDAC1.
The exact mechanism(s) through which HDAC1 and DAXX regulate the GAD65 and GAD67 promoters are not yet fully understood. Neither the GAD67 nor the GAD65 promoter has binding sequences for these factors. One possibility is that HDAC1 and/or DAXX might employ other transcription factors to assist them in the indirect regulation of GAD expression. An example is the transcription factor Egr-1 that has been found to be associated with the regulation of the GAD67 promoter (Luo et al. 2008; Zhang et al. 2010). Consistent with this, our in vitro model demonstrated that inhibition of either HDAC1 or DAXX expression resulted in an increased transcription of Egr-1 mRNA expression (Subburaju et al. 2016). A second possibility is that HDAC1 and DAXX may be a component of an enhancer complex that is operative at sites distal to the GAD67 and GAD65 promoters. Such a regulatory mechanism involving histone deactylases activity has been proposed for the GAD65 promoter (Pan 2012). Future studies will elucidate how HDAC1 and DAXX influence the GAD promoters in hippocampal GABA neurons.
Importantly, clinical data support the hypothesis that increased HDAC1 contributes to GABA neuron dysfunction in SZ. In clinical practice, the non-specific HDAC inhibitor valproic acid is used in combination with the atypical antipsychotic clozapine in the treatment of SZ. In mice, clozapine, together with valproate, increases DNA demethylation of the GAD67 promoter in a synergistic fashion (Guidotti et al. 2011; Gavin and Akbarian 2012). The resulting chromatin remodeling may reduce GABAergic dysfunction in SZ through a deinhibition of the GAD67 promoter.
The data presented here also suggest that HDAC1 and DAXX may engage in interplay with the KAR subunits GluR6 and GluR7. These receptor subunits contribute to early development of the hippocampus by influencing the differentiation of GABA cells and their subsequent participation in the functional integrity of the trisynaptic pathway (Carta et al. 2003; Maingret et al. 2005). A rodent model for postmortem findings has suggested that KARs mediate the synaptic influence of excitatory glutamatergic afferents from the BLA on the electrical properties of GABA neurons in the SO-CA3/2 locus (Martin et al. 1993; Kullmann and Semyanov 2002). In our postmortem study, increased expression of GluR6 and GluR7 subunits has also been reported (Benes et al. 2007) and parallels the increase in transcripts for HDAC1 and DAXX at this site in SZ. In the current study, however, decreased expression of HDAC1 and DAXX was associated with an increase in GluR6 and GluR7 expression. Overall, these data suggest that these two KAR subunits that mediate activation of inhibitory and disinhibitory interneurons in SO-CA3/2 (Gisabella et al. 2012) may be directly or indirectly influenced by HDAC1 or DAXX. However, the exact mechanism(s) of KAR promoter regulation by HDAC1 or DAXX is (are) at present unknown.
Conclusion
Together, these findings support the hypothesis that the epigenetic factors HDAC1 and DAXX are part of a complex network of genes that contribute to the synaptic regulation of GAD67 and GAD65 and help to promote the functional integrity of GABAergic interneurons located within SO-CA3/2 of the hippocampus
Funding
This work was funded by grants to F.M.B. from the National Institutes of Health (MH42261 and MH77175), Takeda Pharmaceuticals Inc, the William P. and Henry B. Test Endowment, and to S.S. from the Brain and Behavior Research Foundation.
Notes
Conflict of Interest: None declared.
