Abstract
Study Objectives:
Although Pierre Robin sequence (PRS) is a major cause of neonatal obstructive sleep apnea (OSA), longitudinal studies reporting evolution with age are lacking. This study aimed to describe changes in sleep-related respiratory parameters and sleep architecture in neonates with PRS treated conservatively (defined for this paper as treatment without tracheostomy or mandibular distraction).
Methods:
A retrospective, 14-year, single-institution study of neonates with PRS who underwent diagnostic polysomnography (PSG) and at least one follow-up PSG. Those treated with surgery were excluded. Data were analyzed using a mixed-effects model with subject-specific random effect.
Results:
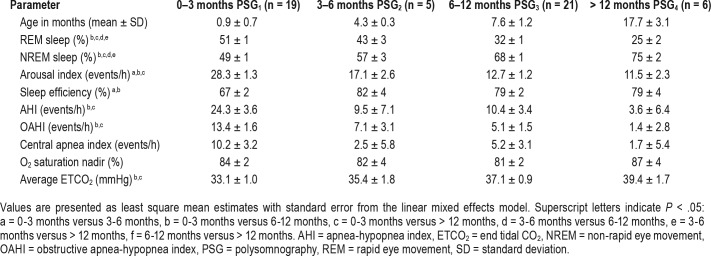
In a cohort of 21 infants, baseline PSG (mean age 0.9 ± 0.7 months) showed a total apnea-hypopnea index (AHI) of 24.3 ± 3.6 events/h, obstructive apnea-hypopnea index (OAHI) of 13.4 ± 1.6 events/h, central apnea index of 10.2 ± 3.2 events/h, and an arousal index of 28.3 ± 1.3 events/h (variables reported as least square means ± standard error of the mean). There was a significant reduction in AHI, OAHI, arousal index, and percentage of REM sleep with advancing age. Although 71% of infants achieved full oral feeds by one month of age, some infants remained underweight during infancy.
Conclusions:
These neonates with PRS and OSA, treated conservatively, had an improvement in OAHI with advancing age with the median age of OSA resolution at 15 months. Factors potentially responsible include craniofacial growth and maturational changes of respiratory control. Further studies are necessary to determine the long-term effects of conservative management on growth and neurodevelopmental outcomes in these infants.
Citation:
Ehsan Z, Kurian C, Weaver KN, Pan BS, Huang G, Hossain MM, Simakajornboon N. Longitudinal sleep outcomes in neonates with Pierre Robin sequence treated conservatively. J Clin Sleep Med. 2019;15(3):477–482.
Keywords: infant, OSA, Pierre Robin sequence
BRIEF SUMMARY
Current Knowledge/Study Rationale: Although Pierre Robin sequence (PRS) is a major cause of neonatal obstructive sleep apnea (OSA), most studies have focused on surgical management outcomes. The natural evolution of severe OSA over the first 2 years of life is not well understood.
Study Impact: This study reports on the largest cohort of symptomatically treated neonates with PRS and moderate to severe OSA, showing improvement in OSA over the first 2 years of life. These results help inform providers regarding the natural history of OSA in neonates with PRS and may affect risk stratification and medical decision making in this population.
INTRODUCTION
Pierre Robin sequence (PRS) involves a clinical triad of micrognathia, glossoptosis, and upper airway obstruction. The incidence of PRS in the United States is approximately 1 per 5,000 to 7,000 live births.1 Between 46% and 85% of neonates with PRS have severe upper airway obstruction and resultant obstructive sleep apnea (OSA).2–4 Although these neonates have improvement in clinical symptoms over time, literature reporting objective improvement of sleep-disordered breathing during the first few years of life is limited. This has hindered development of standardized algorithms for the diagnosis and management of OSA in this population.5
The purpose of the study was to report longitudinal outcomes of sleep-disordered breathing in a cohort of neonates with PRS who were treated conservatively (nonsurgical management).
