Abstract
Study Objectives:
Investigation into sleep and coronary heart disease (CHD) has predominantly been focused on sleep disturbances as a risk factor for developing CHD. Objectively measured and self-reported sleep at a patient level has only been sparsely and not systematically reported. Therefore, we set out to review the literature for studies using objectively measured and self-reported sleep in patients with CHD. The review focuses on patients with acute coronary syndrome (ACS) and stable CHD.
Methods:
A systematic review performed in four databases adhering to the PRISMA guidelines applying a qualitative synthesis of evidence.
Results:
Following ACS, we found sleep architecture to be significantly disturbed with changes normalizing over a period of up to 6 months. With increasing severity of CHD, sleep disturbances were more pronounced; however, the modulating effects of sleep-disordered breathing and ejection fraction on sleep in patients with CHD are conflicting. Overall, studies were predominantly cross-sectional in design and of low methodological quality. Polysomnography was the predominant outcome assessment tool and validated self-reported assessment tools were limited.
Conclusions:
Future investigations in sleep and CHD applying both a longitudinal design and investigating objective and self-reported sleep assessments are warranted.
Systematic Review Registration:
Registry: PROSPERO, Title: Sleep measures in relation to coronary heart disease: a systematic review, Identifier: CRD42017056377, URL: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=56377
Citation:
Madsen MT, Huang C, Zangger G, Zwisler AD, Gögenur I. Sleep disturbances in patients with coronary heart disease: a systematic review. J Clin Sleep Med. 2019;15(3):489–504.
Keywords: actigraphy, acute coronary syndrome, coronary heart disease, insomnia severity index, Pittsburgh Sleep Quality Index, polysomnography
INTRODUCTION
Sleep is an essential part of the human homeostasis, and sleep disturbances are associated with several pathological states.1,2 In spite of this little research has been performed on the prevalence and pattern of sleep disturbances in patients with coronary heart disease (CHD).3 Sleep disturbances are associated with recurrence of cardiac events4 and are an independent prognostic marker for cardiac prognosis.5
An abundance of research has been performed on the association between length of nighttime sleep and development of CHD.6 In a recent meta-analysis,6 nighttime sleep and the risk of CHD showed a U-formed relationship, whereby both sleep disturbances of too much and too little sleep were associated with increased risk of CHD. Seven hours of sleep was shown to be the optimal amount of nighttime sleep. For each increased hour of sleep, the relative risk (RR) of CHD was increased by 11%, and for each hour of reduced sleep the RR was increased with 7%. However, objective sleep assessment tools were lacking in the included studies of this meta-analysis, as most studies were based on patient questionnaires.6 Furthermore, a recent cohort study of 400,000 Taiwanese adults7 confirmed the meta-analysis and showed that sleeping less than 4 hours resulted in an increased risk of dying from CHD by 34% and sleeping more than 8 hours resulted in a 35% increase in the risk of dying from CHD.
Investigation of sleep disturbances in patients with CHD is problematic as these co-occur with both anxiety, depression, and sleep-disordered breathing (SDB).8–11 With regard to sleep disturbances in patients with CHD, it has been shown that sleep continuity is an independent risk factor for the development of cardiovascular disease.9 Regarding depression, the current literature supports the notion that depression is an independent risk factor for the development of cardiovascular disease (true for sleep continuity but not for sleep duration).9 The predominance of literature investigating the relationship between the sleep disturbances in CHD have been performed in relation to SDB.8,10 SDB is a risk factor for the development of CHD in male patients; however, the relationship in female patients is unclear.8 SDB is treated with continuous positive airway pressure (CPAP) machines which have shown to reduce hypertension.8 The ability of CPAP in preventing cardiovascular events in patients with SDB has only recently been investigated in the SAVE and RICCADSA trials.12,13 The RICCADSA trial showed no preventive effect of CPAP on CHD events in the intention-to-treat (ITT) analysis.13 Likewise, the SAVE trial did not show a preventive effect on cardiovascular events in patients with moderate to severe obstructive sleep apnea and establish cardiovascular disease.12 Considering this, sleep disturbances in patients with CHD cannot be explained by co-occurring disease and sleep disturbances are likely an independent prognostic factor for CHD.
