Abstract
Achalasia is a rare esophageal motility disorder that necessitates the disruption of the lower esophageal sphincter. Patients with achalasia should be evaluated in a systematic, multidisciplinary fashion. Workup should include upper endoscopy, esophagography, and high-resolution manometry. The gold standard for surgical treatment is laparoscopic Heller myotomy with partial fundoplication. Per-oral esophageal myotomy is a novel endoscopic technique that has gained considerable traction over the past decade. The procedure includes the creation of a submucosal tunnel and a selective circular myotomy of the lower esophageal sphincter. Common intra-operative hazards include bleeding within the submucosal tunnel and capnoperitoneum. Significant complications are rare. Patients experience excellent dysphagia relief that is on par with laparoscopic Heller myotomy at moderate-term follow up. Post-operative gastroesophageal reflux disease occurs in greater than one-third of patients, and the vast majority of cases are readily controlled with an anti-secretory medication. Although data is sparse, there is a growing body of literature that supports the long-term durability of per-oral esophageal myotomy.
Keywords: Endoscopic Submucosal Dissection, Motility, Foregut Surgery, Esophagus, Endoscopic Surgery
1. Introduction
Idiopathic achalasia is a rare esophageal motility disorder that affects 1 – 2 / 100,000 individuals worldwide1. Derived from the Greek a-khalasis (without loosening), the disorder is characterized by a failure of lower esophageal sphincter relaxation and absent or highly disordered peristalsis. The etiology of achalasia is unknown. The pathophysiology is highlighted by a functional loss of myenteric plexus ganglion cells in the distal esophagus and lower esophageal sphincter1, 2. Patients present with months to years of dysphagia and regurgitation. Chest pain and rapid weight loss may also occur.3
The gold standard surgical treatment for achalasia is a palliative division of the lower esophageal sphincter via laparoscopic Heller myotomy4–6. In 2010, Inoue and colleagues described their experience with per-oral esophageal myotomy (POEM), a novel technique that incorporates principles of endoscopic submucosal dissection (ESD)7. The procedure has since been utilized worldwide for the treatment of all achalasia subtypes and other achalasia variants8, 9. Although a plethora of short- and moderate-term data are available, there are few studies that examine the long-term outcomes of POEM10–15. Examinations of laparoscopic Heller myotomy versus POEM are often comparative, single-institution studies that employ historical controls16. Despite these limitations, the technique remains an important tool in the procedural armamentarium. Herein, we discuss the pre-operative patient evaluation, procedural details, and outcomes associated with POEM for idiopathic achalasia.
2. Pre-Operative Evaluation
2.1. Institutional Experience
Given the rarity of achalasia, evaluation and management should take place in a tertiary or quaternary medical center with significant achalasia experience and volume3. A multidisciplinary team of gastroenterologists, gastrointestinal and/or thoracic surgeons, and ancillary staff should be utilized throughout all phases of care. Complex cases should be reviewed on a regular basis by representatives from each care team. At our institution, challenging cases are reviewed at a biweekly multidisciplinary conference, wherein multiple members of each care team share their experience and recommendations. We have found this method to be of critical importance in providing comprehensive evidenced-based care to our patients.
2.2. Diagnostic Tests
The most common validated symptom assessment for patients with achalasia is the Eckardt Score17 (Table 1). The method utilizes a 3-point scale to note the frequency of four symptom domains: dysphagia, regurgitation, chest pain, and weight loss. Each domain is scored from 0 to 3, with higher scores representing worse disease severity. Treatment success is most often defined as a score ≤3 and correlates with physiologic outcomes18. Although commonly distributed, this scoring system is not immune to test reliability limitations, most notably in the chest pain and weight loss domains. A recent study by Taft et al demonstrated that weight loss and chest pain each account for ~ 10% of the variance seen in Eckardt Score19. These figures, coupled with modest overall validity and reliability, suggest that this long-used assessment may require revision in the near future.
