Abstract
BACKGROUND:
Estimates of blood collection and use in the United States derived from the National Blood Collection and Utilization Survey (NBCUS) call for application of robust statistical methods in the analysis of survey data. Since 1993, annual inpatient surgical volume has been used as the main stratification variable for sampling and estimation. However, recent NBCUS results have shown a decrease in blood use in surgical settings, raising the possibility that inpatient surgical volume may no longer be the optimal stratification variable. The objective of this study is to explore factors affecting hospital blood utilization.
STUDY DESIGN AND METHODS:
A multivariate generalized linear regression with a negative binomial distribution was used to determine which hospital characteristics best explained allogeneic red blood cell (RBC) use, using data from the 2015 NBCUS to determine hospital blood use and the 2013 annual American Hospital Association database to identify hospital characteristics.
RESULTS:
Annual inpatient surgical volume explained the most variation in allogeneic RBC use among hospitals (pseudo-R2 of 70.8%). Additional variables, such as presence of an oncology service, were also statistically significant in the models but explained little additional variability in blood use.
CONCLUSION:
These findings suggest that annual inpatient surgical volume is an appropriate indicator for estimating blood utilization in the United States. As trends in blood utilization continue to evolve, ongoing analytic efforts to understand indicators of blood use are necessary.
The biennial National Blood Collection and Utilization Survey (NBCUS) is a primary data source for estimating blood use in the United States. The analytic design of this survey is based on stratification of hospitals by inpatient surgical volume, which is used as an indicator for blood usage within hospitals. Relying on inpatient surgical volume for facility sampling has been largely unchanged since the 1989 survey, in which annual inpatient surgical volume was compared with total number of hospital beds (which was used before 1989 for sample design) for use as a stratification variable for estimation of red blood cell (RBC) use.1 Measures that reflect hospital size, such as number of beds, may be correlated with blood use because larger hospitals could have a larger number of patients overall, as well as the capacity to treat patients with more complex conditions who may require higher rates of transfusion. Therefore, before 1989, the total number of hospital beds was used for stratifying hospitals within NBCUS. In 1993, a regression analysis was used to study the association between RBC use in transfusing facilities to the total number of hospital beds or annual inpatient surgical volume at each facility.1 That analysis found that annual inpatient surgical volume explained more of the variation in hospital RBC use than total number of hospital beds and would therefore be a better indicator for estimating blood use for national surveys. Inpatient surgical volume may reflect both patient volume and the complexity of care requiring blood use.1 Since this 1993 report, annual inpatient surgical volume has been used as the primary stratification variable for each NBCUS.
Blood use in the United States has substantially changed over the past 3 decades due to evolving clinical practices and the introduction of new technologies. Since 2008, a significant decline in RBC transfusions has been observed in the United States.2–5 There was a 12.2% overall decline in hospital RBC use from 2013 to 2015, and the decline was most pronounced in hospitals performing 100 to 999 inpatient surgical procedures annually.2 The number of RBC units transfused in surgical procedures decreased by an estimated 41.5% from 2013 to 2015.6 RBC use has remained steady in high-acuity areas such as emergency departments and critical care settings.6–8 The large decline in RBC use in surgical settings raises the question of whether annual inpatient surgical volume remains the optimal stratification variable for predicting blood utilization.
We analyzed data from the 2015 NBCUS, together with additional hospital characteristics reported in the annual American Hospital Association (AHA) survey, to determine whether inpatient surgical volume is still the most accurate indicator of allogeneic RBC use. Allogeneic RBCs were the only blood component considered because they are the most commonly used blood product,2 and past NBCUS sampling methods have centered on correctly estimating allogeneic RBC use. The main analytic objective was to determine sampling variables that lead to the most accurate estimate of hospital allogeneic RBC use. Additionally, we attempted to forecast the demand for RBC use through 2020. Implications for modeling future demand for blood products are also discussed.
