Abstract
Osteoarthritis (OA) is a painful and debilitating disease. A striking feature of OA is the dramatic increase in vascular endothelial growth factor (VEGF) levels and in new blood vessel formation in the joints, both of which correlate with the severity of OA pain. Our aim was to determine whether anti-VEGF monoclonal antibodies (mAbs) – MF-1 (mAb to VEGFR1) and DC101 (mAb to VEGFR2) – can reduce OA pain and can do so by targeting VEGF signaling pathways such as Flt-1 (VEGFR1) and Flk-1 (VEGFR2).
After IACUC approval, OA was induced by partial medial meniscectomy (PMM) in C57/BL6 mice (20 g). ln the first experiment, for validation of VEGFR1 in DRG, the mouse dorsal root ganglion (DRG) was stimulated with NGF for 48 hours to find the relative gene induction for VEGFR1 vs. 18S by RT-PCR. In the second experiment, Biotin-conjugated VEGFA (1 µg/knee joint) was administered in the left knee joint of mice with advanced OA in order to characterization of VEGFR1 and VEGFR2. pVEGFR1/VEGFR2 was detected by immunostaining in DRGs. Finally, MF-1 and DC101 were administered in OA mice by both intrathecal (IT) and intraarticular (IA) injections, and the change in paw withdrawal threshold (PWT) was measured.
Retrograde transport of VEGF was confirmed for detection of pVEGFR1/VEGFR2 in the DRG. PMM surgery led to development of OA and mechanical allodynia, with reduced paw withdrawal thresholds (PWT) (P<0.0001). IT injection of MF-1 led to a reduction of allodynia in advanced OA, but injection of DC101 did not. IA injection of MF-1 or DC101 at one week after PMM injury did not reduce allodynia, but when injected in advanced OA mice joints at 12 weeks, both Mabs increased PWT an indicator of analgesia. Our data show that MF-1 (VEGR1 inhibition) decreases pain in advanced OA after IT or IA injection. Activation of MF-1 or DC101 may ameliorate OA-related joint pain.
Keywords: Osteoarthritis, OA, Pain, VEGF, PIGF, Flt-1, Flk-1
Introduction
Osteoarthritis (OA) is associated with cartilage degeneration and articular cartilage degradation, followed by subchondral bone thickening, osteophyte formation, synovial inflammation and joint degeneration (Varela-Eirin et al. 2018). Therefore, OA causes ectopic firing and neuroplasticity changes in peripheral and central nervous systems via modulation of various factors including TGF-β/BMPs, Wnt, and Cx43, or SASP in neuronal and non-neuronal mechanisms that are involved in signaling pathways for developing chronic pain (Das 2015, Gersing et al. 2017, Varela-Eirin et al. 2018). Clinically this pain is very difficult to manage with current medications and creates a sizeable economic burden – $600 billion per annum in the United States – which exceeds the combined annual cost of cancer, heart disease, and diabetes (Sirianni at al. 2015). Nevertheless, acute inflammatory and neuropathic pain can be attenuated or abolished by local treatment with sodium channel blockers and/or opioids, showing that peripheral nociceptive input is dependent on the presence of functional voltage-gated ion channels (Dworkin et al. 2013, Das 2015). But, the lack of selective pharmacological modulators for many types of ion channels is a major barrier to understanding their biological function (Latremoliere and Woolf 2009, Woolf 2011). In 2007, global sales of pain medications were totaled $34 billion, highlighting the pervasive nature of this condition (Dworkin et al. 2013). In many patients, chronic OA pain substantially reduces quality of life, which is especially significant given that the incidence of chronic pain increases to 50% in elderly cohorts (Gersing et al. 2017). Thus, this research addresses National Research Priority 2, Promoting & Maintaining Good Health, with particular relevance to the Priority Goal Ageing well, ageing productively (Varela-Eirin et al. 2018).
Nevertheless, a striking feature of OA is the dramatic increase in vascular endothelial growth factor (VEGF) levels and in new blood vessel formation in the joints, both of which correlate with severity of OA pain (Hamilton et al. 2016, Nagao et al. 2017). More recent genomic studies revealed that vegf is one of the key genes that are strongly associated with OA progression in humans (Hamilton et al. 2016, Nagao et al. 2017). VEGF-family ligands signal via two receptor tyrosine kinases, VEGFR1 (also known as Flt1) and VEGFR2 (also known as Flk1) (Hamilton et al. 2016). VEGF-A activates both Flt1 and Flk1; VEGF-B and placental growth factor (PlGF) activate Flt1; VEGF-E (encoded by viruses) exclusively activates Flk1 (Hamilton et al. 2016). Redundant and compensatory roles among Flt1 ligands (VEGF-B, PlGF, VEGF-A) have been reported (Hamilton et al. 2016, Nagao et al. 2017). Thus, targeting the receptors they converge upon may be more efficacious than individual ligands. Recently, we and our collaborators reported that selective pharmacological inhibition of Flk1 by ZD6474, known as Vandetanib, significantly inhibits pathological progression in an established traumatic injury induced OA animal model that resembles injury induced development of OA in humans (Nagao et al. 2017).
