Abstract
Study Objectives:
Snoring is perceived to be directly proportional to sleep apnea severity, especially obstructive sleep apnea (OSA), but this notion has not been thoroughly and objectively evaluated, despite its popularity in clinical practice. This might lead to overdiagnosis or underdiagnosis of OSA. The goal of this study is to examine this notion and objectively quantify the relationship between sleep apnea and snoring detected using advanced signal processing algorithms.
Methods:
We studied adults referred for polysomnography, from which the apnea-hypopnea index (AHI) was derived. Breath sounds were recorded simultaneously, from which snoring was accurately quantified using acoustic analysis of breath sounds and machine-learning computer algorithms. The snore index (SI) was calculated as the number of snores per hour of sleep.
Results:
In 235 patients, the mean AHI was 20.2 ± 18.8 and mean SI was 320.2 ± 266.7 events/h. On the one hand, the overall correlation between SI and AHI was weak but significant (r = .32, P < .0001). There was a significant stepwise increase in SI with increasing OSA severity, but with a remarkable overlap in SI among OSA severity categories. On the other hand, SI had weak negative correlation with central AHI (r = −.14, P = .035). SI had modest positive and negative predictive values for OSA (0.63 and 0.62 on average, respectively) and good sensitivity but low specificity (0.91 and 0.31 on average, respectively) attributed to the large number of snorers without OSA.
Conclusions:
Snoring on its own is probably of limited usefulness in assessing sleep apnea presence and severity, because of its weak relationship with AHI. Thus, the complaint of snoring should be interpreted with caution to avoid unnecessary referrals for sleep apnea testing. Conversely, clinicians should be aware of the possibility of missing diagnosis of patients with sleep apnea who have minimal snoring.
Citation:
Alshaer H, Hummel R, Mendelson M, Marshal T, Bradley TD. Objective relationship between sleep apnea and frequency of snoring assessed by machine learning. J Clin Sleep Med. 2019;15(3):463–470.
Keywords: apnea-hypopnea index, frequency of snoring, machine learning, sleep apnea
BRIEF SUMMARY
Current Knowledge/Study Rationale: Snoring, along with other symptoms, is routinely used in screening tools for sleep apnea. It is often thought that the more prominent the snoring, the worse the sleep apnea. Most studies that examined this notion relied on self-reporting of snoring. Objective quantification of snoring and its relationship to the presence and severity of sleep apnea have not been thoroughly investigated.
Study Impact: This is the first study to deploy advanced techniques for accurate determination of snoring frequency. Snoring frequency was found to have a modest predictive value for sleep apnea and a tenuous relation with its severity. Our findings suggest that assessment of snoring alone is probably of limited usefulness in screening for obstructive or central sleep apnea, and it should be more appropriately combined with assessment of other features of sleep apnea, such as daytime sleepiness.
INTRODUCTION
Sleep apnea is characterized by recurrent cessation of breathing during sleep. The two main types are obstructive sleep apnea (OSA), which results from upper airway narrowing and collapse, and central sleep apnea (CSA), which results from lack of central respiratory drive and also by an overshoot and undershoot of breathing regulation during periodic breathing. Apneas are frequently terminated by arousals that disrupt sleep and lead to excessive daytime sleepiness and fatigue.1 OSA is the more common of the two conditions and affects approximately 7% of adults2,3 in whom it is independently associated with increased risk of motor vehicle accidents,4 cardiovascular diseases,5 and impaired cognitive function.6 The physiological presence and severity of OSA are most commonly assessed by the frequency of apneas and hypopneas per hour of sleep (apnea-hypopnea index or AHI).
Snoring, the most common symptom of OSA, is reported by up to 94% of patients with OSA.7 Snoring is a harsh respiratory sound caused by passage of air through a narrowed airway resulting in tissue vibration. It is commonly thought that the louder and more frequent the snoring, the worse the OSA. However, a substantial proportion of the general population (10% to 60%) are habitual snorers,8–11 but most do not have OSA.2 This relationship was mostly assessed by self-reporting or by means of OSA screening questionnaires, which are limited by their subjectivity and are likely biased by self-perception, and the presence or absence of a bedmate who witnesses snoring.12
Few studies tried to objectively investigate the relationship between snoring and OSA severity, but such studies have relied mainly on the sound intensity (amplitude) or sound bursts.13,14 This approach assumes that snoring has a higher sound intensity than nonsnoring breath sounds. This approach has limitations; first, snoring is not uniform in intensity. For example, snoring associated with obstructive events is louder than snoring not associated with obstructive events.15 Second, this approach does not take into account that some nonsnoring breath sounds, such as postapneic hyperventilation or gasping, which have high amplitude, might be falsely misclassified as snoring by an amplitude-based snoring detection system. Accordingly, using a sound amplitude threshold to detect snoring can result in missing low amplitude snores or falsely labeling high amplitude breath sounds as snoring. This is manifested by the very low specificity (38.5%) of sound amplitude levels in detecting snoring.16 Thus, the relationship between accurately measured snoring and OSA remains to be fully elucidated. Additionally, snoring has been very sparsely examined in the context of CSA.