Table 1:
The Eckardt Symptom Score. The sum of all four domains produces a score ranging from 0–12, with higher scores denoting worse disease severity.
| Symptom | Score | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Dysphagia | None | Occasional | Daily | With Each Meal |
| Regurgitation | None | Occasional | Daily | With Each Meal |
| Chest Pain | None | Occasional | Daily | Several Times Per Day |
| Weight Loss (kg) | 0 | <5 | 5–10 | >10 |
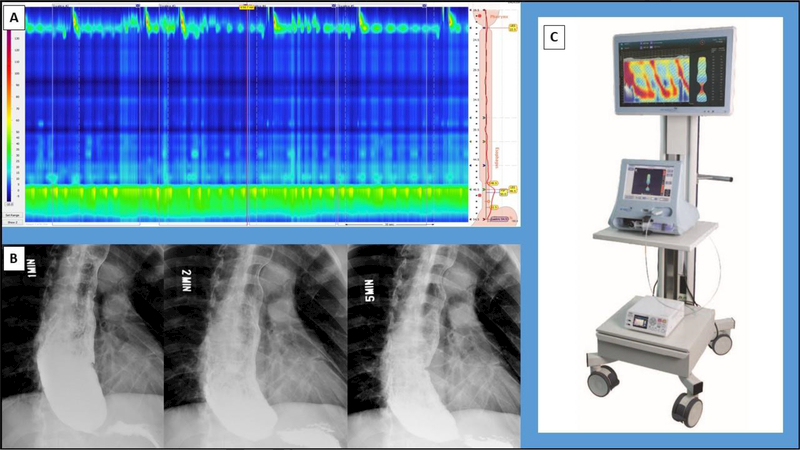
Other diagnostic studies include esophagogastroduodenoscopy (EGD), high-resolution manometry, timed barium esophagram, and the functional lumen imaging probe. Evaluation with EGD is mandatory to exclude alternative diagnoses, such as pseudoachalasia. Data from high-resolution manometry (HRM) is used in conjunction with the Chicago Classification v3.0 for the diagnosis and subtyping of patients with achalasia into meaningful treatment/prognostic groups20, 21 (Figure 1A). High-resolution impedance manometry (HRIM) is an emerging method of manometry that offers novel metrics for the monitoring of post-treatment achalasia patients22 The timed barium esophagram (TBE) provides an objective measure of esophageal emptying, the size/angulation of the esophagus, and the presence of a hiatal hernia23 (Figure 2B). The functional lumen imaging probe is a novel catheter-based device that measures esophagogastric junction distensibility index (DI) in real-time (Figure 2C). Previous studies have demonstrated DI to be a strong predictor of post-treatment clinical recurrence in patients with achalasia24, 25.
Figure 1:
Diagnostic modalities for patients with achalasia. (A) Esophageal pressure topography plot from a high-resolution manometry study of a patient with Type 1 achalasia. (B) Timed barium esophagram from the same patient. (C) EndoFLIP® 2.0 System (Medtronic, Minneapolis, MN).
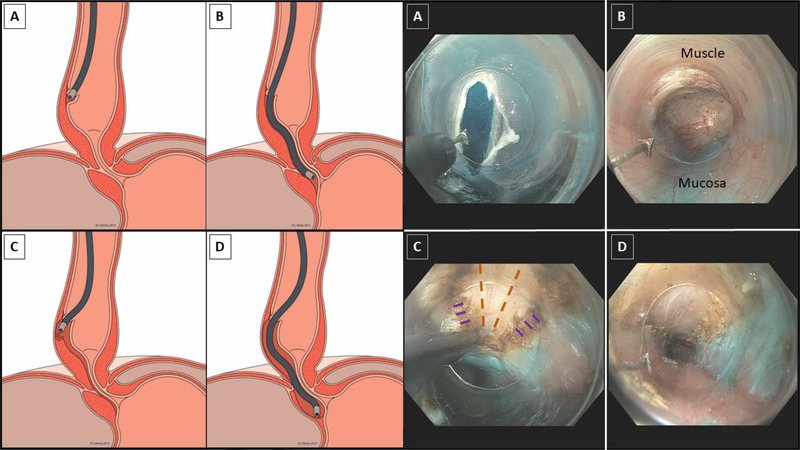
Figure 2:
Animated and endoscopic depictions of per-oral esophageal myotomy. (A) A 1–2 cm mucosotomy is made with an electrocautery knife. (B) Within the submucosal tunnel, the muscle layers are oriented anteriorly and the mucosa is oriented posteriorly. (C) An electrocautery knife is used to fashion a selective circular myotomy, producing the cut circular muscle edges (purple dashes) and revealing the underlying longitudinal muscle (orange dashed lines). (D) Completed myotomy. Note the cut circular muscle edges and intervening longitudinal muscle, which may splay in segments of the myotomy. Animations reprinted with permission of Eric Hungness and David Botts, Northwestern University (25).
