Abstract
Nicotine is harmful to many bodily systems; however, the effects of nicotine on intra-substance tendon healing remain largely unexplored. The purpose of this study was to examine the functional, structural, and biomechanical effects of nicotine on the healing of Achilles tendons in rats after an acute full-thickness injury. Sixty Sprague-Dawley rats were enrolled in this study. Half were exposed to 0.9% saline and half to 61ng/mL of nicotine for 3 months via subcutaneous osmotic pumps. At 3 months, all rats underwent blunt full thickness transection of the left Achilles tendon and were immobilized for one week in plantarflexion. In-vivo assays were conducted prior to injury, at 21 days, and at 42 days post-injury and included the following: functional limb assessment, passive joint mechanics, and vascular evaluation. Rats were sacrificed at 21 and 42 days for biomechanical testing and histologic evaluation. Rats exposed to nicotine demonstrated decreased vascularity, greater alteration in gait mechanics, and increased passive ROM of the ankle joint. Biomechanically, the nicotine tendons failed at lower maximum loads, were less stiff, had smaller cross-sectional areas and had altered viscoelastic properties. Histologically, nicotine tendons demonstrated decreased vessel density at the injury site.
Keywords: Nicotine, tendon healing, biomechanical properties, tendon structure, vascularity
Introduction
The World Health Organization estimates that almost 1.3 billion people consume tobacco products worldwide and that tobacco is responsible for nearly 5.4 million deaths per year.1 One important ingredient in tobacco products is nicotine, which is in large part responsible for the addictive nature of tobacco.1 Concerted international efforts and campaigns led by the Centers for Disease Control and Prevention have increased awareness about the health risks of smoking and decreased smoking rates in the United States over the last decade.2 While smoking rates have declined, the consumption of nicotine products has in fact risen3 as e-cigarettes and similar products are marketed as safer alternatives to tobacco consumption. Particularly in the youth and young adult populations, the use of nicotine has increased at an alarming rate.4 Between 2011 and 2015, high school students saw a 900% increase in nicotine e-cigarette consumption.4 In fact, use of nicotine-only products now surpasses cigarettes, cigars, chewing tobacco, and hookahs combined in younger adults.4 As such, the burden of disease from nicotine is high and measures to curb its use are necessary.
Nicotine has been repeatedly demonstrated as a carcinogenic agent with adverse health effects on the cardiovascular, gastrointestinal, respiratory, immune, and reproductive systems. From a musculoskeletal perspective, nicotine has been shown to delay fracture union, impair ligament healing, and accelerate degeneration of intervertebral discs in the spine.5–7 Given the high incidence of nicotine consumption and its multitude of adverse effects, counseling against nicotine use can serve as an important clinically modifiable risk factor to improve health outcomes.
Despite the ample evidence demonstrating the various negative effects of nicotine, literature describing its impact on tendon healing is lacking. Previous studies have suggested that nicotine delays the healing of tendon to bone8 but the effects of nicotine on intrasubstance tendon healing remain largely unexplored. Basic science studies have also suggested that nicotine alters the material properties of tendons9, but a change in function correlating to the these altered material properties is unknown.
The purpose of this study was to investigate the functional, structural, and mechanical effects of nicotine on tendon healing after full thickness injury of the Achilles tendon in a rat, compared to a non-nicotine control group. Functionally, we hypothesized that nicotine would lead to a greater alteration in gait mechanics and decreased use of the injured limb. Structurally, we hypothesized that nicotine would lead to decreased vascularity and a histologically less organized healing response. Mechanically, we hypothesized that nicotine would lead to decreased maximum load to failure, decreased modulus, decreased stiffness and altered viscoelastic properties.
Materials and Methods
Study Design
Prior to the commencement of the study, Institutional Animal Care and Use Committee (IACUC) approval was obtained. We used 60 adult male Sprague-Dawley rats weighing 400–450g (Charles River Laboratories, Inc, Devault, PA) between 10–13 weeks of age in this study. Rats were housed in a conventional facility with 12-hour light-dark cycles and fed standard rat chow ad libitum. After arrival to the facility, the rats were allowed a one week acclimation period. They were then randomized to receive either 0.9% saline (n=30) or 61mg/mL of nicotine (Nicotine, N3876, Sigma Aldrich, St. Louis, MO, n=30) at a rate of 2.5 µL/hr through subcutaneously implanted osmotic pumps (2ML4; Alzet, Cupertino, CA). The nicotine dose was based on the concentration observed in heavy smokers (1–1.5 packs per day) and users of high-nicotine content e-cigarette liquids, as well as reported concentrations in previous studies, in order to create a predisposed condition within the tendon tissue prior to injury.9–11 To confirm an appropriate exposure to nicotine, serum levels of cotinine, a metabolite of nicotine, were measured before pump implantation and every four weeks thereafter. Cotinine levels were assessed using Enzyme-linked Immunosorbent Assay (ELISA, EA100902, Origene, Rockville, MD) at a light wavelength of 450 nm. Rats were exposed to either saline or nicotine for 3 months (Figure 1) and underwent osmotic pump exchanges every 4 weeks when the osmotic pumps had fully eluted. At 3 months, all rats underwent full thickness blunt transection of the left Achilles tendon (Figure 2) and casting in plantarflexion for one week, similar to previously described12. Regular assessment of perfusion to the toes was performed while the rats were casted. Casts were inspected daily and replaced or patched with polymethylmethacrylate as needed based on damage from chewing or noticeable swelling. Casts were all removed and replaced at post-op day 2 to inspect the underlying surgical wound and skin. Rats continued to be exposed to either saline or nicotine post-injury until time of euthanasia. Half the animals from each group were humanely euthanized at 21 days and the remaining half at 42 days post-injury using carbon dioxide inhalation. At no time were there any obvious differences in animal health, activity, feeding, etc. nor were there any surgical complications attributed to nicotine treatment. At the time of sacrifice, the left Achilles tendons (n = 5 per group per time point) were immediately dissected, fixed in formalin, and processed for histologic analysis. The remaining animals (n=10 per group per timepoint) were frozen at –20°C and thawed for dissection at the time of mechanical testing.
