Abstract
Objective:
To describe 1) obstetrician–gynecologists’ perceptions of the frequency of vaccine refusal among pregnant patients and perceived reasons for refusal; and 2) obstetrician–gynecologists’ strategies used when encountering vaccine refusal and perceived effectiveness of those strategies.
Methods:
We conducted an e-mail and mail survey among a nationally representative network of ob-gyns from March through June 2016.
Results:
The response rate was 69% (331/477). Health care providers perceived that pregnant women more commonly refused influenza vaccine than Tdap vaccine: 62% of respondents reported ≥10% of pregnant women they care for in a typical month refused influenza vaccine compared to 32% reporting this for Tdap vaccine. The most commonly reported reasons for vaccine refusal were patients’ belief that influenza vaccine makes them sick (48%), belief they are unlikely to get a vaccine-preventable disease (38%), general worries about vaccines (32%), desire to maintain a natural pregnancy (31%), and concern that their child could develop autism as a result of maternal vaccination (25%). The most commonly reported strategies obstetrician-gynecologists used to address refusal were stating that it is safe to receive vaccines in pregnancy (96%), explaining that not getting the vaccine puts the fetus or newborn at risk (90%), or that not getting the vaccine puts the pregnant woman’s health at risk (84%). The strategy perceived as most effective was stating that not getting vaccinated puts the fetus or newborn at risk.
Conclusion:
Ob-gyns perceive vaccine refusal among pregnant women as common, and refusal of influenza vaccine as more common than refusal of Tdap vaccine. Emphasizing the risk of disease to the fetus or newborn may be an effective strategy to increase vaccine uptake.
PRECIS:
Obstetricians perceive vaccine refusal among pregnant women as common; emphasizing disease risk to the fetus or newborn may be an effective strategy to increase vaccine uptake.
INTRODUCTION
Pregnant women have increased risk of severe complications from influenza,1–3 and newborns have increased risk of severe disease and death from both influenza4,5 and pertussis.6,7 Vaccination against these diseases is routinely recommended for all pregnant women in each pregnancy.8,9 Evidence is strong for the effectiveness10–12 and safety13,14 of these vaccines in pregnancy.
However, uptake of these vaccines is suboptimal.15 Lack of health care provider recommendation16 and patient concerns about the need for vaccination and vaccine safety in pregnancy have been identified as important barriers.17 Among pregnant women who received but refused a recommendation and an offer for influenza vaccine from a health care provider, the reasons most often cited were that the vaccine would cause influenza, was unsafe for the baby, or was not effective.16 Another barrier is lack of health care provider time to explain the risks and benefits of vaccination.18
Our objectives in this study were to describe, among a national sample of ob-gyns, practices and attitudes regarding vaccination of pregnant women, including barriers to use of standing orders for vaccination; perceived frequency of vaccine refusal among pregnant patients and reasons for refusal; strategies used when encountering vaccine refusal and their perceived effectiveness; and barriers to discussing the risks and benefits of vaccines with pregnant women.
METHODS
Between March and June 2016, we administered an Internet and mail survey to a national network of ob-gyns representative of American College of Obstetricians and Gynecologists (ACOG) membership. The human subjects review board at the University of Colorado Denver approved this study as exempt research not requiring written informed consent.
The Vaccine Policy Collaborative Initiative (VPCI) conducted this study.19 The VPCI is a program designed and implemented collaboratively with the Centers for Disease Control and Prevention (CDC) to perform rapid turnaround surveys assessing physician practices and attitudes about vaccine-related issues. We developed a national network of ob-gyns for this program by recruiting from members of ACOG. To develop the network, we constructed sampling matrices using demographic data from random samples of ACOG membership. We then determined proportions of US ob-gyns falling into each cell of a 3-dimensional matrix that crossed US region (Northeast, South, Midwest, or West), and practice location (urban inner city, suburban, or rural). We applied proportions for each cell in the 12-cell matrix to a total sample size of 475 to create cell-sampling quotas. Power calculations established that 300 completed surveys would yield 80% power with a 5% Type I error rate to detect at least a 16 percentage point difference when comparing dichotomous variables between 2 groups of equal size. Assuming a 60–75% survey response rate, the network was therefore designed to have approximately 475 participants. No more than one health care provider from each individual practice was included in the sample. We previously demonstrated that survey responses from network physicians compared to those of physicians randomly sampled from American Medical Association physician databases had similar demographic characteristics, practice attributes, and attitudes about a range of vaccination issues.19 Ob-gyns who reported that they cared exclusively for non-pregnant patients were excluded from this study.