2.3. Prerequisite Training
The technical aspects of POEM closely resemble an endoscopic submucosal dissection7. Although experience with ESD in not an absolute pre-requisite, the practitioner should have demonstrable advanced endoscopic skills. It is recommended that (s)he spends time in the clinical laboratory with POEM models and/or at an instructed course. Moreover, practitioners new to this technique should enlist the help of an experienced proctor for their initial case.
Several experienced POEM centers have described the technique’s procedural learning curve26–29. Operative metrics that delineate proficiency include the total time of procedure, inadvertent mucosotomies, and total number of clips. Estimates for the case number required to reach the “learning curve plateau” range from 15–60 cases26.
3. Procedure
3.1. Patient and Care Team Preparation
Patients are prescribed a 7-day course of oral fluconazole for the week preceding their myotomy. They are maintained on a clear liquid diet for the final two preoperative days and kept nil per os (NPO) the night before surgery. Anticoagulant and antiplatelet therapy are held according to established protocols30.
POEM is most-often performed with a specialized team of care providers in an operating room or advanced endoscopy suite. Each team member must be familiar with disease-based and procedural needs unique to patients with achalasia. Endoscopic equipment and radiographic images should be prepared prior to the patient entering the room. Of note, tools for rapid decompression of the chest and abdomen should be readily available.
3.2. Per-Oral Esophageal Myotomy
We employ a POEM method that is similar to Inoue and others7. Herein, we describe our institution-specific protocol in detail. Many variants of this technique have been described, and it behooves the novice POEM provider to use methods that are most familiar to their practice.8 As with any procedural endeavor, adherence to fundamental surgical principles is critical. Proficiency with each step of the operation will help deter complications.
In our practice, the patient is brought to the operating room and placed in the supine position. After adequate pre-oxygenation, a “rapid sequence” endotracheal intubation is performed with succinylcholine and propofol. Special attention is given to additional aspiration precautions, including the application of cricoid pressure and liberal oropharyngeal suction. If there is any appreciable concern for a challenging airway, fiberoptic intubation is employed. Esophagoscopy is performed for inspection and removal of any residual debris. The operation is aborted in the presence of Candida esophagitis or significant solid food burden.
An angled dissecting cap is affixed to the distal end of the gastroscope. A submucosal wheal is made 12 cm above the squamocolumnar junction with a sclerotherapy needle. The wheal is placed at a more proximal location in the case of an extended myotomy (e.g. during the treatment of Type III achalasia), as dictated by pre-operative HRM. We use a dilute indigo carmine or methylene blue solution, to which epinephrine is added for the first injection. The vast majority of our POEMs are approached anteriorly, wherein the submucosal bleb is raised at the 1 to 2 o’clock position26. We reserve the posterior approach for redo- or exceptionally challenging cases, while others employ this method on a routine basis31, 32.
A 1–2 cm longitudinal mucosotomy is made with a triangular-tip electrocautery knife and the underlying submucosal connective tissue is cleared away (Figure 2A). Some institutions make use of the T-type hybrid knife, which combines saline injection and electrocautery in one instrument.33 The endoscope is maneuvered into the mucosotomy, revealing the submucosal space. We routinely orient the gastroscope such that the circular muscle is visualized anteriorly and the mucosa lies posteriorly (Figure 2B). The submucosal fibers are divided using a combination of electrocautery and blunt dissection, with dissection erring to the side of the muscle fibers. Visualization is aided by periodic hydrodissection as the tunnel progresses along the length of esophagus. Other centers use through-the-scope dilating balloons to bluntly dissect segments of the submucosal space.34
The esophagogastric junction (EGJ) is identified by a narrowing of the muscle/mucosa interface, palisading or large caliber vessels, and endoscopic measurements8. A meticulous dissection must be carried out, as this is the most common location for inadvertent mucosal perforation29. The distal extent of the EGJ is marked by a sudden widening of the mucosa/muscle interface. The dissection is extended 2–3 cm beyond this point, after which the endoscope is withdrawn from slowly to inspect for bleeding. Tunnel orientation and adequate extension onto the stomach are confirmed via esophagoscopy and retroflexion in the true lumen of the stomach.