METHODS
To determine whether the number of inpatient surgical procedures performed annually is the most appropriate indicator of allogeneic RBC use, we conducted two separate analyses. These analyses were performed to determine the variability in transfusion between facilities that could be attributed to specific facility characteristics. First, we repeated an analytic method previously described for NBCUS sample design that used ordinary least squares (OLS) linear regression, which assumes normally distributed residuals.9 This OLS regression was applied to 2015 NBCUS data using allogeneic RBC transfusion as the dependent variable and total number of hospital beds or annual inpatient surgical volume as independent variables. Based on analyses of the 2015 survey year, the NBCUS blood transfusion estimates at the hospital level are not well approximated by a normal distribution. Therefore, a log-transformation of RBC use was used to obtain adjusted R2 estimates.
Given that NBCUS data are not normally distributed, a generalized linear model (GLM)10 was also used to quantify the relationship between allogeneic RBC use and several explanatory variables (Table 1). GLMs are suited to this task as multiple variables can be assessed in the same model and model selection strategies can be used to determine which variables should be included in the optimal model.10 The number of allogeneic RBC units used in each facility was assumed to have a negative binomial error distribution—a common approach for modeling counts when overdispersion is likely.11–13 Forward model selection based on the Akaike information criterion (AIC)14 was used to determine the optimal metric, although the first step of model selection included only variables related to facility size (Table 1). Variables remained in the model only if they had a p value less than 0.05. We then used the Nagelkerke pseudo-R2 measure to assess the percentage of variability explained by the model.15 The interpretation of pseudo-R2 measures can be challenging because they do not have the same additive properties of the R2 obtained from OLS linear regression and therefore can be used appropriately only between nested models, such as those considered in this forward model selection.15
TABLE 1.
Hospital characteristics, including size proxy variables, service characteristics, and other characteristics expected to have an effect on amount of blood used by a hospital
| Variable description | Number of categories | Variable categories | Justification for inclusion |
|---|---|---|---|
| Size characteristics | |||
| Total number of hospital beds | 6 | 6–99, 100–199, 200–299, 300–399 400–499, 500+ | In the past, total number of hospital beds was used for sampling and weighting in NBCUS analysis. |
| Number of medical/surgical beds | 6 | 1–29, 30–65, 66–101, 102–151, 152–229, 230+ | The majority of blood products are used in inpatient medical settings and surgical settings.6 |
| Annual inpatient surgical volume | 6 | 100–999, 1000–1399, 1400–2399, 2400–4999, 5000–7999, 8000+ | Has been used for sampling and weighting in NBCUS analysis since 1993. Significant amount of blood is used in surgical settings.6 |
| Number of ICU beds | 6 | 1–3, 4–9, 10–13, 14–19, 20–31, 32+ | Transfusion, especially of red blood cells, is common in ICU patients.7 An estimated 9% to 40% of ICU patient receive a platelet transfusion,20 and in one multisite study 12.7% received a plasma transfusion.21 |
| Number of ER visits | 6 | 1 −11,710, 11,710–23,764, 23,765–34,523, 34,524–48,276, 48,277–68,351, 68,352+ | Transfusion of RBCs, platelets, and plasma are common in trauma cases.8 |
| Service characteristics | |||
| Trauma service at hospital | 3 | No trauma service/Level 2–4 trauma service/Level 1 trauma service | Transfusion of RBCs, platelets, and plasma are common in trauma cases.8 |
| Oncology service at hospital | 3 | No oncology service/general oncology service/oncology with bone marrow transplant service | Both cancers and cancer treatments can lead to low blood counts, including anemia.22 Bone marrow transplant patients additionally require extensive blood product transfusion.23 |
| Level 2/3 obstetric unit | 2 | No OB service or Level 1 OB service/Level 2 or 3 OB service | Transfusion of blood products is common practice in more difficult obstetrics cases due to acute blood loss.24 |
| Transplant service at hospital (heart, lung, kidney, liver) | 2 | Yes/No | The majority of blood products are used in inpatient medical settings and surgical settings.6 |
| Other characteristics | |||
| USPHS region | 10 | 10 USPHS regions (See Sapiano et al.6 for states included in each region) | Regional differences may exist in blood transfusion practices. |
| Urban location | 2 | Metro/Division, Rural/Micro | Transfusion practices may vary due to an urban or a rural location. |
| Residency training program | 2 | Yes/No | Teaching hospitals and nonteaching hospitals may differ in blood transfusion practices. |
ER = emergency room; ICU = intensive care unit; NBCUS = National Blood Collection and Utilization Survey; USPHS = US Public Health Service.
