Abstract
Antibodies that target the blood-brain barrier (BBB) in vivo are of particular interest for the treatment of neurological diseases. Here, we screened a phage display single-chain antibody (scFv) library by brain perfusion in an attempt to isolate scFv that target the rat BBB. After four rounds of screening, the resulting antibody pool remained highly complex and discrete clonal sampling did not identify any scFvs capable of binding to the rat BBB. Thus, the heavy chain CDR3 in the resulting pools was subjected to NGS, and the resulting data was used to identify 12 scFv clones that were of high abundance and/or enriched from round 3 to 4, signifying potential hits. Of these, two scFv, denoted scFv 4 and scFv 40, were identified that bound the rat BBB. Neither of these scFvs was identified by discrete sampling, motivating NGS as a tool to identify lead antibodies from complex in vivo screens.
Introduction
Although there has been substantial effort to identify novel blood-brain barrier (BBB) targeting molecules, only a handful of antibodies have been discovered that can successfully target the brain vasculature in vivo 1,2. Most of these antibodies were developed against specific BBB targets having elevated abundance at the BBB, such as the transferrin, insulin and basigin receptors 3–5. However, from gene and protein expression profiling alone, it is impossible to identify every potential protein that will allow targeting of the BBB since many proteins have unknown cellular localization and function 6,7. Thus, as a complement to profiling methods for target identification, combinatorial screens can be used to access these currently unknown targets. For instance, a combinatorial screen on an in vitro BBB model has yielded FC5, a camelid antibody that targets TMEM30A and can deliver pharmacologic amounts of therapeutic to brain tissue8–10. Unfortunately, while there are many in vitro cell-based BBB models that could be used as a screening substrate, there are no assurances that the identified antibodies and targets will have relevance in vivo since many key BBB properties are lost in culture11. For example, a combinatorial screen using an immortalized rat BBB cell line yielded 34 different antibodies that bound the in vitro BBB, but only one antibody was able to recognize an antigen at the BBB in vivo12,13, and the most BBB selective antibody identified in another screen using primary cultured rat brain endothelial cells did not bind the in vivo BBB14. To enhance the in vivo relevance of combinatorial BBB screens, it is possible to perform in vivo screens centered on vascular administration of phage display libraries15. While several BBB binding peptides have been discovered by variations of in vivo phage display1,16, peptides can be limited in their binding affinity and specificity. Despite the fact that antibodies may be preferable for some applications, and that several academic and industrial groups have attempted in vivo phage display screens with antibody libraries, there is only one example of successful in vivo phage antibody screening to identify an antibody that targets the glioblastoma vasculature 1,17.
One particular challenge that can confound brain-targeted in vivo phage display screens is that high amounts of background phage are recovered from the screening process. The problem of high background is exacerbated by the common practice for analyzing selection outputs that consists of post-screen random clone evaluation and assay of as many clones as possible (generally between 20 and 1,000). Thus, many interesting clones may be masked in a high background screen and therefore missed by random pool sampling, leading to a failed screen. However, given the clear advantages of performing these screens in vivo, it would be useful to mine the complex repertoire for signatures of enriching clones that may be masked by a large background. One approach is to use next generation sequencing (NGS) to provide information regarding what antibody clones are in each pool, their abundance and their round-to-round enrichment18–20. In this study, we used transcardial perfusion to introduce a phage displayed scFv library into rat brain. After four rounds of screening, NGS data was generated from phage DNA pools and subsequently analyzed using the Antibody Mining Toolbox21. The diversity and round-to-round enrichment of the resulting phage pools were assessed. After choosing 12 scFv to pursue for further analysis based on the criteria of abundance and enrichment, we found two clones designated scFv 4, and scFv 40 that clearly show binding to the rat BBB.
Methods
Phage and bacteria
The phage display scFv library was a kind gift of Dr. James D. Marks. The library is of human origin and its construction and diversity were previously described22,23. The host bacteria strain was TG1 Escherichia coli (Agilent Technologies). All infections and phage purifications were performed using standard techniques described previously14,24. Briefly, infections were performed by static incubation of the phage with log phase TG1 grown in 2xYT media (16 g/L tryptone, 10 g/L yeast extract, and 5 g/L NaCl, pH=7.0) at 37°C for 30 minutes followed by another incubation with shaking at 37°C for 30 minutes. Phage were precipitated and purified using polyethylene glycol as a precipitant24.