We developed the survey jointly with CDC with input from experts in vaccination and obstetrics and gynecology. The survey was pre-tested with a panel of six ob-gyns and then piloted among 38 ob-gyns from different regions of the country. Questions regarding assessing and administering vaccines and use of evidence-based practices were asked using a series of yes or no questions. Questions regarding frequency of a given practice and barriers to discussion were assessed using 4-point Likert scales (never or rarely, sometimes, often, always). Respondents were asked to estimate the amount of time they spent discussing vaccines in two scenarios: with a pregnant woman with substantial concerns who needs one or more vaccines and with a pregnant woman who does not have concerns and needs one or more vaccines. Response options for these questions included ‘no time or someone else discusses,’ 1–2 minutes, 3–5 minutes, and 6 or more minutes. Response options for questions regarding proportions of women in a typical month who refuse vaccines included none, 1–9%, 10–19%, 20–29%, and 30% or more. Barriers questions also utilized a 4-point Likert scale from ‘not a barrier’ to ‘major barrier.’ Other responses to information questions were either yes/no, with answers that were not mutually exclusive, or selections from a list of possible options. The survey instrument is available in Appendix 1, available online at http://links.lww.com/AOG/B214.
We surveyed physicians via the Internet or, if they preferred, by mail. We used a Web-based program (Verint®, Melville, New York, www.verint.com) to administer Internet surveys, and we sent mail surveys by the U.S. Postal Service. We sent the Internet group an initial e-mail with up to 8 e-mail reminders, and we sent the mail group an initial mailing and up to 2 additional mailed reminders. We sent Internet survey non-respondents a cross-over mail survey in case of problems with e-mail correspondence. Unique IDs were used to assure that duplicate surveys were not received from the same individual. We patterned the mail protocol on Dillman’s tailored design method.20
We pooled Internet and mail surveys together for analyses because other studies have found that physician attitudes are similar when obtained by either method.21 Response options had less than 5% missing answers with exceptions noted. We compared respondents with non-respondents on all available characteristics using Wilcoxon and chi-square analyses. To compare responses of respondents to different questions, we used McNemar’s test to account for the paired nature of the responses.
RESULTS
The response rate was 69% (331/477). Respondents were more likely than non-respondents to be female and had a higher median number of health care providers in their office (Table 1).
Table 1.
Comparison of Responders and Non-responders Among a National Sample of Obstetrician-Gynecologists to a Survey Regarding Vaccine Refusal in Pregnancy.
| Variable | Non-Responder Col % (n) n=146 | Responder Col % (n) n=331 | p value |
|---|---|---|---|
| Gender | |||
| Male | 40.7 (59) | 29.9 (98) | |
| Female | 59.3 (86) | 70.1 (230) | 0.02 |
| Setting | |||
| Private practice | 72.9 (105) | 63.6 (208) | |
| Hospital or clinic | 19.4 (28) | 29.4 (96) | |
| Health maintenance organization | 7.6 (11) | 7.0 (23) | 0.08 |
| Census Location | |||
| Urban | 52.1 (76) | 62.2 (206) | |
| Suburban | 48.0 (70) | 36.9 (122) | |
| Rural | 0.0 (0) | 0.9 (3) | 0.06 |
| Region | |||
| Midwest | 19.9 (29) | 20.5 (68) | |
| Northeast | 25.3 (37) | 19.9 (66) | |
| South | 36.3 (53) | 36.3 (120) | |
| West | 18.5 (27) | 23.3 (77) | 0.48 |
| Decision-making | |||
| Independent | 62.5 (90) | 56.0 (183) | |
| Larger system level | 37.5 (54) | 44.0 (144) | 0.19 |
| Mean (sd) / Median age in years | 50.5 (10.5) / 50.0 | 49.7 (10.9) / 49.0 | 0.48 |
| Mean (sd) / Median number of health care providers | 12.5 (32.2) / 5.0 | 14.7 (29.5) / 7.0 | 0.001* |
Sd; standard deviation.