Figure 1. Overall study design.

Blue bar represents exposure to saline. Yellow bar represents exposure to nicotine.
Figure 2. Surgical Procedures.

(A) Intra-operative view of a rat after right lateral-decubitus positioning and preparation with povidone-iodine. Note insertion of osmotic pump subcutaneously on the dorsolateral surface. (B) Dorsal view of hindfoot after surgical transection of the Achilles tendon. Proximal and distal tendon ends are marked in green.
Contrast Enhanced High Frequency Ultrasound
In-vivo ultrasound was used to assess the vascular status of the left Achilles tendons. Twenty rats (n=10 saline-exposed, n=10 nicotine-exposed) underwent contrast enhanced ultrasound immediately prior to injury, 21 days post-injury, and 42 days post-injury. Only rats that were designated for euthanasia at 42 days underwent in-vivo assays so that these longitudinal measures would be made throughout the full time course of the study.
Animals were anesthetized using isoflurane, hair was removed from the left hindlimb, and a tail vein catheter was inserted for contrast injection. The rat’s ankle was held at 90° of flexion and the animal was placed on a heated mat for the duration of imaging. A 21MHz center frequency ultrasound transducer (MS250, VisualSonics, Toronto, ON) was used in brightness mode. Using the calcaneus as a centering guide, the probe was aligned with the long axis of the Achilles tendon. A focal depth of 7mm and total image depth of 12mm were used when imaging the tendon. During image capture in nonlinear contrast mode, a frame rate of 1 fps and contrast gain of 35dB were used. 100µL of microbubble contrast agent (Definity, Lantheus Medical Imaging, Billerica, MA) was injected through the tail vein catheter and was flushed by 200µL of saline. The inflow and outflow of contrast through the tendon was recorded. After imaging, the catheter was removed, and the animal was placed under a heat lamp for recovery.
The VevoCQ (VisualSonics, Toronto, ON) program was used to analyze the data. Motion artifact was removed and two regions of interest (ROIs) were identified, one including the complete tendon, the other at the site of injury (Figure 3). Each frame of the clip was used to quantify the perfusion of the microbubble contrast. Using linearization, the video data was converted into echo-power data, which directly correlates to the contrast concentration. These data were then processed using a curve-fitting algorithm for a parametric perfusion model as a function of time.
Figure 3. Ultrasound image of a rat Achilles tendon.

The calcaneus is located on the right and the muscle body on the left. Two Regions of Interest (ROIs) are identified - the full tendon and the injury site. The hypoechoic region is identified as the injury site.
Each ultrasound parameter was measured for both the tendon and the injury site – the two ROIs described above. Circular standard deviation (CSD) and degree of echogenicity were used as parameters for assessing alignment. Increased CSD corresponds to lower structural organization of the fibers, while increased echogenicity corresponds to greater structural organization. Perfusion parameters were also derived, including rise time, wash-in rate, and wash-in area under the curve (Supplementary Figure 1), as previously described.13,14 We report Wash-in Area Under the Curve (AUC) rather than overall (wash-in and wash-out) AUC in order to eliminate the variability introduced by venous AUC. Algorithmic calculation of venous AUC is based on extrapolated data, not measured data as is arterial AUC. As such, venous AUC data is considered unreliable, and we do not report Wash-out parameters. All parameters were normalized to pre-injury values.
Quantitative Ambulatory Assessment
Rats were video-recorded as they walked across an instrumented walkway equipped with load cells. Data from the load cells were used to measure ground reaction forces (rate of loading, braking force, propulsion force). Data from the video clips were used to measure speed and stride length.15 Measurements were taken immediately prior to injury, 21 days post-injury, and 42 days post-injury. Parameters were averaged across walks on a given day and measurements were adjusted by animal body weight for each day. All parameters were normalized to pre-injury values.
Passive Joint Mechanics
A custom device was used to measure passive range of motion (ROM) and stiffness, as previously described.16 After being anesthetized with isoflurane the rat’s left hindlimb was secured into a rotating clamp at 90° of ankle flexion. The knee was stabilized manually to isolate tibio-talar motion and the ankle was rotated through the full range of dorsi- and plantar flexion three times. The average of three values for dorsi- and plantar flexion was used to compute the range of motion. Joint stiffness was calculated in both the toe and linear regions of dorsi- and plantar flexion. Measurements were recorded immediately prior to injury, 21 days post-injury, and 42 days post-injury. All parameters were normalized to pre-injury values.
Tendon Histology
Achilles tendons (n = 5 per group, per time point) were harvested at time of euthanasia, fixed in formalin and processed using standard paraffin techniques. After embedding, 7 µm sagittal sections were collected and stained with Hematoxylin and Eosin (H&E). Histological analysis was performed to assess cell shape and cellularity at the injury site. The injury site was defined as the central portion of the fibrous scar tissue.