For these analyses, data related to allogeneic RBC use within hospitals were obtained from 2015 NBCUS survey responses. This survey had a 73.8% response rate for trans-fusing hospitals.2 For hospital-related characteristics, we used the 2013 AHA database, as this was available at the time of 2015 NBCUS dissemination. Of the 6295 facilities in the 2013 AHA, 2428 were excluded due to size (below the minimum threshold of 100 inpatient surgeries annually), location (outside of the 50 US states), ownership (Department of Defense hospitals were not surveyed), or service category (specialty and rehabilitation facilities were excluded). Of the 3867 facilities that met inclusion criteria for the 2015 NBCUS, all facilities performing at least 1000 surgeries in 2013 and a random sample of 40% of facilities performing between 100 and 999 surgeries in 2013 were sampled, with a final sample of 2883. The 40% sample of the smallest annual inpatient surgical volume hospitals was considered appropriate due to the high number of hospitals that were in this category (>1500) and the low mean amount of blood this strata of hospitals has historically used. Of 2138 total respondents, 2050 reported the number of allogeneic RBCs transfused.
In addition to number of hospital beds and annual inpatient surgical volume, other variables available in the 2013 AHA database were considered for inclusion in these analyses. Variables considered in analysis are displayed in Table 1, where they are grouped by type as either size characteristics, service characteristics, or other characteristics. Size characteristics are factors identified as potential indicators of hospital size, such as total number of hospital beds, number of medical/surgical beds, annual inpatient surgical volume, number of critical care (intensive care unit [ICU]) beds, and number of emergency room (ER) visits. Service characteristics denote the presence of hospital services that may affect blood usage, such as presence of a trauma unit and trauma center level designation, oncology (including bone marrow transplant service), obstetrics service level (level of capability to provide care for complex and high-risk pregnancies), and organ transplantation service. Other characteristics considered included region of the country (by US Public Health Service region),6 urban versus rural location, and presence of a residency training program. Continuous variables in AHA (total number of hospital beds, annual inpatient surgical volume, total number of medical/surgical beds, total number of ICU beds, and total number of ER visits) were stratified into six categories for the analysis to maintain consistency with the currently used annual inpatient surgical volume variable in NBCUS. Presence of a trauma service and trauma center level designation were combined into a single variable with three levels (no trauma service, trauma service, Level 1 trauma center). Similarly, presence of an oncology service and presence of a bone marrow transplant service were combined into a single three-level variable (no oncology service, oncology service, bone marrow transplant service). A new variable was created to denote facilities that have any organ transplantation service (heart, kidney, liver, or lung transplantation service). Facilities lacking data for any variable of interest were excluded. A total of 1614 observations were used in modeling allogeneic RBC use.
We further projected RBC use to 2020 based on inputs of blood use indicators. The most recent report of NBCUS results included a comparison of RBC units transfused by hospital location in 2015 and 2013 based on matched facilities that responded in both years.6 Hospital locations included in the analysis were surgery (including transplant), ER, inpatient medicine (including hematology-oncology), obstetrics-gynecology, pediatrics-neonatology, and critical care. An additional category, outpatient and nonacute inpatient settings, was available for 2015 but could not be accurately matched to 2013 data and was therefore not used for analysis. We used this 2-year trend to create projections of RBC use in 2020 under three scenarios and using several different methodological assumptions. A similar methodology was used in previous studies.16–18
Scenario 1 assumes all trends in RBC use continued as they had from 2013 to 2015 (including all increases and decreases). Scenario 2 assumes that only decreases in RBC use occur (any hospital locations with increases in RBC use from 2013 to 2015 will be considered to have no change in blood use to 2020), representing the commonly held view that trends in RBC use come primarily from increased efficiency. Scenario 3 assumes that changes in RBC use are driven only by decreases in RBC use in the surgical setting, which was the only location type included in the survey that showed a statistically significant change between 2013 and 2015. The locations included in NBCUS do not represent all possible locations, so there is a residual between overall RBC use and RBC use in locations included. For each scenario, we considered the case where this residual either had no trend or where the trend in the residual was equivalent to the average trend across all other facilities. Finally, we considered both linear and percentage-by-year trends—the former allows RBC use to reach zero (locations with zero projections were truncated to zero), whereas the latter is more realistic and ensures that RBC use cannot be reduced to zero for a particular location. In total, there were 12 possible combinations: 3 scenarios, each with the 2 assumptions of trends in the residual and the 2 different ways of estimating trends. Not all of these 12 scenarios can be claimed to be realistic, but they are intended to represent the range of plausible values. All analysis was conducted using software (SAS version 9.4, SAS Institute Inc.).