A myotomy is initiated 6 cm proximal to the EGJ, ensuring at least 3 cm of intact mucosa between the mucosotomy and myotomy. The pointed edge of the electrocautery knife is introduced into the muscle and a selective circular myotomy is developed along the intermuscular plane (Figure 2C). The myotomy proceeds in a proximal-to-distal fashion at most institutions. However, some centers routinely employ a distal-to-proximal technique.9 It is imperative to carry the myotomy 2–3 cm past the EGJ, as persistent symptoms are often due to an inadequate distal myotomy35. Splaying of the longitudinal muscle fibers is common, and some areas my exhibit a full-thickness myotomy The total myotomy length is typically 8 to 10 cm for Type I/II patients36. Adequacy of the myotomy can be assessed endoscopically, as indicated by the visual appearance of the intraluminal EGJ and the ease of scope passage (Figure 2D). Intra-operative FLIP can also provide a quantitative measure of improved EGJ distensibility.14, 24. The tunnel is irrigated with an antibiotic solution and the mucosotomy is closed with 5–10 clips37.
3.3. Common Intra-Operative Challenges
Bleeding is a common intraoperative test that necessitates diligence, patience, and skill. The systolic blood pressure should remain below 120 mmHg for the entire case, as even mild hypertension can engorge the friable submucosal vessels. Mild bleeding is controlled with electrocautery, while larger vessels should be prophylactically divided with a coagulation grasper. Bleeding that obscures visualization should prompt the use of an external irrigation system or the withdrawal and application of direct pressure to the tunnel via the endoscope. Installation of a dilute epinephrine solution into the tunnel has also been described38. The application of high-pressure variceal balloons should be avoided in the setting of a new myotomy, given the substantial risk of esophageal perforation.
Capnoperitoneum occurs in 20–40% of cases and should not be considered a complication27, 39. This is most often characterized by progressive abdominal distension despite adequate gastric suctioning. Abdominal decompression with a Veress needle is both quick and effective. Capnothorax is unusual and resulting hemodynamic compromise is exceedingly rare40, 41. Nevertheless, instruments to rapidly decompress the chest should be available at all times.
3.4. Post-Operative Care
Prophylactic anticoagulation is initiated six hours after surgery. Patients are given clear liquids on the evening of surgery if they are not experiencing significant nausea. We no longer perform a routine esophagram in the immediate post-operative period, as we’ve previously demonstrated its low specificity for clinically relevant complications42. However, we recommend that novice practitioners obtain routine esophagography during their early POEM experience. On rare occasion, a motivated patient can be discharged on post-operative day zero; the vast majority leave on post-operative day 1.
Patients are advanced to a soft mechanical diet after one week and solid foods at 3–4 weeks post-operatively. Routine clinic evaluation takes place 2–4 weeks following the procedure. Patients are maintained on a proton-pump inhibitor until they undergo pH testing approximately 6 months post-operatively. EGD, HRIM, TBE, and a clinical symptom assessment are also obtained at this time.
Although rare, significant complications may arise in the early post-operative period. Esophageal perforation, pneumothorax, or any complication requiring re-intervention occur in less than 1% of cases43. The vast majority of intra-operative and post-operative complications arise while the proceduralist is traversing the learning curve26, 29, 38. Special care must be taken to rapidly adopt a standardized protocol during this time.
4. Outcomes
4.1. Symptoms
POEM provides excellent symptom relief on short- and moderate-term follow up, as evidenced by a reduction in the Eckardt symptom score. Success rates (defined as an Eckardt score of ≤3) range from 9095% at 1- to 2-year follow up44, 45 (Table 2). Symptom relief is somewhat attenuated in the long-term but remains well over 80% at five years13. This efficacy is comparable to laparoscopic Heller myotomy16, 39. POEM has also demonstrated similar operative times, post-operative analgesic requirements, and complications, with a significant reduction in hospital length of stay45, 46. Moreover, there is evidence to suggest that POEM offers better symptom relief for patients with Type III achalasia as compared to LHM, likely due to its ability for an extended proximal myotomy47, 48.