METHODS
A 14-year, retrospective cohort study was conducted in all neonates with PRS treated at Cincinnati Children's Hospital and Medical Center (CCHMC) from January 1, 2002 to January 1, 2016. PRS was defined as micrognathia, glossoptosis, and upper airway obstruction with or without cleft palate. We included neonates with PRS who underwent diagnostic polysomnography (PSG1) at age 3 months or younger and at least one follow-up PSG (PSGn) at age 12 months or younger. Those treated with surgery (for example, mandibular distraction or tracheostomy) were excluded. Additionally, we excluded neonates with micrognathia who did not meet diagnostic criteria for PRS, based on information in the medical chart, as determined by a genetics subspecialist consultant (KNW). The medical records and sleep studies of the identified study participants were reviewed. Clinical parameters recorded were: age at PSG, prematurity (defined as < 37 weeks gestation), presence of a genetic syndrome, and treatment provided. Isolated PRS was defined as PRS without a genetic syndrome. This study was approved by the Institutional Review Board of Cincinnati Children's Hospital Medical Center.
At CCHMC, newly diagnosed or transferred newborns with micrognathia and respiratory distress are evaluated by a multi disciplinary team involving neonatology, otolaryngology, genetics, plastic surgery, pediatric pulmonology, and sleep medicine and speech pathology specialists. All neonates undergo standard overnight PSG (detailed in the next paragraphs) and noncontrast computed tomography of the maxillofacial skeleton to assess mandibular form, bone quality, associated malformations of the condyle, and temporomandibular joint ankylosis. Additionally, all neonates undergo bedside nasopharyngoscopy by a pediatric otolaryngologist to confirm base of tongue collapse and assess for additional upper airway abnormalities. Eligibility for surgical versus nonsurgical intervention is determined collaboratively by the multidisciplinary team after this comprehensive evaluation.
The PSG studies were performed in the sleep laboratory at CCHMC in accordance with the American Academy of Sleep Medicine (AASM) guidelines. The standard infant montage was used and the following variables were recorded simultaneously: body position, left and right electrooculogram (ROC/A1, LOC/A2), four-channel electroencephalogram (EEG; O1A2, O2A1, C4A1, C3A2), chin electromyogram, electrocardiogram, pulse oximetry (Masimo Corporation, Irvine, California, averaging time of 3 seconds) and pulse waveform, thoracic and abdominal inductance plethysmography, nasal thermistor and nasal pressure transducer, end-tidal pCO2 monitoring (BCI Capnocheck, Smiths Medical, Minneapolis, Minnesota, United States), and transcutaneous pO2 and pCO2 (Tina TCM4/40; Radiometer, Copenhagen, Denmark). All infants were placed in the supine position for the study. Sleep scoring was performed by a registered PSG technologist and validated by a board-certified pediatric sleep specialist, using standard pediatric criteria recommended by the AASM at the time of the sleep studies were obtained. Studies were analyzed in 30-second epochs and staged as awake, rapid eye movement (REM) sleep, non-rapid eye movement (NREM) sleep. For sleep studies conducted in patients younger than age 2 months, sleep was scored as active, quiet, or indeterminate. For infants between age 2 to 6 months, sleep was scored as REM and NREM. For infants older than 6 months, pediatric sleep scoring was used (NREM1, NREM2, NREM3, and REM). In order to compare outcomes consistently across infancy, they were labeled as: REM (active + indeterminate) and NREM (quiet) sleep. Sleep efficiency was calculated by dividing total sleep time by time in bed and expressing as a percentage. An arousal was defined as a shift in the EEG pattern to frequencies of 8 to 13 Hz, or above 16 Hz, for a minimum of 3 seconds. Arousals in REM sleep were accompanied by a concurrent increase in submental EEG. A respiratory arousal was defined as an arousal occurring during or within 5 seconds following a respiratory event. Apnea was defined as at least a 90% reduction in airflow from baseline over two or more respiratory cycles. An obstructive apnea was defined as apnea in the presence of persistent or increased respiratory effort. A central apnea was defined as apnea with absence of both chest wall and abdominal movements. Hypopnea was defined as a 50% or greater reduction in airflow accompanied by either an oxygen desaturation of at least 3% or arousal. A mixed apnea was defined as an apnea event with absent respiratory effort during one portion and the presence of inspiratory effort in another portion. The central apnea index was defined as the number of central respiratory events per hour. The obstructive apnea-hypopnea index (OAHI) was defined as the number of obstructive apneas, mixed apneas, and obstructive hypopneas per hour. There are no accepted criteria for definition of OSA in children younger than 1 year. We defined OSA as an OAHI > 1 event/h based on the cutoff for children. OSA severity criteria were based on our institutional practice (mild: OAHI 1 to < 5; moderate OAHI 5 to < 10 and severe: OAHI > 10 events/h) and limited available literature.6
Data Analysis
Descriptive clinical characteristics of the cohort are reported. We compared PSG parameters for the entire cohort. Data were analyzed longitudinally to track changes in sleep and respiratory parameters using the linear mixed- effects model with subject-specific random effect and group as a categorical covariate with four levels. The group variable was constructed based on the age at PSG: 0–3 months; 3–6 months; 6–12 months; and older than 12 months. Unless otherwise noted, PSG variables were presented as least square means ± standard error; count and percentage were given for categorical variables. SAS software (version 9.4; Cary, North Carolina, United States) was used for all analyses. A P value ≤ .05 was considered statistically significant.