Three blinded graders evaluated cell shape and cellularity using a semi-quantitative method. Magnification of 200X was used to image the specimens. A custom grading scale was created as previously described.17 Briefly, images were blinded and arranged in order for each histologic property (e.g., for cell shape, images were arranged from most spindle-shaped to roundest). A representative image was selected for each grade of 1, 2, and 3 for cell shape and cellularity, rather than using descriptive terminology to select grades. The images were then randomly organized and blinded prior to being sent to the graders. Each grader compared each histologic image with the preselected representative images and assigned the appropriate grade, as described previously.17,18
Circular standard deviation of collagen fibers was determined by images taken with a polarizing microscope and analyzed with custom software as described previously.18
Vessel density analysis was performed on H&E images taken at a 25X magnification, encompassing a large region of the injury site. Images were blinded and vascular structures were counted using ImageJ (NIH, version 1.48v) Cell Counter command and were normalized to the tissue pixel area of the ROI. Data from two ROIs were averaged to create a single data point for each specimen.
Tendon Mechanics
The Achilles tendons (n=10 per group, per time point) underwent mechanical testing as described previously.19,20 Briefly, all tendons were removed en bloc and grossly stripped of skeletal muscle tissue. The tendons were then finely dissected under a stereomicroscope (Leica Microsystems, Inc. Buffalo Grove, IL) to remove any remaining non-tendinous connective tissue. Verhoeff’s stain was used to create dots on the tendon for optical strain tracking. Using a custom laser device, the cross-sectional area (CSA) of the tendons was measured. Specimens were then prepared for mechanical testing by potting the foot in polymethylmethacrylate and attaching sandpaper proximally. Custom fixtures were used to secure the bone-tendon units in a physiologic orientation into a fatigue testing machine (ElectroPuls E3000, Instron, Norwood, MA). Specimens were submerged in a phosphate buffered saline bath maintained at 37o Celsius to mimic physiologic conditions. The specimens then underwent the following protocol: ten cycles of preconditioning, stress relaxation for 10 minutes at 5% strain, and ramp to failure at 0.1% strain/second. A camera (102F, Basler, Exton, PA) and 200mm lens (Micro-NIKKOR AF, Nikon. Melville, NY) were used to record images at 1 frame per second. From these images, the stained regions were tracked to the time of tendon rupture using a custom code (MATLAB, Mathworks, Natick, MA). Data from biomechanical testing were used to calculate stiffness, percent relaxation, maximum load, maximum stress and modulus. Any tendons that did not fail physiologically (e.g., at the grip where stress is concentrated) were excluded from calculation of maximum load to failure and maximum stress.
Statistics
The Shapiro-Wilk test was used to assess the data sets for normality. Outlier data points, defined as values falling outside two standard deviations from the average of the group, were identified and excluded. One tailed, Student’s t-test was used to compare between the groups at each of the three time points. One-tailed Mann-Whitney tests were used for histologic comparisons due to non-parametric data. No comparisons across time were made. The level of significance was set at p < 0.05. Trends were defined as p-values between 0.05 and 0.1. Data were plotted using Prism (GraphPad Software, La Jolla, CA). Longitudinal data are presented as normalized to pre-injury values with mean ± standard deviation error bars. Significant values are indicated by an asterisk (*) in line plots and a horizontal line in the bar graphs. Trends are indicated by a hashtag (#) in line plots or a horizontal dashed line in the bar graphs. Corresponding p-values are noted for each significant parameter.
Results
Serum Cotinine Levels
No detectable levels of cotinine were found prior to implantation of the osmotic pumps in either group. Sequential measurement of cotinine levels demonstrated an appropriate rise in the nicotine group to a target range of 400–700 ng/mL (Supplementary Figure 2), as determined by previous epidemiological studies.21
Contrast Enhanced High Frequency Ultrasound
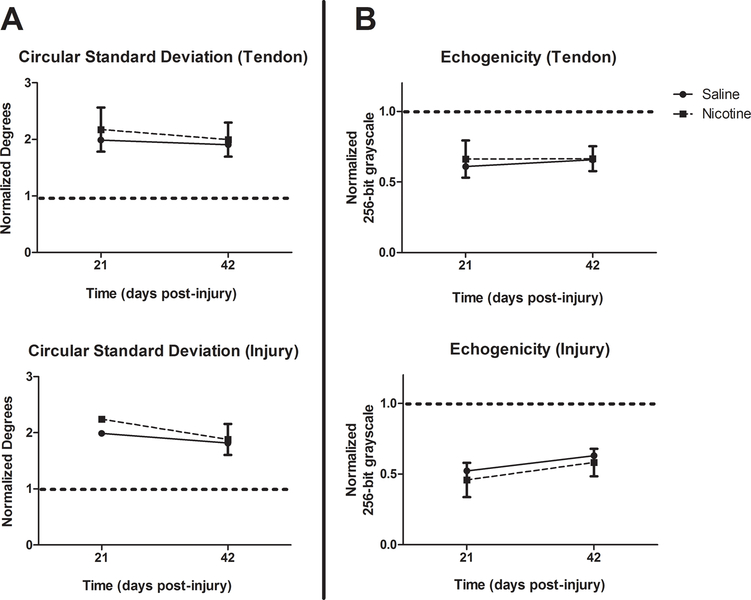
CSD and echogenicity, which are indicators of alignment, were compared and no differences were found between the saline and nicotine groups at any time point for either ROI (Figure 4).
Figure 4. Alignment data.
High frequency ultrasound used to determine the alignment of tendon fibers, as represented by Circular Standard Deviations (A) and Echogenicity (B). No significant differences are seen. ROIs are listed in parentheses following each graph title. Pre-injury values are represented by the dashed horizontal line on the Y-axis. All data are presented as normalized to pre-injury values with mean ± SD.
