Abstract
Mitochondrial encephalomyopathies (ME) are complex, incurable diseases characterized by severe bioenergetic distress that can affect the function of all major organ systems but is especially taxing to neuromuscular tissues. Animal models of MEs are rare, but the Drosophila ATP61 mutant is a stable, well-characterized genetic line that accurately models progressive human mitochondrial diseases such as Maternally-Inherited Leigh Syndrome (MILS), Neuropathy, Ataxia, and Retinitis Pigmentosa (NARP), and Familial Bilateral Striatal Necrosis (FBSN). While it is established that this model exhibits important hallmarks of ME, including excess cellular and mitochondrial reactive oxygen species, shortened lifespan, muscle degeneration, and stress-induced seizures, it is unknown whether it exhibits defects in sleep or circadian function. This is a clinically relevant question, as many neurological and neurodegenerative diseases are characterized by such disturbances, which can exacerbate other symptoms and worsen quality of life. Since Drosophila is highly amenable to sleep and circadian studies, we asked whether we could detect disease phenotypes in the circadian behaviors of ATP61. Indeed, we found that day-time and night-time activity and sleep are altered through disease progression, and that circadian patterns are disrupted at both the behavioral and neuronal levels. These results establish ATP61 as an important model of sleep and circadian disruption in ME that can be studied mechanistically at the molecular, cellular, and behavioral level to uncover underlying pathophysiology and test novel therapies.
Keywords: Mitochondrial disease, Neurodegenerative disease, Drosophila
Highlights
-
•
A Drosophila model of mitochondrial disease (ATP61) displays altered sleep patterns.
-
•
ATP61 sleep quantity and consolidation are reduced in advanced disease.
-
•
ATP61 is behaviorally arrhythmic under conditions of constant darkness.
-
•
Selected neurons of the circadian circuit display altered daily firing rates in ATP61.
1. Introduction
Primary mitochondrial diseases result from mutations in the mitochondrial genome or in the nuclear genome-encoded components of the electron transport chain (ETC). Such mutations typically interfere with oxidative phosphorylation (OXPHOS), the foremost source of ATP in organisms utilizing aerobic respiration, causing severe bioenergetic stress. These progressive metabolic diseases, including Maternally Inherited Leigh Syndrome (MILS), Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-like Episodes (MELAS), and Leber’s Hereditary Optic Neuropathy (LHON), affect up to 1 in 5000 people worldwide, and tend to disproportionately distress the energetically demanding tissues of the nervous and muscular systems, causing symptoms such as neurodegeneration, muscle degeneration or weakness, cognitive or sensory impairment, cardiomyopathy, and seizures (Berardo et al., 2011, Canafoglia et al., 2001, DiMauro and Schon, 2003, DiMauro and Schon, 2008, DiMauro et al., 1989). These diseases are notoriously difficult to treat; with no direct therapies available to directly address the mitochondrial defects themselves, treatment plans are often empirically determined amalgamations of vitamins, antioxidants, nutritional supplements, and pharmacology directed at individual symptoms, such as anti-epileptic drug therapy for seizures.
Because of these difficulties and the devastating impact of these diseases, animal models are crucial for understanding their pathophysiology and developing novel treatments. The Drosophila ATP6 1 genetic line is a well-characterized, stable animal model of mitochondrial encephalomyopathy (ME) (Celotto et al., 2006). Its pathology arises from a glycine to glutamate point mutation (resulting in a G116E substitution) in the mitochondrially encoded ATP6 subunit of Complex V (the ATP synthase), which energetically couples the mitochondrial electrochemical gradient to OXPHOS by passing hydrogen ions down their concentration gradient. ATP61 animals exhibit a progressive symptomatic profile similar to that seen in human ME patients, including muscle degeneration, seizure-like activity, excess cellular levels of lactic acid and of reactive oxygen species (ROS), and a shortened lifespan (Celotto et al., 2006, Celotto et al., 2011, Palladino, 2010). These features make ATP61 an ideal model in which to study ME pathology at a molecular, cellular, and whole-animal behavioral level.
Perturbations in sleep, including night-time insomnia or hyposomnia, fragmented sleep, and day-time hypersomnia are frequently observed in patients living with neurological or neurodegenerative diseases, including Alzheimer's Disease, Parkinson’s Disease, and epilepsy, exacerbating other symptoms and worsening quality of life (Manni et al., 2000, Reddy and O’Neill, 2010, Hogl et al., 2018, Videnovic, 2018, Ju et al., 2014, Musiek et al., 2015). Often, these disruptions manifest in the form of increased latency to sleep or premature waking, suggesting that dysfunction of the circadian clock may underlie some sleep issues. Conversely, disruptions in sleep are known to alter neurological symptoms, including those common to MEs such as seizure frequency and cognitive ability, and metabolic function in diseases such as type II diabetes (Suzuki et al., 1997, Grigg-Damberger and Ralls, 2014, Jain and Kothare, 2015, Nedeltcheva and Scheer, 2014, Guarnieri and Sorbi, 2015). Additionally, it has recently been shown that sleep deprivation alters mitochondrial function and gene expression in response to oxidative stress in Drosophila (Rodrigues et al., 2018). Taken together, these observations suggest an intimate relationship between sleep/circadian health and bioenergetic function. Indeed, a recent survey of clinical literature suggests that sleep disturbance is a clinically underappreciated aspect of mitochondrial disease (Ramezani and Stacpoole, 2014).
Drosophila are extremely well-suited to sleep studies, exhibiting similar characteristics to vertebrate sleep, including inactivity, increased response threshold, and increased sleep drive in response to deprivation (Shaw et al., 2000). Their sleep and circadian systems have a well-described circuitry and there is a highly standardized methodology for studying these behaviors (Tomita et al., 2017, Chatterjee and Rouyer, 2016, Peschel and Helfrich-Forster, 2011, Potdar and Sheeba, 2013). Therefore, we have asked whether the ATP61 mutant exhibited defects in these behaviors due to its mitochondrial dysfunction. We found that sleep is perturbed throughout the ME disease time course, with higher night time levels in early disease but reduced levels in advanced disease, and poorer consolidation throughout.
In circadian experiments, we observed diseased ME flies to have lengthened and weakened circadian periods, with a drastically higher number of arrhythmic individuals as disease progressed. ATP61 flies also exhibited arrhythmic eclosion patterns, and lacked a well-established time-dependent firing pattern in the lateral ventral neurons of the circadian circuit. Overall, these studies suggest that significant sleep and circadian disruption may be a characteristic phenotype of some mitochondrial diseases.
2. Methods
2.1. Fly strains and genetics
Strains, matings, and experimental flies were maintained in standard media comprised of agar, corn meal, dextrose, sucrose, dry yeast, and molasses, with propionic acid, phosphoric acid, and Tegosept added as fungicides. Flies are maintained in a light and temperature controlled room under a 12:12LD schedule.