RESULTS
The adjusted-R2 values obtained from the OLS linear regression of allogeneic RBC use in the 2015 NBCUS data were consistent with those previously reported,1 with an R2 value of 61.6% for annual inpatient surgical volume using 2015 NBCUS data (Table 2) compared to 64% (69%) reported by Wallace and colleagues1 for the 1987 (1989) NBCUS. The adjusted R2 value for total number of hospital beds was 54.0% using the 2015 NBCUS data (Table 2) compared to 51% reported for both the 1989 and 1987 surveys.1 Applying a log-transform to the number of allogeneic RBC units transfused did not significantly change the results (Table 2).
TABLE 2.
Comparison of hospital size proxy variables for explaining allogeneic RBC unit use variability
| Adjusted R2 | Adjusted R2 (log-transformed RBC) | AIC | Pseudo-R2 | |
|---|---|---|---|---|
| Annual inpatient surgical volume | 61.6% | 64.2% | 28,136 | 70.8% |
| Total number of hospital beds | 54.0% | 60.9% | 28,355 | 66.5% |
| Number ICU beds | … | … | 28,863 | 54.2% |
| Number of ER visits | … | … | 29,192 | 43.8% |
| Number of medical/surgical beds | … | … | 28,937 | 52.0% |
AIC = Akaike information criterion; ER = emergency room; ICU = intensive care unit.
Table 2 shows the AIC and pseudo-R2 values obtained from the regression of allogeneic RBC use on the hospital size proxy variables from Table 1, including annual inpatient surgical volume and total number of hospital beds. Annual inpatient surgical volume accounted for 70.8% of the variation in allogeneic RBC use among hospitals, total number of hospital beds accounted for 66.5%, number of ICU beds accounted for 54.2%, number of medical/surgical beds accounted for 52.0%, and number of ER visits accounted for 43.8%. This suggests that annual inpatient surgical volume remains the best predictor of allogeneic RBC use, which is in agreement with the OLS linear regression estimate. Annual inpatient surgical volume was identi-fied as the optimal hospital size variable for inclusion in the model, and all other hospital size proxy variables were excluded from nested models due to high collinearity with annual inpatient surgical volume.
Table 3 shows AIC, p values, and pseudo-R2 values from each step of the forward model selection applied to allogeneic RBC use. As described above, the first variable included in the model was annual inpatient surgical volume, which had a pseudo-R2 value of 70.8%. The next variable added to the model was the three-level oncology service variable. The model with annual inpatient surgical volume and oncology service had a pseudo-R2 value of 73.3%. Although pseudo-R2 values cannot be considered additive in the sense of the traditional R2 measure, this shows that inclusion of oncology provided little additional information when compared to the model that included annual inpatient surgical volume alone. USPHS region, Level 2/3 OB service, trauma service, urban location, organ transplant service, and residency training program were all statistically significant within the model with annual inpatient surgical volume and oncology service, but the addition of these variables resulted in only a minor increase in the pseudo-R2 of the model, to a total of 76.3%. While the full model is statistically significant, it may not offer better predictive power than the model only containing annual inpatient surgical volume.
TABLE 3.
