Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disorder among children in the developed world and can progress to cirrhosis, hepatocellular carcinoma, and liver failure. No evidence-based dietary guidelines exist on the most effective diet prescription to treat NAFLD.
Objective
To compare the effect of a carbohydrate (CHO)-restricted diet vs fat-restricted diet, the current standard of care, on changes in hepatic fat infiltration, body composition, and metabolic health over an 8-week period among overweight and obese children diagnosed with NAFLD.
Methods
In this two-arm, parallel design randomized controlled trial (RCT), 40 participants aged 9 to 18 years were randomized to a CHO restricted diet (<25:>50:25% daily calories from CHO: fat: protein) or control, fat restricted diet (55:20:25% daily calories from CHO: fat: protein). This family-based diet intervention included: (1) a 2-week supply of groceries to feed a four-person household specific to the assigned diet; and (2) extensive education on diet implementation through biweekly, diet-specific group and individualized counseling sessions with participants and one parent or guardian led by a registered dietitian (RD). The primary outcome measure of this study was hepatic lipid, measured using magnetic resonance spectroscopy (MRS). Secondary outcomes included liver transaminases; markers of inflammation (hsCRP, IL-6, TNF-α); body composition; visceral adipose tissue; and insulin resistance. All testing was conducted at baseline and week 8; hepatic transaminases were also measured at weeks 2 and 4. This RCT is registered with ClinicalTrials.gov (ID: NCT02787668).
Keywords: diet intervention, carbohydrate restriction, non-alcoholic fatty liver disease, childhood obesity, adolescent
1. Introduction
The prevalence of obesity among children has reached 17% worldwide and affects nearly 13 million children in the United States (1). In parallel with the rate of childhood obesity, the occurrence of non-alcoholic fatty liver disease (NAFLD) is also increasing in this age group. NAFLD is the most common cause of pediatric chronic liver disease, with prevalence estimates reaching 40% in children with obesity (2–4).
NAFLD refers to a spectrum of liver diseases ranging from simple fat infiltration to non-alcoholic steatohepatitis (NASH), a medical condition involving extensive hepatic inflammation, cellular injury, and potentially fibrosis (5). If left untreated, NASH can progress to cirrhosis, hepatocellular carcinoma, and end-stage liver disease (6–7). NAFLD in children, as in adults, is associated with obesity and metabolic syndrome and involves complex interactions between defects in glucose and lipid metabolism and inflammation in multiple organ systems (5, 8 –11). While primary pharmacotherapies target the metabolic disorders associated with fatty liver, no treatment currently exists to directly reverse hepatic fat infiltration.
Observation of NAFLD is becoming a common occurrence among pediatricians; thus, there is urgent need for evidenced-based guidelines on how to treat NAFLD in children and adolescents. Because obesity increases the risk for the progression of fatty liver, weight loss through caloric restriction and exercise is typically the recommended therapy for children (12). However, the dietary recommendation of calorie restriction alone may not be optimal in a pediatric population for multiple reasons including changes in hormonal milieu, growth velocity, lean mass, and bone mineral density that occur with significant weight loss (13–17). Indeed, adults with significant weight loss through caloric restriction have been shown to develop persistent metabolic adaptations over time causing weight regain (13, 18–19). Moreover, RCT’s examining the effects of lifestyle interventions have shown limited success potentially due to the difficulty in adhering to long-term physical activity and caloric restrictive regimens (20).
There is evidence that suggests a change in diet composition alone can reduce hepatic fat infiltration. Studies in rodent models and humans have shown that reducing intake of CHO sources such as added sugars, high glycemic grains, and fructose may be an effective approach to reverse fatty liver by significantly reducing insulin resistance, inflammation, and, primarily, hepatic de novo lipogenesis (DNL) (21–23). Limiting hepatic DNL, a process that converts dietary CHO into triglyceride in the postprandial state, can reduce the accrual of hepatic lipids and simultaneously enhance their disposal via mitochondrial β-oxidation (5, 24–25). A 2-week randomized clinical trial in adults found that a CHO restricted diet (<20 g/day) compared to a reduced calorie, low-fat diet resulted in similar weight loss but greater reduction in hepatic fat (−55% vs. −28%, p < .001) (26). This suggests a clear metabolic advantage of CHO-restriction, independent of overall weight loss, in adults with NAFLD. To date, studies have not tested CHO restriction on changes in hepatic fat among children with NAFLD.
The primary purpose of this study is to evaluate the effects of a CHO vs. fat restricted diet on changes in hepatic lipid, aminotransferases, markers of inflammation, insulin resistance, body composition, and visceral adiposity over 8 weeks among overweight/obese children with NAFLD.