Therefore, we sought to determine the efficacy of monoclonal antibodies (mAbs) – MF-1 (mAb to VEGFR1) and DC101 (mAb to VEGFR2) – to reduce OA pain by targeting VEGF pathways. This has the potential for development of novel therapeutics for OA pain by targeting VEGFRs including Flt-1 (VEGFR1) and Flk-1 (VEGFR2). Moreover, intra-articular (IA) injections appear to have several potential advantages over systemic delivery, including minimal side effects if any, increased bioavailability, and lower doses (Evans et al. 2013).
Methodology
Validation of VEGFR1 in NGF stimulated DRG neuron culture
Four week old mice (n=10) were sacrificed with 5% isoflurane and sterile (soaked in 70% ethanol) surgical tools. L1–L6 DRGs were harvested and stored in 15 ml of Hanks Balanced Salt Solution (HBSS; Cat#14170-112, Life technology, USA) in a 15 ml conical tube on ice. After that, conical tubes (15 ml) with target tissues were centrifuged at 400 rpm for 30 s to pellet tissues. All of the HBSS was aspirated using autoclaved glass pipettes without disturbing tissue pellets. All of the thawed collagenase was gently added using a 1 ml pipette. Tubes were shaken until the tissue was homogeneously suspended in collagenase A (Cat# 10103578001, Roche, USA). Tissues were incubated in a 37°C water bath for 25 min. Tubes were shaken again at the half-way point (12.5 min) to make sure the tissue was suspended in collagenase A. After 25 min, 15 ml conical tubes containing tissues were centrifuged at 400 rpm for 30 s to pellet the tissue at the bottom of the tube. We then aspirated as much collagenase A as possible using autoclaved glass pipettes without disturbing the tissue pellets. Thereafter, all of the thawed collagenase D (Cat#11088858001, Roche, USA) was added using a 1 ml pipette. Collagenase D should be yellow in color. The tubes were shaken until the tissue was suspended in collagenase D. Tissue was incubated in a 37°C water bath for 20 mins. The tube was shaken again at the half-way point (10 min) to make sure tissue was suspended in collagenase D. During this incubation time, we prepared for dissociation of tissue (Trypsin Inhibitor, Cat#10109886001, Roche, USA and Papin, Cat# P3125-100MG, Sigma, USA at 1:1 ratio) and plating of cells. 50 ml conical tubes were placed in a hood with a cell strainer (70 um). After 20 min, 15 ml conical tubes were centrifuged at 400 rpm for 2 min to pellet tissue at the bottom of the tube. Dissociation solution was added and the suspension was gently dissociated (pipetted up and down about 8–10 times) with a small fire-polished Pasteur pipet or a 1ml pipette until the suspension became homogeneous. The dissociated cell suspension was strained through a Falcon 70 µM cell-strainer, and placed on a 50 ml conical Falcon tube. The sieve was washed 12 times with 1 ml of growth media each time. The pellet was centrifuged at 400 rpm for 4 min and we aspirated as much of the supernatant as possible without disrupting the pellet. We then re-suspend the pellet in growth media (70–100µl/well; Growth Media formulation included 480 ml, Neurobasal Media (Cat#21103049, Thermo Fisher Scientific, USA), L-Glutamine (5 ml; GLUTAMAX I, Cat#A1286001, Thermo Fisher Scientific, USA), Penicillin-Streptomycin solution (5 ml, SV30010, HyClone, Austria) and B-27(10 ml; Cat#17504-044, Life technology, USA) in 500 ml volume of media) by mixing several times. The seed dish was coated with poly-D-lysine by forming a bubble or droplet with 70–100 µl of re-suspended cells at the center of the dish. Cells were incubated at 37°C for at least 2 h. We then flooded the dishes by adding 1 ml of growth media to each well disrupting the droplet. We then placed the dishes in a 37°C incubator and stimulated with NGF (100ng/ml; Recombinant Human beta-NGF Protein, Catalog#CAA36832, R&D Systems, Inc. Minneapolis, USA) for 48 h prior to RNA extraction. At the end of 48 hours, total RNA was isolated using TRIzol reagent (Invitrogen Corp., Carlsbad, CA) according to the manufacturer’s protocol. RT-PCR was done to verify the differential expression of selected genes including VEGFR1 (Fw- TGGCCACCACTCAAGATTAC and Re- TATAGACACCCTCATCCTCCTC) vs. 18S (Fw- GTAACCCGTTGAACCCCATT and Re- CCATCCAATCGGTAGTAGCG), using a Roche Light Cycler system (Roche Diagnostics, GmbH Mannheim, Germany) and the SYBR Green method. Relative gene expression was determined using the comparative CT method.