To overcome these limitations, we have developed a system to detect individual snores based on the acoustic nature of the breath sounds regardless of its amplitude solely.17 This system is capable of accurately identifying individual snores, which are then quantified in order to evaluate their relationship to sleep apnea (both OSA and CSA). Machine learning algorithms have been deployed by other researchers for identifying snoring and examining snoring acoustic features in snorers with and without OSA, in a small set of participants.18 However, to our knowledge, there are no reports on using machine learning–identified snoring to accurately and objectively examine relationship between snoring frequency and the presence and severity of sleep apnea, which is the goal of this study.
METHODS
Participants
We recruited consecutive patients at least 18 years of age referred for polysomnography (PSG) due to a history suggestive of sleep apnea including at least two of the following symptoms: a history of loud habitual snoring, restless sleep, morning headaches, or excessive daytime sleepiness. Exclusion criteria were breath sound recordings less than 3.5 hours or sleep efficiency less than 30% on the PSG. The protocol was approved by the local research ethics board and participants provided written informed consent prior to participation.
Polysomnography
Participants underwent overnight PSG using standard techniques and scoring criteria for sleep stages and arousals from sleep.19,20 Thoracoabdominal movements and tidal volume were measured by respiratory inductance plethysmography (Respitrace, Ambulatory Monitoring Inc, Ardsley, New York, United States). Airflow was measured by nasal pressure cannulae (Binaps, Salter Labs, Arvin, California, United States) and a thermistor (ThermiSense, Salter Labs). Arterial oxyhemoglobin saturation (SaO2) was measured by oximetry (Nellcor, N-200 pulse oximeter, Nellcor Inc, Minneapolis, Minnesota, United States). Signals were recorded on a computerized sleep scoring system (Sandman, Nellcor Inc). An infrared camera recording (Sony PTZ IP/Analog Hybrid Camera) was used to determine body position throughout the night. Apnea was defined as a reduction in the respiratory signals by ≥ 90% lasting ≥ 10 seconds and hypopnea as a reduction by ≥ 30 to 90% lasting ≥ 10 seconds and accompanied by a ≥ 3% desaturation or an arousal from sleep. Respiratory events were classified as obstructive if there was out-of-phase thoracoabdominal motion or flow limitation on the nasal pressure tracing, and central if there was absent or in-phase thoracoabdominal motion without evidence of airflow limitation, during apneas and hypopneas, respectively. The AHI was calculated as the total number of apneas and hypopneas per hour of sleep. Obstructive AHI (AHIO) and central AHI (AHIC) were calculated as the number of obstructive events or central events per hour of sleep, respectively. Sleep apnea was considered to be an OSA disorder if ≥ 50% of events were obstructive, and CSA if ≥ 50% of events were central. Sleep apnea was further classified into mild (AHI 10 to < 20 events/h), moderate (AHI 20 to < 30 events/h), and severe (AHI ≥ 30 events/h).
Identification of Snores
Simultaneous with PSG, breath sounds were captured by a portable monitoring device, BresoDX (BresoTec Inc., Toronto, Ontario, Canada).21,22 It consists of an open lightweight face frame with an embedded electronic module and a microphone as shown in Figure 1, of which the technical details have been described previously.22 Breath sounds from the nose and mouth were sampled at 16 kHz and stored continuously for up to 8 hours on a micro SD card. Upon completion of the overnight study, data were transferred to a central server for acoustic analysis.
Figure 1. Illustration of the BresoDX device.
Illustration of the BresoDX device used to capture breath sounds.
Snores were automatically detected using an algorithm for breath sounds classification that has been previously described and validated against the human ear.17 Briefly, in that study, a human operator, who was blinded to the machine score, manually annotated audio of breath sounds based on this definition: snoring is a harsh, low- pitched sound produced by tissue vibration during passage of air through a partially collapsed upper airway during sleep.23,24 Separately, audio files were segmented into 64 ms, from each 10 acoustic variables (features) that were calculated to be used by the machine learning algorithm. The main features were: periodicity, which detects periodic sounds resulting from vibration of tissues during snoring; signal energy, which is proportional to sound amplitude; ratio of frequencies bands above and below 400 Hz; flatness of the frequency spectrum; uniformity of the signal; and entropy, a measure of signal's uncertainty.17 Subsequently, the acoustic features were fed into a machine learning algorithm known as Random Forest classifier, along with the human annotations as the ground truth. The algorithm learns the statistical distribution of the acoustic features for snoring and other breath sounds in a phase referred to as the training phase. The last phase is the validation phase, in which each participant was treated according to the algorithm as new unseen data and their audio was classified into snoring and nonsnoring classes. The machine classifier output was compared against the human annotations in each participant, and the accuracy of the machine was calculated for each participant. The average accuracy for all participants we reported was 97.5%.17
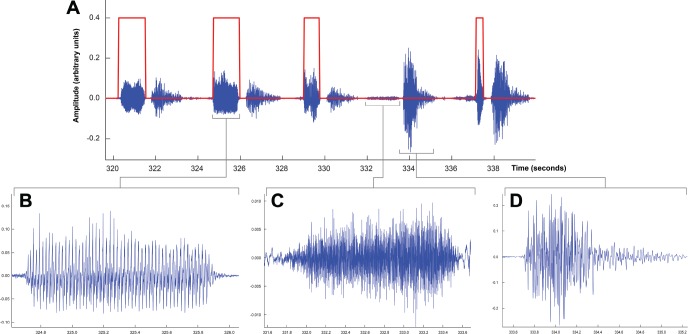
This method is far more sophisticated than detection of snoring by simply relying on sound amplitude (loudness or intensity) because it incorporates other sound features, which makes it sensitive to even low-amplitude snores. For example, Figure 2 shows that although snoring was lower in amplitude than some nonsnoring breath sounds, the algorithm accurately delineated the beginning and end of the snores. Finally, the total number of snores per hour of sleep was considered as the snoring index (SI).