2. Methods
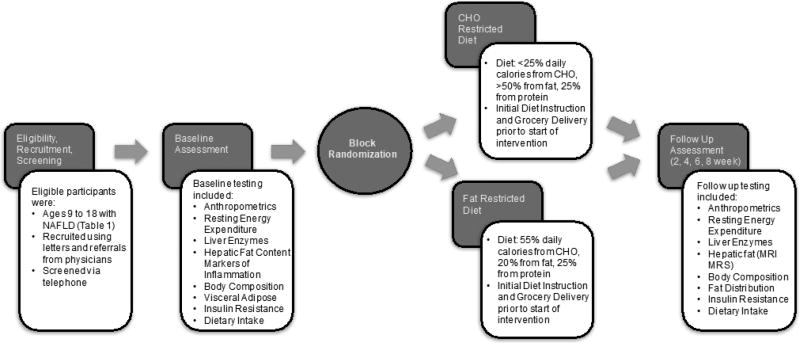
The Consolidated Standards of Reporting Trials (CONSORT) guidelines were referenced to provide comprehensive and clear information about the methodology of this randomized controlled trial (RCT) (27). Key components of the design and delivery of this diet intervention study are shown in Figure 1.
Figure 1.
Flow Chart of Diet Intervention Study
2.1 Study Design
This two-arm, parallel design RCT was designed to compare the effectiveness of a CHO restricted diet (CHO <25%; fat >50%, protein 25%) vs a fat restricted diet (CHO 55%; fat 20%; protein 25%) on improvements in hepatic lipid content among children ages 9 to 18 with NAFLD (n = 40) over an 8-week period. In this family-based diet intervention, each participant and at least one parent or guardian were randomized to one of two diets and received (1) a two week supply of groceries for a four-person household specific to the assigned diet and (2) extensive education on diet implementation through weekly, diet specific group counseling sessions and biweekly individualized dietary counseling sessions led by a registered dietitian (RD). Hepatic lipid content was measured at baseline and week 8 using magnetic resonance imaging (MRI) and MRS. Secondary outcomes include measurement of changes in transaminase levels from a blood draw, body composition via dual-energy x-ray absorptiometry (DXA), visceral adiposity via MRI, insulin resistance (via HOMA-IR), and markers of inflammation (hs-CRP, TNF-a, and IL-6) via fasting blood draw. Average dietary intake and adherence were assessed using 4-day food records at weeks 0, 2, and 4, and analyzed using the Nutrition Data System for Research (NDSR). This RCT is registered with ClinicalTrials.gov (ID: NCT02787668).
2.2 Participants and Eligibility Criteria
Recruitment targeted participants who lived within 200 miles of the clinical testing facility in Birmingham, AL. Eligible participants were those between the ages of 9–18 years at the time of initial screening, English speaking, overweight or obese, and diagnosed with NAFLD through biochemical or radiological testing (Table 1). Exclusion criteria included all other liver diseases, pregnancy, alcohol consumption, history of parenteral nutrition, history of bariatric surgery, and use of medications known to induce steatosis, elevations in liver enzymes, or affect body weight and carbohydrate metabolism. Participants were also excluded if they and their parents/guardians were unwilling or unable to give informed consent, accept random assignment, attend dietary counseling sessions, adhere to treatment prescription, or complete study measures.
Table 1.
Inclusion and Exclusion Criteria for Participants
| Inclusion Criteria |
| Between the ages of 9–18 years at the time of initial screening |
| Overweight or obese (BMI ≥ 85th percentile) |
| Diagnosis of NAFLD through elevations in serum transaminase (AST or ALT) 1.5 times higher than the reference range or radiological findings (ultrasound, computed tomography, or biopsy) consistent with NAFLD |
| Fluency in English (participants and parents/guardians) |
| Exclusion Criteria |
| Pregnant |
| Alcohol consumption |
| History of parenteral nutrition |
| Hepatic virus infections (HCV RNA–polymerase chain reaction negative; hepatitis A, B, C, D, E, and G; cytomegalovirus; and Epstein-Barr virus) |
| Use of medications known to induce steatosis (e.g. valproate, amiodarone, or prednisone), elevation of liver enzymes, or affect body weight and carbohydrate metabolism (within the last 6 months) |
| Autoimmune liver disease, metabolic liver disease, and Wilson’s disease |
| Genetic conditions (e.g. glycogen storage disorder) leading to hepatic steatosis |
| History of Bariatric Surgery |
| Participants and parents/guardians unwilling or unable to give informed consent, accept random assignment, attend dietary counseling sessions, adhere to treatment prescription, or complete study measures |
| Inability to speak and comprehend English (participants and parents/guardians) |
| Currently receiving intense lifestyle modification treatment |
Abbreviations: BMI- Body Mass Index; NAFLD- Non-Alcoholic Fatty Liver Disease; AST- Aspartate Aminotransferase; ALT- Alanine Aminotransferase; HCV- Hepatitis C Virus; RNA- ribonucleic acid
2.3 Recruitment Strategies
Several strategies were employed to recruit participants, including recruitment letters, flyers, and referrals from clinicians. The study team first identified potential participants by searching the Children’s of Alabama electronic medical record (EMR) for patients with ICD-9 billing codes indicating NAFLD (571.8) or elevations in liver transaminases (790.4). Each patient chart with a billing code of interest was then manually reviewed to determine eligibility. All individuals meeting eligibility criteria were mailed a letter with information about the study and how to enroll.