Wilcoxon test
Overall, 90% of ob-gyns reported that their practice currently administered at least one vaccine to pregnant women. Almost all respondents reported strongly recommending both influenza (97%) and Tdap (95%) vaccines to pregnant women. More ob-gyns reported ‘always’ recommending to pregnant women that their household contacts receive Tdap vaccine to protect their newborns than they do for influenza vaccine (Tdap: 68% always; influenza: 53% always; p<0.001, McNemar’s test comparing always to all other responses). Sixty percent of respondents reported they sometimes receive questions from pregnant women about vaccines for their newborn (10% often or always, 31% never or rarely).
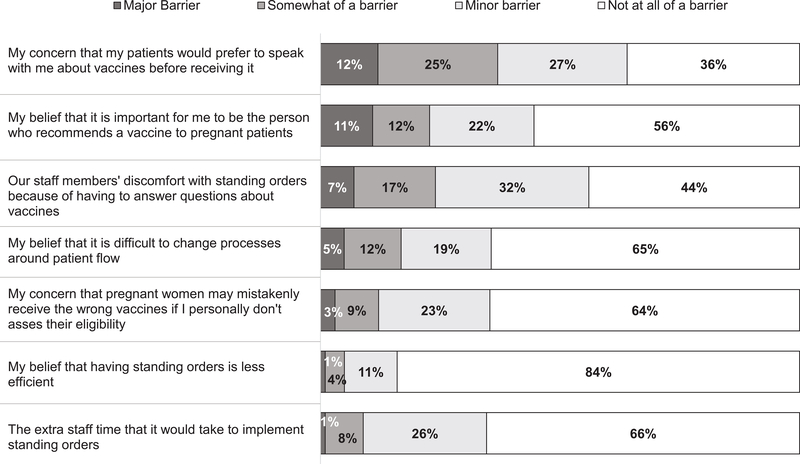
Sixty percent of ob-gyns reported using standing orders for influenza vaccine and 56% for Tdap vaccine. Items most commonly reported as barriers to standing orders included physician concern that patients would prefer to talk with them prior to receiving a vaccine, staff members’ discomfort with having to answer questions, and physician belief that it is important that they be the person to make a vaccine recommendation (Figure 1). Regarding time spent discussing vaccines with a pregnant woman in need of vaccines who had no concerns, 12% of respondents reported ‘no time or someone else discusses,’ 69% reported 1–2 minutes, 17% reported 3–5 minutes, and 3% reported 6 or more minutes. For women with substantial vaccine concerns, 1% reported ‘no time or someone else discusses’, 7% reported 1–2 minutes, 54% reported 3–5 minutes, and 38% reported 6 or more minutes.
Figure 1.