Figure 2. Illustration of breath sounds waveforms.
A 19-second waveform of breath sounds showing 5 breaths. The identification function found by the automatic classifier (shown in red) is high when snoring is present and low otherwise. The second snore (B) is enlarged to show the periodic nature of its waveform as compared with the more aperiodic waveforms of inspiration (C) and expiration (D) that were not considered snores. Although snoring was generally similar or lower in amplitude than other breath sounds such as the expiration in (D), the classifier correctly identified snoring because of incorporation of the acoustic features of the breath sounds. Reprinted from Singh et al. The effect of sitting and calf activity on leg fluid and snoring. Respiratory Physiology & Neurobiology. 2017;240:1-7, copyright 2017, with permission from Elsevier (license number 4458921247048).
Statistical Analysis
The correlation coefficient between SI and AHI was calculated for the entire population. Data normality was tested using Jarque-Bera normality test. Kruskal-Wallis test, the equivalent to analysis of variance, for nonparametric data was performed to detect differences in SIs among subgroups of sleep apnea severity. Post hoc analyses were then performed by the Tukey-Kramer method to compare between-group medians. To evaluate the likelihood of snoring to predict OSA, the positive and negative predictive values (PPV and NPV), sensitivity, and specificity were calculated. Because an SI cutoff that defines a snorer does not exist in the literature, we used two nominal SI cutoffs of 50 and 100 snores/h to define a snorer. Additionally, the receiver operating characteristic (ROC) curves of SI for detecting OSA were plotted, in which sensitivity (the true positive rate) is plotted against 1-specifity (false-positive rate) for different AHI cutoffs. The areas under the ROC curves were calculated as a measure of how useful SI is in distinguishing those with and without OSA.
RESULTS
A total of 235 patients met the inclusion criteria and were included in the study, whose characteristics, AHIs, and SIs distributions are displayed in Table 1. Of those, 82 had no sleep apnea, 69 had mild, 33 had moderate, and 51 had severe sleep apnea. A total of 1,180,156 breaths were identified including 410,949 snores. Figure 3 demonstrates the distribution of 3 of the 10 acoustic features of snoring, normal inspiration, and expiration.
Table 1.
Patient characteristics.
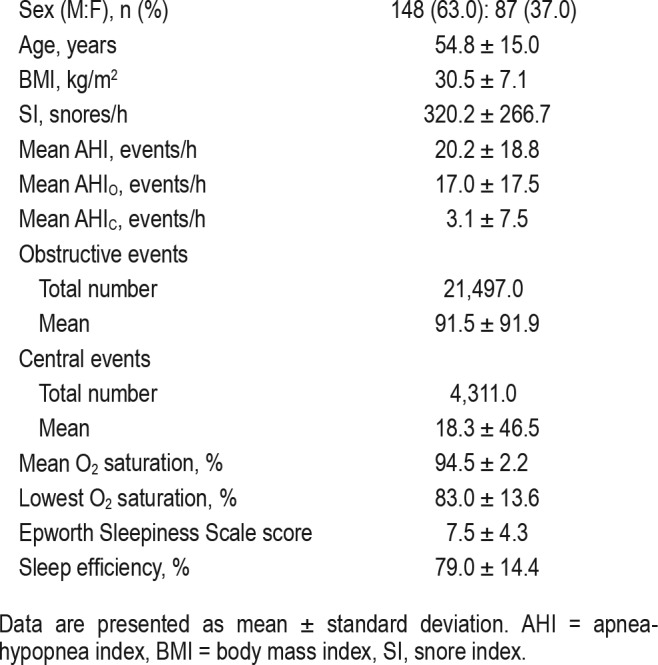
Figure 3. Feature space of breath sounds.
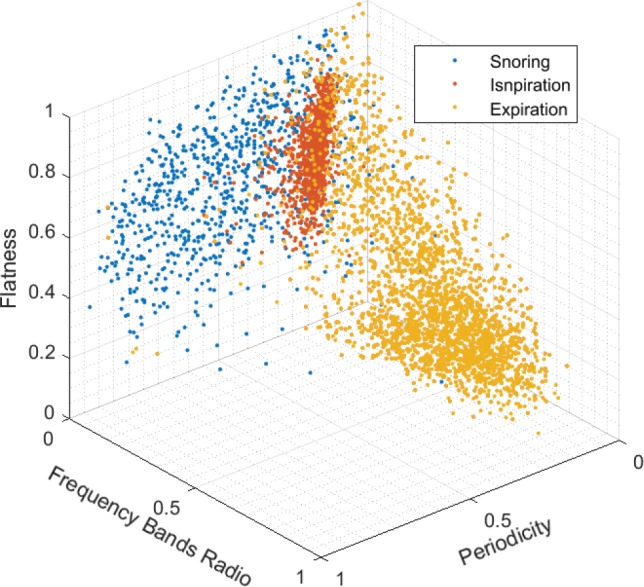
Feature space: three-dimensional distribution of three acoustic features: periodicity, frequency bands ratio, and spectral flatness for three breath sounds, snoring, inspiration, and expiration. All features are normalized between 0–1 with arbitrary units. The three sound classes show distinct clustering of their features, which allows the machine learning algorithm to identify breath sound classes. Each point represents the average value of 500 instances, eg, 1 blue point represents 500 snores.