References
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jones EG. 1995. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 52:258–278. [DOI] [PubMed] [Google Scholar]
- Amin HM, Saeed S, Alkan S. 2001. Histone deacetylase inhibitors induce caspase-dependent apoptosis and downregulation of daxx in acute promyelocytic leukaemia with t(15;17). Br J Haematol. 115:287–297. [DOI] [PubMed] [Google Scholar]
- Bahari-Javan S, Maddalena A, Kerimoglu C, Wittnam J, Held T, Bahr M, Burkhardt S, Delalle I, Kugler S, Fischer A, et al. 2012. HDAC1 regulates fear extinction in mice. J Neurosci. 32:5062–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM. 2010. Amygdalocortical circuitry in schizophrenia: from circuits to molecules. Neuropsychopharmacology. 35:239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM. 2015. Building models for postmortem abnormalities in hippocampus of schizophrenics. Schizophr Res.167:73–83. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. 2001. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 25:1–27. [DOI] [PubMed] [Google Scholar]
- Benes FM, Khan Y, Vincent SL, Wickramasinghe R. 1996. Differences in the subregional and cellular distribution of GABAA receptor binding in the hippocampal formation of schizophrenic brain. Synapse. 22:338–349. [DOI] [PubMed] [Google Scholar]
- Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. 1998. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 44:88–97. [DOI] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Subburaju S, Walsh JP. 2008. Circuitry-based gene expression profiles in GABA cells of the trisynaptic pathway in schizophrenics versus bipolars. Proc Natl Acad Sci USA. 105:20935–20940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. 2007. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci USA. 104:10164–10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Lim B, Subburaju S. 2009. Site-specific regulation of cell cycle and DNA repair in post-mitotic GABA cells in schizophrenic versus bipolars. Proc Natl Acad Sci USA. 106:11731–11736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. 1991. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 48:996–1001. [DOI] [PubMed] [Google Scholar]
- Benes FM, Todtenkopf MS, Logiotatos P, Williams M. 2000. Glutamate decarboxylase(65)-immunoreactive terminals in cingulate and prefrontal cortices of schizophrenic and bipolar brain. J Chem Neuroanat. 20:259–269. [DOI] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Alsterberg G, Bird ED, SanGiovanni JP. 1992. Increased GABAA receptor binding in superficial layers of cingulate cortex in schizophrenics. J Neurosci. 12:924–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Heckers S, Benes FM. 2014. Searching human brain for mechanisms of psychiatric disorders. Implications for studies on schizophrenia. Schizophr Res. 167 (1–3):91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Ariwodola OJ, Weiner JL, Valenzuela CF. 2003. Alcohol potently inhibits the kainate receptor-dependent excitatory drive of hippocampal interneurons. Proc Natl Acad Sci USA. 100:6813–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Moser EI. 2010. Gamma oscillations in the hippocampus. Physiology (Bethesda). 25:319–329. [DOI] [PubMed] [Google Scholar]
- Ecsedy JA, Michaelson JS, Leder P. 2003. Homeodomain-interacting protein kinase 1 modulates Daxx localization, phosphorylation, and transcriptional activity. Mol Cell Biol. 23:950–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. 1996. Interneurons of the hippocampus. Hippocampus. 6:347–470. [DOI] [PubMed] [Google Scholar]
- Gavin DP, Akbarian S. 2012. Epigenetic and post-transcriptional dysregulation of gene expression in schizophrenia and related disease. Neurobiol Dis. 46:255–262. [DOI] [PubMed] [Google Scholar]
- Gisabella B, Bolshakov VY, Benes FM. 2005. Regulation of synaptic plasticity in a schizophrenia model. Proc Natl Acad Sci USA. 102:13301–13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisabella B, Bolshakov VY, Benes FM. 2012. Kainate receptor-mediated modulation of hippocampal fast spiking interneurons in a rat model of schizophrenia. PLoS One. 7:e32483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisabella B, Cunningham MG, Bolshakov VY, Benes FM. 2009. Amygdala-dependent regulation of electrical properties of hippocampal interneurons in a model of schizophrenia. Biol Psychiatry. 65:464–472. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Chen Y, Davis JM, Dong E, Gavin DP, Grayson DR, Matrisciano F, Pinna G, Satta R, et al. 2011. Epigenetic GABAergic targets in schizophrenia and bipolar disorder. Neuropharmacology. 