Rise Time (RT) and Wash-in Rate (WiR) were used as measures of the speed of contrast inflow. At 21 days, the nicotine group demonstrated an increase in RT in the tendon (p=0.001) and injury site (p=0.045). At 42 days, the RT trended towards an increase in the tendon (p=0.07) and injury site (p=0.096) (Figure 5A). WiR was decreased in the nicotine group at 21 days in the tendon (p=0.03) and injury site (p=0.02). It remained decreased in the nicotine group at 42 days in the tendon (p=0.03) and injury site (p=0.04) (Figure 5B).
Figure 5. Vascular data from high frequency ultrasound.
(A) Rise Time is increased in the nicotine group at both timepoints. (B) Wash-in Rate is decreased in the nicotine group at both timepoints. It is calculated as Echo Power/Time. (C) Wash-in Area Under the Curve is increased in the nicotine group. ROIs are listed in parentheses following each graph title. Pre-injury values are represented by the dashed horizontal line on the Y-axis. All data are presented as normalized to pre-injury values with mean ± SD. Significant values are indicated by (*) and trends are indicated by (#). Arbitrary units abbreviated as A.U.
Wash-in Area Under the Curve (WiAUC) was used as a measure of the volume of contrast inflow. WiAUC was increased in the nicotine group at 21 days in the tendon (p=0.03) and injury site (p=0.04). WiAUC was also increased in the nicotine group injury site (p=0.02) at 42 days (Figure 5C).
Quantitative Ambulatory Assessment
The nicotine group demonstrated decreased rate of loading (p=0.01) (Figure 6A), decreased braking force (p<0.001) (Figure 6B), increased propulsion force (p<0.001) (Figure 6B), and decreased anterior-posterior stride width of the injured left hindlimb (p=0.03) at 21 days (Figure 6D).
Figure 6. Quantitative Ambulatory Assessment Data.
(A) Nicotine group demonstrates decreased rate of loading at 21 days post-injury. (B) Assessment of ground reaction forces reveals that the nicotine group has decreased braking force but increased propulsion force. (C) The speed of the injured left hindlimb is decreased in the nicotine group at the late healing stage. (D) Increased medial-lateral stride width demonstrates that the nicotine rats ambulated with a wider stance. The decreased anterior-posterior stride width demonstrates that the nicotine rats ambulated with shorter steps, suggesting alteration of gait mechanics. All data are presented as normalized to pre-injury values with mean ± SD. Significant values are indicated by (*).
At 42 days, the nicotine group demonstrated continued decrease in braking force (p<0.001) (Figure 6B), continued increase in propulsion force (p=0.005) (Figure 6B), and continued decrease in anterior-posterior stride width (p=0.03) (Figure 6D). The nicotine group also demonstrated decreased speed of the injured left hindlimb (p=0.008) (Figure 6C) and increased medial-lateral stride width (p=0.002) at 42 days (Figure 6D).
Passive Joint Mechanics
At 21 days, the nicotine group demonstrated increased total passive ROM (p=0.006) (Figure 7A), decreased dorsiflexion linear region stiffness (p<0.001) (Figure 7B), and decreased plantar flexion linear region stiffness (p<0.001) (Figure 7C).
Figure 7. Passive Joint Mechanics.
(A) The nicotine group demonstrates greater total passive ROM. (B) The nicotine group demonstrates decreased ankle stiffness in dorsiflexion. (C) The nicotine group demonstrates decreased ankle stiffness in plantar-flexion. All data are presented as normalized to pre-injury values with mean ± SD. Significant values are indicated by (*).
At 42 days, the nicotine group demonstrated increased total passive ROM (p<0.001) (Figure 7A), decreased dorsiflexion toe (p=0.003) and linear (p<0.001) region stiffness (Figure 7B), and decreased plantar flexion toe region stiffness (p=0.005) (Figure 7C).
Tendon Histology
Grading of histologic images revealed no difference in cellularity or cell shape between the two groups at either 3 or 6 weeks (Figure 8A, B). Additionally, there were no differences in collagen alignment between groups at either post-operative time point (Fig 8C). However, nicotine tendons contained significantly fewer vascular structures at the injury site than the saline group at 3 weeks, and this difference remained as a trend at 6 weeks (Fig 8D). Representative images of ROI used for cell characteristics grading are shown in Figure 9.
Figure 8. Histologic Assessments.

(A) Cellularity, (B) cell shape, and (C) collagen alignment at the injury site of the tendon were evaluated at 21 and 42 days post-injury. No differences are observed between the groups. (D) Vessel density was significantly decreased in the nicotine group at three weeks, and trended toward decreased in the same group at 6 weeks post-injury. In A and B, bars denote median score, whiskers denote maximum to minimum grading score range, and boxes denote inter-quartile range (25%−75%). In C and D, data is presented as mean ± standard deviation. Significant differences are indicated by a solid horizontal line, and trends are indicated by a hashed horizontal line.
Figure 9. Representative histology images.

Hematoxylin and Eosin (H&E) images at 200X for both groups at each timepoint. No differences in cellularity or cell shape are observed. Scale bar: 100 μm.
Tendon Mechanics
At 21 days, the nicotine group demonstrated increased maximum stress (p=0.02) (Figure 10F). At 42 days, the nicotine group demonstrated decreased cross sectional area (p<0.001) (Figure 10A), decreased maximum load (p=0.02) (Figure 10B), decreased stiffness (p=0.01) (Figure 10D), and decreased stress relaxation (p=0.02) (Figure 10E). No differences in modulus were found at either timepoint (Figure 10C). Two tendons each from the saline and nicotine group failed at the grip at the 42 day timepoint and were excluded from maximum load and maximum stress calculations. The remaining tendons failed at the site of injury.