Sleep disturbances have independently been associated with increased health care cost,14 morbidity,15,16 and mortality.17 Therefore, insight into prevalence and longitudinal development of sleep disturbances in patients with CHD is of high importance. In addition, sleep disturbances may already be present before an acute coronary event and have the potential to affect post-event recovery and participation in cardiac rehabilitation. Therefore, we aimed to systematically review literature reporting on sleep disturbances evaluated objectively and by self-report in patients with CHD in clinical samples. Secondarily, we wished to describe changes in sleep disturbances in relation to an acute cardiac event. Our hypothesis was that sleep disturbances in patients with CHD would be worse compared to healthy controls. Furthermore, we expected CHD events like acute coronary syndrome to result in acute disturbances in sleep outcomes, which gradually would return to normal levels.
METHODS
The current systematic review followed the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines.18 The systematic review was registered prospectively on PROSPERO with the registration number CRD42017056377.19,20
We used the following PICOS (P = population, I = intervention, C = comparator/control, O = outcomes, S = study design)18 when we constructed the eligibility criteria and the search strategy:
P: human adults, age ≥ 18 years, diagnosed with CHD
I: coronary heart disease
C: healthy controls or different CHD diagnosis
O: sleep assessment with valid sleep assessment tool
S: all study types
Case definition of CHD was based on the definition by Anderson et al.21
Inclusion criteria were as follows; the study should be performed in patients with CHD defined as ischaemic heart disease (DI20-25, ICD-10); the study should use a validated objective and/or self-report tool to assess sleep; comparisons with healthy controls were included if data were available; sleep outcomes should be reported independently at an individual outcome level; and all study designs were applicable. Exclusion criteria were as follows; population age < 18 years of age; not published in a peer reviewed journal; published in another language than English; not original study containing original data (no protocol articles, conference abstracts, or thesis allowed); exclusively reporting data on patients undergoing coronary artery bypass grafting; and ongoing trials. Studies investigating associations between sleep disturbances and CHD in the general population were not eligible in the current review.
The search was conducted on January 31, 2017. The search was carried out using MEDLINE, Embase, CINAHL, and the Cochrane Library (MEDLINE: 1966 to search date, Em-base: 1974 to search date, CINAHL: 1981 to search date, Cochrane Library: date of inception to search date). The search terms and strategy were developed in collaboration with a dedicated research librarian and identical search terms were implied in the mentioned databases. No limits were set for language; therefore, all records were manually screened with regard to being published in English. No limits were set for the year of publication. No additional attempts to contact study authors were performed as the choice was made only to report published data. The reference lists of all included studies were manually reviewed to identify additional relevant studies. An example of the search terms used in MEDLINE was:
(exp Myocardial Ischemia/ OR exp Coronary Artery Bypass/ OR exp Percutaneous Coronary Intervention/ OR exp Angioplasty/ OR exp Stents/ OR exp Atherectomy/ OR (myocard* adj5 isch?mia).tw. OR (isch?emi* adj5 heart).tw. OR (coronary adj5 disease*). tw. OR acute coronary syndrom*.tw. OR CHD.tw. OR (myocard* adj5 infarct*).tw. OR (heart adj5 infarct*). tw. OR angina.tw. OR (coronary adj5 (bypass or thrombo* or angioplast* or graft*)).tw. OR (percutaneous coronary adj2 (interven* or revascular*)).tw. OR angioplast*.tw. OR ((coronary or arterial) adj4 dilat*). tw. OR endoluminal repair*.tw. OR stent*.tw. OR (pci or ptca).tw. OR atherectom*.tw.) AND (exp Sleep/ OR exp polysomnography/ OR exp actigraphy/ OR Sleep assessment.tw OR Wrist Actigraphy.mp. OR Actometer. mp. OR Actimeter.mp. OR Actical.mp. OR Actiwatch. mp. OR Actigraphic recording.mp. OR Sleep diary.mp. OR Pittsburgh Sleep Quality Index.tw. OR Insomnia Severity Index.tw OR Epworth Sleepiness Scale.tw OR Berlin Questionnaire.tw OR Karolinska Sleepiness Scale.tw)
All available records were uploaded to the Covidence platform.22 After removal of duplicates two reviewers (MTM and CH) independently screened all titles and abstracts for eligibility. Discrepancies were resolved through discussion until a consensus decision was reached within the author group. At full-text level all studies were evaluated for eligibility and eligible articles went on to data extraction.