Final model for allogeneic RBC use in hospitals
| AIC | p value* | Pseudo-R2 | |
|---|---|---|---|
| Null model | 30,112 | … | 0.0% |
| Step 1: Annual inpatient surgical volume | 28,136 | <0.0001 | 70.8% |
| Step 2: Model from Step 1 + oncology service | 27,993 | <0.0001 | 73.3% |
| Step 3: Model from Step 2 + USPHS region | 27,922 | <0.0001 | 74.8% |
| Step 4: Model from Step 3 + Level 2/3 OB service | 27,873 | <0.0001 | 75.6% |
| Step 5: Model from Step 4 + trauma service | 27,857 | <0.0001 | 75.8% |
| Step 6: Model from Step 5 + urban location | 27,845 | 0.0002 | 76.1% |
| Step 7: Model from Step 6 + organ transplant service | 27,836 | 0.0009 | 76.2% |
| Step 8: Model from Step 7 + residency training program | 27,835 | 0.0490 | 76.3% |
P value refers to significance of last-added variable.
AIC = Akaike information criterion; USPHS = US Public Health Service.
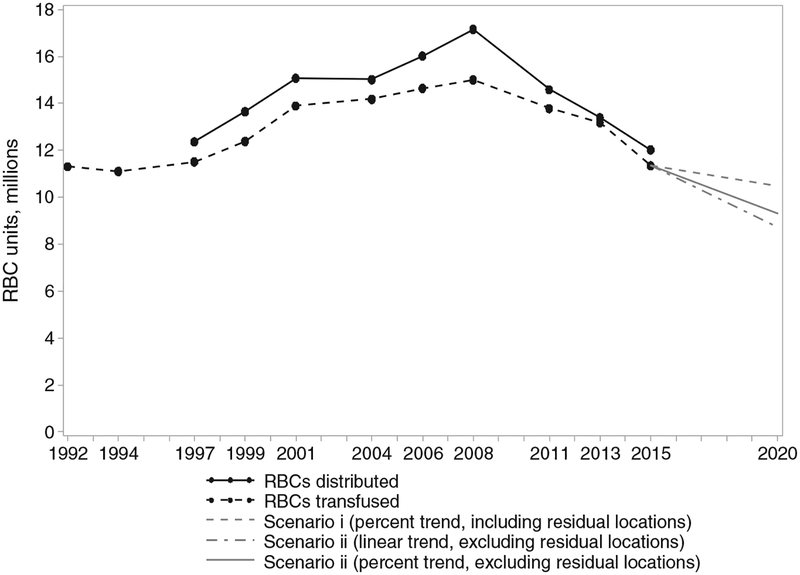
RBC use was projected up to the year 2020 with 12 possible modeled outcomes considered (see Methods). Not all of the modeled outcomes are equally likely but were used to give upper and lower bounds on projected RBC use. Figure 1 shows RBC collections from 1992 to 2015 and transfusions from 1997 to 2015, with 3 of the 12 modeled outcomes (the models with the largest and smallest decreases as well as a third model based on a continuation of previously observed trends in declining surgical RBC use with consistent RBC use across other clinical settings) extending to 2020. The model using Scenario 1 (includes increases/decreases from all locations) with a percentage trend, and assuming locations not included in NBCUS had zero trend, had a 7.6% decrease between 2015 and 2020 and formed the upper bound for our projections of RBC use. The model using Scenario 2 (uses only decreasing RBC trends) with a linear trend and assuming locations not included in the NBCUS had a trend equivalent to the average trend over other locations had a 23.2% decrease in RBC use and formed the lower bound of our projections. This lower bound involved setting surgical setting RBC use to zero.
Fig. 1.
Actual and projected RBC unit use through 2020 for the United States based on the 2015 NBCUS estimates. Black lines indicate distributed units (solid) and transfused units (dashed) up to and including 2015. Gray lines indicate projected RBC transfusion estimates under three possible scenarios.