Experimental animals
Female C57BL6 mice (n=84) (25–30 g each] were randomly assigned to our studies. Mice were allocated to 3 different experiments including (i) retrograde transportation of VEGFA as experimental group-1 (n=6) in advanced OA mice, (ii) intrathecal IT injection as experimental group-2 (n=51) for MF-1 and DC101 analgesic efficacy in advanced OA mice (12–15 weeks post PMM) and (iii) intraarticular (IA) injection as experimental group-3 (n=27) for MF-1 and DC101 analgesic efficacy in OA mice (at 1 and 12 weeks post PMM). Mice were housed under standard laboratory conditions (in a temperature-controlled (21±1°C) room with a normal 12-h light/dark cycle). The study protocols involving animal procedures followed the guidelines of the Rush Institutional Animal Care and Use Committee (IACUC).
Surgical procedure to induce partial medial meniscectomy (PMM) for OA induction
All the surgical operations were done under a microscope in an aseptic setting. Mice were placed in a supine position and anesthetized with 1.5 % isoflurane (Abbott Laboratories, North Chicago, IL, USA) in oxygen via a facemask at a rate of 1 L/minute. The left hind leg hair was shaved, thoroughly scrubbed with a topical antiseptic solution (chlorhexidine gluconate), and draped in sterile fashion. After confirming adequate anesthesia, a 1 cm left knee incision was made with a #15 scalpel blade. The knee joint was identified from the tibia and femur; and the medial menisco-tibial ligament was identified using anatomic landmarks. To induce partial medial meniscectomy (PMM), which destabilizes the ligaments, a microscalpel at a depth of 0.5–0.7 mm was used to remove 1 mm of cartilage at midline (Glasson, Blanchet and Morris 2007, Thysen, Luyten and Lories 2015, Das et al. 2018). The skin incision was then closed with 4-0 vicryl sutures.
Biotin-conjugated VEGFA administered intraarticular (IA) in advanced OA mice for validating the retrograde transportation of VEGFA and characterization of pVEGFR1/VEGFR2 via immunostaining in the DRG
For retrograde VEGFA transport studies, Biotin-conjugated VEGFA (1 µg/knee joint) was administered intraarticularly (IA) in the left knee joint of mice with advanced OA (16 week-post PMM surgery; experimental group-1) or sham surgery (n=3/per group). VEGFA (Cat# 293-VE, R&D systems, Minneapolis) was labeled using Biotin-XX microscale protein labeling Kits (Cat# B30010, Molecular Probes; Oregon). After 3 days, mice were terminally anesthetized in 5% isoflurane and perfused transcardially with 0.9 % saline followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate buffered saline (PBS, pH 7.4). Lumbar dorsal root ganglions (DRGs) were harvested and were processed for immunofluorescence analyses for retrograde transportation of VEGFA. For detection of retrograde transportation of VEGFA-Biotin, sections were then incubated in a 1:20 dilution of Streptavidin-Alexa Fluor 488 (Life Technologies, Carlsbad, CA) for 5 h at room temperature and then mounted in antifading mounting media (Vector Laboratories, Burlingame, CA) and examined using a Nikon Eclipse NiE upright microscope (Nikon Instruments Inc., Melville, NY). In our previous publication we showed retrograde transportation of NGF-Biotin from the knee joint following a similar protocol (Kc et al. 2016).
Pharmacological validation of MF-1 and DC101 localized drug treatment for reducing pain hypersensitivity in OA-induced mice
MF-1 and DC101 localized drug treatment for OA-induced experiment -2 and experiment-3 mice
OA-induced experiment-2–3 mice had two parts: intrathecal and intraarticular injection for MF-1 and DC101. For each part, mice were randomized into three groups: vehicle treated and VEGF antibody treated (MF-1 and DC101). Intrathecal or intraarticular injection was administered using 5 µg drug in 5 µl volume. Intrathecal injection was carried out at 13–15 weeks post PMM at 8:00 AM. However, intraarticular injection was carried out once a week from week 1 to 12 at 4:00 PM each day.
Animal pain behavioral tests
Behavioral testing for reduction of pain in VEGF-antibodies-treated PMM mice (preclinical experimental group-2 mice) was done 2 h after for each mouse and preclinical experimental group-3 mice groups were done at week 1 and week 12 for each mouse.
Mechanical allodynia (von frey testing)
Allodynia was evaluated based on hindpaw withdrawal from mechanical stimuli (Chaplan et al., 1994). After allowing mice to accommodate for 30 min on a wire mesh grid, a calibrated set of von Frey filaments was applied from below to the plantar hind paw to determine the 50% force withdrawal threshold using an iterative method. The filament was applied to the skin with enough pressure to buckle. A brisk lifting of the foot was recorded as a positive response. If no response was observed, the filament with the next highest force was applied, while the filament with the next lowest force was applied after a positive response.
Statistical analysis
Statistical analyses were done using GraphPad Prism (version 5.00, March7, 2007; Chicago, IL, USA) software. All data were presented as mean ± SEM. The statistical significance criterion was P<0.05. The t test was used to assess relative gene induction, PWT and protein levels between 2 groups at one time point (P<0.05). One-way ANOVA with post hoc Dunnett’s Multiple Comparison Test was used to assess PWT for IT and IA injection between 3 or more groups.
