1. Introduction
Child-caregiver attunement reflects the warm and sensitive dyadic relationship that is essential to adaptive and healthy development in children. Attunement requires constant adaptation by both partners in the dyad, in which mother and child act as agents in shaping the dynamics of their mutual interactions (Bornstein, 2013). Starting in pregnancy, maternal-child attunement operates at different levels. Changes in mothers occur in the central nervous system (Kim et al., 2011) and in adjustment at hormonal (Feldman, 2012) and behavioral levels (Bornstein, 2012). Maternal adjustment at all levels is directed towards a common goal of attuning responses to infant signals. The unique adjustments that mothers make in adapting to their own child play a vital role in promoting warm and sensitive care.
The faces of children convey critical information that helps mothers to interact and better understand their children’s needs. Infant faces are a special class of salient social stimuli and elicit feelings of care and empathic approach in adults (Bowlby, 1969; Brosch et al., 2007; Caria et al., 2012; Glocker et al. 2009; Kringelbach et al., 2008; Lorenz, 1971; Leibenluft et al., 2004; Kringelbach et al., 2016; Senese et al., 2013, 2016). In general, infant faces are perceived as rewarding by mothers and non-mothers (Montoya et al., 2012; Pechtel et al., 2013). However, the experience of looking at one’s own child, with whom a warm and meaningful relationship has been established, contrasted to another child, exerts in mothers strong and complex brain responses that involve phylogenetic and ontogenetic mechanisms linked to biological bases of parenting and the affective relationship between mother and her child (Bartels & Zeki, 2004; Atzil et al., 2017).
Several neuroimaging studies have sought to identify unique maternal responses to visual cues in own child contrasted with another child (for fMRI see Pechtel et al., 2013; for EEG see Maupin et al., 2015). For example, EEG findings support the assumption that own infant face is a uniquely salient stimulus that activates in mothers a differentiated brain response in early components (100–170 msec) of the ERP, related to processing of visual features, and late components (around 600 msec), that reflect top-down control, which are more sensitive to the emotional valence and personal significance of one’s own child than other children (Bick et al., 2013; Bornstein et al., 2013; Esposito et al., 2015). fMRI research, the focus of this meta-analysis, has identified specific brain regions activated to own infant images compared to other infant images, and highlighted involvement of neural regions associated with reward and maternal motivation (substantia nigra/ventral tegmental area [SN/VTA], striatum, amygdala), emotion processing (medial prefrontal cortex [mPFC], anterior cingulate cortex [ACC], insula), cognition and learning (PFC), and control of motor responses (Kim et al., 2016; Lonstein et al., 2015; Luo et al., 2015; Piallini et al., 2015; Swain et al., 2014). The growing number of studies on maternal brain response to own child, contrasted to another child, has resulted in the emergence of variability of cortical and subcortical brain regions involved, and not all studies have identified the same brain areas.
Part of the variability in fMRI research derives from differences in the visual (infant) stimuli used across studies. For example, stimuli might differ in duration, emotive valence of facial expressions, or presentation format (pictures and video). These variations in stimulus features can affect subsequent patterns of brain response. Maternal psychology also influences the site of cerebral activity and the magnitude of activation (Barrett et al., 2012). Consequently, these factors mentioned above can potentially influence regions activated in response to the mother’s own child. An enhanced maternal response to own infant’s face occurs in dopaminergic brain regions, such as the SN/VTA, and subcortical nuclei, which are brain areas important for maternal attachment (Bartels & Zeki, 2004; Numan & Young, 2016). Additionally, enhanced activity has been found in the ventral striatum and nucleus accumbens, which, according to animal and human models of parenting, are regions that reinforce maternal motivation and social interaction (Hoekzema et al., 2017; Lonstein et al., 2015; Numan 2012; Numan & Young, 2016). However, not all findings have been systematically reported across studies (Leibenluft et al., 2004; Nitschke et al., 2004; Noriuki et al., 2008; Schechter et al., 2012).
Maternal mood and positive feelings associated with parenting and attachment to the child modulate activity in brain regions that mediate affect and social behavior, influencing the activity of the amygdala, the thalamus, the insula, the ACC, and the PFC (Atzil et al., 2011; Barrett et al., 2012; Laurent & Ablow 2013; Michalska et al., 2014). For example, the amygdala is a critical brain region activated by salience of an infant stimulus, which, together with the reward system, promotes approach behaviors in parenting (Kim et al., 2016; Numan, 2012; Ranote et al., 2004; Strathearn & Kim, 2013; Wan et al., 2014). However, the amygdala shows variability in its involvement; for example, ROI analysis revealed significant amygdala activation with the own child happy face, when contrasted with other child happy face, but no significant amygdala activity modulation was found with sad and neutral affects (Strathearn et al., 2008). Other research with amygdala shows higher deactivation with the own child face than other child (Bartels & Zeki, 2004). In still other findings the amygdala is activated in association with “own” or “other” vs. baseline, and no differences were found from a direct comparison of own child vs other child face (Hoekzema et al., 2017). Given that contributors to research of parenting in non-human and human mammals point to core brain regions that regulate maternal behaviors, it is important to more completely account for observed variability in maternal brain activity and identify regions that converge across studies. Meta-analysis can be used to pinpoint fundamental neural regions involved in maternal responses that reflect the special status of “own child” among typical (healthy) human mothers.
The aim of this meta-analysis was to identify maternal brain regions that are selectively and specifically activated when viewing own child versus other children, independent of the other stimulus modalities (i.e., auditory stimuli as child vocalization or cry). One meta-analysis focused on maternal brain responses to a variety of stimuli, including child’s cries and faces. That cross-modality meta-analysis aimed to reveal which cerebral regions are the target of oxytocin (OT) and activated in mothers by pictures of their own maternal-child interaction or their own child face or cry (Rocchetti et al., 2014). However, it is crucial to examine neural regions engaged by infant cries and faces separately. Looking at neural responses to infant cry might be developmentally appropriate during the first few months postpartum, whereas neural responses to faces can be reliably assessed in mothers with children over a wider age range. Furthermore, responses to infant faces is a good way to assess maternal neural activity during a typical range of everyday mother-infant interactions whereas responses to infant cry are perforce more specific to situations of infant distress. Infant distress can elicit a wide range of maternal brain responses that may reflect, for example, the urgency to respond, the attempt to understand the cause of infant stress, or mothers’ own negative feelings in reaction to cry. Here, we confined our analysis to brain activation in mothers as elicited by their own child’s (versus a stranger child’s) face. Given our interest in the maternal cerebral activation in response to the special status of “own child” (vs. other child), and to avoid specific emotions that would likely elicit specific behaviors of care in mothers, we focused as much as possible on neutral visual stimuli.
In this meta-analysis, we investigated maternal cerebral activations that have been systematically reported across studies of mothers to their own child’s face. We expected that seeing one’s own child, relative to other stranger children, would elicit greater activation in sub-cortical nuclei in the midbrain (ventral tegmental area, substantia nigra) and other dopaminergic structures (the striatum) involved in maternal approach-related motivation and parental motivation (Numan, 2007, 2012). Considering the convergence of fMRI and EEG about the emotional salience of one’s own child (Pechtel et al., 2013; Maupin et al., 2015), we also expected to find greater involvement of amygdala, which plays a critical role in processing the salience of infant cues and promoting positive approach behaviors (Strathearn & Kim, 2013; Kim et al., 2016; Numan, 2012). Maternal behaviors are regulated by emotional appraisal of infant cues that influence maternal responses, and so we also expected to find the involvement of neural areas implicated in social emotion processing, such as the insula and the prefrontal cortex (e.g. Streathern et al., 2008). Understanding which brain regions respond selectively to own infant cues represents an important step toward understanding the special neural bases which bond mother and infant and represent a potential biomarker for parental neglect.