In all reported genotypes, “ATP61” or “ATP6 (Berardo et al., 2011)” refers to the mitochondrially encoded gene mutation in ATP6 and is denoted first, separated from the nuclear chromosomes by standard semi-colon notation. The ATP61 strain used in all experiments was generated by the O’Farrell lab (Ma and O’Farrell, 2015, Ma and O’Farrell, 2016) and described previously (Fogle et al., 2016). Controls were ATP6+;w1118.
For physiology experiments, female ATP61 were mated with the recombinant strain PDFGAL4-UAS-NC1. NC1 is a non-conducting potassium channel GFP fusion for visualization of the large lateral ventral neurons. This mating results in the experimental genotype ATP61; w*;PDFGAL4-UAS-NC1/+. “Wild type” flies were generated by the reciprocal mating, resulting in the same nuclear genome but a wild type mitochondrial ATP6+.
2.2. Sleep/wake, circadian, and arousal behavior
Sleep and 24-h activity rhythm experiments were performed using standard TriKinetics (Waltham, MA) Drosophila activity monitors, with which activity was recorded in one-minute bins. Male flies of control and ME genotypes were collected simultaneously at eclosion, maintained in the 12:12LD 25-degree C incubator for entrainment, then loaded into monitors at age 7–8 days. Three full 32-tube monitors (minus incidental deaths) of ATP61 flies and ATP6+ flies were analyzed for sleep behavior.
For circadian period experiments, flies were collected, maintained, and loaded as above. For testing circadian rhythm in advanced disease, the lights in the incubator were turned off at day 14 and flies were monitored in 24 h of darkness (DD) until day 20. This experiment was run three times with different cohorts of ATP61 and ATP6+ flies. For assessing circadian health in earlier disease, the flies were loaded at 4–5 days of age and the lights were turned off at day 7; this was assessed for ~64 flies per genotype minus incidental deaths.
Eclosion assays were performed by mating ATP61 females with ATP6+;w1118 males or vice versa to produce ATP61 or ATP6+ progeny, respectively. Crosses and developing larvae/pupae were kept in 12:12LD at 25 degrees. As soon as eclosion began, vials were cleared of progeny at lights-on and then at four-hour intervals until lights off. The progeny eclosed during each interval were counted and averaged over a five-day period.
Arousal threshold in response to mechanical stimulus experiments were conducted by securely attaching a TriKinetics monitor to a standard laboratory rocker apparatus and turning the power on for 5 s. Appropriate strength of the stimulus was empirically determined and standardized for values designated “mild,” “moderate,” and “strong.” Flies were analyzed according to whether they were awake or asleep at the time of the pulse, as described in the Results Section 2.4.
2.3. Electrophysiology
Flies for electrophysiology experiments were maintained in 12:12 LD at 25 °C from eclosion. Recordings were performed within four hours of lights-on (ZT 0–4, a.m.) or in the late day (ZT 8–11, p.m.) as indicated. Whole brains were dissected from ice-immobilized aged adult flies (21–24 days) and cleaned in a solution containing 20 units/mL papain dissolved in standard external solution (see below). Brains were transferred to a RC-22C recording chamber (Warner Instruments) and held in place with a platinum-framed nylon mesh. Standard external recording solution (101 mM NaCl, 1 mM CaCl2, 4 mM MgCl2, 3 mM KCl, 5 mM glucose, 1.25 mM NaH2PO4, and 20.7 mM NaHCO3, 250 mOsm, pH 7.2, aerated by a gas mixture of 95% O2–5% CO2) was gravity perfused over the specimen at a rate of ∼1 mL/min. Standard-wall borosilicate capillary tubes were pulled into patch pipettes (11–13 mΩ) by a Narishige PP-83 electrode puller and filled with standard internal solution (102 K-gluconate, 0.085 CaCl2, 1.7 MgCl2, 17 NaCl, 0.94 EGTA, 8.5 HEPES, 235 mOsm, pH 7.2), then guided to GFP+ neurons by a Sutter MP-225 micromanipulator. Whole-cell current-clamp recordings were performed using an Olympus BX51W microscope (Olympus, Lehigh Valley, NJ), CV201A headstage, Axopatch 200 A Integrating Patch-Clamp amplifier, DigiData 1322 A 16-bit Data Acquisition System and PClamp 10 software (Molecular Devices, Sunnyvale, CA). After whole-cell configuration was achieved and the recording stabilized, data were collected for basal firing frequency, which was calculated by hand counts divided by length (in seconds) of the analyzed region, which always 30 s to account for any variability and make sure each recording was represented equally. Analysis of electrophysiological data was performed using ClampFit10 (Molecular Devices/Axon Instruments).
2.4. Analysis and statistics
Daily/nightly activity, hourly sleep patterns, total sleep, bout length, actograms, circadian period, and rhythm strength were calculated/constructed using ShinyR-DAM (Cichewicz and Hirsh, 2018). Data are presented as mean ± standard error. Two-way comparisons were performed by t-test with a two-tailed p-value in SigmaPlot13.