Correlation Between Snoring and AHI
The relationship between SI and overall AHI was weak but significant (r = .32, P < .0001). There was a somewhat stronger relationship between SI and AHIO (r = .40, P < .0001) and very weak negative correlation with AHIC (r = −.14, P = .035) as demonstrated in Figure 4. A total of 136 patients had an AHIO ≥ 10, whose mean SI was 385.6 ± 284.3 snores/h. However, 16 patients had an AHIC ≥ 10 whose mean SI was 220.4 ± 188.8 snores/h, which was significantly lower than that of the former group (P = .016). Because SI is positively related to the AHIO but not AHIC, subsequent results examine the relationship between SI and AHIO only.
Figure 4. Correlation between AHI and SI.
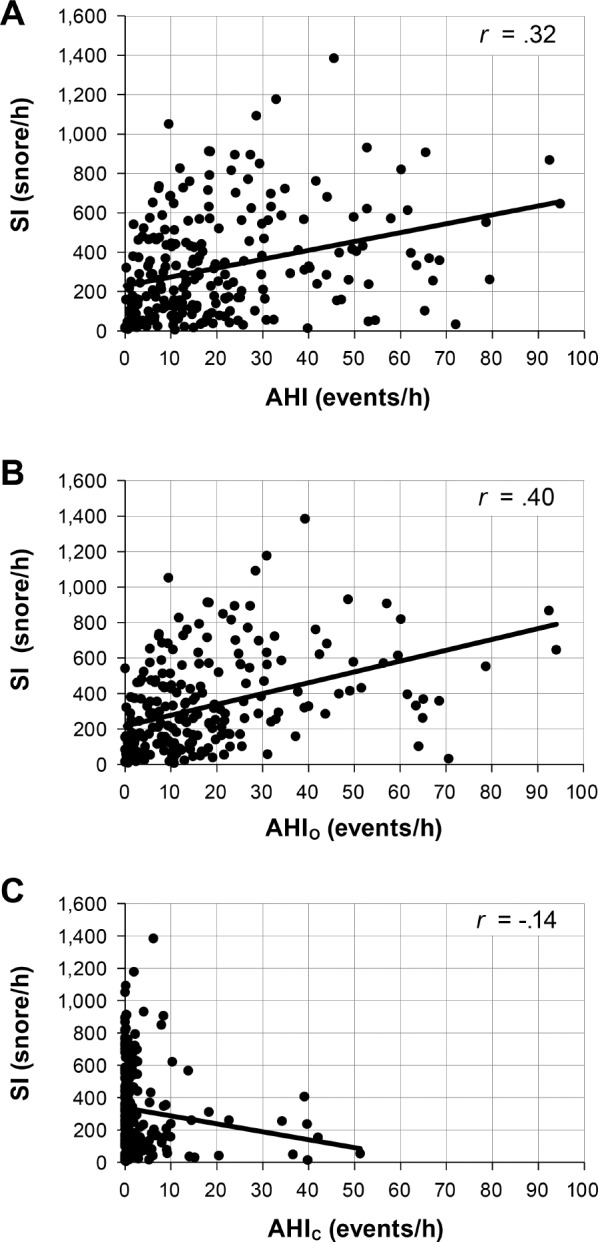
Relationships between the SI and the overall AHI (A), obstructive AHI (B) and central AHI (C). All three panels contain the entire population. In panel (C), most of the data points are skewed to the left because AHIC for most patients was very low. AHI = apnea-hypopnea index, AHIC = central apnea-hypopnea index, AHIO = obstructive apnea-hypopnea index, SI = snore index.