Figure 10. Biomechanical data.

The nicotine group demonstrates decreased (A) CSA, (B) Maximum load to failure, (D) Stiffness, and (E) Stress relaxation at 42 days post-injury. (C) Modulus demonstrates no differences between the two groups. (F) The nicotine group demonstrates increased maximum stress at 21 days post-injury. Data are presented as mean ± SD error bars. Significant values are indicated by a horizontal line.
Discussion
Clinical and basic science research has well established the harmful effects of nicotine on an individual’s health. In tendon specifically, nicotine has been shown to be detrimental in tendon-to-bone healing.8 However, the effects of nicotine on intra-substance tendon healing remain largely unknown. Tendon healing normally occurs by fibrovascular bridging rather than tendon regeneration, resulting in structural and mechanical properties inferior to the original tissue.22 As such, it is paramount that factors that further weaken the tendon be discovered and targeted as modifiable risk factors in the clinical setting. The primary focus of this study was to determine whether nicotine was one such factor that causes inferior tendon healing. Overall, our results suggest that nicotine leads to inferior functional, vascular, and biomechanical properties in tendon healing.
Contrary to our hypothesis, we did not find differences in the structural organization of tendon fibers between the two groups, as revealed by the comparable CSDs and echogenicity levels on ultrasound. Similarly, histologic analysis also revealed no significant differences in cellularity, cell shape, or collagen fiber alignment. Overall, tendons from both groups demonstrated marked cellularity and rounding of tenocytes, consistent with previous literature.23 Given that there was no difference in cellularity or cell shape, the equivalent collagen fiber alignment between groups suggests that maturation of the healing tendon is not delayed in the nicotine treated group. Instead, the effects appear to be simply due to less tissue formation. This may be because cells in the nicotine group are less metabolically active and producing less extracellular matrix.
The contrast ultrasound data reveals the negative effects of nicotine on blood supply to the tendon. The higher RT and lower WiR in the nicotine group at 21 days reveals that nicotine inhibits vascular inflow rate during early healing. Furthermore, the WiR remains significantly low at 42 days, suggesting continued vascular insult from nicotine during late healing. Quantitation of vascular structures in histologic sections also demonstrated a negative effect of nicotine on the vascular response to injury.
The WiAUC is increased in the tendon and the injury site at 21 days, but only at the injury site at 42 days. This suggests that while the rate of inflow of blood is compromised, as discussed above, the rats are able to compensate by increasing the total volume of blood flow. However, this compensation only occurs during early healing. With continued nicotine exposure and vascular insult, the volume of blood flow to the tendon also diminishes, as the rat is no longer able to physiologically compensate. Interestingly, while the tendon as a whole has decreased vascular supply during late healing, the injury site is still able to compensate to some degree, as represented by the increased WiAUC at the injury site at 42 days. When overall blood flow to the tendon is limited by nicotine, the body diverts it to where it is needed most – the injury site within the tendon. This suggests that healing in the nicotine group is incomplete and requires continued metabolic expense to promote blood flow and healing. Clinical literature reveals that nicotine impairs vascular supply by inducing vasoconstriction through α-adrenergic receptors, by causing endothelial dysfunction through oxidative stress, and by increasing thrombus formation through greater platelet adhesion.24–27 Our results are consistent with these data, and the decrease in vascular supply may present an underlying mechanism of how nicotine leads to inferior tendon healing.
Quantitative ambulatory assessment demonstrates that nicotine leads to greater functional impairment in the injured limb, as represented by decreased braking force, decreased rate of loading, decreased stride length, and decreased speed of the injured hindlimb. These changes occur in early healing but persist during late healing, suggesting long-term impairment incurred by the nicotine. Interestingly, nicotine led to increased propulsion force at both timepoints, even though braking force was decreased. Perhaps this represents a difference in tendon function between concentric muscle contraction (plantarflexion during toe off) and eccentric muscle contraction (dorsiflexion after heel strike), with the eccentric contraction being affected more by inferior tendon healing, as has been suggested.28 It is also important to note that nicotine plays a role in the modulation of nociceptive stimuli and may lead to increased pain sensitivity.29,30 As such, the alteration in gait mechanics in the nicotine rats may be a reflection of an increased pain response.
Passive joint mechanics testing reveals that nicotine leads to an overall decrease in ankle stiffness and increased passive ROM globally, both during early and late healing. Nicotine’s vasoconstrictive properties are well known24 and our ultrasound data confirms that nicotine hinders blood flow in tendons. Furthermore, our biomechanical data reveal that nicotine leads to decreased cross sectional area of the injured tendon (i.e., less scar formation at the injury site), in concordance with previous literature.31 Data suggest that nicotine results in less hypertrophic scar and keloid formation, as well as decreased proliferation of myofibroblasts in post-surgical scars.32–34 As such, a link between nicotine, decreased blood flow, and decreased scar formation can be made. The increased ROM of the ankle joint can be explained by this diminished fibrotic response in the nicotine-exposed rats.
Mechanical testing reveals that nicotine leads to inferior viscoelastic and biomechanical properties. Exposure to nicotine reduced the maximum load by 44.7%, stiffness by 35.0%, and stress relaxation by 14.4% during late healing. The reduced stiffness may lead to greater elongation of the tendon and therefore lead to greater functional impairment. Furthermore, the decreased failure load may predispose patients to re-rupture their tendons. Given that nicotine exposure already increases the risk of wound complications after surgical tendon repair, 35,36 re-rupture is particularly problematic as future surgical therapy in that patient population would be at high risk for complications. Importantly, the equivalent modulus, coupled with the equivalent collagen fiber organization discussed above, further reinforces that the overall maturity of the healing tissue does not necessarily differ between groups, and that differences between the groups are a matter of quantity, not quality or material properties.
