Abstract
BACKGROUND:
Neurobiological predictors of antidepressant response may help guide treatment selection and improve response rates to available treatments for major depressive disorder (MDD). Behavioral activation therapy for depression (BATD) is an evidence-based intervention designed to ameliorate core symptoms of MDD by promoting sustained engagement with value-guided, positively-reinforcing activities. The present study examined pre-treatment task-based functional brain connectivity as a predictor of antidepressant response to BATD.
METHODS:
Thirty-three outpatients with MDD and 20 nondepressed controls completed a positive emotion regulation task during fMRI after which participants with MDD received up to 15 sessions of BATD. We used generalized psychophysiological interaction analyses to examine group differences in pre-treatment functional brain connectivity during intentional upregulation of positive emotion to positive images. Hierarchical linear models were used to examine whether group differences in functional connectivity predicted changes in depression and anhedonia over the course of BATD.
RESULTS:
Compared to controls, participants with MDD exhibited decreased connectivity between the left middle frontal gyrus and right temporoparietal regions during upregulation of positive emotion. Within the MDD group, decreased connectivity of these regions predicted greater declines in anhedonia symptoms over treatment.
LIMITATIONS:
Future studies should include comparison treatments and longitudinal follow-up to clarify the unique effects of BATD on neural function and antidepressant response.
CONCLUSIONS:
Results are consistent with previous work showing BATD may be particularly effective for individuals with greater disturbances in brain reward network function, but extend these findings to highlight the importance of frontotemporoparietal connectivity in targeting symptoms of low motivation and engagement.
Introduction
Behavioral Activation Therapy for Depression (BATD) is an intervention designed to ameliorate core symptoms of major depressive disorder (MDD) by promoting systematic engagement in valued activities and reductions in avoidance behaviors. The overarching goal of BATD is to increase contact with potential sources of positive reinforcement (Dimidjian et al., 2011). In line with the emerging science of neuroprediction to better match MDD patients to existing treatments (e.g., Langenecker et al., 2018; Pizzagalli et al., 2018), our recently completed open trial investigated neuroimaging predictors of BATD response (Walsh et al., 2017; Carl et al., 2016; Crowther et al., 2015). In a sample of MDD patients and nondepressed controls, we evaluated group differences in pre-treatment brain activation and connectivity during a reward-based task or at rest. For MDD patients enrolled in BATD, we then examined the extent to which these neuroimaging biomarkers explained the observed decreases in symptoms of depression and anhedonia over the course of treatment. Thus far, we have provided evidence that BATD may be most optimal for MDD patients with deficits in reward-related brain network function, with particularly robust effects on the core symptom of anhedonia.
An exploratory aim of our open trial study was to evaluate prediction of BATD response from pretreatment brain connectivity during an emotion regulation task. MDD is characterized by emotion dysregulation, and much research to date has focused on addressing deficits in regulation of negative mood states. However, given that anhedonia is a defining feature of MDD (American Psychiatric Association, 2013), it is likely that deficits in positive emotion regulation may increase risk for or maintain MDD. Therefore, we hypothesize that positive emotion regulation disturbances may predict treatment outcome in response to BATD.
Methods
Full details of the study protocol and participants are described in Carl et al. (2016) and Walsh et al. (2017). The protocol was approved by local Institutional Review Boards and all participants provided written informed consent.
Participants
Participants with MDD were recruited via participant recruitment registries and listservs at Duke University and the University of North Carolina at Chapel Hill. Participants in the MDD group met DSM-IV criteria for a current episode of MDD using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (First et al., 2002). Control group participants did not meet criteria for a current or lifetime episode mood episode. Exclusion criteria included: 1) history of psychosis or mania; 2) active suicidal ideation, 3) evidence of organicity, 4) magnetic resonance imaging contraindication, 5) history of neurological injury or disease, 6) current pregnancy, and, in the MDD group, 7) current mood, anxiety, psychotic, or substance abuse disorder beyond unipolar MDD or dysthymia.
Thirty-eight outpatients with MDD (29% male; mean age = 33 (range=21–45)) and twenty controls (30% male; mean age = 31 (range=20–44)) were enrolled. Five MDD participants were excluded from analyses; two did not return for therapy after the pre-treatment fMRI session, and three were taking psychoactive medications. The final sample included 33 outpatients with MDD and 20 nondepressed control participants.