3. Results
3.1. Daily activity and sleep patterns are altered in ATP61 disease
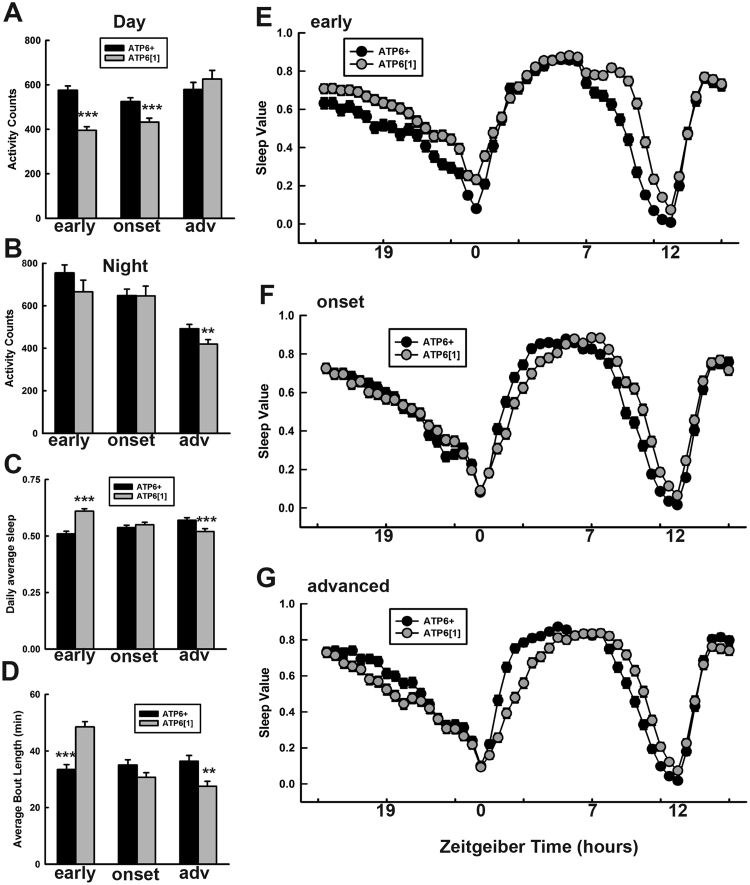
To determine the daily activity patterns of ATP61 vs. ATP6+ nuclear genome-matched control flies, we placed male flies into standard Trikinetics monitors and collected their minute-by-minute activity from ages 7 to 21 days post-eclosion. The two genotypes were collected at the same time and the experiments run in parallel. The monitors were kept in a light controlled incubator at 25 °C in standard 12 h of light: 12 h of darkness conditions (LD). We first tabulated the average daily and nightly activity for flies during early life (days 8–11), disease onset (days 12–15), and advanced disease (days 16–21). We observed that in early life and disease onset, daily activity of ATP61 during the day is reduced compared to normal mitochondrial controls (Fig. 1A), while nightly activity is unaltered until late disease, when it becomes significantly lower (Fig. 1B). Next, we quantified total sleep and average bout length, and found a striking pattern in both properties: both relative amount of sleep (Fig. 1C) and bout length (Fig. 1D) are increased in young, pre-diseased ATP61 flies, but significantly reduced in advanced disease compared to wild type controls. To examine the daily patterns of sleep in more detail, we plotted sleep over the course of the 24-h day in 30-min bins, averaged for early, onset, and advanced flies. In early life (Fig. 1E), sleep levels for ATP61 are elevated compared to ATP6+ for almost the entire night (ZT12-24). ATP61 also stays asleep later into the day, exhibiting sleep behavior while ATP6+ is ramping up its activity in anticipation of evening (ZT8-11). In middle life, when most established disease phenotypes are displaying characteristic onset, sleep behavior also evolves (Fig. 1F). Night time sleep, instead of being elevated, now overlaps almost entirely with ATP6+, while daytime sleep begins to show a “lag” behind ATP6+ after the morning peak of activity. Like in early disease, sleep reduction during evening anticipation is slower than ATP6+. In advanced disease (Fig. 1G), the night time pattern seen in early life has reversed, with ATP61 sleep significantly reduced compared to ATP6+ for most of the night. The slowdown in daytime return to sleep (ZT1-3) is more pronounced than at disease onset, and the evening anticipation delay persists. Overall, these results show that ATP61 experiences complex activity and sleep patterns that differ from those of control animals and evolve throughout the disease time course.
Fig. 1.
ATP61 exhibits altered sleep and activity patterns. A. Average daytime activity for ATP6+ and ATP61 through disease time course. Early (days 8–11, p < .001), onset (days 12–15, p < .001), and advanced disease (days 16–21, n.s.). B. Average night time activity for ATP6+ vs. ATP61 through disease time course. Early (p = .19), onset (p = .44), and advanced disease (p = .014). C. Daily average percentage of sleep. Early and advanced time points, p < .001. D. Quantified average length for bouts of sleep. In early disease, p < .0001; in advanced disease, p = .0014. E. Amount of sleep averaged for early life, days 8–11, in 30-min bins. All time points ZT15-20, ZT22-0.5, and ZT7.5-12 are significantly different with a p value <.01. F. Amount of sleep averaged for mid-life, days 12–15. ZT23 and 23.5, ZT1-4, ZT8-11.5 are significantly different with a p value <.01. G. Amount of sleep averaged for advanced age, days 16–21. Sleep values are significantly (p <. 01) different at ZT17-20.5, ZT0.5-5, ZT8-12.
3.2. ATP61 exhibits abnormal circadian behavior
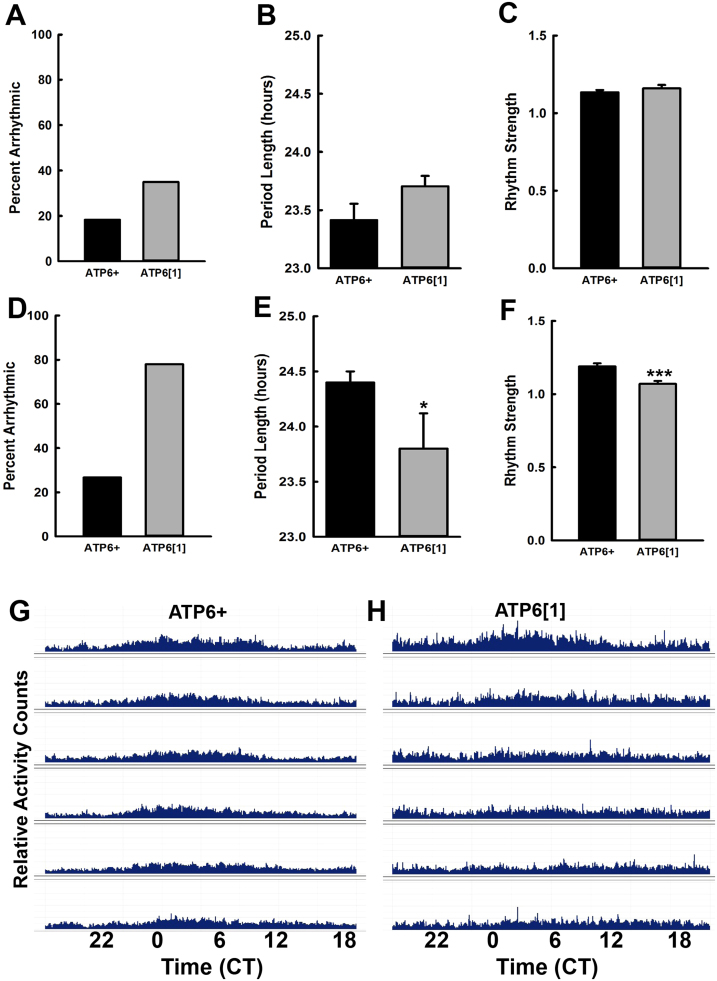
Since sleep and circadian dysfunction often occur in tandem, we wanted to test the ability of ATP61 to maintain its daily circadian rhythm in the absence of external cues. We separated the lifespan into two groups – “early” (days 8–13) and “late” (days 14–20). For the early life group, after entrainment to a standard LD regimen, ATP6+ and ATP61 flies were monitored in constant darkness (DD) from ages 8 to 13 days, and their behavior is quantified in Fig. 2A-C. ATP61 produces more arrhythmic flies, 38%, compared to ATP6+ at 19%. However, neither the circadian period nor the rhythm strength is significantly different between the two genotypes in the early time frame. In contrast, advanced disease does appear to perturb rhythm strength and power, in addition to drastically increasing the number of arrhythmic flies for ATP61. Fig. 2D shows that nearly 80% of ATP61 flies are too arrhythmic to be included in the circadian analysis shown in Fig. 2E and F, while arrhythmicity in ATP6+ only increases about 10% from early to late life. Of the flies that do meet the criteria for circadian analysis, ATP61 has a significantly shorter circadian period than ATP6+, although it should be noted that both still lie within a typical/normal period length range. ATP61 also displays a small but significant reduction in the power of its rhythm strength (Fig. 2F). The ATP6+ actogram from late life in Fig. 2G, which is an average of the activity of approximately 30 flies for circadian analysis, shows that although flies held in DD lack the characteristic strong peaks evoked by light-dark transitions, a clear pattern of daily activity persists throughout the monitoring period. In contrast, the ATP61 average actogram lacks a discernably rhythmic pattern after the first three days in DD (Fig. 2H).