Interestingly, the nicotine tendons demonstrate increased maximum stress at 21 days post-injury. This may be because the rats are able to compensate during early healing, as suggested by the increased blood volume at that timepoint discussed above. It also may explain why the nicotine group did not demonstrate inferior mechanical properties in any parameter during early healing. Furthermore, maximum stress is inversely related to CSA. As such, the nicotine tendons may have experienced greater maximum stress at 21 days due to a smaller CSA – a result of a weaker fibrotic response due to compromised vascularity, as discussed above.
While nicotine may have direct local effects on tendon healing, it can also have indirect effects through the modulation of cytokines and chemokines, given that nicotine consumption is a systemic disease. Previous literature has suggested that nicotine down-regulates the expression of growth factors like VEGF, PDGF, TGF-β1 and TGF-β2 but has a less significant effect on inflammatory markers like TNF, IL-6 and IL-12.37 The downregulation in growth factors, coupled with the decreased vascularity, could explain the decreased quantity of tissue formation in the healing tendons. The less significant effects of nicotine on inflammatory mediators may explain why maturation of healing tissue is affected less, as suggested by our CSA and structural organization data from US and histology above.
Our study has several limitations. Firstly, our experiment involves the use of an animal model that may not completely reflect the clinical presentation in humans. Nonetheless, it is important to note that animal models allow control of more variables including activity level, sex, genetic background, and, in this case, precise exposure to nicotine. In addition, our study focuses on the effects of heavy nicotine use but does not evaluate the effects of light nicotine consumption. More studies with gradations in nicotine dose are needed to determine whether a dose-dependent response exists. Furthermore, our study examined tendon healing only up to 42 days post-injury. Perhaps more healing occurs at later timepoints or perhaps the detrimental effects persist. A study with more timepoints that followed rats for greater than 42 days would be valuable. Our study employed surgical transection of the Achilles tendon to create an injury. This may not mimic degenerative tendon tears seen clinically, and may be more akin to acute traumatic tears. Furthermore, all rats used in our study were male. Our lab has previously shown that gender plays an important role in creating differential tendon properties.38 As such, the effects of nicotine on tendons may be different in female rats. Some assay specific limitations also exist. For instance, our histologic analysis was limited by its semi-quantitative methodology and our mechanical testing was limited by tendons that failed at the grip, as discussed above.
In conclusion, our study reveals the detrimental effects of nicotine on tendon healing, suggesting decreased tissue formation and decreased vascularity as potential mechanisms for inferior healing. Future studies are needed to assess whether cessation of nicotine exposure prior to treatment would allow tendons to heal better than if the subjects had continued to expose themselves to nicotine. This would provide evidence supporting the use of clinical interventions to help patients quit nicotine use prior to undergoing treatment for tendon injuries.
Supplementary Material
The graph represents the inflow and outflow of contrast through the tendon. Rise Time (RT) represents the time it takes for the contrast to reach its peak volume. Wash-in Rate (WiR) is the slope of the line representing the rate of inflow of contrast. Area under the curve (AUC) represents the total volume of contrast that has flown through the tendon. This can be subdivided into the wash-in AUC, or the AUC only during the in-flow phase (i.e., prior to the peak of the curve). Echo Power on the Y-axis is represented by Arbitrary Units (A.U.)
Saline group shows no detectable cotinine levels. Nicotine group shows increasing cotinine levels over time that fell within the target range of 400–700 ng/mL (shaded area).
Statement of Clinical Significance:
This study demonstrates that nicotine leads to worse functional outcomes and biomechanical properties in tendons. The decreased vascularity in the nicotine group may suggest an underlying mechanism for inferior tendon healing. Patients should be counseled that using nicotine products increase their risk of poor tendon healing and may predispose them to tendon re-rupture.
Acknowledgements:
The authors would like to acknowledge Julianne Huegel, PhD, Harina Raja, MS, Ashley Rodriguez, BS and Snehal Shetye, PhD for their contributions to this study. Funding provided by the Orthopaedic Research and Education Foundation Grant (#17-011) and NIH/NIAMS supported Penn Center for Musculoskeletal Disorders Grant (5P30AR069619). The authors report no relevant financial disclosures.
References
- 1.Mishra A, Chaturvedi P, Datta S, Sinukumar S, Joshi P, Garg A. Harmful effects of nicotine. Indian J Med Paediatr Oncol 2015;36(1):24–31. doi: 10.4103/0971-5851.151771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Health CO on S and. Smoking and Tobacco Use; Fact Sheet; Adult Cigarette Smoking in the United States; 2018. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm. Accessed April 24, 2018.
- 3.Hu T, Sung HY, Keeler TE, Marciniak M. Cigarette consumption and sales of nicotine replacement products. Tob Control 2000;9 Suppl 2(suppl 2):II60–3. doi: 10.1136/TC.9.SUPPL_2.II60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Department of Health U, Services H, for Disease Control C, Center for Chronic Disease Prevention N, Promotion H, on Smoking O. E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General https://e-cigarettes.surgeongeneral.gov/documents/2016_sgr_full_report_non-508.pdf. Accessed April 24, 2018.