Change in SI According to OSA Severity
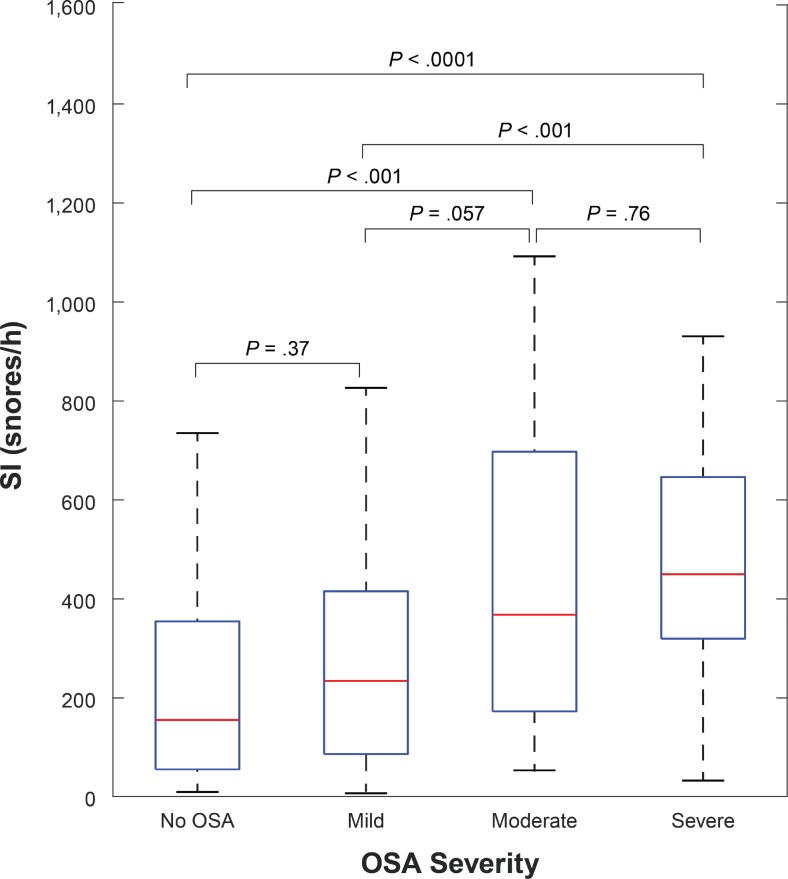
There is a stepwise increase in SI with increasing OSA severity (ie, no sleep apnea, mild, moderate, and severe), but with a substantial overlap in the SI among categories of OSA severity as depicted in Figure 5. The Kruskal-Wallis test showed that the groups' medians are statistically different (P < .001). Post hoc analysis showed that distant groups had statistically different medians whereas adjacent ones did not (Figure 5.)
Figure 5. Difference in SI among OSA severity categories.
Distribution of SI by OSA severity. The boxplots illustrate the distribution of snoring indices across the entire range for each category of OSA severity. OSA = obstructive sleep apnea, SI = snore index.
Snoring Predictive Value for OSA
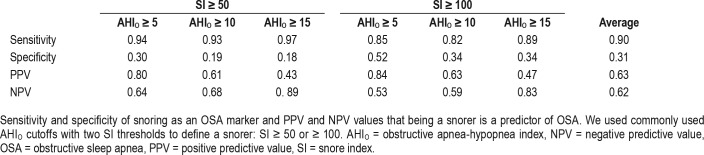
Of patients with OSA and an AHIO ≥ 15 events/h, 3.2% had minimal snoring with an SI < 50 and 10.8% with an SI < 100 snores/h. Conversely, of patients without OSA (AHIO < 15 events/h), 82.4% were snorers with an SI ≥ 50 and 66.2% with SI ≥ 100 snores/h. Sensitivity, specificity, PPV, and NPV of snoring for detecting OSA, defined as AHIO ≥ 5, 10, or 15 events/h, are displayed in Table 2. The area under the ROC of SI for identifying OSA using AHIO cutoffs of ≥ 5, 10, and 15 events/h were 0.78, 0.67, and 0.73 respectively, as depicted in Figure 6.
Table 2.
Positive and negative predictive values of snoring for OSA.
Figure 6. Receiver operating characteristic curves.
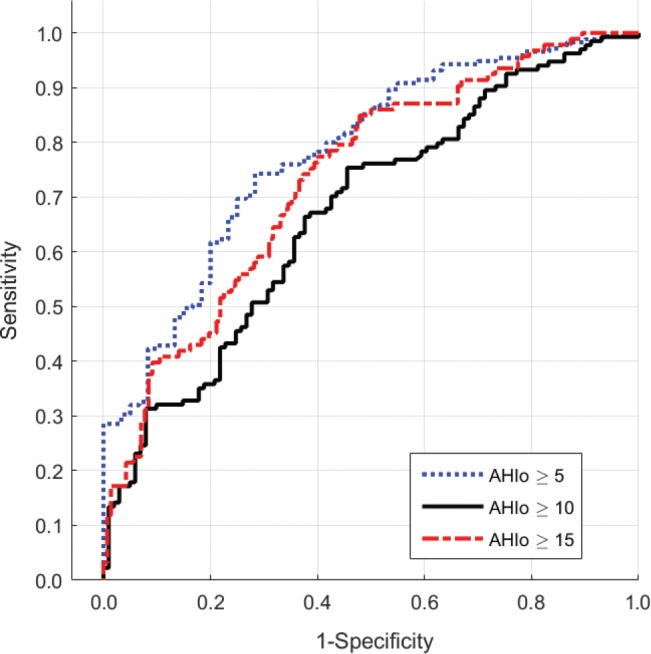
Receiver operating characteristic curves depicting accuracy of SI against AHIO for detecting OSA at three cutoffs: AHIO ≥ 5, ≥ 10, and ≥ 15 events/h. AHIO = obstructive apnea-hypopnea index, OSA = obstructive sleep apnea, SI = snore index.