Barriers to the Use of Standing Orders for Vaccination Reported by Obstetrician-Gynecologists
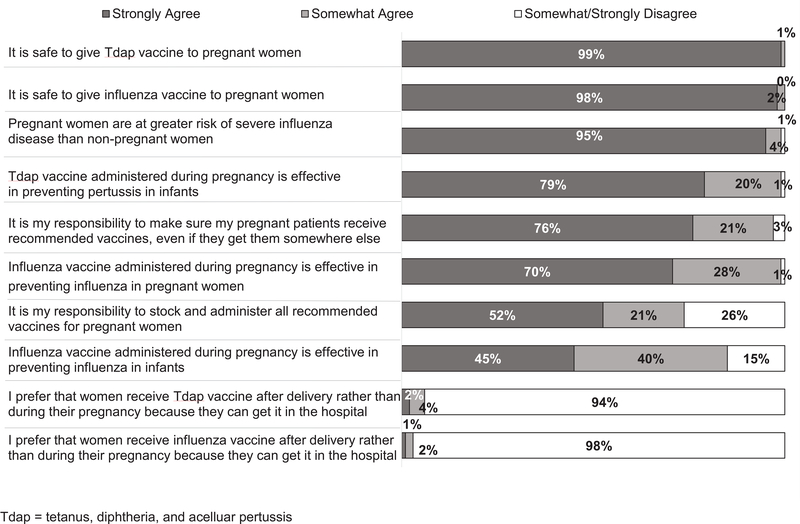
Ob-gyns reported favorable attitudes towards vaccinating their pregnant patients (Figure 2). Almost all strongly agreed that it was safe to give both Tdap and influenza vaccines to pregnant women. Ob-gyns’ attitudes were in concordance with current recommendations. Ob-gyns were also asked about the best time for pregnant patients to receive Tdap vaccine, with 93% responding between 27 and 36 weeks of pregnancy, 3% at a pre-pregnancy visit, 3% anytime during pregnancy, and 1% responding either ‘just prior to delivery’ or ‘after delivery’.
Figure 2.
Obstetrician-Gynecologists’ Attitudes about Vaccination of Pregnant Patients
In general, respondents reported more pregnant women refuse influenza vaccine than Tdap vaccine, although refusal was common for both vaccines (Table 2). Overall, 62% of ob-gyns reported that 10% or more of their pregnant population refuses influenza vaccine versus 32% reporting this for Tdap (p<0.001, χ2, <10% vs ≥10%).
Table 2.
Obstetricians’ Reports of Frequency of Tdap and Influenza Vaccine Refusal Among Pregnant Women.*
| 1–9% | 10–19% | 20–29% | ≥30% | None | |
|---|---|---|---|---|---|
| Influenza vaccine | 37% | 33% | 21% | 8% | 1% |
| Tdap vaccine | 60% | 20% | 10% | 2% | 9% |
p<0.001
χ2, comparing influenza and Tdap vaccine, <10% vs ≥10%
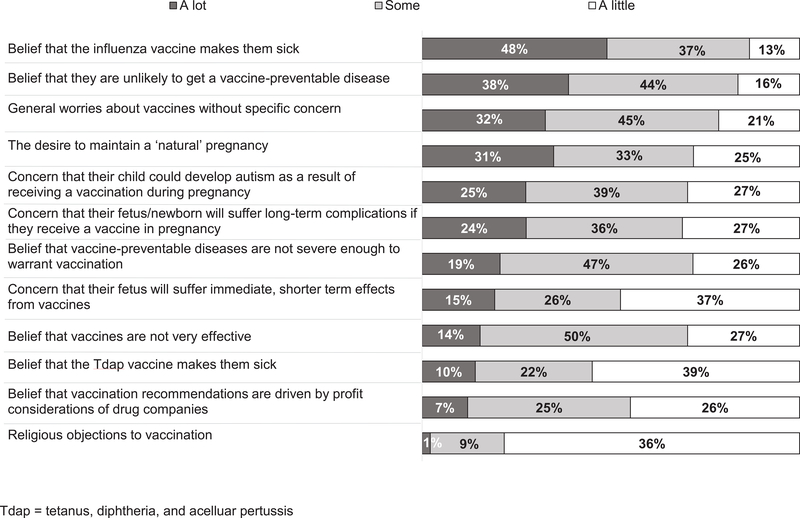
Factors most commonly reported by respondents as contributing ‘a lot’ to refusal included belief that influenza vaccine makes them sick, belief they are unlikely to get a vaccine-preventable disease, general worries about vaccines, the desire to maintain a natural pregnancy, and concern their child could develop autism as a result of receiving a vaccination during pregnancy. (Figure 3.)