Participants were also recruited from several Children’s of Alabama clinics, including the primary care, adolescent, hepatology, endocrinology, and weight management clinics through flyers and clinician referrals. Patients identified by clinicians or study staff as eligible for the study were given the option to contact the study team directly or have a member of the study team contact them at their convenience.
Other recruitment strategies employed included advertisements in the University of Alabama Birmingham (UAB) Reporter, a virtual bulletin board that advertises various clinical trials occurring at UAB, and television. Recruitment flyers were also placed in various locations around the UAB campus and the Jefferson County Department of Public Health.
2.4 Screening
All interested individuals were screened via telephone using a telephone interview script. This script provided general information about the study, including purpose, eligibility criteria, the diet intervention, and all tests and procedures.
All eligible individuals were then scheduled for an in-person interview. During this interview, potential participants and their parents/guardians received additional information about the study, provided informed consent and assent, and had the opportunity to view clinic and testing facilities. Unless the interested participant received a recruitment letter or was referred to the study by a clinician, they were required to provide medical documentation confirming NAFLD. Eligible individuals and their parents consenting to all components of the study, including the diet intervention and all testing, were enrolled into the trial and completed baseline testing.
2.5 Randomization
Participants were randomly assigned to either the fat restricted diet (control) or CHO restricted diet. A blocked randomization scheme was used to ensure an allocation ratio of 1:1. The random allocation sequence was created using a random number generator by PROC PLAN (SAS Version 9.3). Participants were notified of their diet assignment at their initial diet instruction. Because this was a diet intervention study, it was not possible for participants or study personnel to be blinded to group assignment. However, to minimize potential bias, study personnel involved in data analysis were blinded to randomized assignment.
2.6 Diet Intervention
This family-based diet intervention was adapted from the UAB’s medically supervised “EatRight” clinic, a lifestyle-oriented weight management program. Although this study focused solely on outcomes of the child and adolescent participants, parents and guardians were strongly encouraged to adopt the prescribed diet for the entire family, as parental and family involvement can predict successful adherence to diet (28–30).
The participant-parent dyad began the 8-week diet intervention after initial diet instruction. This intervention consisted of bi-weekly, diet specific, individual and group counseling sessions led by the study’s registered dietitian (RD). Individual meetings with the RD focused on diet instruction, meal planning, goal setting, and a review of nutritional resources. Participants, with the help of their parents, were instructed how to record their dietary intake every two weeks using food journals, and to plan their intake using handouts provided by the study team. These handouts provide lists of common foods broken down by servings per macronutrient.
Dietary adherence was assessed using 4-day food records, which were reviewed with participants and parents at the start of each individual counseling session. To encourage dietary adherence, the RD employed behavioral strategies effective in childhood weight management, such as goal setting, review of food journals, and counseling modification based on the participant’s readiness to change (28–30). Diet specific group classes included topics such as label reading, meal planning, healthy substitutions, mindful eating, and other relevant topics important for dietary adherence.
2.6.1 Initial Diet Instruction
Following randomization, participants and their parents/guardians attended an initial diet instruction with the RD. During this meeting, the dietitian educated participants on the amount of macronutrients to be consumed each day using food lists. These lists outlined permissible food items organized by food group and macronutrient content, and included information on portion sizes. Participants were also provided with a 14-day sample menu and corresponding recipes.
2.6.2 Food Provision
Participants received groceries specific to their 14-day meal plan and recipes at the initial diet instruction meeting. A grocery delivery service was used to minimize the initial burden of shopping on the family and study personnel coordinated with participants to choose a time for grocery pick up or delivery. Each household received sufficient groceries to feed a four-person household for two weeks.
2.6.5 Diet Prescriptions
The features of each diet are detailed below with differences highlighted in Table 2.
Table 2.
Example Day of CHO- and Fat-Restricted Diet Meal Plan
| CHO RESTRICTED | FAT RESTRICTED | |
|---|---|---|
| BREAKFAST |
|
|
| LUNCH |
|
|
| DINNER |
|
|
| SNACK |
|
|
Composition based on 1800 calorie meal plan
Carbohydrate Restricted Diet
The CHO-restricted diet was designed to minimize intake of refined CHO sources such as added sugars, high glycemic grains, and fructose and provided ≤25% energy from CHO, 25% energy from protein, and ≥50% energy from fat. CHO sources were primarily derived from leafy greens and non-starchy vegetables. Additional CHO sources included in the diet prescription were nuts and nut butters, unsweetened yogurt, and low-glycemic fruits such as apples and berries. Limited amounts of legumes, root vegetables, and “treats” like dark chocolate were permitted. Protein sources included meat, fish, eggs, poultry, and whey protein if appropriate. Saturated fat intake was limited to <10% total energy/day. Other permitted fat sources included olive oil, walnut oil, and other sources of poly- and monounsaturated fatty acids. A multi-vitamin was also encouraged to ensure all micronutrient requirements were met.