DISCUSSION
Although the complaint of snoring is commonly considered a cardinal feature of sleep apnea, there is little objective evidence to support this self-reported observation. We therefore examined relationships between objectively measured indices of snoring frequency and the presence and severity of sleep apnea assessed by the AHI. The two novel and most important findings of this study in participants with suspected sleep apnea are that: (1) there is only a weak positive correlation between the SI and the AHI, and this relationship only holds for OSA, but not for CSA, in which the correlation is inverse, and (2) although the SI increases with increasing OSA severity, there is substantial overlap in SI among categories of OSA severity. The method for snoring detection used in this study is unique in that it identifies individual snores using sophisticated acoustic features and machine learning algorithms. Therefore, it is sensitive to all snores, even those with very low amplitude, and excludes nonsnoring high-amplitude breath sounds such as gasps and postapneic hyperventilation from the SI. Accordingly, it has advantages over commonly used self-reporting of snoring or use of simple sound amplitude levels as a marker of snoring.
There are many definitions of snoring in the literature. A commonly used objective definition identifies snoring as any spike in sound intensity that exceeds a certain level such as 40 dB25 or 50 dB.12 This definition is susceptible to variation depending on the type and gain of the microphone and distance from the mouth, which have a substantial effect on sound amplitude, and thus, could compromise the accuracy of snoring detection. Using sound amplitude is also subject to contamination by high-amplitude sounds such as gasps and hyperventilation, which might be detected falsely as snores. It is also prone to missing low amplitude snores that indicate upper airway narrowing and vibration. The means of identifying snoring in the current study involved detection of tissue vibration regardless of the sound amplitude. It makes use of a large set of acoustic features that characterize turbulence, periodicity, and vibration.17 Using this method, high-amplitude nonsnoring sounds are not classified as snores, whereas low-amplitude sounds that satisfy the criteria for snoring are classified as snores. Accordingly, our data likely provide a representative picture of the relationship between the frequency of snoring and the presence, type, and severity of sleep apnea.
Habitual snoring is very common among adults, especially men, but reports of its prevalence vary substantially, likely because of variations in the means of quantifying snoring and in the populations studied. In large epidemiological studies, 52.8% of 22,745 American participants8 and 62% of 12,700 Japanese participants self-reported habitual snoring.10 In a French study, 32% of 299 men self-reported snoring,11 whereas in a British study, including 890 men, only 10% self-reported snoring often and 50% self-reported snoring sometimes.9 Despite the wide range in reported prevalence of snoring, it is reasonably clear that a significant proportion of the adult population snore for at least some part of the night. Yet, the relationship between the frequency of snoring and sleep apnea severity has not been thoroughly and objectively examined.
Our work took a more objective approach to this issue by deploying machine learning algorithms for quantifying snoring accurately and its relationship to sleep apnea. We found that the SI correlates weakly and positively with the AHIO, but weakly and negatively with the AHIC. The negative correlation is driven by the high SI in individuals without sleep apnea and the low SI in the 12 patients with an AHIC ≥ 10 events/h (Figure 4C). Although the number of patients with CSA was small, our data are in keeping with the observation that among patients with heart failure and who have CSA, the prevalence of self-reported habitual snoring is much less than that among patients with heart failure and OSA (28% versus 78%).26 These findings are probably attributable to the relative stability of the upper airway, and a lesser propensity to collapse during sleep in patients with CSA compared to those with OSA. However, this property was not examined in the current study and could be the topic of future work. Despite the small number of patients with CSA, to our knowledge this is the first report on objective quantification of snoring in patients with CSA.
In standard clinical practice and in most commonly used OSA screening questionnaires, snoring has been considered one of the cardinal features of OSA.27,28 Our study showed only a modest PPV of snoring for OSA, ranging between 0.47 to 0.84. This is a reflection of the remarkable number of patients without OSA with SIs in the range of and sometimes exceeding that of patients with sleep apnea (Figure 4 and Figure 5). Using objective methods, our study agrees with that of Young et al., who found that the 86.8% of people who self-reported habitual snoring were found to have an AHI < 15 events/h on PSG.2 Therefore, a history of heavy snoring, in the absence of other symptoms of OSA, might yield high scores on OSA questionnaires and lead to unnecessary referral for sleep studies.
Snoring frequency was only a weak indicator of OSA severity as revealed by the low correlation coefficient between SI and AHI and by the lack of significant differences between SI medians of adjacent classes of OSA severity (Figure 5). More importantly, there was a large overlap in snoring frequency between participants with and without OSA (Figure 4 and Figure 5). Of particular significance are patients with sleep apnea with low SI, including a few patients with severe OSA (Figure 4). We found that 10.8% of patients with OSA (AHIO ≥ 15 events/h) had minimal snoring with an SI < 100 snores/h. Such patients could be referred to as “quiet OSA.” Similarly, Young et al. showed that 13% of patients with OSA (AHI ≥ 15 events/h) didn't report snoring. Because of the paucity of snoring in these patients, they are less likely to come to clinical attention for evaluation of sleep apnea. Therefore, relying on a history of snoring might result in missing the diagnosis of OSA in a substantial proportion of people who do not snore, have OSA, and may benefit from treatment. However, most patients with severe OSA (AHI ≥ 30 events/h) were heavy snorers with a SI well above that of those without OSA (Figure 4B). This shows that only when a patient has severe OSA does snoring become a sensitive, although not specific, marker of OSA (Table 2).