2. Methods
2.1. Selection of studies
Different sources were used to identify empirical studies that investigated brain activity in mothers while viewing their own child contrasted with other children. We searched MEDLINE indexes. To achieve an exhaustive resource of studies and reduce the risk of missing articles of interest, we used the following keywords: (((“magnetic resonance imaging”[MeSH Terms] OR (“magnetic”[All Fields] AND “resonance”[All Fields] AND “imaging”[All Fields]) OR “magnetic resonance imaging”[All Fields]) OR (“magnetic resonance imaging”[MeSH Terms] OR (“magnetic”[All Fields] AND “resonance”[All Fields] AND “imaging”[All Fields]) OR “magnetic resonance imaging”[All Fields] OR “fmri”[All Fields])) AND (“mothers”[MeSH Terms] OR “mothers”[All Fields])) AND ((“infant”[MeSH Terms] OR “infant”[All Fields]) OR (“infant, newborn”[MeSH Terms] OR (“infant”[All Fields] AND “newborn”[All Fields]) OR “newborn infant”[All Fields] OR “baby”[All Fields] OR “infant”[MeSH Terms] OR “infant”[All Fields]))). To expand the potential cohort of studies, we also used reference lists in the studies we identified and well-known general review articles (Kim et al., 2016; Luo et al., 2015; Moses-Kolko et al., 2014; Pechtel et al., 2013; Piallini et al., 2015; Swain et al., 2014). Only peer-reviewed studies that focused on maternal responses to visual stimuli were considered. A total of 212 articles published before February 2018 were found. Despite our use of infant and newborn as search keyword terms, the articles retrieved included children aged 1–144 months. Therefore, we use the more appropriate general term child in this meta-analysis. Given that the purpose of the present meta-analysis was to investigate which cerebral regions in mothers are selectively involved when viewing their own child, the following criteria were used: (a) only studies presenting results of contrasts that matched own child’s still pictures or videos versus other stranger children’s still pictures or videos; (b) because many studies tested the effect of covariates on the BOLD response, whole brain analysis results were needed to report brain areas associated with the main contrast own child (OWN) vs. other child (OTHER); (c) participants were healthy mothers (e.g., with no past or present history of psychiatric disorders); and (d) if the article included different sub-groups of mothers, results should also report regions activated for each group (the selected sub-group should meet the criteria in point c). Figure 1 illustrates the selection process based on PRISMA recommendations (Moher et al., 2009). Twelve studies met the criteria and were included in the Multi Kernel Density Analysis MKDA (Table 1).
Figure 1. Flow chart of study selection.
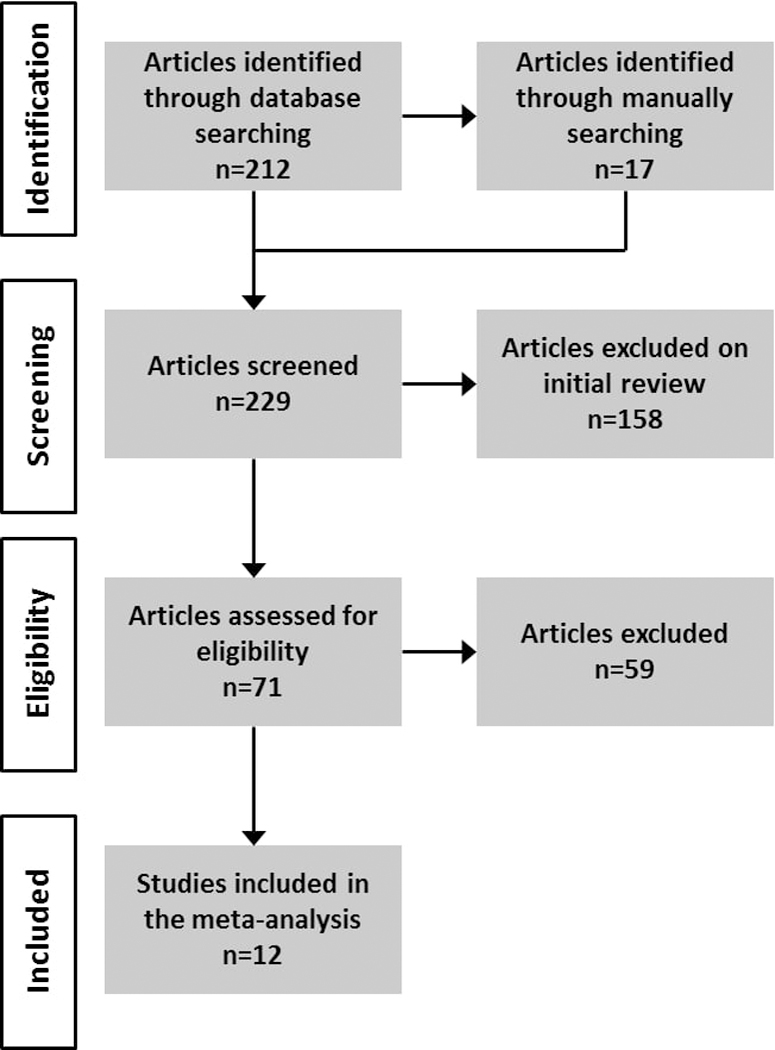
The flow diagram of the study selection process shows the following steps: identification of studies, screening to identify the eligible studies, selection of studies that met all inclusion criteria, and studies included in the meta-analysis.
Table 1.
Descriptions of the samples and stimuli in the selected studies. In the first column we only report the first author’s names and the publication year. Abbreviation: N/D=not defined.
| Study | Mothers (N) |
Primiparous (N) |
Mother Age (years) |
Mother Education years) |
Stimulus Format |
Stimulus Infant Age (months) |
Stimulus Infant Sex (% male) |
Stimulus Duration (sec) |
Social Situation (if specified) |
Part Of Body (stimulus) |
|---|---|---|---|---|---|---|---|---|---|---|
| Atzil et al. 2011 | 23 | N/D | 30 | 12–21 | picture | 3–6 | N/D | 120 | play | head and chest |
| Barret et al. 2012 | 22 | 7 | 30 | N/D | picture | 3 | N/D | 3 | - | face |
| Bartel et al. 2004 | 19 | N/D | 34 | N/D | picture | 9–72 | N/D | 2.5 | - | face |
| Hoekzema et al. 2016 | 20 | 20 | 33 | 12–21 | picture | 1 | 52 | 1.5 | - | face |
| Leibenluft et al. 2004 | 7 | N/D | 30 | N/D | picture | 60–144 | N/D | 1.5 | - | face |
| Michalska et al. 2014 | 34 | N/D | 47 | N/D | picture | 48–72 | 85 | 6 | - | face |
| Nitschke et al. 2004 | 6 | 6 | N/D | N/D | picture | 3–5 | N/D | 6 | - | face |
| Noriuchi et al. 2008 | 13 | 11 | 31 | N/D | video | 16 | 46 | 32 | separation + play | upper body |
| Ranote et al. 2004 | 10 | 3 | 26 | N/D | video | 4–8 | N/D | 40 | - | N/D |
| Schechter et al. 2012 | 9 (1) | N/D | 30 | 14 | picture | 22 | 44 | 40 | - | face |
| Strathearn et al. 2008 | 26 | 26 | 30 | 12–21 | video | 7 | N/D | 2 | play | face and shoulders |
| Wan et al. 2014 | 20 | 60% (2) | 32 | 12–21 | video | 6 | 35 | 30 | play | N/D |
Control group;
The article only reported the ratio between primiparous and multiparous mothers
2.2. Contrast selection
The contrasts of interest were OWN > OTHER and OWN < OTHER, which reflect, respectively, greater activation and less activation in response to own-child’s face when compared to another-child’s face. Five out of the 12 studies did not report negative contrasts as OWN < OTHER (Atzil et al., 2011; Barrett et al., 2012; Hoekzema et al., 2017; Michalska et al., 2014; Strathearn et al., 2008). One of the authors (P.R.) selected the contrasts of interest, and a second author (P.K.) double checked the selected contrasts.
We focused on studies where the own child was clearly recognizable and, therefore, we included only visual stimuli. This analysis focuses on brain activation in mothers as elicited by their own child’s face (picture or video). We assessed contrasts that grouped different emotions from neutral to mild positive to avoid infant negative-emotion activation in response to crying facial expressions. We were interested in stimuli that elicit maternal responses to own child in general but not specific to infant distress cues. In one report, the activated clusters were shown only graphically without a coordinates table (Schechter et al., 2012), so we obtained coordinates of the selected contrasts from the authors. Table 1 reports the study’s first author and year, sample size of mothers, number of primiparous mothers, mother’s age and education, stimulus format (picture or video; studies using the video did not include sounds), stimulus child age and gender, stimulus duration, (if specified) social situation (i.e. play), and which child body parts were captured in the static or dynamic images.
2.3. Participants
Altogether, 209 child-mother dyads were included in this meta-analysis. Demographic details about maternal age, education level, and primiparous status and the gender of children were not systematically reported across studies. Demographic information and percentages of studies that reported (%rep) this information are: Mothers’ M age=32.10 years, SD=5.36 (92% rep), educational level range=12–21 years (42% rep), child range age=1–144 months, (100% rep), % male=35–85 (42% rep).