Fig. 2.
ATP61 is highly arrhythmic under conditions of constant darkness (DD) in advanced disease. A. Percent of individuals that were arrhythmic in the early disease (day 8–13) trials and thus were not factored into the analysis shown in B and C. B. Length of the free-running circadian period for ATP6+ (n = 40 flies) and ATP61 (n = 32 flies) during the DD monitoring period (p = .26). C. Rhythm strength for the circadian period calculated in C. (p = .12). D. Percent of individuals that were arrhythmic in the advanced disease trials and thus were not factored into the analysis shown in E and F. E. Length of the free-running circadian period for ATP6+ (n = 68 flies) and ATP61 (n = 23 flies) during the DD monitoring period (p = .034) in late disease (day 14–20). F. Rhythm strength for the circadian period calculated in E. (p < .001). G. Representative average actogram for ATP6+ for one trial run under DD conditions for ages 14–20 days. H. Representative average actogram for ATP61 for one trial run under DD conditions for ages 14–20 days.
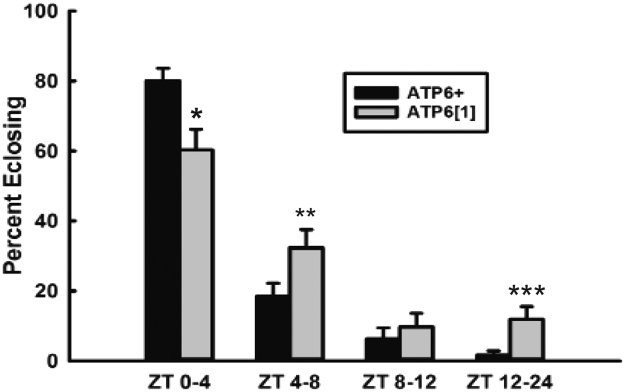
In addition to daily patterns of sleep and wakefulness, eclosion from the pupal case is also a circadian controlled behavior in flies, with most new adults emerging within a few hours of daybreak/lights-on (Sheeba et al., 2001, Di Cara and King-Jones, 2013). Therefore, we assayed eclosion throughout the day for ATP6+ and ATP61 maintained in a temperature-controlled LD incubator. ATP61 ecloses significantly less during the expected ZT 0-4 window and significantly more in the middle of the day (ZT4-8) and overnight (ZT12-24) (Fig. 3), indicating that this behavior, too, is circadian clock compromised in ATP61.
Fig. 3.
Circadian timing of adult eclosion is disrupted in ATP61. Percentage of flies that eclosed during the early day (p = .017), mid-day (ZT4-8, p = .01), late day (ZT8-12, p = .25), and over night (ZT12-24, p = .001). The total number of eclosed flies were n = 485 for ATP6+ and 537 for ATP61.
3.3. ATP61 lacks a well-established neuronal firing pattern that is modified by time of day
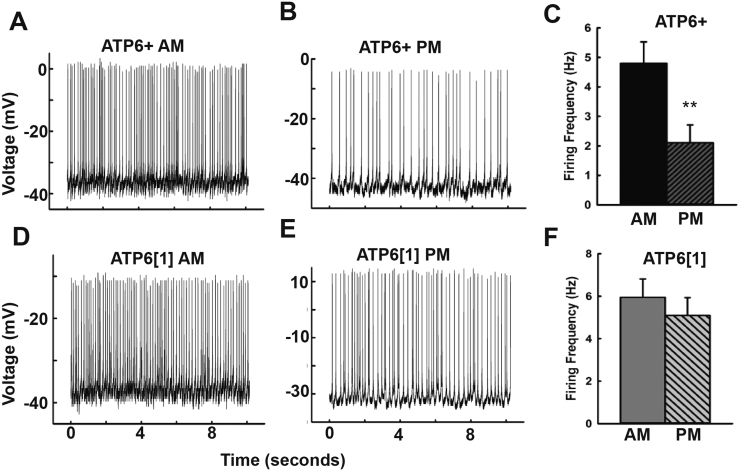
The large lateral ventral neurons (l-LNv) of the Drosophila circadian, sleep, and arousal circuits are well-characterized peptidergic neurons that respond acutely to light cues and contribute to wakefulness and setting the behavioral arousal threshold (Fogle et al., 2015, Fogle et al., 2011, Sheeba et al., 2008, Sheeba et al., 2008, Parisky et al., 2008). These spontaneously-firing neurons have an established daily pattern, firing at higher frequency during the early day and measurably dropping during the late-day activity trough (Sheeba et al., 2008a). We performed whole cell current clamp electrophysiology on these neurons during defined circadian time frames to test whether ATP61;;pdfGAL4-NC1’s maintained this time of day dependent firing pattern. Recordings were performed during the first four hours of the day (ZT0-4) and during the mid/late day (ZT8-11) on age-matched ATP6+;;pdfGAL4-NC1 and ATP61;;pdfGAL4-NC1 flies (21–24 days old) that had been maintained in a controlled LD incubator since eclosion. Representative recordings from ATP6+;;pdfGAL4-NC1 flies performed in the morning (Fig. 4A) vs. the afternoon (Fig. 4B) confirm the daily regulation of l-LNv firing frequency in normal flies, as the average firing frequency in the afternoon is significantly lower (4C). Representative traces from ATP61;;pdfGAL4-NC1 (Figs. 4D, 4E) indicate that while there is some discernable reduction in afternoon firing frequency compared to morning recordings, this effect is minor and does not reach significance (Fig. 4F).
Fig. 4.
The large lateral ventral neurons of the circadian and sleep circuits show weakened characteristic time-dependent changes in firing rate in ATP61. A, B. Representative recordings from ATP6+; w*; PDFGAL4-UAS-NC1/+ in the morning (A) and the late afternoon (B). C. Average spontaneous firing frequency of ATP6+l-LNv recorded in the early day (ZT0-4, n = 8) and late afternoon (ZT8-11, n = 9, p = .011).