Figure 3.
Obstetrician-Gynecologists’ Perceptions of Reasons for Vaccine Refusal Among Pregnant Patients
Strategies physicians reported as always or often used by more than half of health care providers when encountering a pregnant woman refusing a vaccine included stating confidence that it is safe to receive vaccines in pregnancy (96%), explaining that not getting the vaccine puts the fetus or newborn at risk (90%), explaining that not getting the vaccine puts the pregnant woman’s health at risk (84%), discussing outbreaks of vaccine-preventable diseases (72%), informing the patient that not getting the vaccine is against the health care provider’s recommendation (64%), and stating that they personally would get the vaccine or give it to a family member if pregnant (53%) (Table 3). In general, most of these strategies were perceived by a majority of physicians as ‘somewhat effective’ at convincing a pregnant woman who has refused vaccination to choose to be vaccinated. The only strategy that stood out as being perceived as ‘very effective’ by a substantial proportion of physicians (40%) was explaining that not getting the vaccine puts the fetus or newborn at risk.
Table 3.
Strategies Used by Obstetrician-Gynecologists and Perceived Effectiveness of Strategies to Address Vaccine Refusal Among Pregnant Patients
| Frequency Reporting Use, % | Perceived Effectiveness, % | |||||||
|---|---|---|---|---|---|---|---|---|
| Strategy | Always | Often | Sometimes | Never/Rarely | Very effective | Somewhat effective | Not very effective | Not at all effective |
| State that you are confident that it is safe to receive the vaccine during pregnancy | 74 | 22 | 2 | 2 | 19 | 61 | 17 | 4 |
| Explain that not getting the vaccine puts the fetus/newborn’s health at risk | 58 | 33 | 7 | 3 | 40 | 51 | 7 | 2 |
| Explain that not getting the vaccine puts the patient’s own health at risk | 46 | 38 | 13 | 3 | 12 | 64 | 20 | 4 |
| Discuss recent outbreaks of vaccine preventable diseases | 39 | 33 | 22 | 7 | 19 | 52 | 24 | 6 |
| Inform the patient that not getting the vaccine is against your recommendation | 37 | 27 | 24 | 13 | 8 | 47 | 36 | 9 |
| State that you would immunize yourself or your family member if pregnant | 22 | 32 | 27 | 20 | 19 | 61 | 14 | 6 |
| Discuss your personal experiences observing mothers and/or babies with influenza* | 20 | 25 | 30 | 26 | 16 | 54 | 22 | 8 |
| Discuss your personal experiences observing mothers and/or babies with pertussis+ | 12 | 16 | 23 | 49 | 15 | 46 | 23 | 15 |
For perceived effectiveness, this response option had 5.3% missing answers
For perceived effectiveness, this response option had 7.0% missing answers
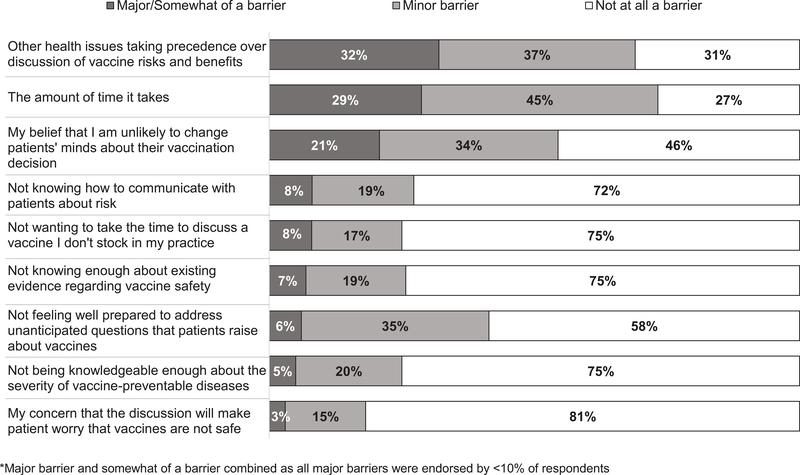
Almost all ob-gyns strongly agreed that they were comfortable discussing influenza and Tdap vaccines with pregnant patients (98% and 96%, respectively). Fewer, however, agreed that they were comfortable addressing questions or concerns about the infant series of vaccines (18% strongly agree, 31% somewhat agree, 34% somewhat disagree, 16% strongly disagree). The most common barriers to discussing the risks and benefits of vaccines with pregnant women were other health issues taking precedence and the amount of time it takes (Figure 4). The only other barrier endorsed as ‘major’ or ‘somewhat’ by more than 10% of respondents was ‘My belief that I am unlikely to change patients’ minds about their vaccination decision.’