Table 2:
Clinical and physiologic outcomes at moderate- to long-term follow up after per-oral esophageal myotomy
| Report (Year) | Patients | Follow Up (months) |
Clinical Successa | EGJ Relaxation Pressure (mmHg) (Pre vs Post) |
TBE Clearance (%) or Column Height (cm) (Pre vs Post) |
Objective GERDb |
|---|---|---|---|---|---|---|
| Inoue (2015) | 500 | >36 | 89% | 25 vs 12 | - | 24% (45/191) |
| Chen (2015) | 45 | 24 | 100% (45/45) |
25 vs 11 | - | - |
| Werner (2015) | 80 | 29 | 79% (62/79) |
32 vs 10 | NR vs 94% | - |
| Hungness (2016) | 112 | 28 | 92% (103/112) |
31 vs 12 | 14.2 cm vs 3.4 cm | 40% (27/68) |
| Teitelbaum (2018) | 36 | 65 | 83% (19/23) |
23 vs 9 | 50% vs 92% |
13% (2/16) |
Abbreviations: EGJ – Esophagogastric Junction; TBE – Timed Barium Esophagram; GERD – Gastroesophageal Reflux Disease
Eckardt Score ≤ 3
As determined by endoscopic evaluation or pH study
4.2. Physiologic Studies
Patients experience a significant reduction in basal EGJ pressure following POEM11–13 (Table 2). There is also a sustained reduction in the EGJ distensibility index, as evidenced by post-operative impedance planimetry18. Barium retention is improved in the post-operative period and correlates with symptom persistence/recurrence10. We strongly encourage the routine monitoring of physiologic parameters postoperatively at 2–3 year intervals, including evaluation with EGD, TBE, HRIM, and a wireless or catheterbased pH study.
4.3. Gastroesophageal Reflux Disease
Post-operative gastroesophageal reflux disease (GERD) following surgical myotomy for achalasia is an oft-debated topic. The drivers of this controversy include an evolving comprehension of reflux pathophysiology, heterogeneous GERD assessment tools, and a paucity of high-quality outcomes data.
An in-depth discussion of GERD pathophysiology is beyond the scope of this manuscript. In short, reflux of gastric contents into the esophagus is a physiologic phenomenon seen in healthy, asymptomatic controls49. Physiologic reflux events are most often caused by transient lower esophageal sphincter relaxations, after which the refluxed contents are propulsed back into the stomach. The anti-reflux barrier itself is a complex mechanism that includes the lower esophageal sphincter, phrenoesophageal membrane, diaphragmatic crura, and the angle of His49. POEM provides a more focused disruption of the anti-reflux barrier, while LHM confers the advantage of a reconstituting fundoplication. However, the long-term physiologic impact on the anti-reflux barrier remains poorly understood. Moreover, post-myotomy reflux may be attributable to multiple causes, including poor acid clearance of normal reflux events, an impaired anti-reflux barrier, and/or visceral hypersensitivity50.
The assessment of GERD in post-treatment achalasia patients may include a basic history, symptom questionnaire, endoscopic evaluation, and a wireless or catheter-based pH study. When used together, these modalities provide a reasonable approximation for the burden of disease in a given patient. However, patient compliance with post-operative physiologic studies remains a universal challenge, with most centers reporting modest success13, 26, 46. Furthermore, most centers preferentially utilize 1–2 evaluative modalities, which creates a heterogeneous data set for the literature at large and hampers institutional cross-comparison.
The most robust long-term POEM studies demonstrate a 20–30% rate of post-operative GERD symptoms, endoscopic esophagitis in 30–56% patients, and positive pH studies in 40–60%38. Although historical studies have cited lower rates of reflux following LHM (8–22%)6, more recent investigations suggest that the rate may be closer to 30%51, 52. Importantly, the majority of post-myotomy reflux is readily controlled with an anti-secretory agent and rarely necessitates intervention during long-term follow-up13, 53.