Procedures and Design
MDD and control groups participated in a pre-treatment MRI scan. Participants completed a number of different imaging protocols, some of which have been published (Walsh et al., 2017; Carl et al., 2016; Crowther et al., 2015). Following the pre-treatment scan, the MDD group began BATD psychotherapy. Up to 15 sessions of BATD were offered; participants received an average of 11.67 (SD=4.40; range: 2–15) weekly sessions.
Positive Emotion Regulation Task
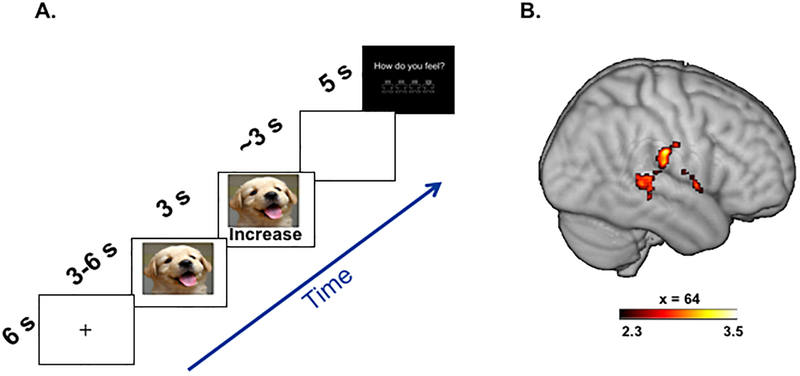
During the scan, participants completed two runs of a positive emotion regulation task (similar to Smoski et al. (2013), but using positive images). Each trial began with a fixation cross (6s) followed by presentation of a positive or neutral picture (Figure 1A depicts timing and content of each trial). After initial picture display without regulation instruction (3–6s, jittered), a visual regulation instruction was superimposed on the bottom of the picture, indicating the regulation strategy to use (3s), followed by a brief delay (~3s). Participants then rated post-trial affect using a visual analog scale (5s; range of 1 = most negative to 4 = most positive). The task included two conditions: Passive Viewing (‘view’) and Positive Upregulation (‘increase’). For the ‘view’ condition, which used both positive and neutral pictures, participants were instructed not to regulate their emotional response (“view images without trying to change the emotions that come”). For the ‘increase’ condition, which occurred only during positive images, participants were instructed to reinterpret the image to increase its positive impact. Specifically, participants were asked to “mentally placing themselves in the scene” or “interpret the image in a way that exaggerates the positive content”. Two runs of 12 trials each were administered (4’42” per run; 24 total trials), and there were 8 trials for each regulation condition.
Figure 1.
(A) Positive emotion regulation task. Each trial consisted of a fixation cross, a positive or neutral image, a regulation cue, a delay, and a query for current affect. (B) The MDD group demonstrated less connectivity between the left middle frontal gyrus (seed) and right temporoparietal regions during viewing of positively-valenced images while upregulating positive emotion compared to no regulation of emotion (Positive Increase > Positive View).
Prior to the scan, participants practiced the regulation strategies with an experimenter until they could implement them without assistance. Task images were drawn from: (i) positive images from the International Affective Picture System based on normative positive ratings (Mikels et al., 2005) and (ii) a normed set of neutral images used in previous MDD imaging studies (e.g., Dichter et al., 2010).
Treatment Outcome Measures
Treatment outcomes were evaluated by the Beck Depression Inventory-II (BDI; Beck et al., 1996), collected at the scan session, every two weeks during treatment, and at the last psychotherapy session. The BDI provides an overall measure of MDD severity and includes items that tap MDD symptom dimensions. We examined BDI total scores, and BDI anhedonia subscale scores derived from items 4, 12, 15, and 21 (Joiner et al., 2003).
Imaging Methods and fMRI Preprocessing
Fully described in Walsh et al. (2017) and Supplement.
fMRI Data Analysis
The general linear model included the following regressors for each task event: “Increase” instructions (positive images), “View” instructions (positive and neutral images), and passive viewing of images (positive and neutral; pre-instructions). For the present study, we were most interested in examining differences in neural responses following instructions to intentionally increase positive emotion during a positive image vs. viewing a positive image without engaging in a specific strategy (Positive Increase Instructions > Positive View Instructions). Temporal derivatives and standard motion parameters (3 rotations, 3 translations) were included as covariates. To further control for excessive motion, we censored volumes that exceeded a framewise displacement threshold of 0.9mm (i.e., head motion displacement occurring from one volume relative to the previous volume summing across linear and rotational displacements (Siegel et al., 2014)).