- 5.Donigan JA, Fredericks DC, Nepola JV., Smucker JD. The Effect of Transdermal Nicotine on Fracture Healing in a Rabbit Model. J Orthop Trauma 2012;26(12):724–727. doi: 10.1097/BOT.0b013e318270466f [DOI] [PubMed] [Google Scholar]
- 6.Kanneganti P, Harris JD, Brophy RH, Carey JL, Lattermann C, Flanigan DC. The effect of smoking on ligament and cartilage surgery in the knee: a systematic review. Am J Sports Med 2012;40(12):2872–2878. doi: 10.1177/0363546512458223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akmal M, Kesani A, Anand B, Singh A, Wiseman M, Goodship A. Effect of nicotine on spinal disc cells: a cellular mechanism for disc degeneration. Spine (Phila Pa 1976) 2004;29(5):568–575. http://www.ncbi.nlm.nih.gov/pubmed/15129075. Accessed April 24, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Galatz LM, Silva MJ, Rothermich SY, Zaegel MA, Havlioglu N, Thomopoulos S. Nicotine Delays Tendon-to-Bone Healing in a Rat Shoulder Model. J Bone Jt Surg 2006;88(9):2027. doi: 10.2106/JBJS.E.00899 [DOI] [PubMed] [Google Scholar]
- 9.Ichinose R, Sano H, Kishimoto KN, Sakamoto N, Sato M, Itoi E. Alteration of the material properties of the normal supraspinatus tendon by nicotine treatment in a rat model. Acta Orthop 2010;81(5):634–638. doi: 10.3109/17453674.2010.524595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldenson NI, Leventhal AM, Stone MD, McConnell RS, Barrington-Trimis JL. Associations of Electronic Cigarette Nicotine Concentration With Subsequent Cigarette Smoking and Vaping Levels in Adolescents. JAMA Pediatr 2017;171(12):1192. doi: 10.1001/jamapediatrics.2017.3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charles FK, Krautter GR, Dixon M, Mariner DC. A comparison of nicotine dose estimates in smokers between filter analysis, salivary cotinine, and urinary excretion of nicotine metabolites. Psychopharmacology (Berl) 2006;189(3):345–354. doi: 10.1007/s00213-006-0586-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng 2003;125(1):106–113. http://www.ncbi.nlm.nih.gov/pubmed/12661203. Accessed August 19, 2018. [DOI] [PubMed] [Google Scholar]
- 13.Chang K-V, Wu C-H, Ding Y-H, Shen H-Y, Wang T-G, Chen W-S. Application of contrast-enhanced sonography with time-intensity curve analysis to explore hypervascularity in Achilles tendinopathy by using a rabbit model. J Ultrasound Med 2012;31(5):737–746. http://www.ncbi.nlm.nih.gov/pubmed/22535721. Accessed May 16, 2018. [DOI] [PubMed] [Google Scholar]
- 14.Needles A, Arditi M, Rognin NG, et al. Nonlinear contrast imaging with an array-based micro-ultrasound system. Ultrasound Med Biol 2010;36(12):2097–2106. doi: 10.1016/j.ultrasmedbio.2010.08.012 [DOI] [PubMed] [Google Scholar]
- 15.Sarver JJ, Dishowitz MI, Kim S-Y, Soslowsky LJ. Transient decreases in forelimb gait and ground reaction forces following rotator cuff injury and repair in a rat model. J Biomech 2010;43(4):778–782. doi: 10.1016/j.jbiomech.2009.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarver JJ, Peltz CD, Dourte L, Reddy S, Williams GR, Soslowsky LJ. After rotator cuff repair, stiffness--but not the loss in range of motion--increased transiently for immobilized shoulders in a rat model. J shoulder Elb Surg 2008;17(1 Suppl):108S–113S. doi: 10.1016/j.jse.2007.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tucker JJ, Cirone JM, Morris TR, et al. Pulsed electromagnetic field therapy improves tendon-to-bone healing in a rat rotator cuff repair model. J Orthop Res 2017;35(4):902–909. doi: 10.1002/jor.23333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res 2003;21(3):413–419. doi: 10.1016/S0736-0266(03)00057-3 [DOI] [PubMed] [Google Scholar]
- 19.Reuther KE, Thomas SJ, Tucker JJ, et al. Disruption of the anterior-posterior rotator cuff force balance alters joint function and leads to joint damage in a rat model. J Orthop Res 2014;32(5):638–644. doi: 10.1002/jor.22586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beason DP, Tucker JJ, Lee CS, Edelstein L, Abboud JA, Soslowsky LJ. Rat rotator cuff tendon-to-bone healing properties are adversely affected by hypercholesterolemia. J Shoulder Elb Surg 2014;23(6):867–872. doi: 10.1016/j.jse.2013.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackford AL, Yang G, Hernandez-Avila M, et al. Cotinine Concentration in Smokers from Different Countries: Relationship with Amount Smoked and Cigarette Type. Cancer Epidemiol Biomarkers Prev 2006;15(10):1799–1804. doi: 10.1158/1055-9965.EPI-06-0427 [DOI] [PubMed] [Google Scholar]