In studies that used sound intensity as a marker for snoring, stronger relationships between SI and AHI (with correlation coefficients between 0.7329 and 0.9114) were observed than between SI and AHI in the current study. However, in one of those studies, only patients with OSA were included; thus, snoring was not examined in patients without OSA.29 In the other study, snoring was defined as amplitude bursts preceded by an apnea or a hypopnea, so that snoring was associated with apneas and hypopneas by design, which would artificially enhance any relationship between snoring and AHI.14 Accordingly, those studies are not comparable to ours, and would overestimate the relationship between snoring and OSA severity, and underestimate the degree of snoring that is not associated with OSA. Our approach, conversely, detects snoring regardless of the presence or absence of apneas and hypopneas, and therefore should provide a more accurate assessment of snoring frequency in patients with and without OSA.
One limitation of this study is that it included patients referred for PSG, and therefore with a high pretest probability for snoring and OSA. Our results may therefore not be directly representative of the general population. Because a significant proportion of our sample had a high SI without OSA, it is likely that this proportion would be higher in the general population. Thus, a complaint of heavy snoring on its own should not necessarily lead to a sleep study unless accompanied by other signs and symptoms of OSA, such as restless sleep, daytime sleepiness, hypertension, obesity, or morning headaches. Another limitation is that we did not quantify the loudness of snoring. Finally, the number of patients with CSA was small, reflecting the relative rarity of this disorder in the general population.30
CONCLUSIONS
Our data demonstrate that the presence and severity of snoring, assessed objectively as the snore index, bear only a weak positive relationship to the presence and severity of OSA, but a negative relationship to CSA. The overlap between the frequency of snoring among those with and without OSA is substantial. Minimal or no snoring does not always rule out OSA. Very frequent snoring is a sensitive but not specific indicator of severe OSA, because a large proportion of patients without OSA snored as frequently as those with severe OSA. Thus, the presence and frequency of snoring on its own is probably of limited usefulness in screening for the possibility of either OSA or CSA.
DISCLOSURE STATEMENT
Work for this study was performed at the Toronto Rehabilitation Institute, University Health Network, Toronto, ON, Canada. All authors have seen and approved the manuscript. This project has been supported by the Ministry of Research and Innovation of Ontario, MaRS Innovation & Ontario Centre of Excellence (MI MSCPoP Ref. No. 2011-0196), Johnson and Johnson Inc, and the Ontario Brain Institute via FedDev, which were granted to Drs. Alshaer and Bradley. Dr. Alshaer received the NSERC scholarship (PGS D3-374148-2009). Dr Bradley is supported by the Clifford Nordal Chair in Sleep Apnea and Rehabilitation Research and The Godfrey S. Pettit Chair in Respiratory Medicine. This study used a medical device, BresoDX, for detection of snoring. Drs. Bradley and Alshaer are inventors of this device and have a financial interest in it. Richard Hummel is an employee of the manufacturer of BresoDX (BresoTec Inc, Toronto, Canada). The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the technicians at the Toronto Rehabilitation Institute-University Health Network sleep research laboratory for their support during data collection; in particular, Fiona Rankin for her help in the interpretation of polysomnography data, and Wen-Hou Tseng for his aid in selecting participants for the study. We would like to thank Maryam Patel for her support in the submission process.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AHIC
central apnea-hypopnea index
- AHIO
obstructive apnea-hypopnea index
- CSA
central sleep apnea
- NPV
negative predictive value
- OSA
obstructive sleep apnea
- PPV
positive predictive value
- PSG
polysomnography
- ROC
receiver operating characteristic
- SI
snore index
REFERENCES
- 1.Kimoff RJ, Cheong TH, Olha AE, et al. Mechanisms of apnea termination in obstructive sleep apnea. Role of chemoreceptor and mechanoreceptor stimuli. Am J Respir Crit Care Med. 1994;149(3 Pt 1):707–714. doi: 10.1164/ajrccm.149.3.8118640. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 4.Young T, Blustein J, Finn L, Palta M. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep. 1997;20(8):608–613. doi: 10.1093/sleep/20.8.608. [DOI] [PubMed] [Google Scholar]
- 5.Kohli P, Balachandran JS, Malhotra A. Obstructive sleep apnea and the risk for cardiovascular disease. Curr Atheroscler Rep. 2011;13(2):138–146. doi: 10.1007/s11883-011-0161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HC, Young T, Matthews CG, Weber SM, Woodward AR, Palta M. Sleep-disordered breathing and neuropsychological deficits. A population-based study. Am J Respir Crit Care Med. 1997;156(6):1813–1819. doi: 10.1164/ajrccm.156.6.9610026. [DOI] [PubMed] [Google Scholar]