RESULTS
In a cohort of 162 neonates with PRS, 104 (64%) underwent surgery (either mandibular distraction osteogenesis [MDO] or tracheostomy) and 58 (36%) were treated conservatively. Of those who were treated conservatively, 21 (36%) were ultimately included. Reasons for exclusion were: absence of diagnostic PSG at age 3 months or younger or inadequate PSG during infancy, no follow-up PSG during infancy, treatment with upper airway surgery between sequential PSG tests (for example, supraglottoplasty or cleft palate repair), and treatment with supplemental oxygen during initial PSG (oxygen titration sleep study or split night). There were no significant differences in sex, prematurity, birth weight, and syndromic diagnosis in infants treated conservatively versus surgically (tracheostomy or MDO). There were no significant differences in age, sex, and baseline OAHI between neonates treated conservatively and excluded versus included. Of those excluded, 19 neonates did not have a diagnostic PSG performed during infancy. Four neonates were treated with supplemental oxygen based on clinical judgement of the provider and underwent a titration PSG without a diagnostic PSG. Five neonates had PSG1 at age 3 months or younger but no follow-up PSG; mean OAHI was 13.8 ± 8.3 events/h. Two neonates had PSG1 at age 6 months or older; mean OAHI was 11.2 ± 0.5 events/h. One neonate had PSGn at age 50 months or older (OAHI at diagnosis was 53.1 events/h). One neonate had two PSG tests within the birth to 3-month age group; OAHI at diagnosis was 25.7 events/h and at follow-up was 9.9 events/h. One neonate did not have OSA based on PSG1 (OAHI = 0.3 events/h). Three infants had supraglottoplasty performed between initial and subsequent PSG tests, with PSG1 OAHI 15.2 ± 1.6 events/h, and one neonate had cleft palate repair between sequentially available PSG tests (OAHI on PSG1 was 3.1 events/h).
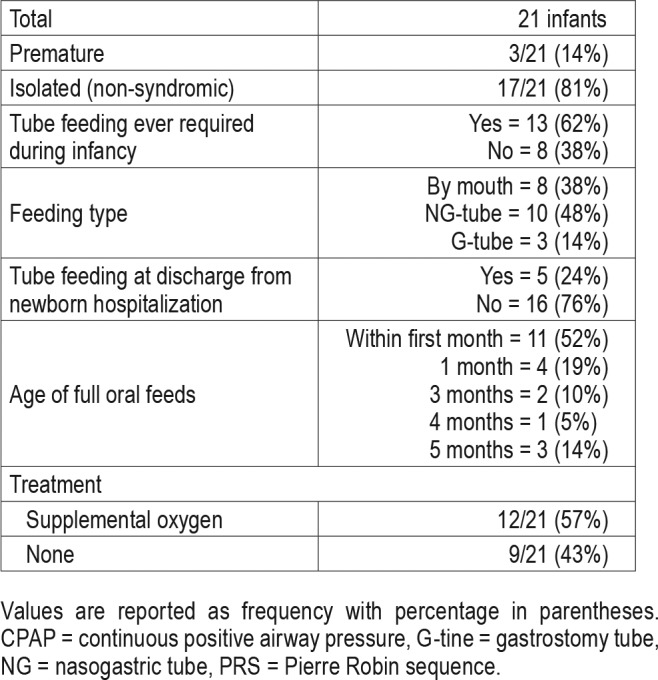
Of the 21 included neonates, 3 (14%) were premature and 17 (74%) had isolated PRS (Table 1). Age at PSG1 was 0.9 ± 0.7 months. Of these 21 neonates, 12 (57%) received supplemental oxygen and 9 (41%) underwent watchful waiting for OSA management. Thirteen neonates (62%) required tube feeding during infancy (either nasogastric tube or gastrostomy tube) (Table 1) with most achieving full oral feeds by 1 month of life (15 neonates [71%]).