Results
Validation of VEGFR1 in NGF stimulated DRG neuron culture
Our RT-PCR data showed that NGF stimulation led to a seven fold increase in VEGFR1 expression in sensory neurons (DRG) as shown in Fig. 1. Quantitative analyses showed that mean (±SEM) relative expression of VEGFR1 in DRG was significantly and substantially increased in expression due to stimulation by NGF (P=0.0236). Therefore, we hypothesized that increased VEGFR1 expression could contribute to increasing various inflammatory mediators during progression of pain.
Figure 1. Validation of VEGFR1 in NGF stimulated DRG neuron culture.
Expression of VEGFR1 targets in the dorsal root ganglia (DRG) following sensory neuron culture treated NGF stimulation for 48 h. Quantitative analyses of VEGFR1 relative gene expression was 7 fold higher than in controls. Values are mean ± SEM and n=3–4.
However, recent reports suggest that VEGF signaling may go beyond ‘angiogenesis’ and transmits ‘pain’ signals. Lin et al reported that decreased expression of Flk1 (VEGFR2) in sensory neurons in dorsal root ganglions (DRGs) attenuates neuropathic pain; Selvaraj et al showed a selective role for Flt1 (VEGFR1) in cancer pain (Lin et al. 2010, Selvaraj et al. 2015). Our previous study demonstrated the selective role of Flt1 in knee joint OA pain transmission (Hamilton et al. 2016, Nagao et al. 2017).
Biotin-conjugated VEGFA administered intraarticular (IA) in advanced OA mice for validating the retrograde transportation of VEGFA and characterization of pVEGFR1/VEGFR2 via immunostaining in the DRG
Immunofluorescence results showed (Fig. 2) significant retrograde transportation of VEGFA-Biotin complexes from peripheral nerve terminals to the soma of L3–5 DRG neurons in mice after PMM surgery (compared to sham control mice). Arrows indicate retrograde transportation of VEGFA in DRG neurons.
Figure 2. Biotin-conjugated VEGFA administered intraarticular (IA) in advanced OA mice for validating the retrograde transportation of VEGFA via immunostaining in the DRG.
Expression of VEGFA-Biotin in the dorsal root ganglia (DRG) following groups of mice including sham, and PMM mice, where single immunofluorescence staining of VEGFA-Biotin (green) in DRGs for both, sham and PMM mice. The scale bar is 10 µm and is shown in white in color for all images. Values are mean±SEM and n=3/per group.
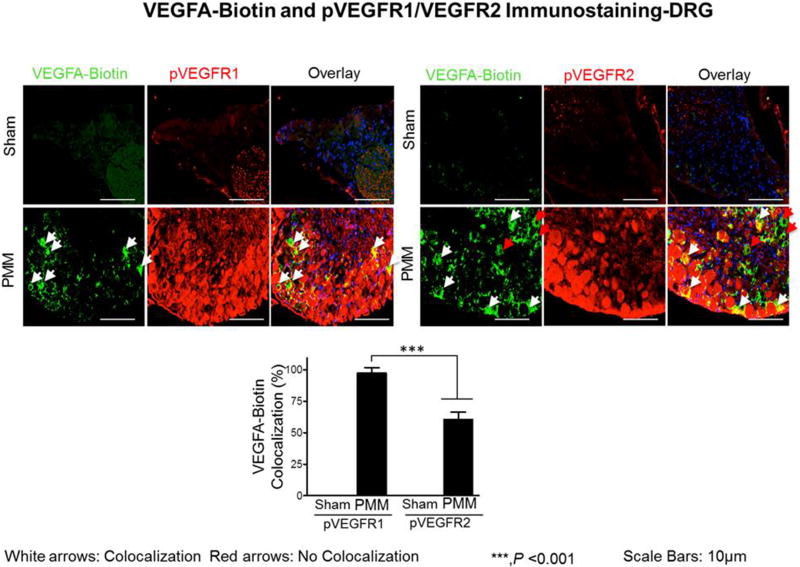
We next examined whether VEGFA retrograde transportation occurs through VEGFR1 (Flt1) or VEGFR2 (Flk1) from peripheral nerve terminals to sensory bodies in the DRG (Fig.3). For this purpose, we did co-localization immunofluorescence studies of VEGFA-Biotin with either phospho-VEGFR1 or phospho-VEGFR2. Our double immunofluorescence results show that retrograde transportation of VEGFA-Biotin complex occurs predominantly through VEGFR1 and not through VEGFR2. The proportion of DRG neurons co-localized with VEGFA-Biotin complex and phosphor-VEGFR1 was 97.44% while the proportion of DRG neurons co-localized with VEGFA-Biotin complex and phosphor-VEGFR2 was 60.74%. (p<0.001; Fig.3). Our data suggest critical roles for VEGFR1 in OA-associated joint pain.