Figure 4.
Barriers Reported by Obstetrician-Gynecologists to Discussing the Risks and Benefits of Vaccines with Pregnant Women*
DISCUSSION
In this study, we provide information regarding ob-gyns’ experiences with vaccine refusal among their pregnant patients, and how they handle refusal. As in other recent work,22 we found few attitudinal barriers regarding vaccination among ob-gyns themselves. However, the majority of U.S. ob-gyns perceive that vaccine refusal among pregnant women is common, particularly for influenza vaccine. They report using a number of different strategies for addressing vaccine refusal, yet only one was perceived as very effective.
While there is a large body of literature regarding parental vaccine refusal for childhood vaccines,23 few prior studies report the prevalence of vaccine refusal among pregnant women. It appears vaccine refusal among pregnant women may be more common than parental refusal of childhood vaccines. In a study of pediatricians and family physicians, 8% of physicians reported parental refusals of vaccination for ≥10% of the children they care for.24 In this study, 62% of ob-gyns reported influenza vaccine refusals for ≥10% of pregnant women and 32% reported Tdap refusals for ≥10%. CDC indirectly reports influenza and Tdap vaccine refusal among pregnant women.25,26 Among pregnant women who received both a recommendation and an offer for influenza vaccine, in the 2016–2017 season, 70.5% reported being vaccinated, implying 29.5% refused, consistent with our data.25 For Tdap, among pregnant women who received both a recommendation and an offer, 69.9% reported vaccination, implying 30.1% refused,26 slightly higher than estimates based on our data. In either case, the conclusion is the same: vaccine refusal among pregnant women is common for both Tdap and influenza vaccines.
Perceived reasons for vaccine refusal among pregnant women include common misconceptions, such as believing influenza vaccine makes them sick, but also included some findings not previously described, such as the concern their child may develop autism. The possibility that childhood vaccines are associated with autism is perhaps the best-studied safety question in the history of vaccination, and the findings are resoundingly clear that vaccines do not cause autism.27 Our finding that fears about autism are linked with vaccination refusal among pregnant women underscores the profound impact that safety information on vaccines, even if erroneous, influences the decisions of pregnant women.
The perceived effectiveness of strategies to address vaccine refusal was low, with almost all strategies being endorsed as ‘very effective’ by less than 20% of respondents, with one exception: 40% of ob-gyns reported that stressing a potential threat to the fetus or newborn by not vaccinating was ‘very effective’ at convincing a woman who had refused vaccination to be vaccinated. In prior work with pediatricians and family physicians, no strategy examined was deemed ‘very effective’ by more than 20% of respondents.28 Further work in this area should explore the vaccination decision-making process from the unique perspective of pregnant women.