2.4. Analysis
The meta-analysis aimed to discern if the distributions of cluster peaks of maternal brain responses in contrasts of OWN vs. OTHER showed specific patterns or a random pattern across studies. The variables included in the database for analysis were the x, y, and z coordinates of cluster peaks, contrast labels, sample sizes, standardized space of coordinates, fixed or random effect analysis, and multi-comparison corrections. The meta-analysis was carried out using the Multi Level Density Analysis tool (MDKA; Kober & Wager, 2010; Wager et al., 2007, 2009) and SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). The MKDA considers that points of activation are not independent of one another, but nested within contrasts within studies. These methodological choices obviate any single study having a large number of peaks that bias the analyses, thus increasing the reliability of the present fMRI findings.
First, peak coordinates reported either in Talairach [TAL] (229 peaks) or Montreal Neurological Institute [MNI] (111 peaks) spaces (Talairach & Tournoux, 1988; standard brain from the Montreal Neurological Institute) were converted to a common MNI space (avg152T1.img). Peak points were convolved with a spherical kernel of 10 mm (blob activation). Peaks that were close together fall in the same spherical kernel. We obtained a contrast activation map of active and inactive voxels, respectively, with values 1 and 0. A density map (statistic of the proportion (P)) for each contrast (OWN > OTHER, OWN<OTHER) was built with the proportion of all contrasts that reported activation within 10 mm of a given voxel by taking a weighted average of contrast activation maps. Voxel-wise significance was obtained via a permutation test (Monte Carlo simulation; n=5000). P was compared to 5000 Monte-Carlo (MC) simulations to identify if the voxel activated above chance. In each MC simulation, the P of activation coordinates from the contrast maps were placed at random locations throughout the brain gray matter (derived from the segmentation of the avg152T1.img template in SPM8), and over the whole brain the maximum across-study P statistic was saved. In the null hypothesis, peaks within each contrast were randomly distributed (P0). After each MC simulation, the largest cluster of contiguous voxels was saved; the cluster extent threshold was then set at the 95th percentile of this value across simulations (cluster extent-based multiple comparison correction; family-wise error rate corrected (FWER) at p<.05), and we report the significant clusters observed at p<.001 and p<.01 (Kober et al., 2008; Mende-Siedlecki et al., 2013; Satpute et al., 2015; Lindquist et al., 2016). The MDKA analysis was performed on 12 contrasts for OWN>OTHER and 7 contrasts for OWN<OTHER. The sum of cluster peaks (points) derived from all contrasts were 239 (OWN>OTHER) and 70 (OWN<OTHER). The resulting coordinates were labeled using the IBASPM 71 within the WFU Pickatlas toolbox (SPM8; Maldjian et al., 2003, 2004).
3. Results
The MDKA analysis aimed to identify which brain regions were systematically activated when mothers view their own child contrasted to another child (OWN>OTHER). Figures 2 and 3 and Table 2 report all significant results that survived cluster extent-based criteria at the primary alpha levels of p < .001 and p < .01 (FWER corrected at p < .05). Viewing their own child, mothers showed greater overall activation in the left hemisphere. Findings showed significant clusters centered in the lateral globus pallidus (extended to the left insula, the putamen, ventral and dorsal nuclei of thalamus, caudate nucleus), in the medial globus pallidus (included the left amygdala and uncus), the bilateral midbrain (substantia nigra [SN] and ventral tegmental area [VTA]), the left insula, the bilateral putamen, the left inferior frontal gyrus BA 45, the bilateral thalamus, the bilateral caudate and the medial globus pallidus), and in the left insula extended to the inferior frontal gyrus BA47. The only significant activation in the contrast OWN<OTHER was found in the right inferior temporal gyrus.
Figure 2. Activation map of OWN > OTHER.
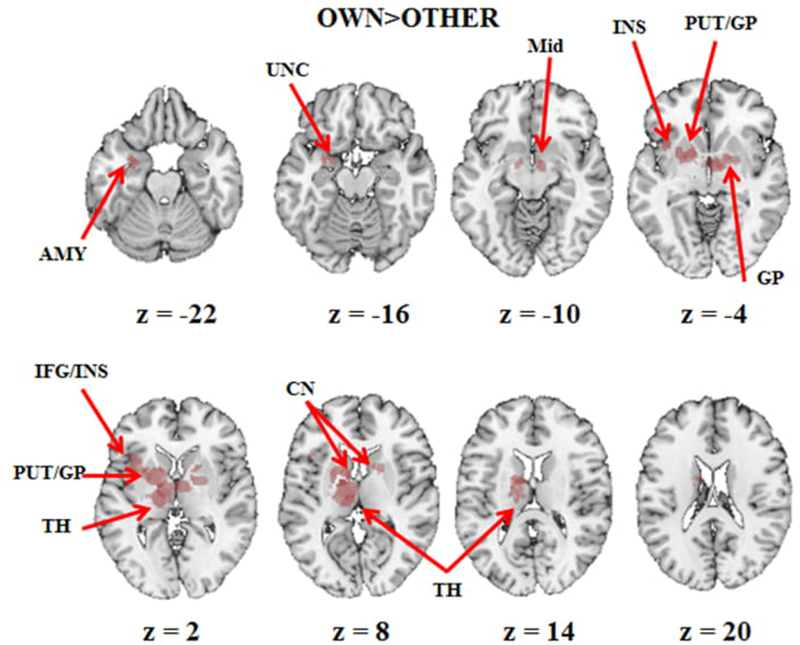
The graphical representation of brain regions consistently activated across studies in the contrast OWN>OTHER. Max cluster size computed at p < .001 and p < .01 (whole brain p(FWER)<.05). Abbreviations: AMY=amygdala, CN=nucleus caudatus, GP=globus pallidus, IFG=inferior frontal gyrus, INS=insula, Mid=midbrain, OTH=other child, OWN=own child, PUT=putamen, TH=thalamus and UNC=Uncus.
Figure 3. Activation map of OTHER>OWN.
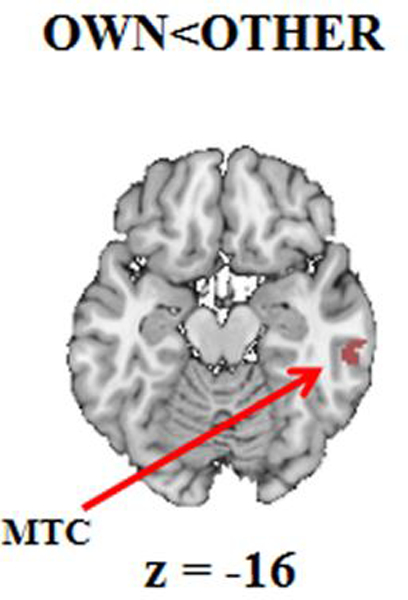
The graphical representation of brain regions consistently activated across studies in the contrast OTHER>OWN. Max cluster size computed at p < .001 and p < .01 (whole brain p(FWER)<.05). Abbreviations: MTC=middle temporal cortex.
Table 2. Activated clusters in OWN > OTHER and OTHER>OWN.
Cluster peak of activity, coordinates, cluster sizes of brain regions resulting from the contrasts OWN > OTHER and OTHER>OWN. Max cluster size was computed at p < .001 and p < .01 (p(FWER)<.05). Abbreviations: SN= substantia nigra, VTA= ventral tegmental area.
