To the Editor
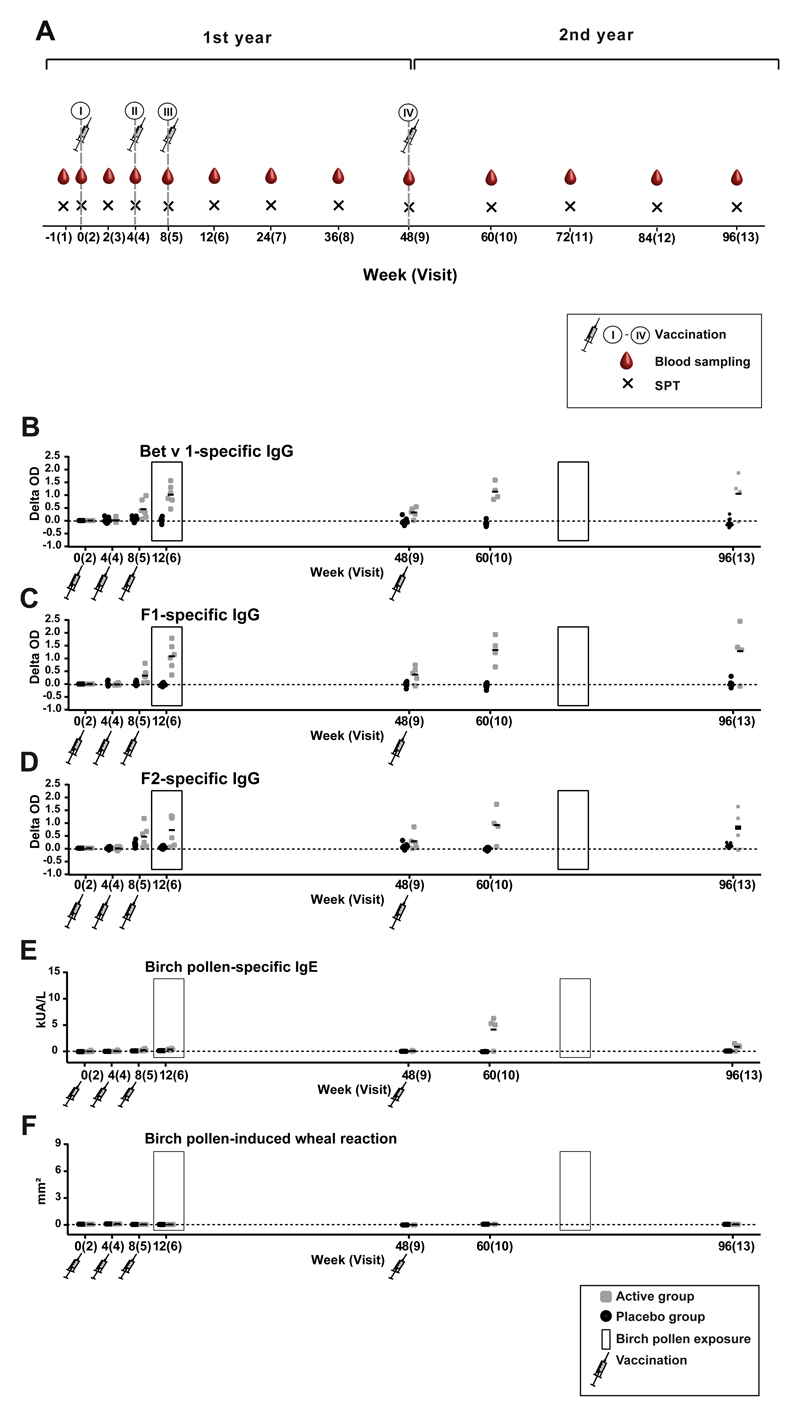
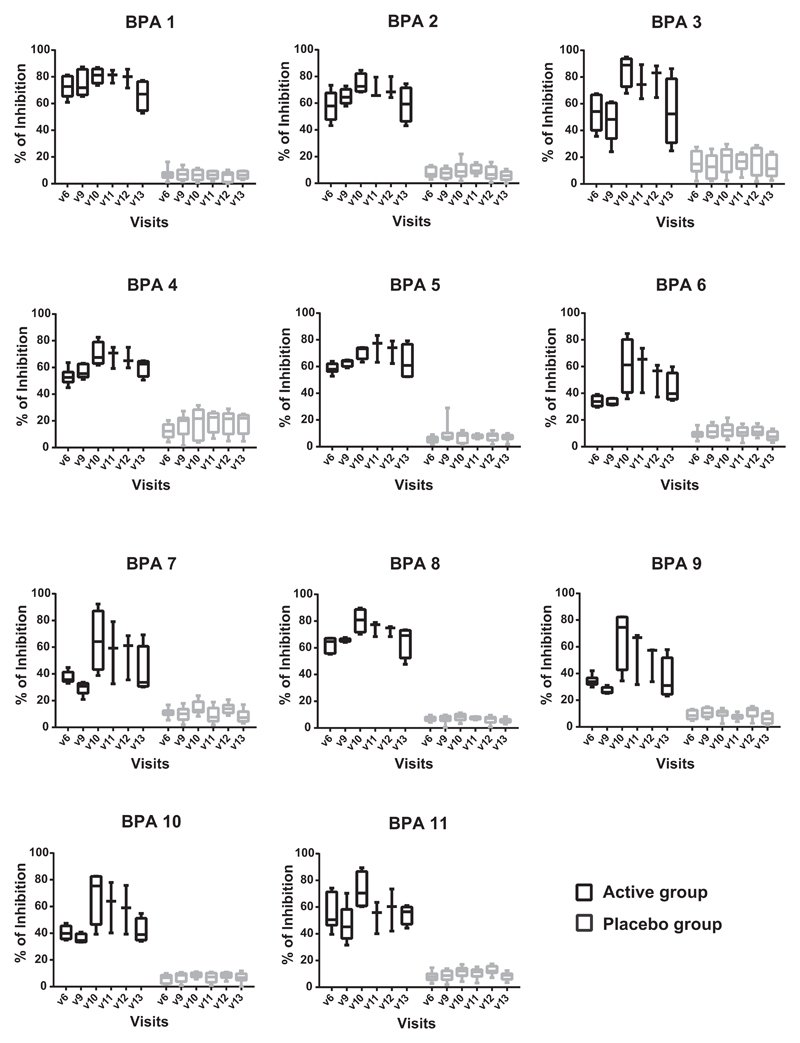
Studies suggested that only allergen-specific immunotherapy (AIT) but not pharmacotherapy can prevent the progression of allergic rhinitis to asthma in children.1 Recently conducted extensive analyses of the evolution of allergic sensitization in birth cohorts with microarrayed allergens demonstrate that silent IgE sensitization often precedes allergic symptoms and that the development of allergic symptoms may be predicted on the basis of IgE recognition of certain allergens already early in life.2 In this context, not only AIT based on allergen extracts but also molecular AIT strategies have been considered for preventing the development of allergy in IgE-sensitized but not yet symptomatic children.3,4 As a first step toward prophylactic allergy vaccination based on a molecular AIT approach we have conducted a doubleblind, placebo-controlled clinical trial, in which nonallergic subjects were vaccinated with recombinant hypoallergenic derivatives of the major birch pollen allergen, Bet v 1.5,6 We screened 44 subjects for the study of whom 16 were randomized, 10 to the placebo group and 6 to the actively treated group (see Fig E1 in this article’s Online Repository at www.jacionline.org). Subjects in the active and placebo groups were similar regarding age and demographic parameters (see Table E1 in this article’s Online Repository at www.jacionline.org). They lacked detectable IgE reactivity to birch pollen, mugwort pollen and grass pollen extract, and cat and house dust mite extract and were negative in skin prick tests with grass pollen, birch pollen, ash pollen, ragweed pollen, mugwort pollen, house dust mites (Dermatophagoides pteronyssinus and Dermatophagoides farinae), molds (Alternaria alternata and Cladosporium herbarum), and cat and dog dander extract (Table E1). During the 2-year study, 3 subcutaneous injections of Alum-adsorbed rBet v 1 fragments or Alum alone (placebo) were administered in monthly intervals before the birch pollen season of the first year (ie, 2013) and a single booster injection was given before the birch pollen season of the second year (ie, 2014). The study design thus allowed monitoring the effects of vaccination and of natural birch pollen exposure on specific immune responses (Fig 1, A-F). The development