Table 1.
Descriptive statistics for cohort of neonates with PRS.
At follow-up, there was a significant decrease in OAHI (PSG1 versus PSG3 [13.49 ± 1.61 versus 5.15 ± 1.54, P < .05], and PSG1 versus PSG4 [13.49 ± 1.61 versus 1.45 ± 2.81, P < .05]) (Figure 1). Additionally, there was a significant decrease in total AHI, reduction in percentage of REM sleep, reduction in the arousal index, improvement in sleep efficiency, and increase in percentage of NREM sleep with advancing age. There were no significant changes in nadir of oxygen saturation and central apnea index, although there were trends toward improvement (Table 2). Although growth indices were not obtained in a systematic fashion for all infants, the available data show that 70% of infants were able to achieve full oral feeds by 1 month of life. Some infants with PRS were underweight and remained so over the first year of life (Figure 2).
Figure 1. Plot depicting longitudinal changes in obstructive apnea-hypopnea index with advancing age.
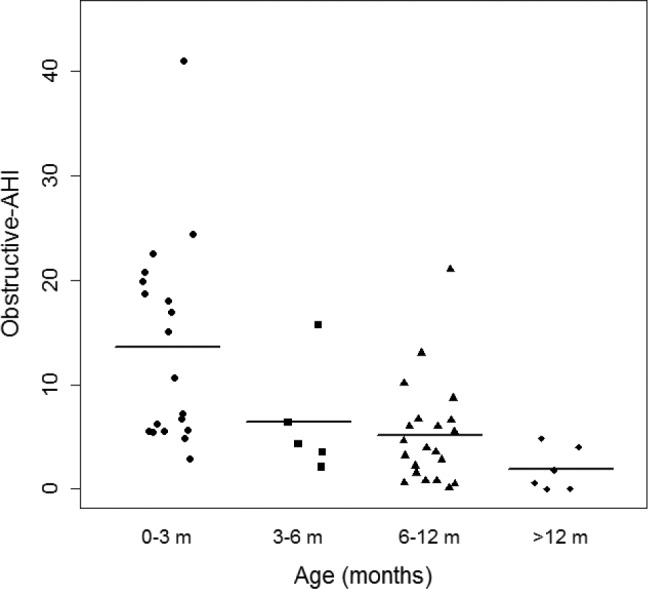
AHI = apnea-hypopnea index.
Table 2.
Changes in sleep and respiratory parameters with age.
Figure 2. Plot depicting longitudinal changes in weight (represented as Z-score) with advancing age.
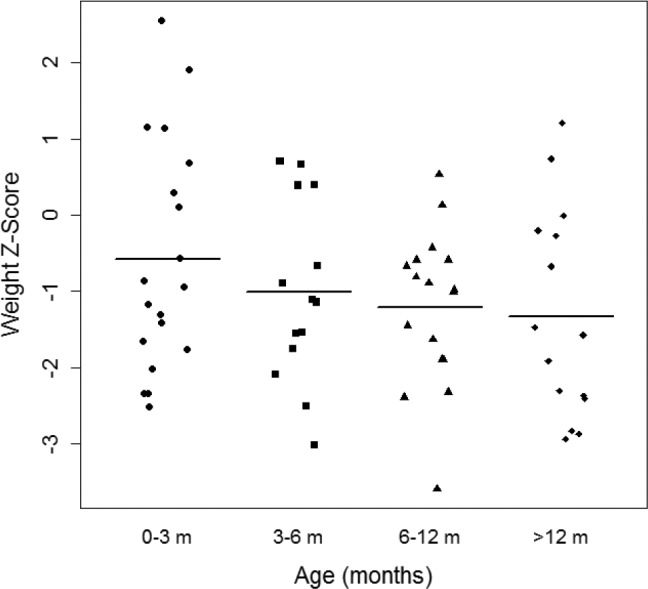
Z-scores have been corrected for gestational age at birth.