Figure 3. Biotin-conjugated VEGFA administered intraarticular (IA) in advanced OA mice for characterization of pVEGFR1 (Flt1)/VEGFR2 (Flk1) via immunostaining in the DRG.
Expression of pVEGFR1 and pVEGFR2 targets in the dorsal root ganglia (DRG) following groups of mice including sham and PMM mice. Double immunofluorescence staining of pVEGFR1 and pVEGFR2 (red) and VEGFA-Biotin (green) in DRGs of sham and PMM mice. Co-localization of the two stains appears yellow. The scale bar is 10 µm and is shown in white in color for all images. Quantitative analyses of pVEGFR1 and pVEGFR2 expression in DRGs (Fig. 3) showed that they were increased in OA mice. Values are mean ± SEM and n=3/per group.
Pharmacological validation of MF-1 and DC101 localized drug treatment for reducing pain hypersensitivity in OA-induced mice
As shown in Fig. 4, induction of OA results in pain hypersensitivity (P<0.0001). Validation of this model to detect pain hypersensitivity in response to OA allowed us to assess the effects of MF-1 and DC101, pharmacological modulators of VEGFR1 and VEGFR2. Using the identical model of OA, treatment of mice with MF-1 (5 µg in 5 µl, IT; Fig.4) significantly reduced the mechanical hyperalgesia (PWT; figure 4). Specifically, mean (±SEM) PWT for pain hypersensitivity was different among groups after 2 h. Importantly, at 2 h (P<0.0001), PWT was higher in MF-1 treated mice than in vehicle-treated mice. However, DC101 (5µg in 5µl) did not elevate PWT. After the IT injection, none of the mice were morbid.
Figure 4. Analgesic activity of MF-1 and DC101 in a model of osteoarthritis induced pain.
Fig. 4 (single intrathecal injection; n=17/group) where, mechanical hyperalgesia was measured using the von Frey filament test. In OA mice, the paw withdrawal threshold (PWT) was elevated – showing analgesia – in the MF-1 and Dc101 treated group vs. the vehicle group. Values are mean ± SEM.
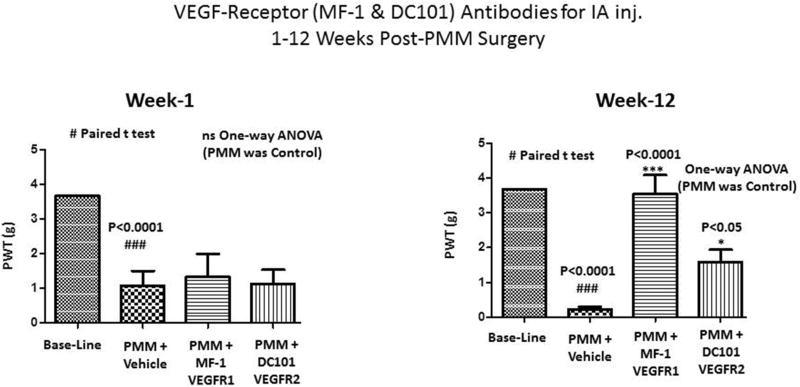
We extended this line of experimentation by examining intraarticular administration of MF-1 and DC101 (5 µg in 5 µl at knee; Fig.5) in the identical OA model, followed by determination of PWT (von Frey) for behavioral assessments. As shown in Fig. 5, mechanical hyperalgesia (PWT) of the MF-1 and DC101-treated groups progressively resolved over time from the 1st to the 12th week. Specifically, mean (±SEM) PWT scores for pain hypersensitivity were different among groups at 12 weeks (P<0.0001). Importantly, at week 12 the PWT was higher in the MF-1 and DC101 groups (P<0.001 and P<0.05) than in vehicle treated PMM mice. During the enter period of the IA injection study, none of the mice were morbid.
Figure 5. Analgesic activity of MF-1 and DC101 in a model of osteoarthritis induced pain.
Fig.5 (multiple intraarticular injections; n=9/group) where, mechanical hyperalgesia was measured using the von Frey filament test. In OA mice, the paw withdrawal threshold (PWT) was elevated in the MF-1 and DC101 treated group vs. the vehicle group. Values are mean ± SEM.
Discussion
Our data clearly demonstrate that MF-1 decreases pain in advanced OA after intrathecal (IT, Fig.4) and intraarticular injection (IA, Fig.5). Because decreases in VEGF are involved, pharmacological activation of MF-1 and DC101 may be an effective method for treating OA-related pain, as well as reducing joint damage associated with this disease. Therefore, this finding will provide additional strategic information on targeting specific VEGFRs for complicated OA conditions - if someone has complex medical problems such as OA pain with diabetic neuropathy, the patient may need to selectively inhibit VEGFR-1/Flt-1 activation at the joint to control OA-related pain, and to prevent ischemia. Previously, we and our collaborators published that a selective chemical inhibitor of Flk-1 (ZD6474) effectively protects cartilage from degeneration (Nagao et al. 2017). In the current study, we report a distinct role of Flt-1, but a lesser role for Flk-1 in OA pain transmission.