Fat Restricted Diet
The standard of care in the dietary management of children with NAFLD is a diet low in fat comprised of foods with low energy density, which served as the basis for the control diet group (31–32). The FRD was based on the USDA MyPlate Daily Food Plan for teenagers and consisted of 55:25:20% energy from carbohydrates: protein: fat (33). Participants were discouraged from consuming foods high in fat such as fried foods, butter, cream cheese, and bacon, while foods such as fruits, vegetables (starchy and non-starchy), whole grains, poultry, lean meats, and low-fat dairy products were permitted. Similar to the CHO restricted group, participants were encouraged to supplement meals with a multivitamin.
2.7 Outcome Measures
A timetable organizing all outcome measures and time points of data collection is provided in
2.7.1 Resting Energy Expenditure (REE)
REE was used to determine participants’ caloric requirements, which in turn was used to estimate intake necessary for a weight maintaining diet. After an overnight fast, participants were tested using in an indirect calorimeter (Vmax ENCORE 29N Systems, SensorMedics Corporation, Yorba Linda, CA) at the Nutrition Obesity Research Center (NORC) Metabolism Core Facility for approximately 30 minutes. A clear, plastic, canopy hood was placed over the head and shoulders, and expired air was collected for 20 min after a 10-min equilibration period. Oxygen consumption and carbon dioxide production were measured continuously during this time to estimate 24-hour REE.
2.7.2 Anthropometric Measurements and Vital Signs
Height, weight, blood pressure and pulse measurements were taken at baseline, and biweekly at diet counseling sessions using a standardized stadiometer, calibrated electronic scale, and automated blood pressure monitor. Height was measured to the nearest 0.25 inch and weight to the nearest 0.1 pound. Using this information, age, and gender of the participant, BMI percentile and z score were calculated (34).
2.7.3 Blood Draw and Laboratory Analyses
Ten milliliters of blood were collected by a phlebotomist at weeks 0, 2, 4, and 8 to measure fasting insulin, glucose, markers of inflammation (hsCRP, IL-6, TNF-α), lipid profile (total cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL), and triglyceride), aminotransferases (ALT, AST) and gamma glutamyl transferase (GGT). Blood draws at baseline and week 8 were conducted following a 10-hour fast of all food and beverages except water. Samples were centrifuged, aliquotted, and sera stored at −85°C; concentrations of serum-derived analytes were assayed at the Diabetes Research Center (DRC) Core Laboratory and the UAB Outreach Laboratory. Insulin Resistance (HOMA-IR) was calculated using measurements for fasting glucose and fasting insulin (35–36). Subjects were instructed to avoid strenuous physical activity the day prior to testing, and to avoid all physical activity on the morning of testing.
2.7.4 Hepatic Lipid (MRI and MRS)
At baseline and week 8, Magnetic Resonance Spectroscopy (MRS) and 3-point M Dixon MRI were performed to assess liver fat. Both MRS and 3-point M Dixon utilize chemical-shift principles to estimate resonant frequencies of water and methylene groups of triglyceride fatty acid chains. MRS data acquisition involved placement of a single large voxel (≈1–8 cm3) in the liver. Post-processing and quantification involved phase correction signal fitting of the peaks within the acquired spectra, and integration to find the area under each spectral peak of interest. Water – and fat-suppressed images obtained from 3-point M Dixon technique were used to assess hepatic lipid percentage by identifying 3 regions of interest (ROI) free of artifacts and vessels. The signal intesity (SI) of the 3 ROIs, which is based on tissue densities, will be averaged and used to calculate the hepatic fat fraction (fat SI/fat SI+water SI).
2.7.5 Body Composition
Body composition was estimated using dual-energy X-ray absorptiometry (DXA) in the NORC/DRC Core Facility. Participants were asked to wear light clothing and lie flat on their backs with arms by their sides during the DXA scan (iDXA; GE Healthcare Lunar, Madison, WI). Total and regional (within the trunk and leg) fat, bone, and lean mass was estimated at baseline and week 8. Girls of childbearing age were required to complete a urine pregnancy test prior to DXA scans. Any female with a positive pregnancy test result were excluded from participating in the study.
2.7.6 Abdominal Fat Distribution
Intra-abdominal adipose tissue (IAAT) and subcutaneous abdominal adipose tissue (SAAT) were measured using magnetic resonance imaging (MRI) using Slice-O-Matic software (version 4.3, Tomovision, Montreal, Canada). IAAT and SAAT were computed from segmented regions of interest on non-fat suppressed axial images of the upper abdominal visceral cavity at baseline and 8 weeks.
2.7.7 Dietary Intake & Adherence
Dietary intake and adherence were measured and analyzed using 4-day food records and the Nutrition Data System for Research (NDSR) Software Version 2012 (37). Participants were asked to complete 4-day food diaries (three weekdays, one weekend day) at weeks 0, 2, and 4. These records were distributed at baseline testing, collected at initial diet instruction to determine typical dietary intake, and reviewed at every biweekly visit with the RD. Detailed instructions of the food records were provided to participants and their parents/guardians at baseline testing. Food records were inputted into NDSR Software (Version 2012, Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN) in order to quantify average macronutrient intake. Typical intake on a CHO restricted vs fat restricted diet is shown in Table 4.