DISCUSSION
In this study we have shown that these neonates with PRS who did not need mechanical ventilation and were mostly fed by mouth within 1 month after birth had spontaneous improvement in obstructive respiratory events during the first year of life, with significant improvement at 6 to 8 months of age and resolution by 15 months of age.
Additionally, there were improvements in sleep efficiency, sleep consolidation, and changes in sleep architecture (increase in NREM and decrease in REM sleep) that paralleled reported developmental changes seen in otherwise healthy infants.7 To our knowledge this is the largest cohort of unique neonates with PRS with objective PSG measures tracked over infancy.
There is still debate over the optimal criteria to determine conservative versus surgical management in neonates with PRS.8–10 Neonates with profound airway obstruction at birth and inability to maintain spontaneous respiration due to airway compromise are obvious candidates for early surgical intervention. In most neonates, however, the decision is not as clear. Management decisions are typically based on severity of respiratory and feeding difficulties as well as presence of underlying syndromes and neurocognitive dysfunction. The spectrum of clinical presentation of sleep-disordered breathing ranges from infrequent episodes of upper airway obstruction (noisy breathing or oxygen desaturations) and feeding difficulties to severe OSA and subsequent failure to thrive. Although conservative (nonsurgical) treatment has long been used for neonates with PRS, objective measures of OSA severity (as obtained by PSG) have not been consistently reported and data on changes in sleep and respiratory parameters with advancing age are limited.11,12 Moreover, there is a lack of criteria for the diagnosis of OSA in infants, with management decisions often based on pediatric criteria. This has hindered development of standardized management guidelines. Although our study was not designed to assess what factors determine outcomes of surgical versus nonsurgical management, our, albeit limited, data suggest that neonates with isolated PRS and OSA (with OAHI < 20 events/h), who do not need a feeding tube by 1 month of age, may have resolution of OSA over the first year of life with conservative treatment.
The noninvasive nature of PSG makes it an effective tool to guide and influence decision making and quantitatively measure the usefulness of both surgical and nonsurgical interventions in neonates with PRS. All neonates in our cohort had OSA in the moderate to severe category at baseline, with improvement in OSA severity on sequentially performed PSG tests with advancing age. Factors potentially responsible for improvement in OSA include craniofacial growth with advancing age and maturational changes of respiratory control. Neonates were treated with close observation in the home, with most foregoing supplemental oxygen or continuous positive airway pressure (CPAP). It is not our institutional practice to recommend prone positioning for neonates with PRS, and positioning at home was left to the discretion of the caregivers. Prone positioning allows for forward movement of the mandible by gravity to increase the oropharyngeal space and is commonly used as first-line treatment for neonates with PRS. Historically, success has been reported with this modality alone in a select cohort of neonates.13 However, despite reported clinical efficacy, given the increased risk of sudden infant death with nonsupine sleeping positions, clinicians are often weary of recommending this approach particularly to infants with underlying sleep-disordered breathing. Moreover, although clinical observation suggests that prone positioning can improve severity of hypoxemic episodes while asleep (often used an indicator for severe obstructive respiratory events), robust objective data on the effect of prone positioning on OSA management in this population is lacking. There is currently only one available study that objectively assessed this and shows that it does not completely resolve obstructive respiratory events, especially in infants with severe OSA.14 It is unclear whether this has affected the surgical rate at our center. Based on a study by Scott and Mader, there may be regional differences in surgical management of infants with PRS, with certain centers (in the Midwest, for example) performing a higher number of MDO procedures.1
Most neonates in our cohort were treated with supportive care or low flow supplemental oxygen for treatment of OSA and had resolution of OSA over time. This is noteworthy as nonsurgical treatment of “severe” OSA is traditionally achieved with CPAP or placement of a nasopharyngeal airway. Although the successful use of noninvasive ventilation with CPAP for management of OSA in infants with PRS has also been previously reported,15 at our institution we have experienced significant barriers to this approach including lack of appropriate mask fit in these infants with significant craniofacial malformations as well as lack of adherence to therapy. Additionally, the success rate of nasopharyngeal airway placement is highly variable.10,16,17
This study has several strengths and limitations. The strengths include the standardized management of these neonates in a systematic fashion and incorporating a multidisciplinary team approach in our institution. The sample size is the largest cohort of neonates with PRS published to date with available objective PSG measures during infancy. Moreover, ours is a homogenous population of neonates with diagnostic and management interventions done at similar (consistent) ages. Additionally, the diagnosis of PRS was confirmed by an attending physician in the Department of Clinical Genetics to determine eligibility for inclusion. We report full PSG parameters and results scored by AASM criteria, making our data robust (most of the current literature involves limited channel or cardiorespiratory studies).