DISCUSSION
Our analyses suggest that annual inpatient surgical volume remains the optimal indicator to explain the variation in allogeneic RBC use, explaining 70.8% of variation in allogeneic RBC use among hospitals. This central finding was evident despite the previously observed decline of blood use in surgical procedures. Annual inpatient surgical volume explained more variation in hospital RBC use than total number of hospital beds, number of medical/surgical beds, number of ICU beds, and number of ER visits. Several other hospital characteristic variables, for example, hospital bed size, were statistically significant in the model for allogeneic RBC use, but they explained little additional variability when considered in the model that included annual inpatient surgical volume. The relative importance of annual inpatient surgical volume as an explanatory factor may be due to this variable serving as a proxy for clinical complexity and, more specifically, operative care requiring transfusions. Further study is necessary.
The findings of this study may provide useful guidance to possible future changes in demand for blood products, particularly given the continued relationship between annual inpatient surgical volume and RBC use. Since 2008, a steep and relatively constant decline in blood use has been noted through NBCUS surveys. One recent description of challenges facing the blood industry has proposed a continued reduction in US blood use over the coming years by a 40% decline by 2020.19 However, the decline, as observed from the NBCUS, appears largely attributable to reduction in transfusions for surgical procedures and was essentially static, from a statistical standpoint, in many settings where blood remains a critical clinical intervention (including ICU, trauma, hematology/oncology).6 Our projections for RBC use in 2020, using the change from 2013 to 2015 for each surgical use category,6 suggest that a decrease in RBC use of between 7.6% and 23.2% by 2020 may be plausible. However, even with the implausible scenario of a total reduction in surgical blood usage to zero, the present analyses project a maximal reduction in RBC use of 23.2% by 2020.
The findings of this study are subject to the following limitations. First, the explanatory variables in the GLM were obtained from the AHA database, and as such the analysis was limited to only those hospital characteristics that were collected by the AHA. It is possible that additional hospital characteristics not captured by the AHA could further explain hospital RBC use more effectively than the variables for which we have access. The impact on the estimates presented here cannot be quantified. Second, the NBCUS and the AHA are both self-reported surveys. The subsequent impact of respondent bias or other inaccuracies in recording or reporting cannot be quantified. However, responses are typically compared to prior years (when available) and subjected to additional logic checks as part of data analyses. Third, the projections of declining RBC use to 2020 are based on assumptions derived from short-term historical trends in blood use. These calculations do not incorporate unforeseen major disruptions in blood demand. These may include emerging new technologies that further obviate the need for blood or factors that may drive new demand for blood (e.g., emerging clinical conditions or therapies requiring transfusion). Finally, the NBCUS does not capture clinical diagnoses for transfusion use, but rather facilities report clinical settings in which blood was transfused. Therefore, it is possible that blood use as reported in surgical settings may not be for surgical procedures. The impact on these estimates cannot be quantified.
In conclusion, our analysis found that annual inpatient surgical volume continues to be the most appropriate indicator of allogeneic RBC use within hospitals, although RBC use in the surgical setting has decreased in recent years.6 As trends in blood use continue to change, and particularly if blood use during inpatient surgical procedures continues a steep decline, continued evaluation of allogeneic RBC use indicators will be necessary. Additionally, our projections for RBC use in 2020 suggest a steady decline in blood use. Further study into blood transfusion trends and clinical impact, including availability of units in surgical and non-surgical settings, is warranted.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC) or the authors’ affiliated institutions. Use of trade names, commercial sources, or private organizations is for identification only and does not imply endorsement by the US Department of Health and Human Services and/or CDC.
ABBREVIATIONS::
- AHA
American Hospital Association
- AIC
Akaike information criterion
- ER
emergency room
- GLM
generalized linear model
- ICU
intensive care unit
- NBCUS
National Blood Collection and Utilization Survey
- OLS
ordinary least squares
Footnotes
CONFLICTS OF INTEREST
The authors have disclosed no conflicts of interest.