While the focus of this manuscript is on vaccine refusal, we also report on use of standing orders, which are among the most effective evidence-based strategies for increasing vaccination uptake.29 In addition to increasing vaccination coverage and efficiency, standing orders may overcome attitudinal barriers. Although we know little about which communication techniques increase uptake of vaccines, science in other areas shows that ‘nudges’ are often effective at overcoming attitudinal resistance to a desired behavior.29 Previous work has demonstrated the importance of social norms in the vaccination decision.30 Standing orders are a clear example of emphasis of a social norm by sending the message to both healthcare professionals and patients that vaccination is the default option. The barriers to use of standing orders are surmountable: for example, patients who prefer to speak with the health care provider prior to vaccination may still do so. Implementation of standing orders may reduce staff discomfort with answering questions and lead to better job satisfaction.29
There are strengths and limitations to this study. It was perfomed in a nationally representative sample of ob-gyns with a high response rate. Respondents’ attitudes and practices, though, may have differed from non-respondents, and the ob-gyns in our survey network may differ from ob-gyns overall, although prior work suggests not.19 Finally, this study examined reported practices and perceptions; actual practices were not observed.
Ob-gyns perceive vaccine refusal as common among pregnant women, and report they spend significant time discussing vaccine concerns with pregnant patients. Ob-gyns perceive that emphasizing the importance of vaccination to protect the fetus or newborn as an effective strategy for addressing vaccine refusal. Future work should focus on testing evidence-based strategies for addressing vaccine refusal in the obstetrical setting and understanding how the unique concerns of pregnant women influence the effectiveness of such strategies.
Supplementary Material
Acknowledgments
FUNDING SOURCE: This investigation was funded by the Centers for Disease Control and Prevention and administered through the Rocky Mountain Prevention Research Center, University of Colorado Anschutz Medical Campus (Grant #1U01IP000849). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
ABBREVIATIONS:
- ACIP
Advisory Committee on Immunization Practices
- Tdap
tetanus, diphtheria and acellular pertussis vaccine
- ACOG
American College of Obstetrics and Gynecology
- CDC
Centers for Disease Control and Prevention
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.Creanga AA, Johnson TF, Graitcer SB, et al. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstet Gynecol. 2010;115(4):717–726. [DOI] [PubMed] [Google Scholar]
- 2.Hartert TV, Neuzil KM, Shintani AK, et al. Maternal morbidity and perinatal outcomes among pregnant women with respiratory hospitalizations during influenza season. Am J Obstet Gynecol. 2003;189(6):1705–1712. [DOI] [PubMed] [Google Scholar]
- 3.Louie JK, Acosta M, Jamieson DJ, Honein MA, California Pandemic Working G. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362(1):27–35. [DOI] [PubMed] [Google Scholar]
- 4.Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000;342(4):232–239. [DOI] [PubMed] [Google Scholar]
- 5.Mullooly JP, Barker WH. Impact of type A influenza on children: a retrospective study. Am J Public Health. 1982;72(9):1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortese MM, Baughman AL, Zhang R, Srivastava PU, Wallace GS. Pertussis hospitalizations among infants in the United States, 1993 to 2004. Pediatrics. 2008;121(3):484–492. [DOI] [PubMed] [Google Scholar]
- 7.Vitek CR, Pascual FB, Baughman AL, Murphy TV. Increase in deaths from pertussis among young infants in the United States in the 1990s. Pediatr Infect Dis J. 2003;22(7):628–634. [DOI] [PubMed] [Google Scholar]
- 8.Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices-United States, 2018–19 Influenza Season. MMWR Recomm Rep. 2018;67(3):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang JL, Tiwari T, Moro P, et al. Prevention of Pertussis, Tetanus, and Diphtheria with Vaccines in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67(2):1–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359(15):1555–1564. [DOI] [PubMed] [Google Scholar]