Task-based functional connectivity was evaluated using a generalized psychophysiological interaction (gPPI) approach (Cisler et al., 2014). Seed regions of interest (ROI) were selected to target canonical positive emotion regulation and reward processing regions (e.g., Kim and Hamann, 2007; Zhang et al., 2013). ROI seeds included the nucleus accumbens, caudate, putamen, frontal medial cortex, frontal pole, and middle frontal gyrus. ROIs were defined using the Harvard-Oxford subcortical and cortical structural probabilistic atlases. For each participant, mean fMRI timecourses (i.e., physiological regressors) were extracted from seed regions using fslmeants, then multiplied by each psychological variable of interest (i.e., task condition) to form the PPI interaction terms. The gPPI model included physiological and psychological regressors, as well as their interaction terms to describe the unique effect of these interactions above and beyond the main effects of seed timecourses and task conditions. Prior to performing group-level analyses, task runs were combined using a fixed-effects model.
To evaluate group differences in seed-based connectivity across the whole brain, we used the FMRIB Local Analysis of Mixed Effects module within FSL. Resulting images were thresholded using a cluster-forming threshold of Z>2.3 and a cluster extent threshold of p<0.05, familywise error (FWE)-corrected using Gaussian random field theory. Cluster localizations were based on Harvard–Oxford cortical and subcortical structural probabilistic atlases in FSLView v3.2.0.
Analytic Plan for Predicting Treatment Response from Functional Connectivity
We used hierarchical linear models (HLMs) to examine whether clusters indicating group differences in connectivity predicted treatment response measured via nine BDI assessments over 15 weeks using SAS PROC MIXED 9.4, with treatment week at level 1 and person at level 2. Treatment week was a continuous time variable and was uncentered. We specified an autoregressive (week-2) covariance structure for within-person errors. Individual coefficients were presented as gamma weights that were analogous to unstandardized beta coefficients in standard regression, representing the estimated change in the dependent variable given a one-unit increase in the predictor. For further details on our HLM approach, see Walsh et al. (2017).
Results
Effects of Treatment Week on Depression Scores
As previously reported (Carl et al. 2016; Walsh et al. 2017), BDI total scores and anhedonia subscale scores significantly decreased from pre- to post-treatment (BDI total pre-treatment mean = 25.27 (SD=8.52), post-treatment mean = 14.73 (SD=9.96), p<0.001); BDI anhedonia pretreatment mean = 4.91 (SD=2.26), post-treatment mean = 2.87 (SD=2.00), p<0.001).
Self-Report of Affect During fMRI
Participants reported greater positive affect following regulation instructions to “increase” compared to “view” for both positive and neutral images. Thus, the task elicited the intended emotional response. See Supplement for detailed information.
Functional Connectivity during Positive Emotion Upregulation and Prediction of Treatment Response
Group differences in seed-based functional connectivity were observed in one ROI seed region during intentional upregulation of positive emotion to positive images (Positive Increase Instructions > Positive View Instructions). Relative to nondepressed controls, participants with MDD exhibited significantly less connectivity between the left middle frontal gyrus (seed) and a large cluster spanning the right temporal and parietal lobes, with peak voxel intensity in the inferior postcentral gyrus (Table 1, Figure 1B). HLM models were then used to evaluate the extent to which pre-treatment functional connectivity between these regions predicted slope of change in BDI total and anhedonia scores over the course of BATD. Within the MDD group, decreased connectivity predicted greater declines in BDI anhedonia subscale scores (γCONNECTIVITYDIFFERENCE*TREATMENTWEEK = .09, SE = .04, t(187) = 2.09, p < .04), but was not associated with BDI total scores (γCONNECTIVITYDIFFERENCE*TREATMENTWEEK = .16, SE = .21, t(186) = .75, p = .45). The direction of this effect reflects that participants with MDD who were more different than nondepressed controls responded most favorably to BATD, while participants who were more similar to nondepressed controls responded the least to BATD.
Table 1.
Group differences in functional connectivity during positive emotion upregulation (Positive Increase > Positive View).