| Cluster Peak | Subcluster | L/R | X | Y | Z | voxels | Volume mm3 |
Maxstat |
|---|---|---|---|---|---|---|---|---|
| Own Child > Other Child | ||||||||
| Lateral Globus Pallidus * | L | −16 | −6 | 4 | 866 | −6928 | 0.44 | |
| Lateral Globus Pallidus (Lentiform Nucleus) | L | −16 | 4 | −2 | 85 | |||
| Insula | L | −38 | 14 | −2 | 74 | |||
| Thalamus (Ventral Anterior Nucleus) | L | −14 | −8 | 4 | 178 | |||
| Putamen | L | −30 | 6 | 0 | 68 | |||
| Thalamus (Medial Dorsal Nucleus) | L | −8 | −16 | 6 | 147 | |||
| Caudate Nucleus | L | −10 | 4 | 8 | 43 | |||
| Caudate Nucleus | L | −14 | −4 | 12 | 116 | |||
| Thalamus (Ventral Posterior Lateral Nucleus) | L | −16 | −16 | 8 | 155 | |||
| Medial Globus Pallidus † | L | −8 | −2 | 0 | 1505 | −12040 | 0.43 | |
| Amygdala | L | −26 | −2 | −22 | 73 | |||
| Uncus | L | −14 | −4 | −12 | 113 | |||
| Uncus | L | −28 | 0 | −14 | 43 | |||
| Brain Stem/Midbrain (SN/VTA) | R | 8 | −4 | −8 | 110 | |||
| Insula | L | −32 | 14 | 2 | 100 | |||
| Putamen (Lentiform Nucleus) | R | 26 | 0 | −2 | 62 | |||
| Lateral Globus Pallidus (Pallidum) | R | 18 | 0 | −2 | 138 | |||
| putamen (Pallidum) | L | −18 | 6 | 0 | 155 | |||
| Thalamus | R | 10 | −8 | −2 | 78 | |||
| Inferior Frontal Gyrus (BA45) | L | −40 | 20 | 4 | 49 | |||
| Thalamus | L | −2 | −8 | 2 | 163 | |||
| Thalamus | L | −8 | −18 | 4 | 49 | |||
| Thalamus (Ventral Posterior Lateral Nucleus) | L | −18 | −20 | 8 | 43 | |||
| Lateral Globus Pallidus (Lentiform Nucleus) | L | −22 | −14 | 4 | 59 | |||
| Putamen (Lentiform Nucleus) | R | 18 | 10 | 4 | 59 | |||
| Putamen (Lentiform Nucleus) | L | −22 | 2 | 6 | 91 | |||
| Caudate Body | L | −8 | 0 | 12 | 44 | |||
| Caudate Body | R | 12 | 12 | 10 | 11 | |||
| Caudate Nucleus | L | −14 | −6 | 18 | 65 | |||
| Insula/IFG pars orbitalis (BA47) † | L | −44 | 16 | 0 | 8 | −64 | 0.29 | |
| Own Child < Other Child | ||||||||
| Inferior Temporal Gyrus * | R | 62 | −30 | −14 | 76 | −608 | 0.58 | |
| Middle temporal Gyrus | R | 62 | −28 | −16 | 52 | |||
| Inferior Temporal Gyrus | R | 64 | −34 | −14 | 24 | |||
Cluster-size significant threshold at
p<.001
p<.01
4. Discussion
Seeing their own child activates cortical and subcortical regions in mothers which likely shape subsequent maternal cognitions and behaviors. Maternal cerebral activation elicited by viewing their own child is a composite response resulting from activation of brain regions which respond to infant features per se, the familiarity of the stimulus, and mothers’ special attachment relationship with their own child (Leibenluft et al., 2004). The specific objective of this meta-analysis was to identify in mothers brain mechanisms that exhibit selective sensitivity to the face of their own child. Several findings emerged. First, this meta-analysis showed that the most consistent brain activation in mothers viewing their own child, rather than other children, is found in the left hemisphere. Second, in line with previous reports of models of parenting (Feldman, 2017; Rilling & Young, 2014), the cerebral regions that systematically show higher activation in response to the own child contra a stranger child are brain structures dedicated to processing salient and rewarding biological stimuli with high personal relevance and neural circuitries that promote parental care in mammals (Feldman, 2017). Results from this meta-analysis showed systematic activation in the midbrain (SN and VTA), the ventrolateral prefrontal cortex, the insula, the amygdala, and the striatum.
Systematic left lateralized brain activity in the ventrolateral prefrontal cortex (BA45/47), the insula, the amygdala, and the striatum, in front of a bilateral activity in the midbrain (SN and VTA), in mothers in response to their own child’s face represents a novel result from the present meta-analysis. Viewing one’s own child elicits more positive affect (emotion) than viewing other stranger children (Barrett et al., 2012). One’s own child is a rewarding stimulus especially for their mothers, and the left hemisphere is typically activated more than the right in approach behaviors toward rewarding social stimuli and processing positive affect (Davidson, 1993; Davidson et al., 1990; Pizzagalli et al., 2003, 2005; Harsay et al., 2011). Indeed, in mothers the degree of left-sided activation is compatible with a motivated approach to their own child, a positive rewarding child cue. For example, depressed mothers show lower activity in the left insula, inferior frontal gyrus, and striatum in response to own infant positive emotion than non-depressed mothers (Laurent et al., 2013); except for left amygdala, left brain areas susceptible to depression reported in Laurent et al. (2013) overlap our findings in the left hemisphere. Instead, activation of the left amygdala in response to own child (vs. other child) is positively associated with maternal psychological well-being (Barrett et al., 2012). In general, plasma oxytocin level, a key hormone for promoting human parenting, correlates extensively with left-sided brain regions including the left insula (Atzil et al., 2012).
However, our interpretation cannot disregard the issue that children’s faces in our meta-analysis did not express distress (cry) but neutral to slightly positive emotions. Notwithstanding a continuing debate, the right hemisphere is usually superior in processing negative emotions, whereas the left hemisphere shows greater involvement in processing positive emotions and affect (Campbell, 1978; Ley & Bryden, 1979; Reuter-Lorenz & Davidson, 1981; Rodway et al., 2003). In this regard, a multimodal fMRI meta-analysis (Rocchetti et al., 2014) showed that, overall, maternal brain responses to positive and negative stimuli from their own baby, compared to control baby stimuli, include extensive bilateral activation in regions involved in emotion, emotional salience processing, and social cognition (PFC, insula, amygdala, striatum, and midbrain). Negative infant cues (i.e., crying facial expressions) seem to activate cerebral regions more specifically associated with behaviors of care oriented to reduce infant distress (Bornstein et al., 2017) than the ownness quality elicited by one’s own child.
There is other evidence that ownness is associated with greater left hemisphere activation. Stoeckel and colleagues (2014) found that, when mothers view their own child and pet, the main effect of cross-species ownness is associated with dominant activation in the left hemisphere, specifically increased brain activation in the left putamen, bilateral thalamus, and VTA/SN linked to emotion, sensory, and reward processing. In relation to the special emotional bond between mothers and children, one’s own child represents a very familiar complex social stimulus. The left hemisphere is more involved in retrieving semantic information and analyzing specific salient details during familiar face perception (Bombari et al., 2014; Brancucci et al., 2009; Marzi & Berlucchi, 1977; Rhodes, 1985). The systematic involvement of the left hemisphere might be interpreted in light of the more positive affect elicited by viewing one’s own child, which promotes approach behaviors toward the child, and the greater familiarity and knowledge associated with him/her, which, together, can contribute to the development of the special quality of ownness in mothers when they view their own child’s face.
Besides the left lateralization of maternal brain response to one’s own child, we observed strong involvement of regions underlying reward and maternal motivation and promoting behavioral approach. We found that visual experience of own child, contrasted to other children, activated the VTA of the midbrain bilaterally, comprising brain areas that are associated with parental care motivation in human and non-human animals (Numan 2015; Numan & Young, 2016). We also found that the own child (vs. other child) activated (predominantly left hemisphere) subcortical regions of the extrapyramidal dopaminergic system, such as the striatum, including the putamen and the caudate, the lateral and medial globus pallidus, and SN of the midbrain. These dopaminergic regions are linked to motor function, reward, and cognitive modulation of behaviors and play a role in responding to and approaching salient stimuli (Champagne, 2004, Arias-Carrión et al., 2010). Dopaminergic neurons are located in midbrain, as the substantia nigra and the VTA, from which originates the mesocorticolimbic dopamine system (MCL-DA system). The MCL-DA system with its projections to limbic structures (important for behavior) and to striatum (a key structure for voluntary movement) have a primary role in guiding approach and avoidance behavior relative to salient stimuli (Numan & Young, 2016, Arias-Carrión et al., 2010). The images depicting the face of the child specifically activate the areas of the midbrain and striatum mutually connected through dopaminergic neurons, involved in cognitive and hedonic mechanisms that lead to the implementation of motivated behaviors (Mirenowicz & Schultz, 1996, Schultz, 1998; Wise, 2004; Barrett & Fleming, 2011). The same regions, directly linked to motherhood and indirectly to the experience of social stimuli, under the influence of hormones such as OT and estrogen, enable and support parental motivation geared to direct infant care (Haber et al., 2000). Moreover, positive thought about one’s own baby is positively associated with the magnitude of plasticity in the midbrain and likely triggers and increases maternal motivation during the early post-partum period (Kim et al., 2010).