Our study has several limitations. One major limitation was the retrospective nature of the study. Moreover, this study was not designed to determine what infants are candidates for conservative versus surgical management and what conservative management option may be best. Longitudinal studies involving a larger sample size will likely be more informative in ascertaining outcomes of OSA in neonates with PRS. Moreover, performing sequential PSG tests across infancy in a more systematic fashion (set time intervals) will enable more robust comparisons across age groups. In our cohort, not all infants underwent PSG at all time points studied. Ours is a highly selective group of neonates based on stringent inclusion criteria (at least two PSG tests during infancy, the first at age 3 months or younger) as our aim was to evaluate changes in PSG parameters over the first few years of life. Additionally, our institutional practice is to lean toward offering surgical management for those neonates with OAHI > 40 events/h at diagnosis. Both of these factors may have biased our results. A prospective study with a larger sample size would enable more robust statistical determinations of OSA resolution over time. Moreover, herein we have primarily focused on PSG parameters in detail across infancy. Growth and neurocognitive development are closely linked to underlying OSA and was not measured in a systematic and consistent fashion in our cohort. Based on limited growth data, our cohort may represent a mild spectrum of infants with PRS as 70% of infants were able to achieve full oral feeds by 1 month of life. However, our data indicate that some of these infants with PRS were underweight and remained so over the first year of life. It is unclear whether improvements in sleep-related parameters were at the expense of weight gain over the first year of life in this conservatively treated cohort. Future, well-designed studies assessing and recording evaluating growth and neurodevelopmental outcomes in a systematic way over the first few years of life and correlating them with PSG parameters will provide more thorough knowledge about the effect of OSA in these neonates. Moreover, outcomes research is needed to determine whether early surgical intervention is helpful in achieving adequate weight gain when compared to conservative management. In conclusion, conservative management of OSA may be considered in a select cohort of neonates with PRS and moderate-severe OSA (OAHI < 20 events/h on baseline PSG). Objective assessment of OSA severity using sequential PSG may be considered to track improvements in sleep and breathing across infancy and assess for residual OSA, particularly at 15 months of age, where resolution may be seen. Future prospective work is needed to determine the long-term effect of OSA on neurodevelopmental outcomes in this population.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. This study was selected for an oral presentation at the Annual Meeting of the Associated Professional Sleep Societies (APSS) in June 2016. This study was conducted at the Cincinnati Children's Hospital, Cincinnati, Ohio. The authors report no financial or corporate interests to disclose associated with this work.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- NREM
non-rapid eye movement
- MDO
mandibular distraction osteogenesis
- OAHI
obstructive apnea-hypopnea index
- OSA
obstructive sleep apnea
- PRS
Pierre Robin sequence
- PSG
polysomnography
- REM
rapid eye movement
REFERENCE
- 1.Scott AR, Mader NS. Regional variations in the presentation and surgical management of Pierre Robin sequence. Laryngoscope. 2014;124(12):2818–2825. doi: 10.1002/lary.24782. [DOI] [PubMed] [Google Scholar]
- 2.Anderson IC, Sedaghat AR, McGinley BM, Redett RJ, Boss EF, Ishman SL. Prevalence and severity of obstructive sleep apnea and snoring in infants with Pierre Robin sequence. Cleft Palate Craniofac J. 2011;48(5):614–618. doi: 10.1597/10-100. [DOI] [PubMed] [Google Scholar]