Table 4.
Macronutrient Composition (grams) by Diet
| Total CHO |
Added Sugar |
Fructose | Total Fat |
Total Fiber (g) |
SFA | Omega 3 | Protein | |
|---|---|---|---|---|---|---|---|---|
| Fat Restriction | 218.9 | 23.2 | 24.2 | 62.7 | 31.5 | 10.1 | 1.9 | 119.9 |
| CHO Restriction | 55.0 | 5.6 | 6.1 | 141.8 | 18.3 | 23.2 | 5.6 | 112.1 |
Composition based on 1800 calorie meal plan
2.8 Adverse Event Monitoring
Adverse events were routinely queried for at each clinic visit through discussions with both participants and their parents/guardians. The study physician, who provided all medical supervision for the intervention, was on call in the case of an adverse event during testing. The adverse event protocol included immediate reporting of the event to the UAB Institutional Review Board (IRB) and review by the investigative team. If any of these individuals and/or the IRB identified any adverse events related to the study protocol, all study testing would be suspended and the protocol would be amended accordingly.
2.9 Sample Size Calculations
The sample size was calculated to detect significant differences in hepatic lipid content. Based on published data by Browning et al, we expected a similar −12.0% absolute reduction in hepatic triglyceride content in the treatment group (low CHO) compared to −5.0% in the control group (low fat) (26). Assuming a standard deviation of 7% and alpha of 0.05, a sample size of 16 in each group will have 80% power to detect a 7% difference in hepatic lipid content between CHO-restricted and fat-restricted diet intervention groups. Allowing for 20% attrition, 20 participants per diet group (total n=40) will be sufficient to detect a statistically significant change in hepatic triglyceride content.
2.10 Statistical Analyses
Descriptive statistics such as means, standard deviations, and frequency counts will be used to characterize the study population. To test the hypothesis that CHO compared to fat restriction will induce reductions in hepatic lipid, comparisons between diet groups will be investigated using analysis of covariance (ANCOVA) with follow-up % hepatic lipid content as the dependent variable and diet group as the independent variable adjusted for baseline % hepatic lipid content. Further adjustments will be made for other relevant confounders (e.g., age, gender, ethnicity, change in body weight). Spearman correlation coefficients will be used to determine associations between levels of change in hepatic fat, transaminases, insulin resistance, markers of inflammation, and body composition among all participants combined and by intervention group. All tests will use a .05 alpha level of significance and will be conducted using SAS Version 9.4.
3. Discussion
In recent years, NAFLD has become the most common liver disorder in children. Lipid accumulation in the liver may contribute to hepatic insulin resistance and other metabolic abnormalities and, over time, can progress to cirrhosis, cancer, and liver failure if not appropriately managed. Given the increased risk of morbidity and all-cause mortality associated with this condition, therapies are urgently needed to reverse hepatic steatosis.
Dietary CHO restriction holds promise as a treatment for pediatric NAFLD. Diets high in CHO stimulate the post-prandial release of insulin, which increases uptake of glucose into the liver. In an energy excess state, this glucose is converted into fatty acids via hepatic DNL. A byproduct of fatty acid synthesis, malonyl co-A, further promotes fat accumulation in the liver by inhibiting transport of fatty acid into the mitochondria for ATP production via β-oxidation (5). By restricting sources of glucose in the diet, a CHO restricted diet may effectively reduce hepatic steatosis directly by decreasing the substrate for lipogenesis and indirectly by reducing concentrations of malonyl CoA.
This study will be the first to prospectively test the effects of CHO restriction on changes in hepatic fat infiltration in children with NAFLD using a family-based intervention design. Some epidemiological studies have investigated the link between sugar consumption, glycemic load, and pediatric NAFLD. Similar to glucose, fructose is a simple sugar that serves as the primary sweetener in corn syrup and sugar sweetened beverages. In a cross-sectional study of 592 Australian adolescents, researchers found a strong relationship between fructose consumption and NAFLD in obese children (38). Indeed, in rodent and human studies, fructose has been strongly linked to hepatic steatosis, inflammation and fibrosis through mechanisms involving hepatic DNL (39–41). There is also research investigating low glycemic load diets designed to limit post prandial serum glucose and insulin concentrations. The effects of a low vs high glycemic load diet on change in liver fat was examined in 16 children with MRS-confirmed NAFLD (42). Results of this diet intervention showed no significant differences between diet groups in liver fat, visceral adipose tissue, BMI, ALT, or insulin resistance over 6 months. Children on the low glycemic load diet were provided 40% of daily total calories from CHO, whereas our study limits CHO to < 25% of total calories per day. It may be critical to not only consume low glycemic CHO sources in the diet, but also limit the total amount of energy from CHO below 40% per day to improve hepatic steatosis and other metabolic outcomes in this population. It is also possible that lack of adherence to the low glycemic load diet or a relatively small sample size affected the ability to detect significant between-group differences in change in primary outcomes (43).