We observed subcortical regions involved in salience processing in response to own infant: the left amygdala and the striatum. It is well known that exposure in mothers to their own infant’s cues elicit a feeling of pleasure and that this experience can increase the salience of own child with respect to other children (Kim et al., 2016; Lonstein et al., 2015; Numan, 2012). Amygdala activation (in particular the basolateral nuclei), in interaction with the reward system, promotes approach and goal-directed maternal behaviors in rodents (Numan, 2010, 2012) and positive maternal dimensions in humans (Lonstein et al., 2015). Moreover, in both non-human animals and humans, the affective influence of the amygdala extends to many processes from sensation and attention to learning and memory (Gallagher & Chiba, 1996; Paré, 2003; Phelps & LeDoux, 2005). In connection with parental responsiveness, animal studies point to a critical role of the amygdala in maternal memory, contributing to mothers maintaining strong attraction to infants, thereby assuring continuing motivation for parental caring (Gur et al., 2014; Keller et al., 2004; Meurisse et al., 2009). Indeed, research reveals that mothers, when exposed to their child during video sessions of alone play, show high dopamine (D2 receptor) response; great D2 responses have been correlated with strong intrinsic connectivity of the medial amygdala with anterior brain midline regions involved in human parental attachment, like as the ventromedial prefrontal and anterior cingulate cortices (Atzil et al., 2017). These findings point to a potential key role of the medial amygdala in mediation of maternal attachment to their own child.
Last, we observed activation of cerebral structures involved in social emotion regulation processing, such as the insula with the inferior frontal gyrus BA45/47 (pars triangularis and orbitalis), the striatum, and the thalamus (Decety, 2010; Kober et al., 2008). The insula is a cerebral structure strongly connected to proximal and distal cerebral lobes and subserves multiple functions underlying sensory and limbic, motor, and visceral processes (Augustine, 1996; Nieuwenhuys, 2012). The anterior insula projects to the amygdala, and together they are involved in emotion regulation and play a role in affective modulation of cognitive processes based on stimulus salience (Cho et al., 2013; Gur et al., 2013; Nieuwenhuys, 2012). In connection with the amygdala and the ventrolateral prefrontal cortex, the anterior insula is involved in social emotions and empathy in parenting (Kim et al., 2016). The inferior frontal gyrus (pars orbitalis and triangularis) is consistently activated with the anterior insula (identified as part of the same functional cortical group) as reported in a meta-analysis focused on functional cortical and subcortical interactions in emotion processing (Kober et al., 2008). Causal modeling shows that the inferior frontal gyrus triggers activation of the anterior insula in empathy elicited by faces (Jabbi & Keysers, 2008) and that both areas represent brain structures involved in attention that can increase the recruitment of cognitive and affective brain resources to elaborate a salient stimulus (Tops & Boksem, 2011). Activity in this functional group anticorrelates with maternal depression in response to own infant positive cues (Laurent & Ablow, 2013). Although referring to another modality (auditory), Swain et al. (2017) reported that the left inferior frontal gyrus activated differently with emotive stimuli when mothers imagined listening to their own cry or their own baby’s cry (contrasted to others). Such findings are pertinent because they suggest a different modulation of the inferior frontal gyrus activation based on the sense of ownness perceived from the emotional stimulus. The bilateral thalamus is a region that mediates and integrates input from and output to subcortical and cortical regions for behavior guidance (Basso et al., 2005). In particular, the anterior and medial nuclei are connected with the limbic system and with brain regions underlying familiarity-based recognition (Aggleton et al., 2011).
Own child’s face (relative to that of other children) elicits diminished cerebral activation in the right infero-middle temporal cortex. The infero-middle temporal cortex is involved in both visual and face processing (Gross et al., 1984; Rossion et al., 2003). But cerebral deactivation of the inferior temporal cortex is not totally consistent with the existing literature. For example, stimulus familiarity typically enhances cerebral activity in the right temporal cortex (Eger et al., 2005; Negro et al., 2014), whereas the left superior temporal gyrus shows activation to unfamiliar stimulation when contrasted with familiar (Ramon et al., 2014). For mothers, the faces of their own children represent of course a very familiar stimulus. It has been reported that the inferior temporal gyrus shows reduced activity in the right hemisphere as an effect of repetition of familiar faces contrasted with unfamiliar ones (Eger et al., 2005). The temporal deactivation that we found might be ascribable to the effect of repetition that suppresses brain responses to the same familiar stimulus.
In light of brain models underlying parental care in humans (Kim, 2016), our findings show that one’s own child’s face, contrasted with faces of unfamiliar children, increases the activation of brain structures involved in (i) detection of salience of the own child, which can influence a series of processes from attention to memory and learning, potentially aimed at the identification and recognition of signals from the child, and the ability to identify such signals as salient; (ii) maternal motivation/reward and approach–avoidance behavior modulation, assuring dedicated/motivated and long-term caregiving to the loved child; (iii) social emotion regulation processes that enable mothers to interact emotionally and flexibly with the child and to express a range of affects that are appropriate to emotional availability to the child. Activated loci that systematically emerged across studies were found to be distributed in the left hemisphere. Therefore, the left lateralization of such neural circuits found in the present study might represent a marker of the quality of ownness perceived by mothers while viewing their own child.
Our findings accord with theories of human parenting from animal models which highlight cerebral mechanisms that may play critical roles in motivation to care for offspring (Levy & Keller, 2008; Numan, 2015; Numan & Young, 2016; Rilling & Young, 2014). Offspring appear very attractive to their mothers (Kringelbach et al., 2016). In accordance with animal models of parenting, the attractiveness and emotional relevance of infants to human mothers are regulated by neural systems underlying parental reward and emotion processes (Levi et al., 1995; Lonstein et al., 2015; Numan & Insel 2003, Numan, 2012). Viewing their own child activates in mothers a subcluster of brain regions that undergo phenomena of plasticity in human new mothers during the first months postpartum and, from recent research, during the transition into motherhood (from pregnancy to the second year postpartum). These brain areas are the striatum, insula, amygdala, thalamus, and inferior frontal gyrus (Kim et al., 2010; Hoekzema, et al., 2017).
Our meta-analysis has limitations, however. We report which brain regions showed systematically higher activation to own child versus other child faces. Cerebral deactivation was not reported in all studies, and only one brain region showed lesser deactivation with own child as compared to other children. Moreover, the selected contrasts own child vs. other child included pictures or videos. Unfortunately, there were not enough studies of each format of stimuli (video and picture) to permit independent meta-analyses and, eventually, comparisons between results from different stimulus formats. This is an important point to consider for interpretation because, even if we were interested in investigating the specific brain regions that systematically respond to the special status of own child (vs. other child) independent of the visual stimulus format, our results might miss potential cerebral activations connected with dynamic characteristics of the own child. Another limitation is the sample size. Despite the amount of data in the literature, many studies have different goals and publish results only considering a priori regions of interest or interactions between BOLD signals and maternal measures; whole brain results of the main contrast (own child vs. other child) are infrequently reported, and so they were excluded from our analysis.
A recommendation arising from this literature review is that future studies publish all activated and deactivated clusters and whole brain analyses to reduce the type 2 error bias given the unpublished uncorrected data (minimally in supplementary materials). Meta-analyses can overcome the type 1 error for uncorrected data. This recommendation would permit comparisons of brain responses to own child in different populations of parents. We focused on responses in mothers, who have been studied most; work on father’s cerebral responses to own children is needed. Moreover, to improve the generalization of findings, future studies should extend investigations of parental brain to members of different cultures to highlight common and specific patterns of brain activation in response to own child in mothers and fathers. Neuroimaging techniques have limitations as well. In this case, the fMRI tells us where more sustained activity takes place with good spatial definition, but transient and early processes are neglected. The present findings reveal sustained and strong conservative cerebral activation across studies.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH/NICHD, USA (DLP and MHB) and an International Research Fellowship in collaboration with the Centre for the Evaluation of Development Policies (EDePO) at the Institute for Fiscal Studies (IFS), London, UK, funded by the European Research Council (ERC) under the Horizon 2020 research and innovation programme (grant agreement No 695300-HKADeC-ERC-2015-AdG) (MHB). This work was also supported by the National Institute of Child Health and Human Development [R01HD090068; R21HD078797] (PI: Kim).