Transcardial perfusion-based screen
A transcardial perfusion-based screen was performed in male Sprague-Dawley rats weighing between 220 and 250 grams purchased from Harlan Laboratories (Indianapolis, IN). The hearts of anesthetized rats were exposed and a hemostat was used to clamp the descending aorta after the branch to the carotid artery to direct perfusate flow to the brain. A 22 gauge catheter was inserted into the left ventricle of the heart and connected to a peristaltic pump. A small incision was made in the right atrium of the heart to allow the outflow of blood and perfusate. First a heparinized perfusate (0.9% NaCl, 0.4% NaNO2, 100 units/mL of heparin, and either 0.1% BSA for first three rounds or 1% goat serum for round 4) was perfused at 10 ml/min for 10 minutes to clear blood from the vasculature, then the phage library was perfused using the phage amounts detailed in Table 1 diluted in 10 ml of heparinized saline and perfused at 2 ml/min. Then, the rat vasculature was washed by perfusing 10 mL of heparinized perfusate containing either BSA or goat serum at 2 ml/minute to wash unbound phage from the vasculature.
Table 1.
Screen Progress Assessed by Phage Input and Recovery
| Round 1 | Round 2 | Round 3 | Round 4–12 | Round 4–11 | Round 4–8 | Round 4–4 | |
|---|---|---|---|---|---|---|---|
| Phage Input | 4.5×1011 | 1.5×1012 | 1.9×1012 | 1.5×1012 | 1.5×1011 | 1.5×108 | 1.5×104 |
| Phage Recovered | 7.3×105 | 2.4×106 | 1.12×107 | 5.39×106 | 1.0×106 | 2.7×104 | 4.0×103 |
| Fraction Recovered | 1.8×10−6 | 2.2×10−6 | 7.8×10−6 | 4.7×10−6 | 9.5×10−6 | 2.3×10−4 | 4.2×10−1 |
After perfusion, the rat brain was removed and placed in phosphate buffered saline (PBS, Sigma # D8537) on ice. Subsequently the cerebellum was removed, and the cortices dissected away from the white matter. Each cortex was then homogenized in the presence of 1 mL of lysis buffer (100 mM Triethanolamine in ddH2O) in a Dounce homogenizer. Finally, each homogenate was incubated with 10 ml of log growth phase TG1 E. Coli for 30 minutes at 37°C, and then shaken in a rotator for 30 minutes at 37°C for phage recovery. 100 μL of the infected bacteria was reserved for titering. The remainder was pelleted, resuspended and plated on 10 cm diameter 2xYT plates with 15 μg/ml tetracycline. The plates were incubated at 37°C overnight. The phage harboring bacteria were recovered and used to inoculate cultures for the next round of selection.
NGS Sample Preparation
Copies of the forward primers designed by D’Angelo et al., 201421 for the amplification of human CDR-H3’s were ordered from Integrated DNA Technologies (Coralville, IA). They were designed for a common annealing temperature and should prime ~94% of all human framework 3 regions on the variable heavy chain if up to four base mismatches are allowed21. We then designed a reverse primer that binds to the conserved linker region with a similar annealing temperature to the forward primers. The sequence was (5’ GAA CCG CCT CCA CCT GAG G 3’), and the resulting amplicon was expected to be around 150 base pairs.
Phage DNA minipreps were performed on 5 mL overnight cultures of the phage pools to be submitted for sequencing (Round 3, Round 4–4, Round 4–8, Round 4–11, and Round 4–12). PCR was performed on each of these libraries using Platinum Pfx DNA polymerase (Life Technologies #11708-0210) according to the manufacturer instructions for 15 cycles with an annealing temperature of 55°C. The DNA was then run on an agarose gel and the band at ~150 base pairs was isolated and recovered using a gel DNA purification kit (Zymo Research # D4008). The amplicons were then submitted to the University of Wisconsin Biotechnology Center for barcoding and synthesis-based sequencing on an Illumina HiSeq 2500 chip with a 150 base pair readout.
Analysis of NGS data using the Antibody Mining Toolbox
Overall quality of the sequence data was assessed by the FastQC program. Detailed quality information can be found in Supplemental Figures 1 and 2. The Antibody Mining Toolbox21 was used for both read quality filtering and CDR-H3 determination. The raw FASTQ files were individually submitted to the cdr3_pipleline.py script. The quality control and accuracy parameters were left on their default settings with the exception of the minimum read length was set to 150 base pairs. A phred score of 20 for each read was set as the quality cutoff. The file containing the binned CDR-H3 data for each of the pools resulting from the pipeline script was fed to cdr3_cluster.py script. This script uses the binned data from two or more screening rounds and collates the data based on the CDR-H3 sequence. One master file was created that compared all five of the sequenced libraries. This was used for Excel-based enrichment analysis. Four more cluster files were generated using cdr3_cluster.py to compare the round 3 library with each subsequent round 4. These four files were fed to the count_pairs.py script to generate four .csv files containing the data graphed in Figure 2. Once the binned data was imported to Excel via the output of cdr3_cluster.py, the data was sorted from most abundant to least abundant in Round 3. Then the number of reads for each CDR-H3 in each library was normalized to the total number of accepted reads in each sequenced DNA pool. This “fractional abundance” was used to calculate the fold change enrichment from Round 3 to the various Round pools.