- 11.Poehling KA, Szilagyi PG, Staat MA, et al. Impact of maternal immunization on influenza hospitalizations in infants. Am J Obstet Gynecol. 2011;204(6 Suppl 1):S141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skoff TH, Blain AE, Watt J, et al. Impact of the US Maternal Tetanus, Diphtheria, and Acellular Pertussis Vaccination Program on Preventing Pertussis in Infants <2 Months of Age: A Case-Control Evaluation. Clin Infect Dis. 2017;65(12):1977–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nordin JD, Kharbanda EO, Benitez GV, et al. Maternal safety of trivalent inactivated influenza vaccine in pregnant women. Obstet Gynecol. 2013;121(3):519–525. [DOI] [PubMed] [Google Scholar]
- 14.Munoz FM, Bond NH, Maccato M, et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. JAMA. 2014;311(17):1760–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahn KE, Black CL, Ding H, et al. Influenza and Tdap Vaccination Coverage Among Pregnant Women - United States, April 2018. MMWR Morb Mortal Wkly Rep. 2018;67(38):1055–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease C, Prevention. Influenza vaccination coverage among pregnant women: 2011–12 influenza season, United States. MMWR Morb Mortal Wkly Rep. 2012;61:758–763. [PubMed] [Google Scholar]
- 17.O’Leary ST, Pyrzanowski J, Brewer SE, et al. Influenza and Pertussis Vaccination Among Pregnant Women and Their Infants’ Close Contacts: Reported Practices and Attitudes. Pediatr Infect Dis J. 2015;34(11):1244–1249. [DOI] [PubMed] [Google Scholar]
- 18.Link-Gelles R, Chamberlain AT, Schulkin J, et al. Missed opportunities: a national survey of obstetricians about attitudes on maternal and infant immunization. Matern Child Health J. 2012;16(9):1743–1747. [DOI] [PubMed] [Google Scholar]
- 19.Crane LA, Daley MF, Barrow J, et al. Sentinel physician networks as a technique for rapid immunization policy surveys. Eval Health Prof. 2008;31(1):43–64. [DOI] [PubMed] [Google Scholar]
- 20.Dillman DA, Smyth J, Christian LM. Internet, Mail and Mixed-Mode Surveys: The Tailored Desgin Method, 3rd Edition. Vol 3rd New York, NY: John Wiley Co.; 2009. [Google Scholar]
- 21.McMahon SR, Iwamoto M, Massoudi MS, et al. Comparison of e-mail, fax, and postal surveys of pediatricians. Pediatrics. 2003;111(4 Pt 1):e299–303. [DOI] [PubMed] [Google Scholar]
- 22.O’Leary ST, Riley LE, Lindley MC, et al. Immunization Practices of U.S. Obstetrician/Gynecologists for Pregnant Patients. Am J Prev Med. 2018;54(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salmon DA, Dudley MZ, Glanz JM, Omer SB. Vaccine Hesitancy: Causes, Consequences, and a Call to Action. Am J Prev Med. 2015;49(6 Suppl 4):S391–398. [DOI] [PubMed] [Google Scholar]
- 24.Kempe A, Daley MF, McCauley MM, et al. Prevalence of parental concerns about childhood vaccines: the experience of primary care physicians. Am J Prev Med. 2011;40(5):548–555. [DOI] [PubMed] [Google Scholar]
- 25.Ding H, Black CL, Ball S, et al. Influenza Vaccination Coverage Among Pregnant Women - United States, 2016–17 Influenza Season. MMWR Morb Mortal Wkly Rep. 2017;66(38):1016–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Pregnant Women and Tdap Vaccination, Internet Panel Survey, United States, April 2016. 2018; https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/tdap-report-2016.html. Accessed May 3, 2018.
- 27.Taylor LE, Swerdfeger AL, Eslick GD. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine. 2014;32(29):3623–3629. [DOI] [PubMed] [Google Scholar]
- 28.Kempe A, O’Leary ST, Kennedy A, et al. Physician response to parental requests to spread out the recommended vaccine schedule. Pediatrics. 2015;135(4):666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnard JG, Dempsey AF, Brewer SE, Pyrzanowski J, Mazzoni SE, O’Leary ST. Facilitators and barriers to the use of standing orders for vaccination in obstetrics and gynecology settings. Am J Obstet Gynecol. 2017;216(1):69 e61–69 e67. [DOI] [PubMed] [Google Scholar]
- 30.Merrill MH, Hollister AC, Gibbens SF, Haynes AW. Attitudes of Californians toward poliomyelitis vaccination. Am J Public Health Nations Health. 1958;48(2):146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.