This study will be among the first to rigorously test the independent effects of diet composition without caloric restriction on hepatic fat content in children with NAFLD. While inevitable in most diet studies, weight loss can confound the ability to observe differences of macronutrient manipulation on total fat loss and distribution. Moreover, intentional weight loss has been shown to affect growth, puberty, lean mass, bone mineral density in growing children. To address the confounding effects of weight loss, our study utilizes indirect calorimetry to precisely estimate total daily caloric requirements of each participant and tailors each diet to be weight maintaining. This study is also among the first to use MRS imaging to quantify changes in hepatic fat in children on CHO and fat restriction. Although this technology is not currently used in clinical practice, it holds promise as a more accurate and less invasive way of diagnosing and managing NAFLD than standard techniques (e.g. ultrasound and liver biopsy) (44). Additional strengths of this study include attention to a condition with high clinical and public health significance, randomized controlled study design, family-based intervention, and provision of meals to assist with dietary adherence.
To date, there are no evidenced-based dietary guidelines for the standard of care in children and adolescents with NAFLD. The results of this RCT will provide insight into the effects of a reduced CHO dietary pattern on hepatic lipid changes, as well as on insulin resistance and inflammation in adolescents with obesity at high risk of developing type 2 diabetes, metabolic syndrome, and severe liver disease.
Table 3.
Outcome Measures
| Measure | Baseline | Week 2 | Week 4 | Week 6 | Week 8 |
|---|---|---|---|---|---|
| Resting Energy Expenditure | X | X | |||
| Anthropometries | X | X | X | X | X |
| Liver Enzymes | X | X | X | X | |
| Alanine Am inotrasnferase (AST) | |||||
| Aspartate Am inotransferase (ALT) | |||||
| Gamma Glutamyltransferase(GGT) | |||||
| Insulin Resistance | X | X | |||
| Markers of Inflammation (hsCRP, IL-6, TNF-α) | X | X | |||
| Lipid Profile (TC, LDL, HDL, Triglyceride) | X | X | |||
| Hepatic Fat (MRI and MRS) | X | X | |||
| Body Composition (DXA) | X | X | |||
| Visceral Fat (MRI) | X | X | |||
| Dietary Intake and Adherence | X | X | X | X |
Acknowledgments
Funding
Research reported in this publication was supported by the Thrasher Research Fund, the National Institute of Diabetes and Digestive and Kidney Diseases (DK079626), the National Center for Advancing Translational Sciences (1TL1TR001418-01), and the National Institute of General Medical Sciences (NIH T32GM008361). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Thrasher Research Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014 Feb 26;311(8):806–14. doi: 10.1001/jama.2014.732. PubMed PMID: 24570244; PubMed Central PMCID: PMC4770258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shneider BL, González-Peralta R, Roberts EA. Controversies in the management of pediatric liver disease: Hepatitis B, C and NAFLD: Summary of a single topic conference. Hepatology. 2006 Nov;44(5):1344–54. doi: 10.1002/hep.21373. PubMed PMID: 17058223. [DOI] [PubMed] [Google Scholar]
- 3.Nobili V, Alkhouri N, Alisi A, Della Corte C, Fitzpatrick E, Raponi M, Dhawan A. Nonalcoholic fatty liver disease: a challenge for pediatricians. JAMA Pediatr. 2015 Feb;169(2):170–6. doi: 10.1001/jamapediatrics.2014.2702. Review. PubMed PMID: 25506780. [DOI] [PubMed] [Google Scholar]
- 4.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006 Oct;118(4):1388–93. doi: 10.1542/peds.2006-1212. PubMed PMID: 17015527. [DOI] [PubMed] [Google Scholar]
- 5.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004 Jul;114(2):147–52. doi: 10.1172/JCI22422. Review. PubMed PMID: 15254578; PubMed Central PMCID: PMC449757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, Clark JM. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011 Nov 18;343:d6891. doi: 10.1136/bmj.d6891. PubMed PMID: 22102439; PubMed Central PMCID: PMC3220620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009 Nov;58(11):1538–44. doi: 10.1136/gut.2008.171280. Epub 2009 Jul 21. PubMed PMID: 19625277; PubMed Central PMCID: PMC2792743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manco M, Marcellini M, Devito R, Comparcola D, Sartorelli MR, Nobili V. Metabolic syndrome and liver histology in paediatric non-alcoholic steatohepatitis. Int J Obes (Lond) 2008 Feb;32(2):381–7. doi: 10.1038/sj.ijo.0803711. Epub 2007 Dec 18. PubMed PMID: 18087267. [DOI] [PubMed] [Google Scholar]