Figure 2. Comparison of NGS data between screening rounds.
The number of sequencing reads for each individual CDR-H3 in the Round 3 pool is compared to each Round 4 pool. The color gradient represents the number of unique CDR-H3’s that possess that number of reads (e.g. red is 10 or greater CDR-H3s, purple is 1 CDR-H3). It should also be noted that the data points in the lower left of each graph most likely represent the errors in base calls, or errors due to PCR amplification.
Recovery and production of scFvs
Twelve CDR-H3’s were selected for further analysis. To recover the whole scFv containing these CDR-H3’s, a two-step PCR scheme was designed. First, a forward primer was designed that bound specifically to the unique CDR-H3 of interest. The forward primer that primes in the CDR-H3 was used in a PCR reaction with the reverse primer (5′-GAATTTTCTGTATGAGGTTTTGCTAAA-3′) binding to a constant region 3’ of the variable light chain sequence in phage backbone. In parallel, the reverse complement of the CDR-H3 primer was used in conjunction with a forward primer (5′-TTTTTGGAGATTTTCAACGTGA-3′) that binds to the phage backbone 5’ to the variable heavy chain encoding region. These two amplicons were gel purified and then assembled in a second PCR reaction using the forward and reverse PCR primers that hybridize to the vector backbone. The reaction product was again run on a 1% agarose gel and the band that appeared at ~750 base pairs was purified. The assembled product was double digested using the Fast Digest enzymes NcoI and NotI (Life Technologies), according to the manufacturer’s instructions and ligated into the similarly cut bacterial expression vector pSYN1 which contains the c-myc and His6 epitope tags. This was then transformed into TG1 bacteria and sanger sequenced to confirm the presence of the correct insert. Soluble scFv were prepared by bacterial expression, periplasmic extraction and HisPur Ni-NTA purification (Thermo Scientific) exactly as previously described14. The scFv concentrations were quantified using UV 280 absorbance and extinction coefficients generated by ExPASy (http://web.expasy.org/protparam/) in conjunction with SDS-PAGE stained with coomassie blue.
ScFv immunolabeling of rat brain tissue
Rat brains were snap frozen and 7 μm tissue sections were generated. Sections were immunolabeled as previously described14. Briefly, sections were thawed and air dried for 20 minutes. Sections were blocked in PBSG (PBS with 40% goat serum) for 30 minutes. ScFvs were artificially dimerized at a concentration of 50 μg/mL in PBSG via the c-myc tag with the monoclonal antibody 9E10 (Fisher Scientific) and incubated on the sections for 30 minutes on ice12. After two washes with PBS, sections were incubated with anti-mouse Alexa594 (Life Technologies) and LEA-FITC (Sigma) for 1 hour in PBSG. The sections were washed 3 times in PBS and then fixed for 10 minutes in 4% paraformaldehyde in PBS.
For fresh and unfrozen sections, a vibratome 1000 was used to generate 150 μm thick brain sections. These sections were blocked with 40% goat serum in PBS for 30 minutes. Afterwards, pre-dimerized scFvs at a concentration of 50 μg/mL in PBSG were incubated with the sections for 2.5 hours on ice. The sections were washed three times with PBS and then incubated with anti-mouse Alexa594 (Life Technologies) and LEA-FITC (Sigma) for 1 hour in PBSG. The sections were washed 3 times in PBS and then fixed for 10 minutes in 4% paraformaldehyde in PBS. Fixed sections (frozen or fresh) were washed 3 additional times and mounted with ProLong Gold mounting media containing DAPI (Life Technologies # 36935) and a coverslip. The finished slides were visualized on an Olympus fluorescence microscope connected to a Diagnostic Instruments camera run by MetaVue.