D,E. Representative recordings from ATP61; w*; PDFGAL4-UAS-NC1/+ in the morning (D) and late afternoon (E). A. Average spontaneous firing frequency of ATP61l-LNv recorded in the early day (ZT0-4, n = 10) and late afternoon (ZT8-11, n = 9, p = .49).
3.4. ATP61 shows an altered response to a stressful stimulus
As described above, the l-LNv contribute to setting the threshold of the fly’s response to an arousing stimulus (Sheeba et al., 2008, Parisky et al., 2008). When the l-LNv are genetically modified to exhibit hyperexcitation, flies require a higher stimulus to evoke a behavioral response (increased activity/arousal to movement). Because we observed a qualitatively higher firing frequency in the l-LNv in the morning and a significantly higher firing frequency in the afternoon for ATP61, we decided to test whether it was more difficult to evoke a behavioral response to a mechanical stimulus in these ME flies, establishing a correlated behavior with the abnormalities at the neuronal level. These experiments were performed in the late afternoon (ZT8-10), when the firing frequency difference in the l-LNv is large. Flies in advanced disease (day 19) were given a mild, moderate, and strong mechanical stimulus with appropriate recovery time in between, and their responses were analyzed using standard TriKinetics activity monitoring. We found that a significantly higher percentage of ATP6+ flies were sleeping (as determined by standard inactivity parameters) than ATP61 during the experiment before any stimulus was given (Fig. 5A). We next calculated the percentage of sleeping flies that were awakened by each stimulus. After strong and moderate stimulation, all of the flies of both genotypes were awakened (data not shown). After mild stimulation, however, all of the ATP61 flies were roused into mobility, while fewer than half of the ATP6+ flies did so (Fig. 5B). We subsequently analyzed the behavior of the flies that were already awake and behaving at the time of the stimuli. To measure the responses, we calculated post-pulse activity over pre-pulse activity to see whether activity was increased, decreased, or unchanged. During the five minute window after a mild pulse, both ATP6+ and ATP61 flies increased their activity approximately two-fold, while a moderate pulse increased their activity up to three-fold, with no significant differences in behavior between the genotypes (Fig. 5C). In response to the strongest stimulus, however, ATP6+ and ATP61 flies respond differently. The activity of the former decreases slightly, perhaps indicating a “freezing” response to a startling stimulus, while ATP61 again increases its activity two-fold. These results do not indicate that elevated l-LNv firing ablates ATP61’s response to arousing stimuli, but instead may demonstrate a hyper-excitatory response to mechanical stress in the ME disease state.
Fig. 5.
ATP61 shows a heightened excitatory behavioral response to mechanical stimulus. A. Quantified percentage of flies that were asleep at the time of each pulse for ATP6+ and ATP61 (n = 6 pulses, p = .001). B. The percentage of previously-sleeping flies that awakened in response to the mildest pulse administered to evoke arousal (n = 2 pulses; p = .044). C. Quantified, normalized behavioral activity (post-pulse/pre-post) evoked by the mild (p = .98), medium (p = .54) and strong (p < .001) mechanical stimulus on flies that were already awake at the time of the pulse ((ATP6+ n = 17 flies, ATP61 n = 27 flies).
4. Discussion
Mitochondrial encephalomyopathies are multifaceted metabolic diseases with a range of neurological manifestations including seizures, cognitive impairment, sensory deterioration, and developmental delay. Therefore, it is perhaps unsurprising that like in other neurodegenerative diseases, sleep disturbances have been reported in ME patients. Often these disturbances take the form of obstructive or central sleep apnea (Sakaue et al., 2002, Tan and Goy, 2013, Sembrano et al., 1997, Yasaki et al., 2001), which in most cases result from weakness in the diaphragm or other respiratory muscles. However, other patients exhibit sleep issues that are likely to be neurological in nature, including delayed sleep phase syndrome (Suzuki et al., 1997), chronic insomnia (Suzuki et al., 1997), increased duration of wakefulness after sleep onset (Ramezani and Stacpoole, 2014), hypnic myoclonus (Pincherle et al., 2006), and decreased ventilatory drive (Barohn et al., 1990). We have found robust sleep and circadian dysfunction in a Drosophila model of human mitochondrial disease, suggesting that this may be an under-recognized but clinically relevant aspect of ME. We show that ATP61 flies with advanced disease experience shorter bouts of sleep, which is a correlate of fragmented and poor-quality sleep. We also show that overall amount of sleep is reduced in late disease, and that its patterns are altered compared to nuclear-genome and age-matched control flies, with ATP61 showing increased latency to day time sleep and slower evening anticipatory sleep reduction. Interestingly, these patterns only evolved when ME has advanced; young ATP61 flies actually exhibit much more night time sleep, longer bout length, and higher overall sleep values while at the same time their day time activity is reduced. This complexity likely reflects a dynamically changing metabolic flux throughout disease time course. One possibility for the observed pattern may be energy conservation in early life that fails in the face of neuronal hyperexcitability when the ME becomes severe.
The neurological basis of sleep is complex, but in Drosophila, as in humans, is known to involve dopaminergic, octopaminergic (adrenergic), GABAergic, and peptidergic signaling in several defined key circuits (Tomita et al., 2017, Artiushin and Sehgal, 2017). The large lateral ventral neurons of the circadian circuit, which secrete the clock-synchronizing peptide Pigment Dispersing Factor (PDF), are wake-promoting and must be inhibited by ionotropic GABAergic signaling for normal sleep (Parisky et al., 2008); when these neurons are hyperexcited, night-time sleep is reduced (Sheeba et al., 2008b). We have shown here that their firing patterns are abnormal in advanced ATP61 disease (although it should be noted this genotype, modified to express GFP in the LNv, was not tested behaviorally in this study). In the central complex, the structure known as the dorsal fan-shaped body is sleep-promoting unless it receives sustained dopaminergic tone. The sleep-promoting properties of this structure are due to the dominance of Kv1 (Shaker) and Kv2 (Shab) currents (Artiushin and Sehgal, 2017). The former is profoundly modulated by a functional aldoketoreductase beta subunit Kvβ2 (Hyperkinetic) which possesses an NADP(H) cofactor, rendering it highly sensitive to cellular redox state (Tipparaju et al., 2007, Wang and Wu, 1996, Weng et al., 2006, Yao and Wu, 1999). In the pars intercerebralis, insulin-producing cells are wake-promoting due to octopaminergic signaling which, via cAMP, modulates the calcium-sensitive K current (slowpoke) (Artiushin and Sehgal, 2017). The function of this channel is known to be redox sensitive (DiChiara and Reinhart, 1997). These known links between sleep physiology, redox state, and metabolic state may prove to be fruitful areas of study for understanding how mitochondrial disease results in sleep dysfunction.