- 3.Bravo G, Ysunza A, Arrieta J, Pamplona MC. Videonasopharyngoscopy is useful for identifying children with Pierre Robin sequence and severe obstructive sleep apnea. Int J Pediatr Otorhinolaryngol. 2005;69(1):27–33. doi: 10.1016/j.ijporl.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Gilhooly JT, Smith JD, Howell LL, Deschaine BL, Richey SL. Bedside polysomnography as an adjunct in the management of infants with Robin sequence. Plast Reconstr Surg. 1993;92(1):23–27. doi: 10.1097/00006534-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 5.van Lieshout MJ, Joosten KF, Mathijssen IM, et al. Robin sequence: a European survey on current practice patterns. J Craniomaxillofac Surg. 2015;43(8):1626–1631. doi: 10.1016/j.jcms.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Daniel M, Bailey S, Walker K, et al. Airway, feeding and growth in infants with Robin sequence and sleep apnoea. Int J Pediatr Otorhinolaryngol. 2013;77(4):499–503. doi: 10.1016/j.ijporl.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 7.MacLean JE, Fitzgerald DA, Waters KA. Developmental changes in sleep and breathing across infancy and childhood. Paediatr Respir Rev. 2015;16(4):276–284. doi: 10.1016/j.prrv.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Basart H, Kruisinga FH, Breugem CC, Don Griot JP, Hennekam RC, Van der Horst CM. Will the right Robin patient rise, please? Definitions and criteria during management of Robin sequence patients in the Netherlands and Belgium. J Craniomaxillofac Surg. 2015;43(1):92–96. doi: 10.1016/j.jcms.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Albino FP, Wood BC, Han KD, et al. Clinical factors associated with the non-operative airway management of patients with Robin sequence. Arch Plast Surg. 2016;43(6):506–511. doi: 10.5999/aps.2016.43.6.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Samkari HT, Kane AA, Molter DW, Vachharajani A. Neonatal outcomes of Pierre Robin sequence: an institutional experience. Clin Pediatr (Phila) 2010;49(12):1117–1122. doi: 10.1177/0009922810379040. [DOI] [PubMed] [Google Scholar]
- 11.Reddy VS. Evaluation of upper airway obstruction in infants with Pierre Robin sequence and the role of polysomnography--review of current evidence. Paediatr Respir Rev. 2016;17:80–87. doi: 10.1016/j.prrv.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Lee JJ, Thottam PJ, Ford MD, Jabbour N. Characteristics of sleep apnea in infants with Pierre-Robin sequence: is there improvement with advancing age? Int J Pediatr Otorhinolaryngol. 2015;79(12):2059–2067. doi: 10.1016/j.ijporl.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Rathe M, Rayyan M, Schoenaers J, et al. Pierre Robin sequence: management of respiratory and feeding complications during the first year of life in a tertiary referral centre. Int J Pediatr Otorhinolaryngol. 2015;79(8):1206–1212. doi: 10.1016/j.ijporl.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Kimple AJ, Baldassari CM, Cohen AP, Landry A, Ishman SL. Polysomnographic results of prone versus supine positioning in micrognathia. Int J Pediatr Otorhinolaryngol. 2014;78(12):2056–2059. doi: 10.1016/j.ijporl.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 15.Amaddeo A, Abadie V, Chalouhi C, et al. Continuous positive airway pressure for upper airway obstruction in infants with Pierre Robin sequence. Plast Reconstr Surg. 2016;137(2):609–612. doi: 10.1097/01.prs.0000475799.07597.23. [DOI] [PubMed] [Google Scholar]
- 16.Evans AK, Rahbar R, Rogers GF, Mulliken JB, Volk MS. Robin sequence: a retrospective review of 115 patients. Int J Pediatr Otorhinolaryngol. 2006;70(6):973–980. doi: 10.1016/j.ijporl.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Glynn F, Fitzgerald D, Earley MJ, Rowley H. Pierre Robin sequence: an institutional experience in the multidisciplinary management of airway, feeding and serous otitis media challenges. Int J Pediatr Otorhinolaryngol. 2011;75(9):1152–1155. doi: 10.1016/j.ijporl.2011.06.009. [DOI] [PubMed] [Google Scholar]