Results
Design and implementation of brain perfusion screen
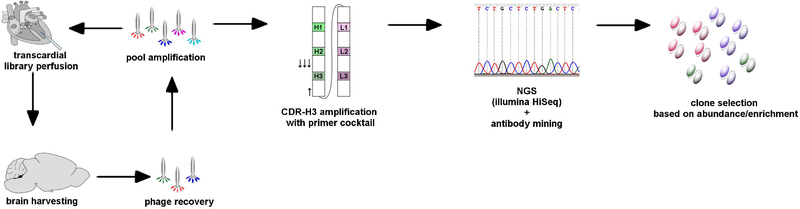
The nonimmune antibody library used in this study was comprised of human scFvs displayed on the surface of the filamentous phage fd-tet with an estimated diversity of 5×108 scFv clones22,23,25. While phage display is a viable option for in vivo screening, phage are quickly scavenged from the blood stream after intravenous administration, resulting in a serum half-life of only 15 minutes in rats17,26,27. To combat this problem, we instead implemented a perfusion-based screen in which the phage displayed libraries were transcardially perfused into rat brain by clamping the descending aorta (See Figure 1 for workflow). This approach also allowed brain vascular sampling of the full library diversity without having to pass through peripheral organs. Prior to the library perfusion, a heparinized saline solution was perfused to clear the circulatory system of blood (See Materials and Methods for details). For the first screening round, 4.5×1011 colony forming units (cfu) of the library were perfused, followed by a saline wash to limit the non-specific phage remaining in the vasculature. The brain was removed, and the cortex dissected from the cerebellum and white matter. The cortex was mechanically homogenized in triethanolamine-containing lysis buffer and the homogenate used to infect bacteria for phage recovery and expansion. For subsequent rounds, the titers of perfused phage are detailed in Table 1. Initially, four rounds of screening were performed. The titer of phage perfused as well as the amount recovered, and the fraction of the input phage that was recovered can be seen in Table 1. The absolute fractional recovery was quite low as has been observed previously with intravenously administered phage libraries15, likely a consequence of perfusion rates limiting the interaction of phage with the brain vasculature. However, the amount of phage recovered from round to round follows the expected pattern of low initial recovery in the first round, with two rounds of increasing phage recovery, and finally an attenuation of phage recovery in the fourth round (4–12).
Figure1. Brain perfusion screening workflow.
Phage display library pools were introduced into brain by transcardial perfusion. Brain tissue was removed, dissected and bound phage recovered. The recovered phage pools were amplified in bacteria and prepped for the next brain perfusion round. A total of four perfusion rounds were performed. NGS was then used to analyze Round 3 and various Round 4 outputs by sequencing CDR-H3 regions. Eighteen forward primers that bind to the various germline sequences in the framework 3 region on the variable heavy chain were used with a single reverse primer that binds to the conserved linker region to amplify the CDR-H3 region of the construct for use in NGS. The CDR-H3 abundance and enrichment were evaluated using the antibody mining toolbox to identify clones with favorable abundance and enrichment patterns. These clones were produced and assessed for brain tissue binding.
Noting the large number of phage recovered from brain cortices, even after Round 1, where there would likely be few true binders, it was apparent that a high non-specific phage recovery could be a problem for clonal analysis techniques. Thirty-eight random clones from the R4–12 library were selected for sequencing-based analysis. Of 36 insert-containing phage clones sequenced, there were no duplicated clones. These phage recovery and diversity results indicated that the complexity of the library was still very high after 4 rounds of perfusion screening, likely as a result of the high non-specific phage recovery. In an attempt to increase the stringency of the screen and lessen the impact of non-specific phage recovery, Round 4 of screening was repeated with lower numbers of perfused phage (Table 1). These are designated R4–11 (1.5×1011 phage), R4–8 (1.5×108 phage) and R4–4 (1.5×104 phage) with R4–12 (1.5×1012 phage) being the original Round 4. It is significant to note that the lower the input phage titer, the higher the fraction of phage recovered, perhaps suggesting a specific enrichment of BBB binders (Table 1). However, upon sequencing of approximately 30 random clones from each of the new R4 (90 clones total), the libraries were still of high complexity with very few duplicated sequences suggesting that while the fraction of phage recovered was high, the screen background was also high.
NGS characterization of library pools
To better explore the sequence diversity in the various pools and identify particular antibody clones that may warrant further investigation, NGS was used. Since the heavy chain CDR3 (CDR-H3) can often be used as representative of the antigen binding specificity of the scFv28, we sequenced CDR-H3 as a proxy for tracking an individual scFv from round to round. A previously described set of 18 forward primers that target the third variable heavy chain framework region in human antibody germline sequences21 was used along with a reverse primer in the conserved sequence of the (Gly4-Ser)3 linker (Figure 1). This suite of primers was used in multiplex PCR reactions to amplify the CDR-H3’s from the Round 3 pool and all of the subsequent Round 4 pools. Each of these pools of amplicons was barcoded and sequenced using the Illumina HiSeq2500 platform, with enough reads to oversample all of the estimated diversity within each pool. The overall quality of the sequencing run along with the per base quality for each barcoded pool submitted was analyzed. The majority of the sequencing reads had an average phred score between 28 and 36 (Supplemental Figure 1). The average phred score at each base position was above 20 for each pool, and was above 28 at most positions along the 150 base read indicating sequencing runs of acceptable quality (Supplemental Figure 2).