We have also shown that ATP61 exhibits perturbed circadian behavior, including increased arrhythmicity in prolonged constant darkness and aberrant patterns of eclosion. Drosophila possess a core circadian molecular clock characterized by a transcription-translation negative feedback loop (TTFL), which is highly analogous to its counterpart in mammalian cells. In flies, the transcription factors Clock (CLK) and Cycle (CYC) promote transcription of the proteins Period (PER) and Timeless (TIM) that dimerize in the cytoplasm, undergo a series of phosphorylation events, and are translocated to the nucleus where they inhibit further PER/TIM transcription (Dubowy and Sehgal, 2017, Tataroglu and Emery, 2015). This TTFL is synchronized to daily light cues by the involvement of cryptochrome (CRY), a blue light-sensitive protein that binds to TIM and targets it for proteasomal degradation (Emery et al., 2000, Yoshii et al., 2015, Peschel et al., 2009). Several of these core clock components are known to be affected by redox state. For example, CRY is a highly redox-sensitive protein, possessing a flavin cofactor that is crucial for its ability to transduce light and effect its downstream biological functions (Fogle et al., 2015, Vaidya et al., 2013, Yoshii et al., 2009, Fedele et al., 2014, Ozturk et al., 2011). In mammals, the DNA binding activity of BMAL/CLOCK (the vertebrate counterparts of CLK/CYC) is modified by the redox environment via the ratio of NAD/NADH and NADP/NADPH, exhibiting increased binding ability in a reduced environment (Rutter et al., 2001). Relatedly, the pentose phosphate pathway has been shown to regulate the circadian clock; its inhibition prolongs circadian period in human cells, mouse tissue, and flies (Rey et al., 2016). Furthermore, treatment of flies with hydrogen peroxide or the genetic over-expression of the superoxide dismutase enzyme that produces it suppresses daily locomotor rhythms (Grover et al., 2009).
The above findings provide ample evidence that redox state can affect the core circadian clock. But it is also clear that redox control in the cell is itself under circadian regulation. The transcription of the reactive oxygen species-responsive gene Nrf2, for example, is circadian regulated in mouse lung, liver, and β cells (Pekovic-Vaughan et al., 2014). Cellular ratios of NADP+:NADPH and GSH:GSSG have been demonstrated to undergo circadian cycling in the liver (Isaacs and Binkley, 1977, Kaminsky et al., 1984), and daily rhythmic ratios of oxidized FAD:NADPH have been uncovered in the mouse suprachiasmatic nucleus, the central pacemaking center of the brain (Wang et al., 2012). Additionally, the transcription factors BMAL/CLOCK regulate the expression of nicotinamide phosphoribosyltransferase, a crucial enzyme in the biosynthesis of NAD+. The resulting daily cycle of NAD+ has been shown to exert control of oscillations in oxidative metabolism in mitochondria (Peek et al., 2013). Taken together, it is clear that redox status, ROS generation and the cellular response to it, and the core circadian clock are mutually influential. Thus, the occurrence of circadian behavioral and neuronal defects we have reported here could be indicative of disruption of the core molecular clock, possibly due to the characteristic excess ROS of ATP61 disease and resulting redox imbalance.
However, the links between redox control and circadian function are not limited to the core TTFL. An independent daily loop of oxidation and reduction that meets the criteria of a circadian phenomenon (including persistence and entrainability) among the enzymes of the peroxiredoxin H2O2 scavenger family has been uncovered in mice, flies, worms, bacteria, and archaea (Edgar et al., 2012, Rey and Reddy, 2015). These enzymes utilize a highly reactive cysteine to neutralize hydrogen peroxide and consume glutathione to regenerate the reduced form. Over a period of hours, over-oxidized and hyper-oxidized forms accumulate before being proteasomally degraded with a half-life of several more hours, completing the daily redox loop. This cycle is accompanied by daily cycles of NAD(P)H and ATP, and intriguingly, exists even in enucleated cells including mouse and human erythrocytes and in green algae, which do not possess core clock machinery (O’Neill et al., 2011). While it is not immediately clear how disruption of this cycle could result in the behavioral circadian defects that we have reported here, it is reasonable to hypothesize that inability to properly scavenge H2O2 could have deleterious effects, especially in the context of a mitochondrial encephalomyopathy. It is possible that pathological buildup of ROS is affecting both the TTFL and the peroxiredoxin cycles, and that these effects compound each other. Future studies will determine whether the peroxiredoxin cycle is perturbed in ATP61 and whether it has discernable effects on sleep or circadian behavior.
Despite these intriguing links between redox state and circadian cycles, we cannot rule out the possibility that the defects reported here are not the result of a circadian disruption per se, and instead reflect a hyperexcitability phenotype which “overrides” circadian control of neuronal firing and behavior. Such a phenomenon could be the result of non-clock dysfunction, such as ion channel, transporter, or synaptic pathophysiology. This would align with our results from the arousal experiments (Fig. 5), wherein elevated firing frequency in the l-LNv was not sufficient to mute ATP61’s stimulus response as has been reported previously in non-ME flies, and instead appears to demonstrate a general hyper-responsive state. Future studies will be undertaken to establish whether the core TTFL or other circadian cycles are perturbed in ATP61.
Overall, establishing distinct, quantifiable sleep and circadian defects in a model of ME is a significant finding, because sleep disturbance can exacerbate other ME symptoms such as seizures, cognitive decline, and cardiac problems, as well as worsen overall quality of life for patients with these complex diseases. The results presented here establish Drosophila ATP61 as a useful model for elucidating the pathophysiological basis of these sleep and circadian phenotypes.
Acknowledgements
We wish to thank Dr. Stacy Hrizo for technical advice and helpful discussion, Molly Novak and Joseph Ho for project support, Drs. Hansong Ma and Patrick O’Farrell – ATP61 recombinant line, and Dr. Todd Holmes for the PDF::GAL4 driver line. Additionally we acknowledge financial support from American Society for Pharmacology and Experimental Therapeutics (ASPET), the University of Pittsburgh Department of Pharmacology & Chemical Biology and NIH grants R21AG059386, R01GM108073, and R21 NS095614.
Acknowledgments
Declarations of interest
None.