of birch pollen–specific antibody, cellular and cytokine responses as well as skin sensitivity was repeatedly recorded throughout the 2 years as indicated in Fig 1, A. Fig E2 in this article’s Online Repository at www.jacionline.org shows the development of IgG responses toward Bet v 1 and the 2 Bet v 1 fragments, F1 and F2, for each of subjects in the active (Fig E2, A) and placebo groups (Fig E2, B) during the 2 years of the study. The quantitative measurement of Bet v 1–specific IgG1 and IgG4 subclass levels is shown in Fig E3, A and B, in this article’s Online Repository at www.jacionline.org. Subjects from the active group as well as the placebo group showed preexisting IgG responses to Bet v 1 and Bet v 1 fragments already before the first vaccination. Most of the subjects vaccinated with the rBet v 1 fragments developed IgG against Bet v 1 and both fragments after the second injection and a significant increase in Bet v 1–specific IgG was found in all 6 actively treated subjects after the third injection (Fig E2, A; see Fig E4 in this article’s Online Repository at www.jacionline.org). Thus, the primary end point of the study was reached (Online Repository: ClinicalTrials.gov Identifier: NCT01353924). No significant increases in Bet v 1–specific IgG levels were found in the placebo group at the same time point (ie, visit 6) (Fig E4). Natural exposure to birch pollen during the first pollen season of year 1 commencing at approximately visit 6 did not induce relevant boosts of Bet v 1–specific IgG levels in the placebo group (Fig E2, B, and Fig E4). Bet v 1–specific IgG antibodies induced by vaccination with the fragments decreased after visit 7 and were low at visit 9 (week 48) when the fourth injection (ie, booster injection) was given (Fig E2, A, and Fig E4). For all actively treated patients with available serum samples after visit 9 (ie, A1, A2, A3, A5, A6), we found a strong and significant increase in Bet v 1– and fragment-specific IgG responses after the booster injection at visit 10 (week 60) (Fig E2, A, and Fig E4). No increases were found in the placebo-treated subjects at this time point. Only 1 placebo subject (ie, P8) showed a transient increase in Bet v 1–specific IgG at visit 11 after the birch pollen season (Fig E2, B), which may be explained by the fact that seasonal allergen exposure can boost allergen-specific