5. Conclusion
Idiopathic achalasia is the most common esophageal motility disorder. POEM is a novel technique that applies principles of endoscopic submucosal dissection for the palliative disruption of the lower esophageal sphincter. Short-term efficacy and safety data are promising, with symptomatic outcomes comparable to laparoscopic Heller myotomy. Although the data is limited, encouraging long-term results are emerging from several centers of excellence. Additional studies are needed to further solidify POEM’s position in the armamentarium of achalasia treatment.
Highlights:
Per-oral esophageal myotomy (POEM) utilizes endoscopic submucosal dissection techniques to palliate the lower esophageal sphincter in patients with achalasia.
The evaluation, diagnosis, and surgical treatment of patients with achalasia is complex and should occur at centers with considerable experience in esophageal disorders.
POEM provides symptom relief that is comparable to laparoscopic Heller myotomy on short- and moderate-term follow up.
Acknowledgements:
Dr. Campagna is supported by T32DK101363 from the National Institutes of Health
Abbreviations:
- POEM
Per-Oral Esophageal Myotomy
- ESD
Endoscopic Submucosal Dissection
- EGD
Esophagogastroduodenoscopy
- HRM
High-Resolution Manometry
- HRIM
High-Resolution Impedance Manometry
- TBE
Time Barium Esophagram
- DI
Distensibility Index
- NPO
Nil Per Os
- EGJ
Esophagogastric Junction
- GERD
Gastroesophageal Reflux Disease
Footnotes
Conflict of Interest Statement: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Pandolfino JE, Gawron AJ. Achalasia: A systematic review. JAMA 313:1841–1852, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Goldblum JR WR, Orringer MB, Appelman HD. Achalasia. A morphologic study of 42 resected specimens. Am J Surg Pathol 4:327–337, 1994 [PubMed] [Google Scholar]
- 3.Boeckxstaens GE, Zaninotto G, Richter JE. Achalasia. The Lancet 383:83–93, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Patti MG, Pellegrini CA, Horgan S, et al. Minimally Invasive Surgery for Achalasia: An 8-Year Experience With 168 Patients. Annals of Surgery 230:587, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaninotto G, Costantini M, Molena D, et al. Minimally Invasive Surgery for Esophageal Achalasia. Journal of Laparoendoscopic & Advanced Surgical Techniques 11:351–359, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Allaix ME, Patti MG. Heller Myotomy for Achalasia. From the Open to the Laparoscopic Approach. World Journal of Surgery 39:1603–1607, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 42:265–271, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Stavropoulos SN, Desilets DJ, Fuchs K-H, et al. Per-oral endoscopic myotomy white paper summary. Gastrointestinal Endoscopy 80:1–15, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Stavropoulos SN, Modayil RJ, Friedel D, Savides T. T he International Per Oral Endoscopic Myotomy Survey (IPOEMS): a snapshot of the global POEM experience. Surgical Endoscopy 27:3322–3338, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Akintoye E, Kumar N, Obaitan I, Alayo QA, Thompson CC. Peroral endoscopic myotomy: a meta-analysis. Endoscopy 48:1059–1068, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Costamagna G, Marchese M, Familiari P, Tringali A, Inoue H, Perri V. Peroral endoscopic myotomy (POEM) for oesophageal achalasia: Preliminary results in humans. Digestive and Liver Disease 44:827–832, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Swanstrom LL, Kurian A, Dunst CM, Sharata A, Bhayani N, Rieder E. Long-term outcomes of an endoscopic myotomy for achalasia: the POEM procedure. Ann Surg 256:659–667, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Teitelbaum EN, Dunst CM, Reavis KM, et al. Clinical outcomes five years after POEM for treatment of primary esophageal motility disorders. Surg Endosc 32:421–427, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Teitelbaum EN, Soper NJ, Santos BF, et al. Symptomatic and physiologic outcomes one year after peroral esophageal myotomy (POEM) for treatment of achalasia. Surgical Endoscopy 28:3359–3365, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Werner YB, Costamagna G, Swanström LL, et al. Clinical response to peroral endoscopic myotomy in patients with idiopathic achalasia at a minimum follow-up of 2 years. Gut 65:899–906, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Bhayani NH, Kurian AA, Dunst CM, Sharata AM, Rieder E, Swanstrom LL. A Comparative Study on Comprehensive, Objective Outcomes of Laparoscopic Heller Myotomy With Per-Oral Endoscopic Myotomy (POEM) for Achalasia. Annals of Surgery 259:1098–1103, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Eckardt VF. Clinical presentation and complications of achalasia. Gastrointestinal Endoscopy Clinics of North America 11:281–292, 2011 [PubMed] [Google Scholar]