- 9.Schwimmer JB, Deutsch R, Rauch JB, Behling C, Newbury R, Lavine JE. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr. 2003 Oct;143(4):500–5. doi: 10.1067/S0022-3476(03)00325-1. PubMed PMID: 14571229. [DOI] [PubMed] [Google Scholar]
- 10.Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation. 2008 Jul 15;118(3):277–83. doi: 10.1161/CIRCULATIONAHA.107.739920. Epub 2008 Jun 30. PubMed PMID: 18591439; PubMed Central PMCID: PMC2996820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lomonaco R, Sunny NE, Bril F, Cusi K. Nonalcoholic fatty liver disease: current issues and novel treatment approaches. Drugs. 2013 Jan;73(1):1–14. doi: 10.1007/s40265-012-0004-0. Review. PubMed PMID: 23329465. [DOI] [PubMed] [Google Scholar]
- 12.Yang M, Gong S, Ye SQ, Lyman B, Geng L, Chen P, Li DY. Non-alcoholic fatty liver disease in children: focus on nutritional interventions. Nutrients. 2014 Oct 28;6(11):4691–705. doi: 10.3390/nu6114691. Review. PubMed PMID: 25353664; PubMed Central PMCID: PMC4245557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011 Oct 27;365(17):1597–604. doi: 10.1056/NEJMoa1105816. PubMed PMID: 22029981. [DOI] [PubMed] [Google Scholar]
- 14.Amador M, Ramos LT, Moroño M, Hermelo MP. Growth rate reduction during energy restriction in obese adolescents. Exp Clin Endocrinol. 1990 Sep;96(1):73–82. doi: 10.1055/s-0029-1210991. PubMed PMID: 2279528. [DOI] [PubMed] [Google Scholar]
- 15.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Ehsani AA, Holloszy JO Washington University School of Medicine CALERIE Group. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol (1985) 2007 Feb;102(2):634–40. doi: 10.1152/japplphysiol.00853.2006. PubMed PMID: 17095635; PubMed Central PMCID: PMC4376253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen LB, Quaade F, Sørensen OH. Bone loss accompanying voluntary weight loss in obese humans. J Bone Miner Res. 1994 Apr;9(4):459–63. doi: 10.1002/jbmr.5650090404. PubMed PMID: 8030433. [DOI] [PubMed] [Google Scholar]
- 17.Compston JE, Laskey MA, Croucher PI, Coxon A, Kreitzman S. Effect of diet-induced weight loss on total body bone mass. Clin Sci (Lond) 1992 Apr;82(4):429–32. doi: 10.1042/cs0820429. PubMed PMID: 1315653. [DOI] [PubMed] [Google Scholar]
- 18.Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, Chen KY, Skarulis MC, Walter M, Walter PJ, Hall KD. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity (Silver Spring) 2016 Aug;24(8):1612–9. doi: 10.1002/oby.21538. Epub 2016 May 2. PubMed PMID: 27136388; PubMed Central PMCID: PMC4989512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blomain ES, Dirhan DA, Valentino MA, Kim GW, Waldman SA. Mechanisms of Weight Regain following Weight Loss. ISRN Obesity. 2013 Apr;2013:210524. doi: 10.1155/2013/210524. Review. PubMed PMCID: PMC3901982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraschnewski JL, Boan J, Esposito J, Sherwood NE, Lehman EB, Kephart DK, Sciamanna CN. Long-term weight loss maintenance in the United States. Int J Obes (Lond) 2010 Nov;34(11):1644–54. doi: 10.1038/ijo.2010.94. Epub 2010 May 18. PubMed PMID: 20479763; PubMed Central PMCID: PMC3671378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen-Urstad AP, Semenkovich CF. Fatty acid synthase and liver triglyceride metabolism: housekeeper or messenger? Biochim Biophys Acta. 2012 May;1821(5):747–53. doi: 10.1016/j.bbalip.2011.09.017. Epub 2011 Oct 8. Review. PubMed PMID: 22009142; PubMed Central PMCID: PMC3288544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003 Nov;29(5):478–85. doi: 10.1016/s1262-3636(07)70061-7. PubMed PMID: 14631324. [DOI] [PubMed] [Google Scholar]
- 23.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005 May;115(5):1343–51. doi: 10.1172/JCI23621. PubMed PMID: 15864352; PubMed Central PMCID: PMC1087172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. Review. PubMed PMID: 6157353. [DOI] [PubMed] [Google Scholar]
- 25.Ide T, Nakazawa T, Mochizuki T, Murakami K. Tissue-specific actions of antidiabetic thiazolidinediones on the reduced fatty acid oxidation in skeletal muscle and liver of Zucker diabetic fatty rats. Metabolism. 2000 Apr;49(4):521–5. doi: 10.1016/s0026-0495(00)80019-0. PubMed PMID: 10778879. [DOI] [PubMed] [Google Scholar]