- 7.Mattei A, Tabbia G, Baldi S. Diagnosis of sleep apnea. Minerva Med. 2004;95(3):213–231. [PubMed] [Google Scholar]
- 8.Bhattacharyya N. Sleep and health implications of snoring: a populational analysis. Laryngoscope. 2015;125(10):2413–2416. doi: 10.1002/lary.25346. [DOI] [PubMed] [Google Scholar]
- 9.Stradling JR, Crosby JH. Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle aged men. Thorax. 1991;46(2):85–90. doi: 10.1136/thx.46.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikematsu T. Clinical study of snoring for the past 30 years. In: Chouard CH, editor. Chronic Rhoncopathy: Proceedings of the 1st International Congress on Chronic Rhonchopathy; 6-8 July 1987; Paris. Montrouge, France: John Libbey Eurotext; 1988. pp. 5–14. [Google Scholar]
- 11.Teculescu D, Hannhart B, Cornette A, Montaut-Verient B, Virion JM, Michaely JP. Prevalence of habitual snoring in a sample of french males. Role of “minor” nose-throat abnormalities. Respiration. 2001;68(4):365–370. doi: 10.1159/000050528. [DOI] [PubMed] [Google Scholar]
- 12.Hoffstein V, Mateika S, Anderson D. Snoring: is it in the ear of the beholder? Sleep. 1994;17(6):522–526. doi: 10.1093/sleep/17.6.522. [DOI] [PubMed] [Google Scholar]
- 13.Maimon N, Hanly PJ. Does snoring intensity correlate with the severity of obstructive sleep apnea? J Clin Sleep Med. 2010;6(5):475–478. [PMC free article] [PubMed] [Google Scholar]
- 14.Wu HT, Pan WY, Liu AB, et al. Vibration signals of snoring as a simple severity predictor for obstructive sleep apnea. Clin Respir J. 2016;10(4):440–448. doi: 10.1111/crj.12237. [DOI] [PubMed] [Google Scholar]
- 15.Herzog M, Plossl S, Glien A, et al. Evaluation of acoustic characteristics of snoring sounds obtained during drug-induced sleep endoscopy. Sleep Breath. 2015;19(3):1011–1019. doi: 10.1007/s11325-014-1085-7. [DOI] [PubMed] [Google Scholar]
- 16.Rohrmeier C, Herzog M, Ettl T, Kuehnel TS. Distinguishing snoring sounds from breath sounds: a straightforward matter? Sleep Breath. 2014;18(1):169–176. doi: 10.1007/s11325-013-0866-8. [DOI] [PubMed] [Google Scholar]
- 17.Alshaer H, Pandya A, Bradley TD, Rudzicz R. Subject independent identification of breath sounds components using multiple classifiers. Presented at: 2014 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP); May 4-9, 2014; Florence, Italy. [Google Scholar]
- 18.Fiz JA, Jane R, Sola-Soler J, Abad J, Garcia MA, Morera J. Continuous analysis and monitoring of snores and their relationship to the apneahypopnea index. Laryngoscope. 2010;120(4):854–862. doi: 10.1002/lary.20815. [DOI] [PubMed] [Google Scholar]
- 19.EEG arousals: scoring rules and examples: a preliminary report from the sleep disorders atlas task force of the American Sleep Disorders Association. Sleep. 1992;15(2):173–184. [PubMed] [Google Scholar]
- 20.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 21.Alshaer H, Fernie GR, Maki E, Bradley TD. Validation of an automated algorithm for detecting apneas and hypopneas by acoustic analysis of breath sounds. Sleep Med. 2013;14(6):562–571. doi: 10.1016/j.sleep.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Alshaer H, Levchenko A, Bradley TD, Pong S, Tseng WH, Fernie GR. A system for portable sleep apnea diagnosis using an embedded data capturing module. J Clin Monit Comput. 2013;27(3):303–311. doi: 10.1007/s10877-013-9435-8. [DOI] [PubMed] [Google Scholar]
- 23.Alshaer H. Toronto, Canada: Institute of Biomaterials and Biomedical Engineering (IBBME), University of Toronto; 2013. A Single-Channel Acoustic Method for Portable Diagnosis of Sleep Apnea [PhD thesis] [Google Scholar]
- 24.Alshaer H. New technologies for the diagnosis of sleep apnea. Curr Hypertens Rev. 2016;12(1):48–56. doi: 10.2174/1573402112666160114094337. [DOI] [PubMed] [Google Scholar]
- 25.Koutsourelakis I, Perraki E, Zakynthinos G, Minaritzoglou A, Vagiakis E, Zakynthinos S. Clinical and polysomnographic determinants of snoring. J Sleep Res. 2012;21(6):693–699. doi: 10.1111/j.1365-2869.2012.01018.x. [DOI] [PubMed] [Google Scholar]
- 26.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97(21):2154–2159. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 27.Kim B, Lee EM, Chung YS, Kim WS, Lee SA. The utility of three screening questionnaires for obstructive sleep apnea in a sleep clinic setting. Yonsei Med. J. 2015;56(3):684–690. doi: 10.3349/ymj.2015.56.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth. 2010;57(5):423–438. doi: 10.1007/s12630-010-9280-x. [DOI] [PubMed] [Google Scholar]
- 29.Acar M, Yazici D, Bayar Muluk N, Hanci D, Seren E, Cingi C. Is there a relationship between snoring sound intensity and frequency and osas severity? Ann Otol Rhinol Laryngol. 2016;125(1):31–36. doi: 10.1177/0003489415595640. [DOI] [PubMed] [Google Scholar]
- 30.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157(1):144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]