- 18.Rohof WO, Hirsch DP, Kessing BF, Boeckxstaens GE. Efficacy of Treatment for Patients With Achalasia Depends on the Distensibility of the Esophagogastric Junction. Gastroenterology 143:328–335, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Taft TH, Carlson DA, Triggs J, et al. Evaluating the reliability and construct validity of the Eckardt symptom score as a measure of achalasia severity. Neurogastroenterology and Motility 30:e13287, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bredenoord AJ, Fox M, Kahrilas PJ, Pandolfino JE, Schwizer W, Smout AJPM Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography1. Neurogastroenterology & Motility 24:57–65 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterology & Motility 27:160–174 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlson DA, Lin Z, Kahrilas PJ, et al. High-Resolution Impedance Manometry Metrics of the Esophagogastric Junction for the Assessment of Treatment Response in Achalasia. The American Journal Of Gastroenterology 111:1702, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kachala SS, Rice TW, Baker ME, et al. Value of routine timed barium esophagram follow-up in achalasia after myotomy. The Journal of Thoracic and Cardiovascular Surgery 2018 [DOI] [PubMed] [Google Scholar]
- 24.Teitelbaum EN, Soper NJ, Pandolfino JE, et al. Esophagogastric junction distensibility measurements during Heller myotomy and POEM for achalasia predict postoperative symptomatic outcomes. Surgical Endoscopy 29:522–528, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlson DA, Hirano I. Application of the Functional Lumen Imaging Probe to Esophageal Disorders. Curr Treat Options Gastroenterol 15:10–25, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Hungness ES, Sternbach JM, Teitelbaum EN, Kahrilas PJ, Pandolfino JE, Soper NJ. Per-oral Endoscopic Myotomy (POEM) After the Learning Curve: Durable Long-term Results With a Low Complication Rate. Annals of Surgery 264:508–517, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Kurian AA, Dunst CM, Sharata A, Bhayani NH, Reavis KM, Swanström LL. Peroral endoscopic esophageal myotomy: defining the learning curve. Gastrointestinal Endoscopy 77:719–725, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Patel KS, Calixte R, Modayil RJ, Friedel D, Brathwaite CE, Stavropoulos SN. The light at the end of the tunnel: a single-operator learning curve analysis for per oral endoscopic myotomy. Gastrointestinal Endoscopy 81:1181–1187, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Teitelbaum EN, Soper NJ, Arafat FO, et al. Analysis of a Learning Curve and Predictors of Intraoperative Difficulty for Peroral Esophageal Myotomy (POEM). Journal of Gastrointestinal Surgery 18:92–99, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Acosta RD, Abraham NS, Chandrasekhara V, et al. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointestinal Endoscopy 83:3–16, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Ren Z, Zhong Y, Zhou P, et al. Perioperative management and treatment for complications during and after peroral endoscopic myotomy (POEM) for esophageal achalasia (EA) (data from 119 cases). Surgical Endoscopy 26:3267–3272, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Tan Y, Lv L, Wang X, et al. Efficacy of anterior versus posterior per-oral endoscopic myotomy for treating achalasia: a randomized, prospective study. Gastrointestinal Endoscopy 2018 [DOI] [PubMed] [Google Scholar]