- 26.Browning JD, Baker JA, Rogers T, Davis J, Satapati S, Burgess SC. Short-term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. Am J Clin Nutr. 2011 May;93(5):1048–52. doi: 10.3945/ajcn.110.007674. Epub 2011 Mar 2. PubMed PMID: 21367948; PubMed Central PMCID: PMC3076656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulz KF, Altman DG, Moher D CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010 Mar 23;340:c332. doi: 10.1136/bmj.c332. PubMed PMID: 20332509; PubMed Central PMCID: PMC2844940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epstein LH. Family-based behavioural intervention for obese children. Int J Obes Relat Metab Disord. 1996 Feb;20(Suppl 1):S14–21. PubMed PMID: 8646260. [PubMed] [Google Scholar]
- 29.Epstein LH, Wing RR, Koeske R, Andrasik F, Ossip DJ. Child and parent weight loss in family-based behavior modification programs. J Consult Clin Psychol. 1981 Oct;49(5):674–85. doi: 10.1037//0022-006x.49.5.674. PubMed PMID: 7287977. [DOI] [PubMed] [Google Scholar]
- 30.Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-based obesity treatment, then and now: twenty-five years of pediatric obesity treatment. Health Psychol. 2007 Jul;26(4):381–91. doi: 10.1037/0278-6133.26.4.381. PubMed PMID: 17605557; PubMed Central PMCID:PMC2387251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barlow SE Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007 Dec;120(Suppl 4):S164–92. doi: 10.1542/peds.2007-2329C. PubMed PMID: 18055651. [DOI] [PubMed] [Google Scholar]
- 32.Mitchel EB, Lavine JE. Review article: the management of paediatric nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014 Nov;40(10):1155–70. doi: 10.1111/apt.12972. Epub 2014 Sep 29. Review. PubMed PMID: 25267322. [DOI] [PubMed] [Google Scholar]
- 33.United States Department of Agriculture: Center for Nutrition Policy and Promotion. [Accessed March 10, 2017];MyPlate Background. http://choosemyplate.gov/food-groups/downloads/results/MyDailyFoodPlan_1800_18plusyr.pdf.
- 34.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002 May;11(246):1–190. PubMed PMID: 12043359. [PubMed] [Google Scholar]
- 35.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999 Sep;22(9):1462–70. doi: 10.2337/diacare.22.9.1462. PubMed PMID: 10480510. [DOI] [PubMed] [Google Scholar]
- 36.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28(7):412–9. doi: 10.1007/BF00280883. PubMed PMID: 3899825. [DOI] [PubMed] [Google Scholar]
- 37.Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988 Oct;88(10):1268–71. PubMed PMID: 3171020. [PubMed] [Google Scholar]
- 38.OʼSullivan TA, Oddy WH, Bremner AP, Sherriff JL, Ayonrinde OT, Olynyk JK, Beilin LJ, Mori TA, Adams LA. Lower fructose intake may help protect against development of nonalcoholic fatty liver in adolescents with obesity. J Pediatr Gastroenterol Nutr. 2014 May;58(5):624–31. doi: 10.1097/MPG.0000000000000267. PubMed PMID: 24345826. [DOI] [PubMed] [Google Scholar]
- 39.Jin R, Le NA, Liu S, Farkas Epperson M, Ziegler TR, Welsh JA, Jones DP, McClain CJ, Vos MB. Children with NAFLD are more sensitive to the adverse metabolic effects of fructose beverages than children without NAFLD. J Clin Endocrinol Metab. 2012 Jul;97(7):E1088–98. doi: 10.1210/jc.2012-1370. Epub 2012 Apr 27. PubMed PMID: 22544914; PubMed Central PMCID: PMC3387406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawasaki T, Igarashi K, Koeda T, Sugimoto K, Nakagawa K, Hayashi S, Yamaji R, Inui H, Fukusato T, Yamanouchi T. Rats fed fructose-enriched diets have characteristics of nonalcoholic hepatic steatosis. J Nutr. 2009 Nov;139(11):2067–71. doi: 10.3945/jn.109.105858. Epub 2009 Sep 23. PubMed PMID: 19776184. [DOI] [PubMed] [Google Scholar]
- 41.Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM Nonalcoholic Steatohepatitis Clinical Research Network. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010 Jun;51(6):1961–71. doi: 10.1002/hep.23535. PubMed PMID: 20301112; PubMed Central PMCID: PMC2922495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramon-Krauel M, Salsberg SL, Ebbeling CB, Voss SD, Mulkern RV, Apura MM, Cooke EA, Sarao K, Jonas MM, Ludwig DS. A low-glycemic-load versus low-fat diet in the treatment of fatty liver in obese children. Child Obes. 2013 Jun;9(3):252–60. doi: 10.1089/chi.2013.0022. Epub 2013 May 24. PubMed PMID: 23705885; PubMed Central PMCID: PMC3675832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taddeo D, Egedy M, Frappier JY. Adherence to treatment in adolescents. Paediatr Child Health. 2008 Jan;13(1):19–24. doi: 10.1093/pch/13.1.19. PubMed PMID: 19119348; PubMed Central PMCID: PMC2528818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee SS, Park SH. Radiologic evaluation of nonalcoholic fatty liver disease. World J Gastroenterol. 2014 Jun 21;20(23):7392–402. doi: 10.3748/wjg.v20.i23.7392. Review. PubMed PMID: 24966609; PubMed Central PMCID: PMC4064084. [DOI] [PMC free article] [PubMed] [Google Scholar]