Next, the cdr3_pipline script from the antibody mining toolbox21 was used to exclude reads with an average phred score of less than 20, as well as to identify CDR-H3’s. The number of usable reads comprised >74% of the total reads for each pool (Table 2). The cdr3_pipline script identifies CDR-H3’s by keying on conserved sequences surrounding CDR-H3; namely, the cysteine that appears before the CDR-H3 and the tryptophan that appears after each CDR-H3. The total number of unique CDR-H3’s discovered across all libraries was 5.9×106. The number of unique CDR-H3’s found in each pool decreased somewhat with the decreasing amount of phage input, but the overall numbers remained high and ruled out any possibility of even marginally comprehensive discrete sampling. Next, to compare how each Round 4 pool compared to the Round 3 parent pool, the number of reads for each CDR-H3 in the Round 3 pool were compared to that in each Round 4 pool. On a pool-wide basis, there was clear similarity between the Round 3 and Round 4 pools (Figure 2). Divergence from the Round 3 pool was more substantial as the input phage titer in the screen was reduced (correlation coefficients: Round 4–4=0.40, Round 4–8=0.65, Round 4–11=0.82, and Round 4–12=0.81), suggestive of a benefit in screen stringency with reduced input titer. In addition, each Round 4 pool also exhibited an enriching tail of transcripts at high abundance. These could potentially represent binding scFv clones or alternatively, those that drive a phage growth bias, allowing their non-specific enrichment from round to round.
Table 2.
Next Generation Quality Control Overview
| Library | Usable Reads | Total Reads | % Sequence Accepted | Unique CDR-H3 |
|---|---|---|---|---|
| Round 3 | 1.6×107 | 1.9×107 | 81.6 | 3.28×105 |
| Round 4–12 | 1.6×107 | 2.2×107 | 74.3 | 1.86×105 |
| Round 4–11 | 2.0×107 | 2.5×107 | 79.7 | 1.63×105 |
| Round 4–8 | 1.4×107 | 1.7×107 | 84.2 | 1.06×105 |
| Round 4–4 | 1.7×107 | 2.3×107 | 76.2 | 8.60×104 |
In order to select individual CDR-H3’s from the various pools for further analysis, two criteria were evaluated: Abundance within each pool, and fold change from round 3 to round 4. The cdr3_cluster script was used to compare all of pools associating the CDR-H3 amino acid sequence with its abundance in each pool, and the data was then sorted according to number of reads in Round 3 (Figure 3). For a successful screen, one would expect to see high abundance and high enrichment clones exhibit rat BBB binding. Therefore, a subset of CDR-H3’s that satisfied these criteria were selected for further analysis (Figure 3). Twelve clones were selected that were of high abundance and enriched in each Round 4 screen. Clones 8 and 10 were selected as examples where the potentially more stringent Round 4–4 screen exhibited an enhanced enrichment. The selected CDR-H3 sequences and associated NGS data can be found in Table 3. It is interesting to note that even though the selected clones were present in the various Round 4 pools at an abundance ranging between ~0.5% and 5% of each population, only clones 2, 6, 10 and 11 were also found in the 128 clones that were randomly chosen and individually sequenced from the Round 4 pools.
Figure 3. Analysis of CDR-H3 abundance and enrichment.
The Round 3 to Round 4 enrichments for the 60 most abundant CDR-H3s are presented as fold increase for each Round 4 pool as denoted in the inset legend. Clones are arranged from most to least abundant in the Round 3 pool (1 to 60). The red wedges denote the CDR-H3s that were selected for further analysis.
Table 3.