- 33.Cai M-Y, Zhou P-H, Yao L-Q, et al. Peroral endoscopic myotomy for idiopathic achalasia: randomized comparison of water-jet assisted versus conventional dissection technique. Surgical Endoscopy 28:1158–1165, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Stavropoulos SN, Harris MD, Hida S, Brathwaite C, Demetriou C, Grendell J. Endoscopic submucosal myotomy for the treatment of achalasia (with video). Gastrointestinal Endoscopy 72:1309–1311, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Wright AS, Williams CW, Pellegrini CA, Oelschlager BK. Long-term outcomes confirm the superior efficacy of extended Heller myotomy with Toupet fundoplication for achalasia. Surgical Endoscopy 21:713–718, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Stavropoulos SN, Modayil R, Friedel D. Achalasia. Gastrointestinal Endoscopy Clinics of North America; 23:53–75, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Inoue H, Tianle KM, Ikeda H, et al. Peroral Endoscopic Myotomy for Esophageal Achalasia: Technique, Indication, and Outcomes. Thoracic Surgery Clinics 21:519–525, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Hungness ES, Jorge JM. Per-Oral Esophageal Myotomy: Is It a Safe and Durable Procedure for Achalasia? Adv Surg 51:193–205, 2017 [DOI] [PubMed] [Google Scholar]
- 39.Hungness ES, Teitelbaum EN, Santos BF, et al. Comparison of Perioperative Outcomes Between Peroral Esophageal Myotomy (POEM) and Laparoscopic Heller Myotomy. Journal of Gastrointestinal Surgery 17:228–235, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Von Renteln D, Fuchs KH, Fockens P, et al. Peroral Endoscopic Myotomy for the Treatment of Achalasia: An International Prospective Multicenter Study. Gastroenterology 145:309–311.e303, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Von Renteln D, Inoue H, Minami H, et al. Peroral Endoscopic Myotomy for the Treatment of Achalasia: A Prospective Single Center Study. The American Journal Of Gastroenterology 107:411, 2011 [DOI] [PubMed] [Google Scholar]
- 42.El Khoury R, Teitelbaum EN, Sternbach JM, et al. Evaluation of the need for routine esophagram after peroral endoscopic myotomy (POEM). Surgical Endoscopy 30:2969–2974, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Barbieri LA, Hassan C, Rosati R, Romario UF, Correale L, Repici A. Systematic review and meta-analysis: Efficacy and safety of POEM for achalasia. United European Gastroenterology Journal 3:325–334, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X, Li Q-p, Ji G-z, et al. Two-year follow-up for 45 patients with achalasia who underwent peroral endoscopic myotomy. European Journal of Cardio-Thoracic Surgery 47:890–896, 2015 [DOI] [PubMed] [Google Scholar]
- 45.Marano L, Pallabazzer G, Solito B, et al. S urgery or Peroral Esophageal Myotomy for Achalasia: A Systematic Review and Meta-Analysis. Medicine (Baltimore 95:e3001, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Talukdar R, Inoue H, Reddy DN. Efficacy of peroral endoscopic myotomy (POEM) in the treatment of achalasia: a systematic review and meta-analysis. Surgical Endoscopy 29:3030–3046, 2015 [DOI] [PubMed] [Google Scholar]
- 47.Kumbhari V, Tieu AH, Onimaru M, et al. Peroral endoscopic myotomy (POEM) vs laparoscopic Heller myotomy (LHM) for the treatment of Type III achalasia in 75 patients: a multicenter comparative study. Endosc Int Open 3:E195–E201, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rohof WO, Salvador R, Annese V, et al. Outcomes of Treatment for Achalasia Depend on Manometric Subtype. Gastroenterology 144:718–725, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Bredenoord AJ, Pandolfino JE, Smout AJPM. Gastro-oesophageal reflux disease. The Lancet 381:1933–1942, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Farmer Adam D, Aziz Q. Mechanisms of visceral pain in health and functional gastrointestinal disorders. Scandinavian Journal of Pain. Vol 52017:51. [DOI] [PubMed] [Google Scholar]
- 51.Rawlings A, Soper NJ, Oelschlager B, et al. Laparoscopic Dor versus Toupet fundoplication following Heller myotomy for achalasia: results of a multicenter, prospective, randomized-controlled trial. Surgical Endoscopy 26:18–26, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Moonen A, Annese V, Belmans A, et al. Long-term results of the European achalasia trial: a multicentre randomised controlled trial comparing pneumatic dilation versus laparoscopic Heller myotomy. Gut. 65:732–739, 2016 [DOI] [PubMed] [Google Scholar]
- 53.Inoue H, Sato H, Ikeda H, et al. Per-Oral Endoscopic Myotomy: A Series of 500 Patients. Journal of the American College of Surgeons 221:256–264, 2014 [DOI] [PubMed] [Google Scholar]