REFERENCES
- 1.Wallace EL, Surgenor DM, Hao HS, et al. Collection and trans-fusion of blood and blood components in the United States, 1989. Transfusion 1993;33:139–44. [DOI] [PubMed] [Google Scholar]
- 2.Ellingson KD, Sapiano MRP, Haass KA, et al. Continued decline in blood collection and transfusion in the United States-2015. Transfusion 2017;57(Suppl 2):1588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung KW, Basavaraju SV, Mu Y, et al. Declining blood collection and utilization in the United States. Transfusion 2016;56: 2184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Services. UDoHaH. The 2011 National Blood Collection and Utilization Report. Washington (DC): US Department of Health and Human Services; 2013. [Google Scholar]
- 5.Services RotUDoHaH. The 2009 national blood collection and utilization survey report. Washington (DC): US Department of Health and Human Services; 2011. [Google Scholar]
- 6.Sapiano MRP, Savinkina AA, Ellingson KD, et al. Supplemental findings from the National Blood Collection and Utilization Surveys, 2013 and 2015. Transfusion 2017;57(Suppl 2):1599–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lelubre C, Vincent JL, Taccone FS. Red blood cell transfusion strategies in critically ill patients: lessons from recent randomized clinical studies. Minerva Anestesiol 2016;82:1010–6. [PubMed] [Google Scholar]
- 8.Kaur P, Basu S, Kaur G, et al. Transfusion protocol in trauma. J Emerg Trauma Shock 2011;4:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long RG. The crux of the method: assumptions in ordinary least squares and logistic regression. Psychol Rep 2008;103:431–4. [DOI] [PubMed] [Google Scholar]
- 10.McCullagh P, Nelder JA. Generalized linear models. 2nd ed Boca Raton (FL): Chapman & Hall/CRC; 1998. [Google Scholar]
- 11.Oviedo M, Pilar Munoz M, Dominguez A, et al. A statistical model to estimate the impact of a hepatitis A vaccination programme. Vaccine 2008;26:6157–64. [DOI] [PubMed] [Google Scholar]
- 12.Elmi M, Mahar A, Kagedan D, et al. The impact of blood trans-fusion on perioperative outcomes following gastric cancer resection: an analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Can J Surg 2016;59:322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayeems RZ, Miller FA, Vermeulen M, et al. False-positive newborn screening for cystic fibrosis and health care use. Pediatrics 2017;140:e20170604. [DOI] [PubMed] [Google Scholar]
- 14.Akaike H A New Look at the Statistical Model Identification In: Parzen E, Tanabe K, Kitagawa G (eds) Selected Papers of Hirotugu Akaike. Springer Series in Statistics (Perspectives in Statistics). New York, NY: Springer; 1974. [Google Scholar]
- 15.Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika 1991;78:691–2. [Google Scholar]
- 16.Elani HW, Starr JR, Da Silva JD, et al. Trends in dental implant use in the U.S., 1999–2016, and projections to 2026. J Dent Res 2018;97:1424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kharrazi H, Gonzalez CP, Lowe KB, et al. Forecasting the maturation of electronic health record functions among US hospitals: retrospective analysis and predictive model. J Med Internet Res 2018;20:e10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Best AF, Haozous EA, de Gonzalez AB, et al. Premature mortality projections in the USA through 2030: a modelling study. Lancet Public Health 2018;3:e374–e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein HG, Hrouda JC, Epstein JS. Crisis in the sustainability of the U.S. blood system. N Engl J Med 2017;377:1485–8. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman L, Bercovitz RS, Sholapur NS, et al. Platelet transfusions for critically ill patients with thrombocytopenia. Blood 2014;123:1146–51 quiz 280. [DOI] [PubMed] [Google Scholar]
- 21.Stanworth SJ, Walsh TS, Prescott RJ, et al. A national study of plasma use in critical care: clinical indications, dose and effect on prothrombin time. Crit Care 2011;15:R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz RN. Anemia in patients with cancer: incidence, causes, impact, management, and use of treatment guidelines and protocols. Am J Health Syst Pharm 2007;64:S5–13 quiz S28–30. [DOI] [PubMed] [Google Scholar]
- 23.Gajewski JL, Johnson VV, Sandler SG, et al. A review of transfusion practice before, during, and after hematopoietic progenitor cell transplantation. Blood 2008;112:3036–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nigam A, Prakash A, Saxena P. Blood transfusion in obstetrics. Kathmandu Univ Med J (KUMJ) 2013;11:355–9. [DOI] [PubMed] [Google Scholar]