Angiogenesis is a counterbalanced regulated process in the extracellular matrix (ECM) including vascular endothelial growth factors (VEGFs), angiopoietins, transforming growth factor, fibroblast growth factor and platelet-derived growth factor (PDGF) within the cellular system (Chung et al. 2006, Selvaraj et al. 2015, Hamilton et al. 2016). Therefore, it is associated with much pathology, such as atherosclerosis, diabetic retinopathy, arthritis and neurodegenerative disease such as amyotrophic lateral sclerosis (ALS). Although structurally very similar, the different VEGFs display distinct properties and bind to specific subtypes of VEGF receptors to promote endothelial cell growth, migration and survival, but also regulate blood vessel permeability and vasodilation (Baldanzi et al. 2004, Hamilton et al. 2016). In mammals VEGF-A is expressed in many variants including VEGF-A121, VEGF-A165, VEGF-A189 and VEGF-A206 (Baldanzi et al. 2004). Among these, the longer versions of VEGF-A bind to additional receptors such as neuropilin-1 and -2, which play a role in cell adhesion and in nerve and vessel guidance (Baldanzi et al. 2004, Lin et al. 2010, Selvaraj et al. 2015). Increased activation of VEGFR-2 in the presence of heparin sulfate (HS) and neuropilin-1 or -2 has been observed for VEGF-A165, the isoform most prominently expressed in mammals (Cebe Suarez et al. 2006). However, another isoform VEGF-A206 is proteolytically cleaved and released from the ECM by metalloproteinases in the bone (Cébe Suarez et al. 2006, Lin et al. 2010).
However, VEGFs signal through cell surface receptor tyrosine kinases related to the PDGF receptor family (Lemmon and Schlessinger 2010). VEGF-A binds to both VEGF receptor 1 (VEGFR-1) and -2 (VEGFR-2) and VEGF-B exclusively binds to VEGFR-1 (Hamilton et al. 2016). VEGF-C and VEGF-D are specific ligands for VEGFR-2 and VEGFR-3, which regulate both blood and lymphatic vessel development (Cébe Suarez et al. 2006). This protein binds VEGF receptors with the same affinity as VEGFA165, shows reduced binding to HS, does not bind neuropilin-1, and displays altered signaling properties through VEGFR-2 and ERK kinases (Cébe Suarez et al. 2006).
Therefore, we and our collaborators recently found that: (i) VEGF is a chondrocyte survival factor for bone formation, skeletal growth and postnatal homeostatic processes (ii) increased VEGF expression significantly increased OA severity, (iii) in surgically induced knee OA in mice, a model of post-traumatic OA in humans, increased expression of VEGF is associated with catabolic processes in chondrocytes and synovial cells, (iv) recent advances in development of conditional knockdown of Vegf attenuates induction of OA, (v) intra-articular anti-VEGF antibodies suppress pathological changes in OA progression via reducing the levels of phosphorylated VEGFR2 in articular chondrocytes and synovial cells and reduce levels of phosphorylated VEGFR1 in dorsal root ganglia and (vi) oral administration of the VEGFR2 kinase inhibitor Vandetanib attenuates OA progression (Hamilton et al. 2016, Nagao et al. 2017).
Therefore, we sought to determine the efficacy of monoclonal antibody (mAb) to MF-1 (mAb to VEGFR1) and DC101 (mAb to VEGFR2) to reduce OA pain by targeting VEGF pathways such as Flt-1 (VEGFR1) and Flk-1 (VEGFR2) as novel therapeutics. Interestingly, inhibition of Flt-1 and Flk-1 signaling could reduce joint pain and inhibit progression of joint pathology via intra-articular (IA) drug delivery with minimal side effects during OA-associated pain. However, the treatment of chronic pain (osteoarthritis), and in particular neuropathic pain, is often recalcitrant to existing analgesics (Lin et al. 2010, Selvaraj et al. 2015, Das 2015, Hamilton et al. 2016, Gersing et al. 2017, Nagao et al. 2017, Varela-Eirin et al. 2018).
Nevertheless, how osteoarthritis (OA) develops and progresses to painful and debilitating disease is unclear. A striking feature of OA is the dramatic increase in vascular endothelial growth factor (VEGF) levels and in new blood vessel formation in the joints, both of which correlate with OA severity (Lin et al. 2010, Selvaraj et al. 2015, Hamilton et al. 2016, Nagao et al. 2017). US Food & Drug Administration (FDA) approval of several inhibitors of the VEGF pathway has enabled significant advances in the therapy of diseases related to pathological angiogenesis (Hamilton et al. 2016). Unfortunately, there are multiple VEGF ligands with redundant and compensatory roles that may contribute to OA progression and pain (Hamilton et al. 2016, Nagao et al. 2017). Thus, targeting individual ligands may be less efficacious than targeting the receptor(s) they converge upon. Specifically, VEGF ligands signal via two receptors, VEGFR-1 (known as Flt-1) and VEGFR-2 (known as Flk-1).