IgG.7 Interestingly, Bet v 1–specific IgG levels, in particular IgG4 levels, remained elevated in 3 (ie, A1, A2, and A3) of the 4 actively treated subjects with available serum samples even 48 weeks after the single booster injection at visit 13 (week 96) (Fig 1, A; Fig E2, A, and Fig E3). Competition ELISA experiments performed with sera from patients allergic to birch pollen (patients 1-11) (see this article’s Methods section in the Online Repository at www.jacionline.org) showed that IgG antibodies induced by vaccination with rBet v 1 fragments in actively treated subjects, but not IgG antibodies from placebo-treated subjects, strongly inhibited IgE binding to Bet v 1 even at visit 13, suggesting that a sustained IgG antibody response blocking IgE binding to IgE epitopes of Bet v 1 was established (Fig 2). Bet v 1–specific IgG antibody responses in the actively treated subjects were accompanied by a Bet v 1–specific T-cell response and the induction of a mixed cytokine response consisting of TH2 (eg, Il-4 and IL-5), tolerogenic (ie, IL-10), and TH1 cytokines (IFN-γ) (see Fig E5, A and B, in this article’s Online Repository at www.jacionline.org), which was not observed in the placebo group.
Fig. 1.
Scheme, time course of study, and development of specific IgG responses. A, The study was conducted over a period of 2 years (x-axis: weeks). The 13 study visits are indicated as well as the time points of vaccination (I-IV), blood sampling, and skin testing. Development of specific IgG responses (y-axes: OD increases compared with baseline) against, Bet v 1 (B), F1 (C), F2 (D) birch pollen–specific IgE (E) (y-axis: kUA/L), and birch pollen–induced wheal reactions (F) (y-axis: mm2) during the 2 years of the study (x-axes: wk/visits) in the active group (grey) and the placebo group (black). Time points of vaccination and pollen seasons (boxed) are indicated. SPT, Skin prick test.
Fig. 2.
Inhibition of IgE binding to Bet v 1 by treatment-induced antibodies in patients with allergy. Shown are the percentages of inhibition as box plots with median and lower and upper quartiles of IgE binding of patients (BPA 1-11) obtained by preincubation of ELISA plate-bound Bet v 1 with sera from actively treated (black) or placebo-treated subjects (grey) taken at the different visits indicated (x-axes) (Table E5). BPA, Birch pollen allergic patient.