References
- Artiushin G., Sehgal A. The Drosophila circuitry of sleep-wake regulation. Curr. Opin. Neurobiol. 2017;44:243–250. doi: 10.1016/j.conb.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barohn R.J. Recurrent respiratory insufficiency and depressed ventilatory drive complicating mitochondrial myopathies. Neurology. 1990;40(1):103–106. doi: 10.1212/wnl.40.1.103. [DOI] [PubMed] [Google Scholar]
- Berardo A., Musumeci O., Toscano A. Cardiological manifestations of mitochondrial respiratory chain disorders. Acta Myol. 2011;30(1):9–15. [PMC free article] [PubMed] [Google Scholar]
- Canafoglia L. Epileptic phenotypes associated with mitochondrial disorders. Neurology. 2001;56(10):1340–1346. doi: 10.1212/wnl.56.10.1340. [DOI] [PubMed] [Google Scholar]
- Celotto A.M. Mitochondrial encephalomyopathy in Drosophila. J. Neurosci. 2006;26(3):810–820. doi: 10.1523/JNEUROSCI.4162-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celotto A.M. Modes of metabolic compensation during mitochondrial disease using the Drosophila model of ATP6 dysfunction. PLoS One. 2011;6(10):e25823. doi: 10.1371/journal.pone.0025823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee, A., Rouyer, F., Control of sleep-wake cycles in Drosophila. In: Sassone-Corsi, P., Christen, Y., (Eds.). A Time for Metabolism and Hormones, 2016, Cham (CH), pp. 71–78. [PubMed]
- Cichewicz K., Hirsh J. ShinyR-DAM: a program analyzing Drosophila activity, sleep and circadian rhythms. Commun. Biol. 2018;1 doi: 10.1038/s42003-018-0031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cara F., King-Jones K. How clocks and hormones act in concert to control the timing of insect development. Curr. Top. Dev. Biol. 2013;105:1–36. doi: 10.1016/B978-0-12-396968-2.00001-4. [DOI] [PubMed] [Google Scholar]
- DiChiara T.J., Reinhart P.H. Redox modulation of hslo Ca2+-activated K+ channels. J. Neurosci. 1997;17(13):4942–4955. doi: 10.1523/JNEUROSCI.17-13-04942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMauro S. Mitochondrial encephalomyopathies. Prog. Clin. Biol. Res. 1989;306:117–128. [PubMed] [Google Scholar]
- DiMauro S., Schon E.A. Mitochondrial respiratory-chain diseases. N. Engl. J. Med. 2003;348(26):2656–2668. doi: 10.1056/NEJMra022567. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Schon E.A. Mitochondrial disorders in the nervous system. Annu. Rev. Neurosci. 2008;31:91–123. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- Dubowy C., Sehgal A. Circadian Rhythms and Sleep in Drosophila melanogaster. Genetics. 2017;205(4):1373–1397. doi: 10.1534/genetics.115.185157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.S. Peroxiredoxins are conserved markers of circadian rhythms. Nature. 2012;485(7399):459–464. doi: 10.1038/nature11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P. Drosophila CRY is a deep brain circadian photoreceptor. Neuron. 2000;26(2):493–504. doi: 10.1016/s0896-6273(00)81181-2. [DOI] [PubMed] [Google Scholar]
- Fedele G. An electromagnetic field disrupts negative geotaxis in Drosophila via a CRY-dependent pathway. Nat. Commun. 2014;5:4391. doi: 10.1038/ncomms5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogle K.J. CRYPTOCHROME is a blue-light sensor that regulates neuronal firing rate. Science. 2011;331(6023):1409–1413. doi: 10.1126/science.1199702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogle K.J. CRYPTOCHROME-mediated phototransduction by modulation of the potassium ion channel beta-subunit redox sensor. Proc. Natl. Acad. Sci. USA. 2015;112(7):2245–2250. doi: 10.1073/pnas.1416586112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogle K.J. The ATP-sensitive K channel is seizure protective and required for effective dietary therapy in a model of mitochondrial encephalomyopathy. J. Neurogenet. 2016;30(3-4):247–258. doi: 10.1080/01677063.2016.1252765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg-Damberger M.M., Ralls F. Sleep disorders in adults with epilepsy: past, present, and future directions. Curr. Opin. Pulm. Med. 2014;20(6):542–549. doi: 10.1097/MCP.0000000000000101. [DOI] [PubMed] [Google Scholar]
- Grover D. Hydrogen peroxide stimulates activity and alters behavior in Drosophila melanogaster. PLoS One. 2009;4(10):e7580. doi: 10.1371/journal.pone.0007580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri B., Sorbi S. Sleep and cognitive decline: a strong bidirectional relationship. It is time for specific recommendations on routine assessment and the management of sleep disorders in patients with mild cognitive impairment and dementia. Eur. Neurol. 2015;74(1–2):43–48. doi: 10.1159/000434629. [DOI] [PubMed] [Google Scholar]
- Hogl B., Stefani A., Videnovic A. Idiopathic REM sleep behaviour disorder and neurodegeneration - an update. Nat. Rev. Neurol. 2018;14(1):40–55. doi: 10.1038/nrneurol.2017.157. [DOI] [PubMed] [Google Scholar]
- Isaacs J., Binkley F. Glutathione dependent control of protein disulfide-sulfhydryl content by subcellular fractions of hepatic tissue. Biochim. Biophys. Acta. 1977;497(1):192–204. doi: 10.1016/0304-4165(77)90152-0. [DOI] [PubMed] [Google Scholar]
- Jain S.V., Kothare S.V. Sleep and epilepsy. Semin. Pediatr. Neurol. 2015;22(2):86–92. doi: 10.1016/j.spen.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Ju Y.E., Lucey B.P., Holtzman D.M. Sleep and Alzheimer disease pathology--a bidirectional relationship. Nat. Rev. Neurol. 2014;10(2):115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky Y.G., Kosenko E.A., Kondrashova M.N. Analysis of the circadian rhythm in energy metabolism of rat liver. Int. J. Biochem. 1984;16(6):629–639. doi: 10.1016/0020-711x(84)90032-6. [DOI] [PubMed] [Google Scholar]
- Ma H., O’Farrell P.H. Selections that isolate recombinant mitochondrial genomes in animals. Elife. 2015:4. doi: 10.7554/eLife.07247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., O’Farrell P.H. Selfish drive can trump function when animal mitochondrial genomes compete. Nat. Genet. 2016;48(7):798–802. doi: 10.1038/ng.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manni R. Daytime sleepiness in epilepsy patients: evaluation by means of the Epworth sleepiness scale. J. Neurol. 2000;247(9):716–717. doi: 10.1007/s004150070120. [DOI] [PubMed] [Google Scholar]