Data on participant characteristics, interventions, and trial methodology were extracted independently by the two reviewers into pre-designed datasheets. In the current manuscript “sleep disturbance” was considered a symptom of altered sleep or a change in self-reported or objectively measured sleep outcomes. “Insomnia” was referenced as a disorder based on either an established cutoff (dichotomous outcome) or nomenclature used in original publications. Sleep outcomes were presented as mean values presented in the publications and intergroup comparisons were noted. With regard to reported polysomnography outcomes (when available) they were total sleep time (TST), sleep efficiency (SE), sleep latency (SL), wake after sleep onset (WASO), stage N1 sleep (S1), stage N2 sleep (S2), slow wave sleep (SWS)—comprising stage N3 and N4 sleep, and REM sleep. Outcomes reported for actigraphy were (when available) TST, SE, SL, and WASO. Self-reported sleep outcomes, ie, Pittsburgh Sleep Quality Index (PSQI) and Insomnia Severity Index (ISI) were reported as total scores. Our sleep outcomes were study level sleep data measured via objective and/or validated self-reported sleep assessment tools (Appendix 1). Risk of bias assessment was performed using the instrument developed by Downs and Black,23 which was developed to assess both randomized and non-randomized trials. Downs and Black evaluate study quality on five sub-scales, ie, reporting, external validity, internal validity (bias), internal validity (confounding), and power which are summarized into a total score with a maximum of 28 points. Two reviewers assessed the methodological quality independently. Discrepancies were resolved through discussion until a consensus decision was reached within the author group.
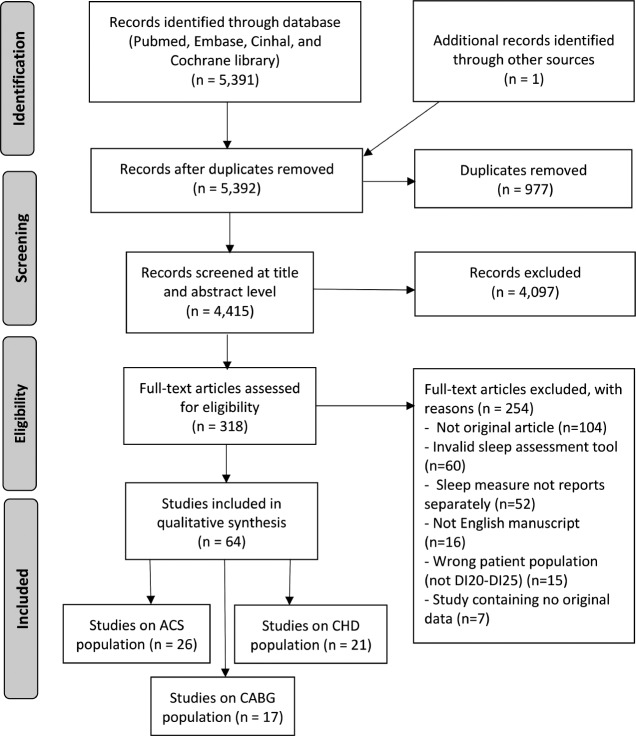
After full text evaluation and data extraction, a post hoc choice was made to evaluate participants undergoing coronary artery bypass grafting (CABG) separately. This meant that the 17 studies reporting only on CABG were not included in the current review (Figure 1).