*Note: Thresholded at p<0.05 (whole brain, FWE corrected); MNI coordinates reflect the maximum voxel of intensity for the top five local maxima; k = number of voxels; Peak z=z-score at peak coordinates; R: right, L: left; MFG=Middle Frontal Gyrus; MDD=Major Depressive Disorder Group; CON=Nondepressed Control Group.
| Seed / Region | MNI coordinates | ||||
|---|---|---|---|---|---|
| MDD<CON | X | Y | Z | k | Peak z |
| L MFG to R Postcentral Gyrus | 64 | −18 | 28 | 620 | 3.48 |
| to R Superior Temporal Gyrus | 60 | −32 | 4 | 3.46 | |
| to R Parietal Operculum Cortex | 52 | −22 | 14 | 3.44 | |
| to R Postcentral Gyrus | 62 | −18 | 20 | 3.15 | |
| to R Central Opercular Cortex | 60 | 4 | 4 | 3.02 | |
Discussion
The present study found that compared to nondepressed controls, participants with MDD exhibited decreased connectivity between the left middle frontal gyrus and right temporoparietal regions during intentional upregulation of positive emotion to positively-valenced stimuli. Further, task-modulated connectivity of these regions differentially predicted BATD response: MDD participants with decreased connectivity showed the greatest reductions in anhedonia symptoms over time.
The middle frontal gyrus is implicated in a range of processes including emotion regulation and selective attention, and shows functional deficits in MDD (e.g., Smoski et al., 2013). The cluster functionally connected to the left middle frontal gyrus is within the anterior temporoparietal junction (Mars et al., 2012). Collectively, these frontotemporoparietal regions are believed to comprise the salience/ventral attention network which is activated when orienting attention to behaviorally relevant stimuli, particularly to stimuli that are unexpected or occur less frequently in the environment (Corbetta and Shulman, 2002).
For individuals with greater disruptions in left middle frontal gyrus and right temporoparietal connectivity, BATD may remediate this dysconnectivity pattern through increased contact and regulation of attention to potentially rewarding activities. Increased exposure to activities may enhance attentional regulation to positive emotion and related sensory experiences, thereby altering appraisals of events and future goal-directed behavior, and decrease anhedonia over time (Quoidbach et al., 2015). However, BATD may be less effective for individuals with intact frontotemporoparietal connectivity—they may already possess attentional and behavioral capacities taught in BATD and therefore be less responsive to this treatment.
Several limitations are worth noting: 1) This was an open trial and lacked a comparator treatment, thus does not exclude the possibility that the observed therapeutic response was due to non-specific, placebo-like factors (Fava et al., 2017) and 2) No post-treatment scans were obtained. In order to clarify the unique effects of BATD on neural function and antidepressant response, future studies should include comparison treatment(s), consideration of non-specific factors that may influence treatment response, and longitudinal follow-up.
Results are consistent with our previous work (Walsh et al., 2017) suggesting BATD may be particularly effective for individuals with greater disturbances in brain reward network function compared to individuals with greater preservation of networks. These findings extend our work by highlighting the importance of salience/attentional networks in targeting symptoms of low motivation and engagement, and are consistent with recent results demonstrating the robust ability of frontocingulate function in predicting antidepressant response (Pizzagalli et al., 2018). In sum, these findings contribute to the growing body of literature addressing neuroimaging endophenotypes as predictors of treatment response in MDD (Langenecker et al., 2018).
Supplementary Material
Acknowledgements:
The authors thank MRI technologists Susan Music, Natalie Goutkin, and Luke Poole for assistance with data acquisition, and BIAC Director Allen Song for assistance with various aspects of this project. Portions of these results were presented at the 2015 Association of Behavioral and Cognitive Therapies annual convention in Chicago, IL.