- 22.Galatz LM, Gerstenfeld L, Heber-Katz E, Rodeo SA. Tendon regeneration and scar formation: The concept of scarless healing. J Orthop Res 2015;33(6):823–831. doi: 10.1002/jor.22853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundgreen K, Lian OB, Scott A, Nassab P, Fearon A, Engebretsen L. Rotator cuff tear degeneration and cell apoptosis in smokers versus nonsmokers. Arthroscopy 2014;30(8):936–941. doi: 10.1016/j.arthro.2014.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Black CE, Huang N, Neligan PC, et al. Effect of nicotine on vasoconstrictor and vasodilator responses in human skin vasculature. Am J Physiol Integr Comp Physiol 2001;281(4):R1097–R1104. doi: 10.1152/ajpregu.2001.281.4.R1097 [DOI] [PubMed] [Google Scholar]
- 25.Nicod P, Rehr R, Winniford MD, Campbell WB, Firth BG, Hillis LD. Acute systemic and coronary hemodynamic and serologic responses to cigarette smoking in long-term smokers with atherosclerotic coronary artery disease. J Am Coll Cardiol 1984;4(5):964–971. http://www.ncbi.nlm.nih.gov/pubmed/6548482. Accessed May 18, 2018. [DOI] [PubMed] [Google Scholar]
- 26.Puranik R, Celermajer DS. Smoking and endothelial function. Prog Cardiovasc Dis 2003;45(6):443–458. doi: 10.1053/pcad.2003.YPCAD13 [DOI] [PubMed] [Google Scholar]
- 27.Burke A, Fitzgerald GA. Oxidative stress and smoking-induced vascular injury. Prog Cardiovasc Dis 46(1):79–90. http://www.ncbi.nlm.nih.gov/pubmed/12920701. Accessed May 18, 2018. [DOI] [PubMed] [Google Scholar]
- 28.Mündel T, Machal M, Cochrane DJ, Barnes MJ. A Randomised, Placebo-Controlled, Crossover Study Investigating the Effects of Nicotine Gum on Strength, Power and Anaerobic Performance in Nicotine-Naïve, Active Males. Sport Med - open 2017;3(1):5. doi: 10.1186/s40798-016-0074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakajima M, Al’Absi M. Nicotine withdrawal and stress-induced changes in pain sensitivity: A cross-sectional investigation between abstinent smokers and nonsmokers. Psychophysiology 2014;51(10):1015–1022. doi: 10.1111/psyp.12241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Z, Liu X-W, Lu S-F, Yu A-L, Zhang Z-W. Effect of nicotine withdrawal on pain sensitivity in rats to mechanical stimulation and thermal stimulation. Eur Rev Med Pharmacol Sci 2014;18(18):2759–2765. http://www.ncbi.nlm.nih.gov/pubmed/25317814. Accessed August 19, 2018. [PubMed] [Google Scholar]
- 31.Ağladıoğlu K, Akkaya N, Güngör HR, Akkaya S, Ök N, Özçakar L. Effects of Cigarette Smoking on Elastographic Strain Ratio Measurements of Patellar and Achilles Tendons. J Ultrasound Med 2016;35(11):2431–2438. doi: 10.7863/ultra.15.11050 [DOI] [PubMed] [Google Scholar]
- 32.Campos ACL, Alves MR, Ioshii SO, Moraes-Junior H, Sakamoto D, Gortz LW. Influência da nicotina na proliferação de miofibroblastos e de vasos sanguíneos no tecido cicatricial da parede abdominal de ratos lactentes: estudo imunoistoquímico. ABCD Arq Bras Cir Dig (São Paulo) 2010;23(4):222–227. doi: 10.1590/S0102-67202010000400003 [DOI] [Google Scholar]
- 33.Deliaert AEK, Van den Kerckhove E, Tuinder S, Noordzij SMJS, Dormaar TS, van der Hulst RRWJ. Smoking and its effect on scar healing. Eur J Plast Surg 2012;35(6):421–424. doi: 10.1007/s00238-011-0661-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jay T, Dip Rad N. The Effects of Nicotine in the Treatment of Keloids: A Pilot Study Aim and Objective of Study doi: 10.15406/mojs.2017.04.00096 [DOI] [Google Scholar]
- 35.Silverstein P. Smoking and wound healing. Am J Med 1992;93(1A):22S–24S. http://www.ncbi.nlm.nih.gov/pubmed/1323208. Accessed May 18, 2018. [DOI] [PubMed] [Google Scholar]
- 36.Bruggeman NB, Turner NS, Dahm DL, et al. Wound complications after open Achilles tendon repair: an analysis of risk factors. Clin Orthop Relat Res 2004;(427):63–66. http://www.ncbi.nlm.nih.gov/pubmed/15552138. Accessed May 18, 2018. [DOI] [PubMed] [Google Scholar]
- 37.Xanthoulea S, Deliaert A, Romano A, Rensen SS, Buurman WA, van der Hulst RR. Nicotine effect on inflammatory and growth factor responses in murine cutaneous wound healing. Int Immunopharmacol 2013;17(4):1155–1164. doi: 10.1016/j.intimp.2013.10.022 [DOI] [PubMed] [Google Scholar]
- 38.Pardes AM, Freedman BR, Fryhofer GW, Salka NS, Bhatt PR, Soslowsky LJ. Males have Inferior Achilles Tendon Material Properties Compared to Females in a Rodent Model. Ann Biomed Eng 2016;44(10):2901–2910. doi: 10.1007/s10439-016-1635-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The graph represents the inflow and outflow of contrast through the tendon. Rise Time (RT) represents the time it takes for the contrast to reach its peak volume. Wash-in Rate (WiR) is the slope of the line representing the rate of inflow of contrast. Area under the curve (AUC) represents the total volume of contrast that has flown through the tendon. This can be subdivided into the wash-in AUC, or the AUC only during the in-flow phase (i.e., prior to the peak of the curve). Echo Power on the Y-axis is represented by Arbitrary Units (A.U.)
Saline group shows no detectable cotinine levels. Nicotine group shows increasing cotinine levels over time that fell within the target range of 400–700 ng/mL (shaded area).