Figure 1. PRISMA flow diagram.
ACS = acute coronary syndrome, CABG = coronary artery bypass grafting, CHD = coronary heart disease.
RESULTS
A total of 64 articles were eligible for the qualitative synthesis of which 26 investigate ACS and 21 investigate stable CHD. The screening process is depicted in Figure 1.
Study Characteristics
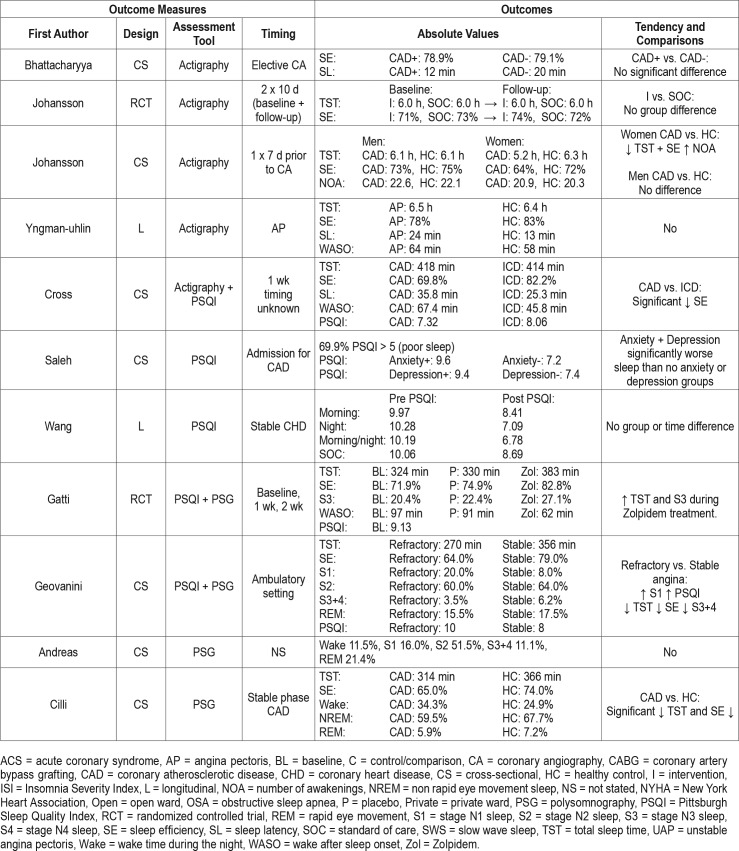
As evident from Table 1 there is a great amount of heterogeneity among the 47 included studies (Table 1). The included studies were published from 1969–2016. The entire span of CHD diagnosis was present including ST-elevation myocardial infarction (STEMI), non-ST-elevation myocardial infarction (NSTEMI), unstable angina pectoris (UAP), and angina pectoris (AP). The population varied from 4 to 3,017 patients with a mean age range from 49.5–68.0 years, although the predominance of studies had a mean age of approximately 60 years. Male participants were more prevalent in the studies; however, female participants were more common in recently published studies. Three studies were randomized control trials, 10 studies were longitudinal (repeated measure design), and 34 studies were cross-sectional (single outcome assessment).
Table 1.
Participant characteristics, study design, and intervention details.
Six studies assessed sleep outcomes in the coronary care unit (CCU), 9 in a hospital setting, 9 in a sleep laboratory, 9 in an ambulatory or home setting, 1 study applied both hospital and home measurement, and 12 studies did not state the setting of sleep measurement. Sleep was objectively measured by polysomnography (PSG) in 30 studies, by actigraphy in 6, both actigraphy and the PSQI in 1 study, and PSG and ISI in 1 study. As for self-reported sleep, 6 studies measured the PSQI and 3 studies the ISI.