Funding:
The project was funded by R21MH094781 and R21MH094781 S1 to GSD and MJS, by K23MH087754 to MJS, and by K23MH081285 to GSD. EW was supported by KL2TR001109 and K23MH113733. TEM was supported by T32MH093315 and K99MH109667. JB was supported by HD079124. This research was also supported by the Duke Brain Imaging and Analysis Center (BIAC) under award S10OD021480. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- American Psychiatric Association, 2013. DSM 5, American Journal of Psychiatry. 10.1176/appi.books.9780890425596.744053 [DOI] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Manual for Beck Depression Inventory-II. Psychological Corporation, San Antonio, TX. [Google Scholar]
- Carl H, Walsh E, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T, Gibbs D, Petty C, Bizzell J, Dichter GS, Smoski MJ, 2016. Sustained anterior cingulate cortex activation during reward processing predicts response to psychotherapy in major depressive disorder. J. Affect. Disord 203, 204–12. 10.1016/j.jad.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Bush K, Steele JS, 2014. A comparison of statistical methods for detecting context-modulated functional connectivity in fMRI. Neuroimage 84, 1042–52. 10.1016/j.neuroimage.2013.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, 2002. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci 3, 201–215. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Crowther A, Smoski MJ, Minkel J, Moore T, Gibbs D, Petty C, Bizzell J, Schiller CE, Sideris J, Carl H, Dichter GS, 2015. Resting-state connectivity predictors of response to psychotherapy in major depressive disorder. Neuropsychopharmacology 40, 1659–73. 10.1038/npp.2015.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Smoski MJ, 2010. The effects of Brief Behavioral Activation Therapy for Depression on cognitive control in affective contexts: An fMRI investigation. J. Affect. Disord 126, 236–44. 10.1016/j.jad.2010.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimidjian S, Barrera M, Martell C, Muñoz RF, Lewinsohn PM, 2011. The Origins and Current Status of Behavioral Activation Treatments for Depression. Annu. Rev. Clin. Psychol 7, 1–38. 10.1146/annurev-clinpsy-032210-104535 [DOI] [PubMed] [Google Scholar]
- Fava GA, Guidi J, Rafanelli C, Rickels K, 2017. The Clinical Inadequacy of the Placebo Model and the Development of an Alternative Conceptual Framework. Psychother. Psychosom 86, 332–340. 10.1159/000480038 [DOI] [PubMed] [Google Scholar]
- First MB Spitzer et, Gibbon RL, Williams M, W. JB, 2002. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV), for DSMIV.
- Joiner TE, Brown JS, Metalsky GI, 2003. A test of the tripartite model’s prediction of anhedonia’s specificity to depression: patients with major depression versus patients with schizophrenia. Psychiatry Res 119, 243–50. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S, 2007. Neural Correlates of Positive and Negative Emotion Regulation. J. Cogn. Neurosci 19, 776–798. 10.1162/jocn.2007.19.5.776 [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Crane NA, Jenkins LM, Phan KL, Klumpp H, 2018. Pathways to Neuroprediction: Opportunities and Challenges to Prediction of Treatment Response in Depression. Curr. Behav. Neurosci. Reports 1–13. 10.1007/s40473-018-0140-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Sallet J, Schüffelgen U, Jbabdi S, Toni I, Rushworth MFS, 2012. Connectivity-based subdivisions of the human right “temporoparietal junction area”: Evidence for different areas participating in different cortical networks. Cereb. Cortex 22, 1894–1903. 10.1093/cercor/bhr268 [DOI] [PubMed] [Google Scholar]
- Mikels JA, Fredrickson BL, Larkin GR, Lindberg CM, Maglio SJ, Reuter-Lorenz PA, 2005. Emotional category data on images from the International Affective Picture System. Behav. Res. Methods 37, 626–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Webb CA, Dillon DG, Tenke CE, Kayser J, Goer F, Fava M, McGrath P, Weissman M, Parsey R, Adams P, Trombello J, Cooper C, Deldin P, Oquendo MA, McInnis MG, Carmody T, Bruder G, Trivedi MH, 2018. Pretreatment Rostral Anterior Cingulate Cortex Theta Activity in Relation to Symptom Improvement in Depression. JAMA Psychiatry 75, 547 10.1001/jamapsychiatry.2018.0252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quoidbach J, Mikolajczak M, Gross JJ, 2015. Positive Interventions: An Emotion Regulation Perspective. Psychol. Bull 10.1037/a0038648 [DOI] [PubMed] [Google Scholar]
- Siegel JS, Power JD, Dubis JW, Vogel AC, Church JA, Schlaggar BL, Petersen SE, 2014. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Hum. Brain Mapp 35, 1981–1996. 10.1002/hbm.22307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski MJ, Keng S-L, Schiller CE, Minkel J, Dichter GS, 2013. Neural mechanisms of cognitive reappraisal in remitted major depressive disorder. J. Affect. Disord 151, 171–7. 10.1016/j.jad.2013.05.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E, Carl H, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T, Gibbs D, Petty C, Bizzell J, Smoski MJ, Dichter GS, 2017. Attenuation of Frontostriatal Connectivity During Reward Processing Predicts Response to Psychotherapy in Major Depressive Disorder. Neuropsychopharmacology 42, 831–843. 10.1038/npp.2016.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W-N, Chang S-H, Guo L-Y, Zhang K-L, Wang J, 2013. The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J. Affect. Disord 151, 531–9. 10.1016/j.jad.2013.06.039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.