- Musiek E.S., Xiong D.D., Holtzman D.M. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp. Mol. Med. 2015;47:e148. doi: 10.1038/emm.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedeltcheva A.V., Scheer F.A. Metabolic effects of sleep disruption, links to obesity and diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2014;21(4):293–298. doi: 10.1097/MED.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J.S. Circadian rhythms persist without transcription in a eukaryote. Nature. 2011;469(7331):554–558. doi: 10.1038/nature09654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk N. Reaction mechanism of Drosophila cryptochrome. Proc. Natl. Acad. Sci. USA. 2011;108(2):516–521. doi: 10.1073/pnas.1017093108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino M.J. Modeling mitochondrial encephalomyopathy in Drosophila. Neurobiol. Dis. 2010;40(1):40–45. doi: 10.1016/j.nbd.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisky K.M. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60(4):672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek C.B. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342(6158):1243417. doi: 10.1126/science.1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekovic-Vaughan V. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 2014;28(6):548–560. doi: 10.1101/gad.237081.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel N. Light-dependent interactions between the Drosophila circadian clock factors cryptochrome, jetlag, and timeless. Curr. Biol. 2009;19(3):241–247. doi: 10.1016/j.cub.2008.12.042. [DOI] [PubMed] [Google Scholar]
- Peschel N., Helfrich-Forster C. Setting the clock--by nature: circadian rhythm in the fruitfly Drosophila melanogaster. FEBS Lett. 2011;585(10):1435–1442. doi: 10.1016/j.febslet.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Pincherle A. Excessive fragmentary hypnic myoclonus in a patient affected by a mitochondrial encephalomyopathy. Sleep Med. 2006;7(8):663. doi: 10.1016/j.sleep.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Potdar S., Sheeba V. Lessons from sleeping flies: insights from Drosophila melanogaster on the neuronal circuitry and importance of sleep. J. Neurogenet. 2013;27(1–2):23–42. doi: 10.3109/01677063.2013.791692. [DOI] [PubMed] [Google Scholar]
- Ramezani R.J., Stacpoole P.W. Sleep disorders associated with primary mitochondrial diseases. J. Clin. Sleep Med. 2014;10(11):1233–1239. doi: 10.5664/jcsm.4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A.B., O’Neill J.S. Healthy clocks, healthy body, healthy mind. Trends Cell Biol. 2010;20(1):36–44. doi: 10.1016/j.tcb.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey G. The pentose phosphate pathway regulates the circadian clock. Cell Metab. 2016;24(3):462–473. doi: 10.1016/j.cmet.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey G., Reddy A.B. Interplay between cellular redox oscillations and circadian clocks. Diabetes Obes. Metab. 2015;17(Suppl. 1):S55–S64. doi: 10.1111/dom.12519. [DOI] [PubMed] [Google Scholar]
- Rodrigues N.R. Short-term sleep deprivation with exposure to nocturnal light alters mitochondrial bioenergetics in Drosophila. Free Radic. Biol. Med. 2018;120:395–406. doi: 10.1016/j.freeradbiomed.2018.04.549. [DOI] [PubMed] [Google Scholar]
- Rutter J. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293(5529):510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- Sakaue S. A case of diabetes, deafness, cardiomyopathy, and central sleep apnea: novel mitochondrial DNA polymorphisms. Tohoku J. Exp. Med. 2002;196(3):203–211. doi: 10.1620/tjem.196.203. [DOI] [PubMed] [Google Scholar]
- Sembrano E. Polysomnographic findings in a patient with the mitochondrial encephalomyopathy NARP. Neurology. 1997;49(6):1714–1717. doi: 10.1212/wnl.49.6.1714. [DOI] [PubMed] [Google Scholar]
- Shaw P.J. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287(5459):1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- Sheeba V. Does the difference in the timing of eclosion of the fruit fly Drosophila melanogaster reflect differences in the circadian organization? Chronobiol. Int. 2001;18(4):601–612. doi: 10.1081/cbi-100106075. [DOI] [PubMed] [Google Scholar]
- Sheeba V. Circadian- and light-dependent regulation of resting membrane potential and spontaneous action potential firing of Drosophila circadian pacemaker neurons. J. Neurophysiol. 2008;99(2):976–988. doi: 10.1152/jn.00930.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeba V. Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr. Biol. 2008;18(20):1537–1545. doi: 10.1016/j.cub.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y. Sleep-wake dysrhythm in mitochondrial diabetes mellitus. Diabetes Res. Clin. Pract. 1997;35(1):61–62. doi: 10.1016/s0168-8227(96)01358-7. [DOI] [PubMed] [Google Scholar]
- Tan A.L., Goy R. Anaesthetic management of a patient with Leigh’s syndrome with central hypoventilation and obstructive sleep apnoea. Singap. Med. J. 2013;54(12):e250–e253. doi: 10.11622/smedj.2013252. [DOI] [PubMed] [Google Scholar]
- Tataroglu O., Emery P. The molecular ticks of the Drosophila circadian clock. Curr. Opin. Insect Sci. 2015;7:51–57. doi: 10.1016/j.cois.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipparaju S.M. NADPH binding to beta-subunit regulates inactivation of voltage-gated K(+) channels. Biochem. Biophys. Res. Commun. 2007;359(2):269–276. doi: 10.1016/j.bbrc.2007.05.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita J., Ban G., Kume K. Genes and neural circuits for sleep of the fruit fly. Neurosci. Res. 2017;118:82–91. doi: 10.1016/j.neures.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Vaidya A.T. Flavin reduction activates Drosophila cryptochrome. Proc. Natl. Acad. Sci. USA. 2013;110(51):20455–20460. doi: 10.1073/pnas.1313336110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnovic A. Disturbances of sleep and alertness in Parkinson’s disease. Curr. Neurol. Neurosci. Rep. 2018;18(6):29. doi: 10.1007/s11910-018-0838-2. [DOI] [PubMed] [Google Scholar]
- Wang J.W., Wu C.F. In vivo functional role of the Drosophila hyperkinetic beta subunit in gating and inactivation of Shaker K+ channels. Biophys. J. 1996;71(6):3167–3176. doi: 10.1016/S0006-3495(96)79510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.A. Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons. Science. 2012;337(6096):839–842. doi: 10.1126/science.1222826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J. Modulation of voltage-dependent Shaker family potassium channels by an aldo-keto reductase. J. Biol. Chem. 2006;281(22):15194–15200. doi: 10.1074/jbc.M513809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W.D., Wu C.F. Auxiliary Hyperkinetic beta subunit of K+ channels: regulation of firing properties and K+ currents in Drosophila neurons. J. Neurophysiol. 1999;81(5):2472–2484. doi: 10.1152/jn.1999.81.5.2472. [DOI] [PubMed] [Google Scholar]
- Yasaki E. Characteristics of breathing abnormality in Leigh and its overlap syndromes. Neuropediatrics. 2001;32(6):299–306. doi: 10.1055/s-2001-20405. [DOI] [PubMed] [Google Scholar]
- Yoshii T. Cryptochrome-dependent and -independent circadian entrainment circuits in Drosophila. J. Neurosci. 2015;35(15):6131–6141. doi: 10.1523/JNEUROSCI.0070-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii T., Ahmad M., Helfrich-Forster C. Cryptochrome mediates light-dependent magnetosensitivity of Drosophila’s circadian clock. PLoS Biol. 2009;7(4):e1000086. doi: 10.1371/journal.pbio.1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]