We also monitored the development of allergen-specific IgE antibodies as well as of skin sensitivity and compared it with allergen-specific IgG responses (Fig 1, B-F). After the first course of 3 vaccinations in year 1, 3 of the 6 actively treated subjects (ie, A1, A3, and A4) developed Bet v 1–specific IgE antibody levels slightly above the cutoff of the ImmunoCAP at visit 6 (week 12), which disappeared already at visit 7. After the booster injection given at visit 9, Bet v 1– and birch pollen–specific IgE levels increased in 4 of the 5 actively treated subjects (ie, A1, A2, A3, and A5) for whom sera had been available. In contrast to Bet v 1–specific IgG levels, which remained high 48 weeks after the booster injection (ie, visit 13; week 96) (Fig 1, B, and Fig E2, A), birch pollen–specific IgE had strongly declined (Fig 1, E). Importantly, none of the actively treated subjects developed skin reactions to birch pollen at any time when tested by skin prick testing with birch pollen extract. The lack of birch pollen–specific skin reactions despite the presence of Bet v 1–specific IgE antibodies is most likely due to the protective effects of treatment-induced IgG antibodies, which were shown to also block IgE binding to Bet v 1 in patients with allergy (Fig 2). Thus, treatment of nonallergic subjects seems to have induced only clinically silent IgE sensitization. A summary of adverse events observed during the 2 years of the study is presented in Table E3 in this article’s Online Repository at www.jacionline.org. These events were mild and classified as not related to the treatment. The only treatment-related side effects were mild (ie, local injection site reactions; n 5 4), which occurred in 3 of the actively treated subjects (Table E3). Thus, vaccination of nonallergic subjects with recombinant hypoallergenic Bet v 1 derivatives was safe and well tolerated.
We are aware of only 1 other study in which a chemical conjugate consisting of a viral particle and a synthetic peptide derived from the major house dust mite allergen, Der p 1, was used to vaccinate nonallergic subjects.8 However, this study was conducted only over a period of 1 year and contained no placebo group, whereas our study reported that the synthetic vaccine induced allergen-specific IgG antibodies without inducing symptomatic allergic sensitization. One possible next first step could be to vaccinate children who have a clinically silent IgE sensitization and to assess whether this vaccination can prevent the development of allergic symptoms later in life. Our study is a small pilot study but may be considered as a first molecular approach toward preventive vaccination.
Acknowledgments
This study was supported by project F4605 of the Austrian Science Fund (FWF) and by a Megagrant of the Government of the Russian Federation (grant no. 14.W03.31.0024 to R.V.).
Footnotes
We thank DorisWerjant for editorial help with the manuscript preparation.
Disclosure of potential conflict of interest: P. Zieglmayer reports personal fees from Thermo Fisher Scientific, United States. R. Valenta received research grants from the Austrian Science Fund, Biomay AG, Vienna, Austria, and Viravaxx, Vienna, Austria, and serves as a consultant for the latter 2 companies. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Host A, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007;62:943–8. doi: 10.1111/j.1398-9995.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 2.Wickman M, Lupinek C, Andersson N, Belgrave D, Asarnoj A, Benet M, et al. Detection of IgE reactivity to a handful of allergen molecules in early childhood predicts respiratory allergy in adolescence. EBioMedicine. 2017;26:91–9. doi: 10.1016/j.ebiom.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szepfalusi Z, Bannert C, Ronceray L, Mayer E, Hassler M, Wissmann E, et al. Preventive sublingual immunotherapy in preschool children: first evidence for safety and pro-tolerogenic effects. Pediatr Allergy Immunol. 2014;25:788–95. doi: 10.1111/pai.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valenta R, Campana R, Marth K, van Hage M. Allergen-specific immunotherapy: from therapeutic vaccines to prophylactic approaches. J Intern Med. 2012;272:144–57. doi: 10.1111/j.1365-2796.2012.02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vrtala S, Hirtenlehner K, Vangelista L, Pastore A, Eichler HG, Sperr WR, et al. Conversion of the major birch pollen allergen, Bet v 1, into two nonanaphylactic T cell epitope-containing fragments: candidates for a novel form of specific immunotherapy. J Clin Invest. 1997;99:1673–81. doi: 10.1172/JCI119330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niederberger V, Horak F, Vrtala S, Spitzauer S, Krauth MT, Valent P, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci U S A. 2004;101:14677–82. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niederberger V, Ring J, Rakoski J, Jager S, Spitzauer S, Valent P, et al. Antigens drive memory IgE responses in human allergy via the nasal mucosa. Int Arch Allergy Immunol. 2007;142:133–44. doi: 10.1159/000096439. [DOI] [PubMed] [Google Scholar]
- 8.Kundig TM, Senti G, Schnetzler G, Wolf C, Prinz Vavricka BM, Fulurija A, et al. Der p 1 peptide on virus-like particles is safe and highly immunogenic in healthy adults. J Allergy Clin Immunol. 2006;117:1470–6. doi: 10.1016/j.jaci.2006.01.040. [DOI] [PubMed] [Google Scholar]