Sleep in Relation to Acute Coronary Syndrome
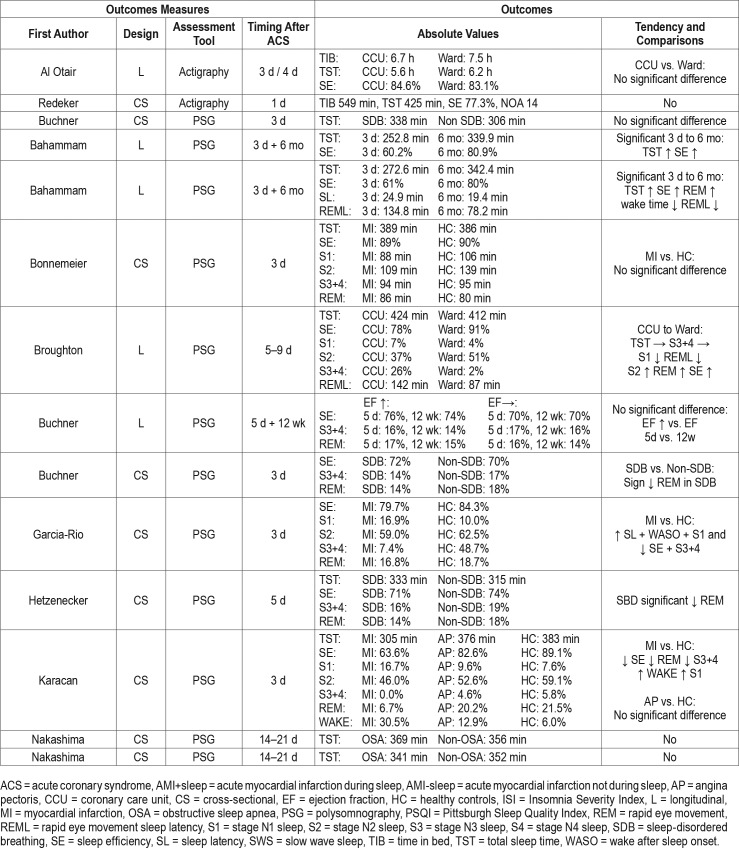
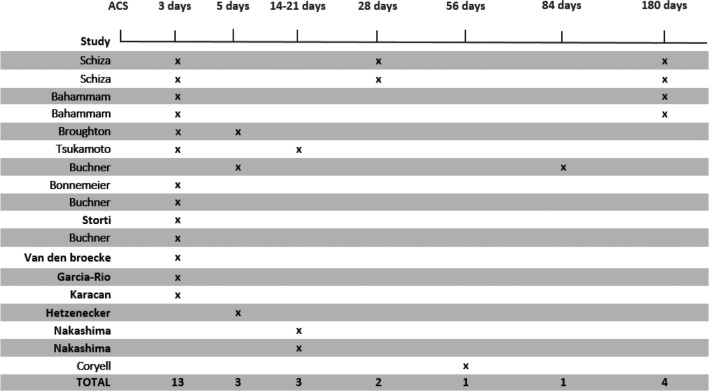
A total of 26 studies investigated sleep in relation to ACS (Table 2). Eighteen of these measuring sleep using PSG and measurements were performed from immediately after the ACS with a follow-up within 6 months.24–41 Thirteen studies24–28,30,32,34,37–41 measured within the first 3 days, 3 studies28,29,33 measured 5 days following ACS, 3 studies measured 2–3 weeks after ACS.35,36,40 Furthermore, 2 studies measured 1 month following ACS,37,38 1 study measured 8 weeks following ACS,31 1 study29 measured 12 weeks following ACS, and 4 studies25,26,37,38 measured 6 months after ACS (Figure 2).
Table 2.
Sleep outcomes in studies of acute coronary syndrome.
Figure 2. Timing of measurement of polysomnography following acute coronary syndrome.
The timing of polysomnography assessment in relation to acute coronary syndrome.