ABBREVIATIONS
- ACC
anterior cingulate cortex
- mPFC
medial prefrontal cortex
- SN/VTA
substantia nigra/ventral tegmental area
- OT
oxytocin
- fMRI
Functional Magnetic Resonance Imaging
- EEG
Electroencephalography
- ERP
Event Related Potentials
References
(* Studies selected in the present meta-analysis)
- Aggleton JP, Dumont JR, & Warburton EC (2011). Unraveling the contributions of the diencephalon to recognition memory: a review. Learning & Memory, 18(6), 384–400. doi: 10.1101/lm.1884611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Carrión O, Stamelou M, Murillo-Rodríguez E, Menéndez-González M, & Pöppel E (2010). Dopaminergic reward system: a short integrative review. International Archives of Medicine, 3:24. doi: 10.1186/1755-7682-3-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Atzil S, Hendler T, & Feldman R (2011). Specifying the Neurobiological Basis of Human Attachment: Brain, Hormones, and Behavior in Synchronous and Intrusive Mothers. Neuropsychopharmacology, 36, 2603–2615. doi: 10.1038/npp.2011.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzil S, Touroutoglou A, Rudy T, Salcedo S, & Feldman R, Hooker JM, Dickerson BC, Catana C, Barrett LF (2017). Dopamine in the medial amygdala network mediates human bonding. Proceedings of the National Academy of Sciences, 114:9, 2361–2366. 10.1073/pnas.1612233114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine JR (1996). Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research Reviews, 22(3), 229–244. 10.1016/S0165-0173(96)00011-2 [DOI] [PubMed] [Google Scholar]
- Barrett J, Fleming AS (2011). Annual Research Review: All mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. Journal of Child Psychology and Psychiatry, 52(4), 368–397. doi: 10.1111/j.1469-7610.2010.02306.x [DOI] [PubMed] [Google Scholar]
- *Barrett J Wonch KE, Gonzalez A, Ali N, Steiner M, Hall GB, & Fleming AS (2012). Maternal affect and quality of parenting experiences are related to amygdala response to infant faces. Social Neuroscience, 7(3), 252–68 doi: 10.1080/17470919.2011.609907 [DOI] [PubMed] [Google Scholar]
- *Bartels A, & Zeki S (2004). The neural correlates of maternal and romantic love. NeuroImage, 21(3), 1155–1166. 10.1016/j.neuroimage.2003.11.003 [DOI] [PubMed] [Google Scholar]
- Basso MA, Uhlrich D, & Bickford ME (2005). Cortical function: A view from the thalamus. Neuron, 45(4), 485–488. 10.1016/j.neuron.2005.01.035 [DOI] [PubMed] [Google Scholar]
- Bick J, Dozier M, Bernard K, Grasso D, & Simons R (2013). Foster mother-infant bonding: associations between foster mothers’ oxytocin production, electrophysiological brain activity, feelings of commitment, and caregiving quality. Child Development, 84(3), 826–40. doi: 10.1111/cdev.12008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombari D, Preuss N, & Mast FW (2014). Lateralized processing of faces: The role of features, configurations, and familiarity. Swiss Journal of Psychology, 73(4), 215–224. 10.1024/1421-0185/a000140 [DOI] [Google Scholar]
- Bornstein MH (2012). Cultural Approaches to Parenting. Parenting: Science and Practice, 12(2–3): 212–221. doi: 10.1080/15295192.2012.683359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH (2013). Mother-infant attunement: A multilevel approach via body, brain, and behavior In Legerstee M, Haley DW, & Bornstein MH (Eds.), The Infant Mind: Origins of the Social Brain (pp. 266–298). New York: Guilford. [Google Scholar]
- Bornstein MH, Arterberry ME, & Mash C (2013). Differentiated brain activity in response to faces of “own” versus “unfamiliar” babies in primipara mothers: an electrophysiological study. Developmental Neuropsychology, 38(6), 365–85. doi: 10.1080/87565641.2013.804923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Putnick DL, Rigo P, Esposito G, Swain JE, Suwalsky JTD, … Venuti, P. (2017). Neurobiology of culturally common maternal responses to infant cry. Proceedings of the National Academy of Sciences. 10.1073/pnas.1712022114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J (1969). Attachment and loss, Volume 1: Attachment. Attachment (Vol. 1). [Google Scholar]
- Brancucci A, Lucci G, Mazzatenta A, & Tommasi L (2009). Asymmetries of the human social brain in the visual, auditory and chemical modalities. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 364(1519), 895–914. doi: 10.1098/rstb.2008.0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch T, Sander D, & Scherer KR (2007). That baby caught my eye... attention capture by infant faces. Emotion, 7(3), 685–689. 10.1037/1528-3542.7.3.685 [DOI] [PubMed] [Google Scholar]
- Cahill L (2003). Sex- and hemisphere-related influences on the neurobiology of emotionally influenced memory. In Progress in Neuro-Psychopharmacology and Biological Psychiatry, 27(8), 1235–1241. 10.1016/j.pnpbp.2003.09.019 [DOI] [PubMed] [Google Scholar]
- Campbell R (1978). Asymmetries in interpreting and expressing a posed facial expression. Cortex, 14(3), 327–342. doi: 10.1016/S0010-9452(78)80061-6 [DOI] [PubMed] [Google Scholar]
- Caria A, Falco S, De, Venuti P, Lee S, Esposito G, Rigo P, … Bornstein MH (2012). Species-specific response to human infant faces in the premotor cortex. NeuroImage, 60(2), 884–893. doi: 10.1016/j.neuroimage.2011.12.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA (2004). Variations in Nucleus Accumbens Dopamine Associated with Individual Differences in Maternal Behavior in the Rat. Journal of Neuroscience, 24(17), 4113–4123. 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YT, Ernst M, & Fudge JL (2013). Cortico-amygdala-striatal circuits are organized as hierarchical subsystems through the primate amygdala. Journal of Neuroscience, 33(35), 14017–14030. doi: 10.1523/JNEUROSCI.0170-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Ekman P, Saron CD, Senulis JA, & Friesen WV (1990). Approach-withdrawal and cerebral asymmetry: emotional expression and brain physiology I. Journal of Personality and Social Psychology, 58(2): 330–341. 10.1037/0022-3514.58.2.330 [DOI] [PubMed] [Google Scholar]
- Davidson RJ (1993). Parsing affective space: Perspectives from neuropsychology and psychophysiology. Neuropsychology, 7(4), 464–475. [Google Scholar]
- Decety J (2015). The neural pathways, development and functions of empathy. Current Opinion in Behavioral Sciences, 3:1–6. 10.1016/j.cobeha.2014.12.001 [DOI] [Google Scholar]
- Eger E, Schweinberger SR, Dolan RJ, & Henson RN (2005). Familiarity enhances invariance of face representations in human ventral visual cortex: fMRI evidence. NeuroImage, 26, 1128–1139. 10.1016/j.neuroimage.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Esposito G, Valenzi S, Islam T, Mash C, and Bornstein MH (2015). Immediate and selective maternal brain responses to own infant faces. Brain Behavioral Research, 278, 40–43. doi: 10.1016/j.bbr.2014.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R (2012). Oxytocin and social affiliation in humans. Hormones and Behavior, 61, 380–391. doi: 10.1016/j.yhbeh.2012.01.008 [DOI] [PubMed] [Google Scholar]
- Feldman R (2017). The Neurobiology of Human Attachments. Trends in Cognitive Sciences, 21(2), 80–99. doi: 10.1016/j.tics.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Gallagher M, & Chiba AA (1996). The amygdala and emotion. Current Opinion in Neurobiology, 6(2), 221–227. 10.1016/S0959-4388(96)80076-6 [DOI] [PubMed] [Google Scholar]
- Glocker ML, Langleben DD, Ruparel K, Loughead JW, Valdez JN, Griffin MD, … Gur RC (2009). Baby schema modulates the brain reward system in nulliparous women. Proceedings of the National Academy of Sciences of the United States of America, 106(22), 9115–9119. doi: 10.1073/pnas.0811620106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecucci A, Giorgetta C, Bonini N, Sanfey AG (2013). Reappraising social emotions: the role of inferior frontal gyrus, temporo-parietal junction and insula in interpersonal emotion regulation. Frontiers in Human Neuroscience, 7, 1–12. doi: 10.3389/fnhum.2013.00523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CG, Desimone R, Albright TD, & Schwartz EL (1984). Inferior temporal cortex as a visual integration area In Reinoso-Suarez F and Ajmone Marsan C. (Eds.), Basic Archicortical & Cortical Association Levels of Neural Integration (pp. 291–315). New York: Raven Press. [Google Scholar]