Summary Table for Selected scFv Clones
| Clone | CDR-H3 | % of R3 | % of R4–4 | % of R4–8 | % of R4–11 | % of R4–12 | Expressed | BBB Bind |
|---|---|---|---|---|---|---|---|---|
| 1 | GALQSGSYYPPGY | 1.59 | 4.29 | 2.50 | 5.46 | 4.60 | - | - |
| 2 | DNGEY | 0.83 | 2.78 | 2.69 | 3.30 | 3.35 | + | - |
| 4 | AWDSYSRKPDY | 0.81 | 2.42 | 1.77 | 3.64 | 3.01 | + | + |
| 6 | ADGGNSDY | 0.66 | 4.25 | 3.17 | 2.96 | 2.96 | + | - |
| 8 | GSMVRGPYPRFDP | 0.48 | 8.75 | 1.67 | 2.74 | 2.55 | - | - |
| 10 | ETGAYYYYGMDV | 0.48 | 4.84 | 0.68 | 0.95 | 0.86 | + | - |
| 11 | DRWLQNWERPFDY | 0.43 | 3.24 | 3.16 | 2.77 | 3.05 | + | - |
| 17 | YYYDSSAFMTGN | 0.37 | 1.97 | 2.40 | 1.34 | 1.24 | - | - |
| 18 | DLYDSSGNLHGNWFDP | 0.36 | 4.16 | 1.45 | 2.20 | 1.69 | - | - |
| 23 | DLHLSIAAAGTGVFKTPLRDY | 0.26 | 1.37 | 1.06 | 1.17 | 1.31 | + | - |
| 40 | LHSEDSSGWGVFDI | 0.17 | 4.98 | 2.66 | 1.00 | 1.22 | + | + |
| 59 | RLGYSAPGDY | 0.13 | 0.42 | 0.87 | 0.61 | 0.69 | + | - |
Recovery, production and analysis of selected scFvs
To recover the entire scFv coding region that is associated with each CDR-H3, primers designed specifically for each CDR-H3 were used to PCR amplify each of the 12 selected scFvs. The open reading frames were subsequently cloned into a bacterial expression vector for production as soluble scFvs. Four individual bacterial transformants resulting from each CDR-H3 based PCR recovery process were sequenced. In all 12 cases, at least one transformant had the exact CDR-H3 sequence that appeared in the NGS analysis, and these clones were used for scFv production and purification. Of the twelve scFvs, only 8 expressed at suitable levels for binding analysis (scFvs 2, 4, 6, 10, 11, 23, 40 and 59) (Figure 4).
Figure 4. Expression of scFv clones.
ScFv clones were expressed and purified from bacterial lysates as described in the methods section and normalized for total protein concentration. Western blotting for the epitope tag using an anti-c-myc antibody was then used to assay for protein production. ScFv clones are listed by their abundance number and ABN is a negative control scFv that recognizes botulinum neurotoxin. The primary scFv band was found at ~28 kDa at the expected size of the intact scFv, with the smaller bands likely being unpaired variable chains and other truncated products.
To determine if any of the selected scFvs bound to the rat BBB, scFvs were used to immunolabel either fresh frozen brain tissue sections (Figure 5) or fresh, never frozen, vibratome cut rat brain tissue to ensure that antigens were not disrupted by processing since the screen was performed with living tissue (Supplemental Figure 3). Of the 8 scFvs tested, 6 (scFvs 2, 6, 10, 11, 23, 59) did not exhibit binding to the rat BBB in either brain tissue section format. However, 2 scFvs (4 and 40) clearly bound antigens in rat brain in both frozen and fresh brain tissue sections (Figure 5 and supplemental Figure 3). ScFv 40 bound specifically to brain microvessels with continuous vessel labeling pattern similar to that of the anti-transferrin receptor antibody, OX26. However, unlike OX26 labeling which can be found throughout the brain, scFv 40 labeling was found only in small ventral brain regions, and infrequently appeared elsewhere throughout the brain cortex. In contrast to scFv 40, scFv 4 bound to both brain microvessels and to extended cellular processes that wrapped brain blood vessels, likely astrocytes (Figure 5 and supplemental Figure 3). Also, scFv4 binding could be observed throughout the brain. These two scFvs were not identified in the discrete sampling of 128 clones noted above, indicating the value of combining NGS with clonal evaluation.
Figure 5. Immunohistochemical analysis of selected scFv.
Freshly frozen 7μm rat brain tissue sections were immunolabeled with 50 μg/mL of scFv that had been pre-dimerized with anti c-myc antibody. Because scFv 40 could not be made in large quantities, a 4 μg/mL concentration was used. OX26 anti-transferrin receptor antibody was used as a positive binding control and ABN was used as a negative binding control. The sections were co-labeled with LEA-FITC to identify the brain microvessels. Enlarged inset is included to demonstrate that both the vascular wall and the cell processes are recognized by scFv40. The scale bar is 100 μm for each of the image panels, and the inset scale bar is 25 μm.
Discussion
In this study, we sought to identify rat BBB targeting scFv using in vivo phage display methods. While in vivo screens represent the highest likelihood for discovery of novel brain targeting molecules, these screens have had limited success, likely because of inherently high background. Indeed, we observed a high background of non-specific phage accumulation that made discrete sampling of potential brain targeting scFvs infeasible. However, NGS was used to address this issue and identify scFvs that were enriched by screening round to round and that spanned a spectrum of abundance. These approaches resulted in the identification of scFv 4 and scFv 40 that bind to the rat BBB.