However, pain is the major driver of OA patients seeking medical help. For symptomatic OA patients, the best option to remedy the fundamental joint problem is (i) immediate pain relief with (ii) gradual cartilage regeneration (Gersing et al. 2017, Varela-Eirin et al. 2018). The ideal OA-disease-modifying-drug (OADMD) should elicit these two simultaneous effects including (1) Simultaneous inhibition of Flt-1 and Flk-1 signaling will immediately reduce joint pain and inhibit progression of joint pathology and (2) Intra-articular (IA) drug delivery will minimize side effects (Lin et al. 2010, Selvaraj et al. 2015). Using technology that causes slow drug release, which prolongs drug effects, will reduce the number and frequency of IA treatments needed for a therapeutic effect.
FDA approval of several inhibitors of the VEGF pathway has enabled significant advances in the therapy of diseases related to pathological angiogenesis, including OA (Hamilton et al. 2016, Nagao et al. 2017). Therefore, these findings will provide essential information critical to basic and clinical OA research fields that will lead to clinical trials of IA mAb therapy, targeting Flt-1 and Flk-1 in symptomatic OA patients.
Conclusion
We used our validated pre-clinical murine OA model, partial medial meniscectomy (PMM), in which the clinical presentation resembles human OA and provides a unique opportunity to assess, over time, OA progression: pain centralization; and joint pathology (Das et al, 2018).
Therefore, we hypothesize that pharmacological targeting of VEGF monoclonal antibody including MF-1 and DC101 may lead to the development of novel therapeutics to treat chronic pain (osteoarthritis) and stress in relation to pain. Importantly, our findings relating to MF-1 and DC101 modulatory effects in pain (during osteoarthritis) have been validated by introducing an osteoarthritis model in mice. Our hypothesis is supported by our results that the pharmacological activator (MF-1) reduces pain significantly in mice through modulating the angiogenesis in the VEGF pathway.
Acknowledgments
Funding: This work was supported by a VA BLD&R Merit Review Award (I01BC002647) to HJI; an NIH NIAMS R01 (AR062136) and R21 grant (AR067935) to HJI; Arthritis Foundation to HJI.
The authors thank VA BLD&R Merit Review Award (I01BC002647) to HJI; an NIH NIAMS R01 (AR062136) and R21 grant (AR067935) to H.J.I.; Arthritis Foundation to HJI for supporting and Eli Lilly Co. for providing MF-1 and DC101 monoclonal antibody mAbs for the pharmacological targeting of VEGF signaling components to find novel therapeutics to treat OA pain the in future.
Abbreviations list
- OA
osteoarthritis
- PMM
partial medial meniscectomy
- h
hour
- PWT
paw withdrawal threshold
- PTT
paw thermal threshold
- DRG
dorsal root ganglion
- IT
intrathecal
- IA
intraarticular
- RT-PCR
Real-time polymerase chain reaction
- P
probability
- SEM
standard error of the mean
- mAbs
monoclonal antibodies
- VEGF
vascular endothelial growth factor
- Flt-1
VEGFR1
- Flk-1
VEGFR2
- IACUC
Institutional Animal Care and Use Committee
- NGF
nerve growth factor
- PIGF
placental growth factor
- TGF-β/BMPs
transforming growth factor beta/Bone Morphogenetic Proteins
- Wnt
group of signal transduction pathways made of proteins that pass signals into a cell through cell surface receptors
- Cx43
connexin43 or Gap junction alpha-1 protein
- SASP
senescence-associated secretory phenotype
- USA
United States of America
- HBSS
Hanks Balanced Salt Solution
- L1–L6
lumber 1– lumber 6
- PDGF
platelet-derived growth factor
- ECM
extracellular matrix
- HS
heparin sulfate
- OADMD
OA-disease-modifying-drug
- FDA
Food & Drug Administration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: None declared.