- Gur R, Tendler A, & Wagner S (2014). Long-term social recognition memory is mediated by oxytocin-dependent synaptic plasticity in the medial amygdala. Biological Psychiatry, 76(5), 377–386. doi: 10.1016/j.biopsych.2014.03.022 [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, & McFarland NR (2000). Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. The Journal of Neuroscience, 20(6), 2369–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay HA, Cohen MX, Oosterhof NN, Forstmann BU, Mars RB, & Ridderinkhof KR (2011). Functional connectivity of the striatum links motivation to action control in humans. Journal of Neuroscience, 31, 10701–10711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Hoekzema E, Barba-Müller E, Pozzobon C, Picado M, Lucco F, García-García D, Soliva JC, Tobeña A, Desco M, Crone EA, Ballesteros A, Carmona S, & Vilarroya O (2017). Pregnancy leads to long-lasting changes in human brain structure. Nature Neuroscience, 20(2), 287–296. doi: 10.1038/nn.4458 [DOI] [PubMed] [Google Scholar]
- Insel TR (2010). The Challenge of Translation in Social Neuroscience: A Review of Oxytocin, Vasopressin, and Affiliative Behavior. Neuron, 65(6), 768–779. doi: 10.1016/j.neuron.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M, Swart M, & Keysers C (2008). Inferior frontal gyrus activity triggers anterior insula response to emotional facial expressions. Emotion, 8, 775–780. [DOI] [PubMed] [Google Scholar]
- Keller M, Perrin G, Meurisse M, Ferreira G, & Lévy F (2004). Cortical and medial amygdala are both involved in the formation of olfactory offspring memory in sheep. European Journal of Neuroscience, 20(12), 3433–3441. doi: 10.1111/j.1460-9568.2004.03812.x [DOI] [PubMed] [Google Scholar]
- Kim P (2016). Human maternal brain plasticity: adaptation to parenting. New Directions for Child and Adolescent Development, 153, 47–48. doi: 10.1002/cad.20168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, & Swain JE (2010). The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behavioral Neuroscience, 124(5), 695–700. doi: 10.1037/a0020884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Strathearn L, & Swain JE (2016). The maternal brain and its plasticity in humans. Hormones and Behavior, 77, 113–123. doi: 10.1016/j.yhbeh.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, & Wager TD (2008). Functional grouping and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. NeuroImage, 42(2), 998–1031. doi: 10.1016/j.neuroimage.2008.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, & Wager TD (2010). Meta-analysis of neuroimaging data. Wiley Interdisciplinary Reviews: Cognitive Science, 1(2), 293–300. doi: 10.1002/wcs.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Lehtonen A, Squire S, Harvey AG, Craske MG, Holliday IE, … Stein, A. (2008). A specific and rapid neural signature for parental instinct. PloS One, 3(2), e1664. doi: 10.1371/journal.pone.0001664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Stark EA, Alexander C, Bornstein MH, Stein A (2016). On cuteness: Unlocking the parental brain and beyond. Trends in Cognitive Sciences, 20(7), 545–558. doi: 10.1016/j.tics.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, & Ablow JC (2013). A face a mother could love: Depression-related maternal neural responses to infant emotion faces. Social Neuroscience, 8(3), 228–239, doi: 10.1080/17470919.2012.762039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Leibenluft E, Gobbini MI, Harrison T, & Haxby JV (2004). Mothers’ neural activation in response to pictures of their children and other children. Biological Psychiatry, 56(4), 225–232. doi: 10.1016/j.biopsych.2004.05.017 [DOI] [PubMed] [Google Scholar]
- Lenzi D, Trentini C, Pantano P, MacAluso E, Iacoboni M, Lenzi GL, & Ammaniti M (2009). Neural basis of maternal communication and emotional expression processing during infant preverbal stage. Cerebral Cortex, 19(5), 1124–1133. doi: 10.1093/cercor/bhn153 [DOI] [PubMed] [Google Scholar]
- Lévy F, & Keller M (2008). Chapter 8 Neurobiology of Maternal Behavior in Sheep. Advances in the Study of Behavior, 38, 399–437. doi: 10.1016/S0065-3454(08)00008-9 [DOI] [Google Scholar]
- Ley RG, & Bryden MP (1979). Hemispheric differences in processing emotions and faces. Brain and Language, 7(1), 127–138. 10.1016/0093-934X(79)90010-5 [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Satpute AB, Wager TD, Weber J, & Barrett LF (2016). The brain basis of positive and negative affect: Evidence from a meta-analysis of the human neuroimaging literature. Cerebral Cortex, 26(5), 1910–1922. doi: 10.1093/cercor/bhv001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, Lévy F, Fleming AS (2015). Common and divergent psychobiological mechanisms underlying maternal behaviors in non-human and human mammals. Hormones and Behavior, 73, 156–185. doi: 10.1016/j.yhbeh.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz K (1971). Studies in Animal and Human Behavior In Methuen (Ed.), Studies in Animal and Human Behavior (Vol. II, M). London: Methuen. [Google Scholar]
- Luo L, Ma X, Zheng X, Zhao W, Xu L, Becker B, & Kendrick KM (2015). Neural systems and hormones mediating attraction to infant and child faces. Frontiers in Psychology, 6, 970. doi: 10.3389/fpsyg.2015.00970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, & Burdette JH (2004). Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage, 21, 450–455. 10.1016/j.neuroimage.2003.09.032 [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, & Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage;19, 1233–1239. 10.1016/S1053-8119(03)00169-1 [DOI] [PubMed] [Google Scholar]
- Marzi CA, & Berlucchi G (1977). Right visual field superiority for accuracy of recognition of famous faces in normals. Neuropsychologia, 15(6), 751–756. 10.1016/0028-3932(77)90005-7 [DOI] [PubMed] [Google Scholar]
- Maupin AN, Hayes NJ, Mayes LC, & Rutherford HJV (2015). The Application of Electroencephalography to Investigate the Neural Bases of Parenting: A Review. Parenting, Science and Practice, 15(1), 9–23. doi: 10.1080/15295192.2015.992735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mende-Siedlecki P, Said CP, & Todorov A (2013). The social evaluation of faces: a meta-analysis of functional neuroimaging studies. Social Cognitive and Affective Neuroscience, 8, 285–299. doi: 10.1093/scan/nsr090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurisse M, Chaillou E, & Lévy F (2009). Afferent and efferent connections of the cortical and medial nuclei of the amygdala in sheep. Journal of Chemical Neuroanatomy, 37(2), 87–97. doi: 10.1016/j.jchemneu.2008.09.001 [DOI] [PubMed] [Google Scholar]
- *Michalska KJ, Decety J, Liu C, Chen Q, Martz ME, Jacob S, … Lahey BB (2014). Genetic imaging of the association of oxytocin receptor gene (OXTR) polymorphisms with positive maternal parenting. Frontiers in Behavioral Neuroscience, 8, 21. doi: 10.3389/fnbeh.2014.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirenowicz J, & Schultz W (1996). Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature, 379(6564), 449–451. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, & The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7), e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya JL, Landi N, Kober H, Worhunsky PD, Rutherford HJV, Mencl WE, … Potenza MN (2012). Regional brain responses in nulliparous women to emotional infant stimuli. PLoS ONE, 7(5), e36270. doi: 10.1371/journal.pone.0036270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Horner MS, Phillips ML, Hipwell AE, & Swain JE (2014). In search of neural endophenotypes of postpartum psychopathology and disrupted maternal caregiving. Journal of Neuroendocrinology, 26(10), 665–684. doi: 10.1111/jne.12183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negro E, D’Agata F, Caroppo P, Coriasco M, Ferrio F, Celeghin A, et al. (2015). Neurofunctional Signature of Hyperfamiliarity for Unknown Faces. PLoS ONE 10(7), e0129970. doi: 10.1371/journal.pone.0129970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R (2012). Chapter 7 - The insular cortex: A review. In M.A.H. and D.F.B.T.-P in Research B (Ed.), Evolution of the Primate Brain (Vol. 195, pp. 123–163). Amsterdam: Elsevier. [DOI] [PubMed] [Google Scholar]
- *Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, & Davidson RJ (2004). Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. NeuroImage, 21(2), 583–592. 10.1016/j.neuroimage.2003.10.005 [DOI] [PubMed] [Google Scholar]
- *Noriuchi M, Kikuchi Y, & Senoo A (2008). The Functional Neuroanatomy of Maternal Love: Mother’s Response to Infant’s Attachment Behaviors. Biological Psychiatry, 63(4), 415–423. 10.1016/j.biopsych.2007.05.018 [DOI] [PubMed] [Google Scholar]
- Numan M(2007).Motivational systems and the neural circuitry of maternal behavior in the rat. Developmental Psychobiology, 49(1), 12–21. doi: 10.1002/dev.20198 [DOI] [PubMed] [Google Scholar]