The use of NGS to follow the progress of combinatorial screening efforts is becoming more widely utilized29,30. Traditionally, it has been very difficult to track round-by-round clonal enrichment during a screen without substantial cost and effort. NGS now enables the monitoring of individual sequences across a screen at a combinatorial level, ultimately aiding in the assessment and success of the screen20. In our case, NGS allowed the intelligent selection of specific scFv clones for downstream phenotypic analysis. Often when NGS is applied to combinatorial screens such as antibody affinity maturation, the most abundant sequences are often those with the most desirable properties, and NGS assures that random sampling of clones will not miss these desirable clones. For example, scFv 4 was abundant in our screen, but was not sampled in 128 randomly picked scFvs. However, some of the most abundant clones such as scFv2 did not bind the rat BBB. Thus, NGS also allows researchers to navigate the high background of an in vivo phage display screen to examine clones that may be much lower in abundance, but that are still enriching from round to round. For example, scFv 40 was a clone that enriched from Round 3 to Round 4 but had a comparatively low abundance that also was not observed in the randomly picked clones. It is also important to note that we did not sample the library diversity comprehensively, but assayed the scFv that, in our estimation, represented the highest likelihood of binding to the BBB. Thus, it is likely that phenotypically interesting scFvs may be in the pools at lower abundance. One approach to identify additional scFvs may be to further mine the NGS data exploring scFvs lower on the abundance scale than just the top 60 most represented CDR-H3’s analyzed here, and selecting those that display the most significant fold change between Round 3 and the various Round 4 screens.
To our knowledge scFv 4 and scFv 40 are the first BBB binding antibody fragments to have been recovered from an in vivo screen. While there are a variety of approaches to screen for targeting antibodies, in vivo phage display can be a powerful approach to target relevant antigens in their natural cellular and structural environment. For instance, it has been shown that an antibody which may bind in vitro, may not work well in vivo if the specific epitope is too close to the cell surface as to be sterically inaccessible31,32. Given the nature of the perfusion screen and limited contact time between phage and the BBB, the scFv binders are likely not additionally filtered for their endocytosis or transcytosis capacity. However, it has been shown that a subset of scFvs isolated in BBB binding screens can also endocytose into brain endothelial cells12. Thus, if one were to further examine the BBB binding repertoire described here (scFv 4, scFv 40 others in Figure 3), it is likely that a subset of scFvs with trafficking properties would be identified. In conclusion, historically difficult in vivo phage display screens can be combined with NGS and data mining tools to identify new targeting antibodies against the accessible BBB proteome.
Supplementary Material
Acknowledgments
We would like to thank Dr. James D. Marks for the kind gift of the phage display scFv library. This work was supported by grants from the National Institutes of Health (NS071513 and NS099158) and the Defense Threat Reduction Agency (HDTRA1-15-1-0012).
Literature Cited
- 1.Stutz C, Zhang X, Shusta E. Combinatorial approaches for the identification of bran drug delivery targets. Current Pharmaceutical Design. 2014;20(10):1564–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goulatis LI, Shusta EV. Protein engineering approaches for regulating blood-brain barrier transcytosis. Current opinion in structural biology. 2017;45:109–115. [DOI] [PubMed] [Google Scholar]
- 3.Yu YJ, Zhang Y, Kenrick M, et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Science Translational Medicine. 2011;3(84). [DOI] [PubMed] [Google Scholar]
- 4.Boado RJ, Zhang YF, Zhang Y, Pardridge WM. Humanization of anti-human insulin receptor antibody for drug targeting across the human blood-brain barrier. Biotechnology and Bioengineering. 2007;96(2):381–391. [DOI] [PubMed] [Google Scholar]
- 5.Zuchero YJ, Chen X, Bien-Ly N, et al. Discovery of Novel Blood-Brain Barrier Targets to Enhance Brain Uptake of Therapeutic Antibodies. Neuron. 2016;89(1):70–82. [DOI] [PubMed] [Google Scholar]
- 6.Enerson BE, Drewes LR. The rat blood-brain barrier transcriptome. Journal of Cerebral Blood Flow and Metabolism. 2006;26(7):959–973. [DOI] [PubMed] [Google Scholar]
- 7.Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: A new resource for understanding the development and function of brain endothelial cells. Plos One. 2010;5(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muruganandam A, Tanha J, Narang S, Stanimirovic D. Selection of phage-displayed llama single-domain antibodies that transmigrate across human blood-brain barrier endothelium. FASEB Journal. 2001;15(14):240. [DOI] [PubMed] [Google Scholar]