References
- Baldanzi G, Mitola S, Cutrupi S, Filigheddu N, van Blitterswijk WJ, Sinigaglia F, Bussolino F, Graziani A. Activation of diacylglycerol kinase α is required for VEGF-induced angiogenic signaling in vitro. Oncogene. 2004;23:4828. doi: 10.1038/sj.onc.1207633. [DOI] [PubMed] [Google Scholar]
- Cébe Suarez S, Pieren M, Cariolato L, Arn S, Hoffmann U, Bogucki A, Manlius C, Wood J, Ballmer-Hofer K. A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2. Cellular and Molecular Life Sciences CMLS. 2006;63:2067–2077. doi: 10.1007/s00018-006-6254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung GG, Yoon HH, Zerkowski MP, Ghosh S, Thomas L, Harigopal M, Charette LA, Salem RR, Camp RL, Rimm DL, Burtness BA. Vascular endothelial growth factor, FLT-1, and FLK-1 analysis in a pancreatic cancer tissue microarray. Cancer. 2006;106:1677–1684. doi: 10.1002/cncr.21783. [DOI] [PubMed] [Google Scholar]
- Das V. Chapter One - An Introduction to Pain Pathways and Pain “Targets”. In: Theodore JP, Gregory D, editors. Progress in Molecular Biology and Translational Science. Academic Press; 2015. pp. 1–30. [DOI] [PubMed] [Google Scholar]
- Das V, Kroin JS, Moric M, Buvanendran A. Biochemical and Pharmacological Characterization of a Mice Model of Complex Regional Pain Syndrome. Regional Anesthesia and Pain Medicine. 2017;42:507–516. doi: 10.1097/AAP.0000000000000622. [DOI] [PubMed] [Google Scholar]
- Das V, Kc R, Li X, Qiu S, Kroin JS, Forsyth CB, Keshavarizian A, van Wijnen AJ, Park TJ, Stein GS, O-Sullivan I, Burris TP, Im Hee-Jeong. Pharmacological Targeting of the Mammalian Clock Reveals a Novel Analgesic for Osteoarthritis-Induced Pain. Gene. 2018;655:1–12. doi: 10.1016/j.gene.2018.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH, O’Connor AB, Kent J, Mackey SC, Raja SN, Stacey BR, Levy RM, Backonja M, Baron R, Harke H, Loeser JD, Treede R-D, Turk DC, Wells CD. Interventional management of neuropathic pain: NeuPSIG recommendations. PAIN®. 2013;154:2249–2261. doi: 10.1016/j.pain.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CH, Kraus VB, Setton LA. Progress in intra-articular therapy. Nature Reviews Rheumatology. 2013;10:11. doi: 10.1038/nrrheum.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersing AS, Schwaiger BJ, Nevitt MC, Joseph GB, Chanchek N, Guimaraes JB, Mbapte Wamba J, Facchetti L, McCulloch CE, Link TM. Is Weight Loss Associated with Less Progression of Changes in Knee Articular Cartilage among Obese and Overweight Patients as Assessed with MR Imaging over 48 Months? Data from the Osteoarthritis Initiative. Radiology. 2017a;284:508–520. doi: 10.1148/radiol.2017161005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis and Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Hamilton JL, Nagao M, Levine BR, Chen D, Olsen BR, Im HJ. Targeting VEGF and Its Receptors for the Treatment of Osteoarthritis and Associated Pain. J Bone Miner Res. 2016;31:911–24. doi: 10.1002/jbmr.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kc R, Li X, Kroin JS, Liu Z, Chen D, Xiao G, Levine B, Li J, Hamilton JL, van Wijnen AJ, Piel M, Shelly DA, Brass D, Kolb E, Im H-J. <em>PKCδ</em>null mutations in a mouse model of osteoarthritis alter osteoarthritic pain independently of joint pathology by augmenting NGF/TrkA-induced axonal outgrowth. Annals of the Rheumatic Diseases. 2016;75:2133–2141. doi: 10.1136/annrheumdis-2015-208444. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J. Cell signaling by receptor-tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Li G, Den X, Xu C, Liu S, Gao Y, Liu H, Zhang J, Li X, Liang S. VEGF and its receptor-2 involved in neuropathic pain transmission mediated by P2X2/3 receptor of primary sensory neurons. Brain Research Bulletin. 2010;83:284–291. doi: 10.1016/j.brainresbull.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Nagao M, Hamilton JL, Kc R, Berendsen AD, Duan X, Cheong CW, Li X, Im H-J, Olsen BR. Vascular Endothelial Growth Factor in Cartilage Development and Osteoarthritis. Scientific Reports. 2017;7:13027. doi: 10.1038/s41598-017-13417-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj D, Gangadharan V, Michalski Christoph W, Kurejova M, Stösser S, Srivastava K, Schweizerhof M, Waltenberger J, Ferrara N, Heppenstall P, Shibuya M, Augustin Hellmut G, Kuner R. A Functional Role for VEGFR1 Expressed in Peripheral Sensory Neurons in Cancer Pain. Cancer Cell. 2015;27:780–796. doi: 10.1016/j.ccell.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirianni J, Ibrahim M, Patwardhan A. Chapter Nineteen - Chronic Pain Syndromes, Mechanisms, and Current Treatments. In: Price TJ, Dussor G, editors. Progress in Molecular Biology and Translational Science. Academic Press; 2015. pp. 565–611. [DOI] [PubMed] [Google Scholar]
- Thysen S, Luyten FP, Lories RJU. Targets, models and challenges in osteoarthritis research. Disease Models & Mechanisms. 2015;8:17–30. doi: 10.1242/dmm.016881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela-Eirin M, Loureiro J, Fonseca E, Corrochano S, Caeiro JR, Collado M, Mayan MD. Cartilage regeneration and ageing: Targeting cellular plasticity in osteoarthritis. Ageing Research Reviews. 2018;42:56–71. doi: 10.1016/j.arr.2017.12.006. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. PAIN. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]