60:1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, et al. 2000. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 57:1061–1069. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. 2003. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 23:6315–6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Akbarian S. 2007. GAD1 mRNA expression and DNA methylation in prefrontal cortex of subjects with schizophrenia. PLoS One. 2:e809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Matevossian A, Whittle C, Kim SY, Schumacher A, Baker SP, Akbarian S. 2007. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J Neurosci. 27:11254–11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski M, Bharadwaj R, Straubhaar J, Gao G, Gavin DP, Jakovcevski I, Mitchell AC, Akbarian S. 2013. Prefrontal cortical dysfunction after overexpression of histone deacetylase 1. Biol Psychiatry. 74:696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. 2007. Chromatin modifications and their function. Cell. 128:693–705. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Semyanov A. 2002. Glutamatergic modulation of GABAergic signaling among hippocampal interneurons: novel mechanisms regulating hippocampal excitability. Epilepsia. 43(Suppl 5):174–178. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Pierri JN, Volk DW, Melchitzky DS, Woo TU. 1999. Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biol Psychiatry. 46:616–626. [DOI] [PubMed] [Google Scholar]
- Luo Y, Lathia J, Mughal M, Mattson MP. 2008. SDF1alpha/CXCR4 signaling, via ERKs and the transcription factor Egr1, induces expression of a 67-kDa form of glutamic acid decarboxylase in embryonic hippocampal neurons. J Biol Chem. 283:24789–24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Lauri SE, Taira T, Isaac JT. 2005. Profound regulation of neonatal CA1 rat hippocampal GABAergic transmission by functionally distinct kainate receptor populations. J Physiol. 567:131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Thompson MA, Nadler JV. 1993. The neuroprotective agent riluzole inhibits release of glutamate and aspartate from slices of hippocampal area CA1. Eur J Pharmacol. 250:473–476. [DOI] [PubMed] [Google Scholar]
- Pan ZZ. 2012. Transcriptional control of Gad2. Transcription. 3:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfranz PJ, Cunningham MG, McKay RD. 1991. Region-specific differentiation of the hippocampal stem cell line HiB5 upon implantation into the developing mammalian brain. Cell. 66:713–729. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Czudek C, Andrews HB. 1990. Deficit and hemispheric asymmetry of GABA uptake sites in the hippocampus in schizophrenia. Biol Psychiatry. 27:1038–1044. [DOI] [PubMed] [Google Scholar]
- Rotaru DC, Lewis DA, Gonzalez-Burgos G. 2012. The role of glutamatergic inputs onto parvalbumin-positive interneurons: relevance for schizophrenia. Rev Neurosci. 23:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka WB, Zhubi A, Veldic M, Grayson DR, Costa E, Guidotti A. 2007. Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol Psychiatry. 12:385–397. [DOI] [PubMed] [Google Scholar]
- Simpson MD, Slater P, Deakin JF, Royston MC, Skan WJ. 1989. Reduced GABA uptake sites in the temporal lobe in schizophrenia. Neurosci Lett. 107:211–215. [DOI] [PubMed] [Google Scholar]
- Subburaju S, Benes FM. 2012. Induction of the GABA cell phenotype: an in vitro model for studying neurodevelopmental disorders. PloS One. 7:e33352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subburaju S, Coleman AJ, Ruzicka WB, Benes FM. 2016. Toward dissecting the etiology of schizophrenia: HDAC1 and DAXX regulate GAD67 expression in an in vitro hippocampal GABA neuron model. Transl Psychiatry. 6:e723 10.1038/tp.2015.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Benes FM. 1998. Distribution of glutamate decarboxylase65 immunoreactive puncta on pyramidal and nonpyramidal neurons in hippocampus of schizophrenic brain. Synapse. 29:323–332. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. 2010. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 11:100–113. [DOI] [PubMed] [Google Scholar]
- Veldic M, Caruncho HJ, Liu WS, Davis J, Satta R, Grayson DR, Guidotti A, Costa E. 2004. DNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proc Natl Acad Sci USA. 101:348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TY, Hellstrom IC, Bagot RC, Wen X, Diorio J, Meaney MJ. 2010. Maternal care and DNA methylation of a glutamic acid decarboxylase 1 promoter in rat hippocampus. J Neurosci. 30:13130–13137. [DOI] [PMC free article] [PubMed] [Google Scholar]