- 9.Abulrob A, Sprong H, Henegouwen P, Stanimirovic D. The blood-brain barrier transmigrating single domain antibody: mechanisms of transport and antigenic epitopes in human brain endothelial cells. Journal of Neurochemistry. 2005;95(4):1201–1214. [DOI] [PubMed] [Google Scholar]
- 10.Farrington GK, Caram-Salas N, Haqqani AS, et al. A novel platform for engineering blood-brain barrier-crossing bispecific biologics. FASEB Journal. 2014;28(11):4764–4778. [DOI] [PubMed] [Google Scholar]
- 11.Helms HC, Abbott NJ, Burek M, et al. In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. Journal of Cerebral Blood Flow and Metabolism. 2016;36(5):862–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang XX, Cho YK, Shusta EV. Mining a yeast library for brain endothelial cell-binding antibodies. Nature Methods. 2007;4(2):143–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Wang XX, Shusta EV. Creation and Evaluation of a Single-chain Antibody Tetramer that Targets Brain Endothelial Cells. AIChE Journal. 2014;60(4):1245–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones A, Stutz C, Zhou Y, Marks J, Shusta E. Identifying blood-brain barrier selective single-chain antibody fragments. Biotechnology Journal. 2014;5:664–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996;380(6572):364–366. [DOI] [PubMed] [Google Scholar]
- 16.Urich E, Schmucki R, Ruderisch N, et al. Cargo Delivery into the Brain by in vivo identified Transport Peptides. Scientific Reports. 2015;5:14104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roodink I, Franssen M, Zuidscherwoude M, et al. Isolation of targeting nanobodies against co-opted tumor vasculature. Laboratory Investigation. 2010;90(1):61–67. [DOI] [PubMed] [Google Scholar]
- 18.Niedringhaus TP, Milanova D, Kerby MB, Snyder MP, Barron AE. Landscape of next-generation sequencing technologies. Analytical Chemistry. 2011;83(12):4327–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georgiou G, Ippolito GC, Beausang J, Busse CE, Wardemann H, Quake SR. The promise and challenge of high-throughput sequencing of the antibody repertoire. Nature Biotechnology. 2014;32(2):158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravn U, Didelot G, Venet S, et al. Deep sequencing of phage display libraries to support antibody discovery. Methods. 2013;60(1):99–110. [DOI] [PubMed] [Google Scholar]
- 21.D’Angelo S, Glanville J, Ferrara F, et al. The antibody mining toolbox: An open source tool for the rapid analysis of antibody repertoires. Mabs. 2014;6(1):160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Connell D, Becerril B, Roy-Burman A, Daws M, Marks JD. Phage versus phagemid libraries for generation of human monoclonal antibodies. Journal of Molecular Biology. 2002;321(1):49–56. [DOI] [PubMed] [Google Scholar]
- 23.Sheets MD, Amersdorfer P, Finnern R, et al. Efficient construction of a large nonimmune phage antibody library: The production of high-affinity human single-chain antibodies to protein antigens. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(11):6157–6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Y, Marks JD. Identification of target and function specific antibodies for effective drug delivery In: Dimitrov A, ed. Therapeutic Antibodies: Methods and Protocols. Totowa: Humana Press; 2009:145–160. [DOI] [PubMed] [Google Scholar]
- 25.Poul MA, Becerril B, Nielsen UB, Morisson P, Marks JD. Selection of tumor-specific internalizing human antibodies from phage libraries. Journal of Molecular Biology. 2000;301(5):1149–1161. [DOI] [PubMed] [Google Scholar]
- 26.Chen YH, Chang M, Davidson BL. Molecular signatures of disease brain endothelia provide new sites for CNS-directed enzyme therapy. Nat Med. 2009;15(10):1215–U1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valadon P, Garnett JD, Testa JE, Bauerle M, Oh P, Schnitzer JE. Screening phage display libraries for organ-specific vascular immunotargeting in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(2):407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu JL, Davis MM. Diversity in the CDR3 region of V-H is sufficient for most antibody specificities. Immunity. 2000;13(1):37–45. [DOI] [PubMed] [Google Scholar]
- 29.Hu D, Hu S, Wan W, et al. Effective Optimization of Antibody Affinity by Phage Display Integrated with High-Throughput DNA Synthesis and Sequencing Technologies. PLoS One. 2015;10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemadou A, Giudicelli V, Smith ML, et al. Pacific Biosciences Sequencing and IMGT/HighV-QUEST Analysis of Full-Length Single Chain Fragment Variable from an In Vivo Selected Phage-Display Combinatorial Library. Frontiers in immunology. 2017;8:1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones AR, Shusta EV. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharmaceutical Research. 2007;24(9):1759–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muro S Challenges in design and characterization of ligand-targeted drug delivery systems. Journal of Controlled Release. 2012;164(2):125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.