- Numan M (2012). Maternal Behavior: Neural Circuits, Stimulus Valence, and Motivational Processes. Parenting: Science and Practice, 12(2–3), 105–114. 10.1080/15295192.2012.680406 [DOI] [Google Scholar]
- Numan M (2015). Neurobiology of Social Behavior: Toward an Understanding of the Prosocial and Antisocial Brain, London: Academic Press. [Google Scholar]
- Numan M, Bress JA, Ranker LR, Gary AJ, DeNicola AL, Bettis JK, & Knapp SE (2010). The importance of the basolateral/basomedial amygdala for goal-directed maternal responses in postpartum rats. Behavioural Brain Research, 214(2), 368–376. doi: 10.1016/j.bbr.2010.06.006 [DOI] [PubMed] [Google Scholar]
- Numan M, & Insel H (2003). The Neurobiology of Parental Behavior (Springer). New York: Springer-Verlag New York, Inc. [Google Scholar]
- Numan M, & Young LJ (2016). Neural mechanisms of mother-infant bonding and pair bonding: Similarities, differences, and broader implications. Hormones and Behavior, 77, 98–112. doi: 10.1016/j.yhbeh.2015.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D (2003). Role of the basolateral amygdala in memory consolidation. Progress in Neurobiology, 70(5), 409–420. 10.1016/S0301-0082(03)00104-7 [DOI] [PubMed] [Google Scholar]
- Pechtel P, Murray LMM, Brumariu LE, & Lyons-Ruth K (2013). Reactivity, regulation, and reward responses to infant cues among mothers with and without psychopathology: an fMRI review. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 52(4), 1233–42. 10.3402/tdp.v1i0.19673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, & LeDoux JE (2005). Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron, 48(2), 175–187. doi: 10.1016/j.neuron.2005.09.025 [DOI] [PubMed] [Google Scholar]
- Piallini G, De Palo F, Simonelli A (2015). Parental brain: Cerebral areas activated by infant cries and faces. A comparison between different populations of parents and not. Frontiers in Psychology, 6, 1625. doi: 10.3389/fpsyg.2015.01625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D, Sherwood RJ, Henriques JB, & Davidson RJ (2005). Frontal Brain Asymmetry and Reward Responsiveness. Psychological Science, 16(10), 805–813. doi: 10.1111/j.1467-9280.2005.01618.x [DOI] [PubMed] [Google Scholar]
- Pizzagalli D, Shackman AJ, & Davidson RJ (2003). The functional neuroimaging of human emotion: Asymmetric contributions of cortical and subcortical circuitry In Hugdahl K & Davidson RJ (Eds.), The asymmetrical brain (pp. 511–532). MIT Press. [Google Scholar]
- Ramon M, Vizioli L, Liu-Shuang J, Rossion B (2015). Neural microgenesis of personally familiar face recognition. Proceedings of the National Academy of Sciences, 112(35), E4835–E4844. doi: 10.1073/pnas.1414929112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Ranote S, Elliott R, Abel KM, Mitchell R, Deakin JFW, & Appleby L (2004). The neural basis of maternal responsiveness to infants: an fMRI study. Neuroreport, 15(11), 1825–1829. doi: 10.1097/01.wnr.0000137078.64128.6a [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P, & Davidson RJ (1981). Differential contributions of the two cerebral hemispheres to the perception of happy and sad faces. Neuropsychologia, 19(4), 609–613. 10.1016/0028-3932(81)90030-0 [DOI] [PubMed] [Google Scholar]
- Rhodes G (1985). Lateralized processes in face recognition. British Journal of Psychology, 76(2), 249–271. doi: 10.1111/j.2044-8295.1985.tb01949.x [DOI] [PubMed] [Google Scholar]
- Rilling JK, & Young LJ (2014). The biology of mammalian parenting and its effect on offspring social development. Science, 345(6198), 771–776. doi: 10.1126/science.1252723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchetti M, Radua J, Paloyelis Y, Xenaki LA, Frascarelli M, Caverzasi E, … Fusar-Poli P (2014). Neurofunctional maps of the “maternal brain” and the effects of oxytocin: A multimodal voxel-based meta-analysis. Psychiatry and Clinical Neurosciences, 68(10), 733–751. doi: 10.1111/pcn.12185 [DOI] [PubMed] [Google Scholar]
- Rossion B, Caldara R, Seghier M, Schuller AM, Lazeyras F, Mayer E A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain, 126, 2381–2395. doi: 10.1093/brain/awg241 [DOI] [PubMed] [Google Scholar]
- Rodway P, Wright L, Hardie S (2003). The valence-specific laterality effect in free viewing conditions: The influence of sex, handedness, and response bias. Brain and Cognition,53(3): 452–463. 10.1016/S0278-2626(03)00217-3 [DOI] [PubMed] [Google Scholar]
- Satpute AB, Kang J, Bickart KC, Yardley H, Wager TD, Barrett LF (2015). Involvement of Sensory Regions in Affective Experience: A Meta-Analysis. Frontiers in Psychology, 6, 1860. doi: 10.3389/fpsyg.2015.01860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Schechter DS, Moser DA, Wang Z, Marsh R, Hao X, Duan Y, … Peterson BS (2012). An fMRI study of the brain responses of traumatized mothers to viewing their toddlers during separation and play. Social Cognitive and Affective Neuroscience, 7(8), 969–979. doi: 10.1093/scan/nsr069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W (1998). Predictive Reward Signal of Dopamine Neurons. Journal of Neurophysiology, 80(1), 1–27. 10.1152/jn.1998.80.1.1 [DOI] [PubMed] [Google Scholar]
- Seifritz E, Esposito F, Neuhoff JG, Lüthi A, Mustovic H, Dammann G, … Di Salle F (2003). Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biological Psychiatry, 54(12), 1367–1375. doi: 10.1016/S0006-3223(03)00697-8 [DOI] [PubMed] [Google Scholar]
- Senese VP, De Falco S, Bornstein MH, Caria A, Buffolino S, & Venuti P (2013). Human infant faces provoke implicit positive affective responses in parents and non-parents alike. PLoS ONE, 8(11). doi: 10.1371/journal.pone.0080379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senese PS, Shinohara K, Esposito G, Doi H, Venuti P, Bornstein MH (2016). Implicit association to infant faces: Genetics, early care experiences, and cultural factors influence caregiving propensities. Behavioural Brain Research, 325, 163–172. doi: 10.1016/j.bbr.2016.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Palley LS, Gollub RL, Niemi SM, & Evins AE (2014). Patterns of brain activation when mothers view their own child and dog: An fMRI study. PLoS ONE, 9(10). doi: 10.1371/journal.pone.0107205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, & Kim S (2013). Mothers’ amygdala response to positive or negative infant affect is modulated by personal relevance. Frontiers in Neuroscience, 7, 176 10.3389/fnins.2013.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Strathearn L, Li J, Fonagy P, & Montague PR (2008). What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics, 122(1), 40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Kim P, Spicer J, Ho SS, Dayton CJ, Elmadih A, & Abel KM (2014). Approaching the biology of human parental attachment: Brain imaging, oxytocin and coordinated assessments of mothers and fathers. Brain Research, 1580, 78–101. doi: 10.1016/j.brainres.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Ho SS, Rosenblum KL, Morelen D, Dayton CJ, Muzik M (2017). “Parent–child intervention decreases stress and increases maternal brain activity and connectivity during own baby-cry: An exploratory study.” Development and Psychopathology, 29(2), 535–553. doi: 10.1017/S0954579417000165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, & Tournoux P (1988). Co-Planar Stereotaxis Atlas of the Human Brain. Thieme, New York. [Google Scholar]
- Tops M, Boksem MA (2011). A potential role of the inferior frontal gyrus and anterior insula in cognitive control, brain rhythms, and event-related potentials. Frontiers in Psychology, 2:330, doi: 10.3389/fpsyg.2011.00330,pmid:22084637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience, 16, 55–61. doi: 10.1038/nrn3857 [DOI] [PubMed] [Google Scholar]
- Wager TD, Lindquist MA, Nichols TE, Kober H, & Van Snellenberg JX (2009). Evaluating the consistency and specificity of neuroimaging data using meta-analysis. NeuroImage, 45(1), S210–S221. doi: 10.1016/j.neuroimage.2008.10.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Lindquist M, & Kaplan L (2007). Meta-analysis of functional neuroimaging data: Current and future directions. Social Cognitive and Affective Neuroscience, 2(2), 150–158. doi: 10.1093/scan/nsm015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Wan MW, Downey D, Strachan H, Elliott R, Williams SR, & Abel KM (2014). The neural basis of maternal bonding. PLoS ONE, 9(3), e88436 10.1371/journal.pone.0088436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (2004). Dopamine, learning and motivation. Nature Reviews Neuroscience, 5(6), 483–494. 10.1038/nrn1406 [DOI] [PubMed] [Google Scholar]