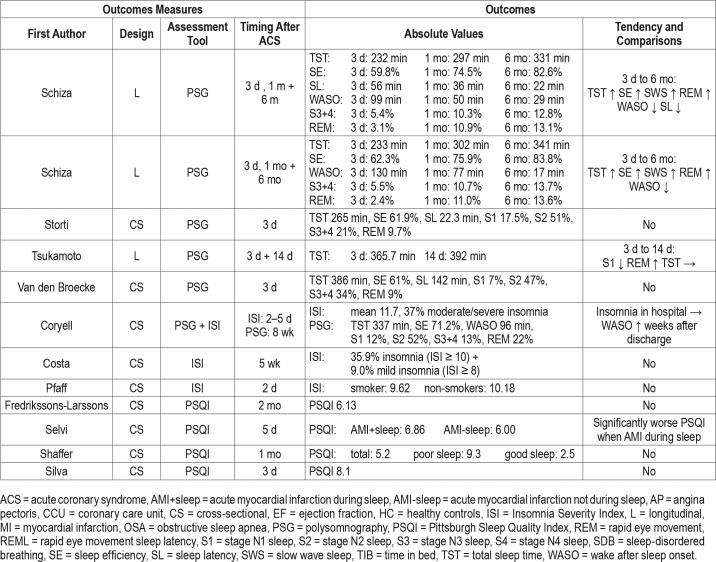
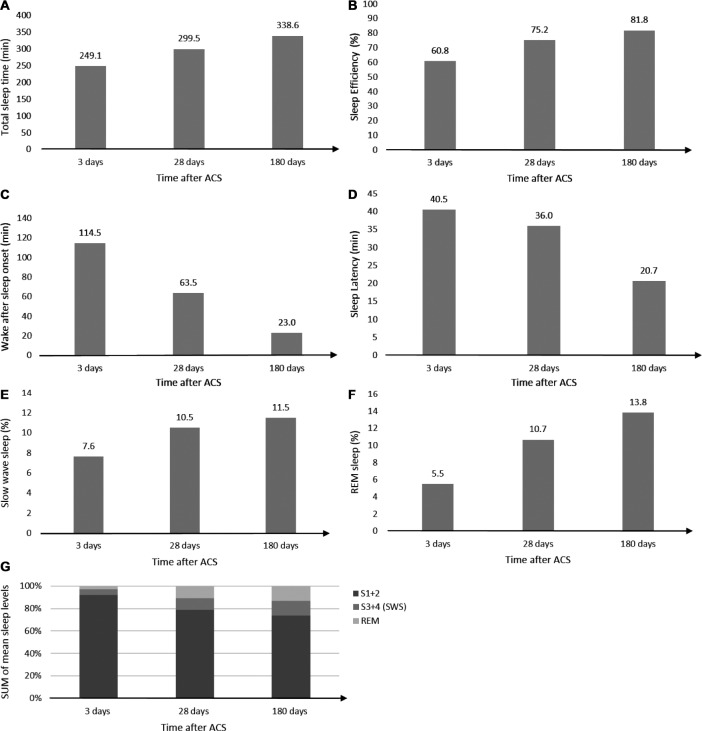
A total of 8 studies25,26,28,29,37,38,40,42 applied a longitudinal design. From these studies, it was evident that TST, SE, SWS, and REM sleep were reduced immediately following ACS and were all significantly increased at 6-month follow-up (Figure 3). On the other hand, WASO and SL were increased immediately following ACS and were significantly shorter at 6-month follow-up (Figure 3). Measurements performed 1 month following ACS had intermediate values between 3 days and 6 months post-ACS, ie, representing a gradual normalization over time.37,38 The reduction in SWS and REM sleep after ACS were associated with an increase in stage N1 sleep and stage N2 sleep, however, these pathological changes seemed to normalize gradually in the following months (Figure 3). The changes and development of sleep outcomes was present in patients with and without obstructive sleep apnea. Patients stratified based on positive or negative change in ejection fraction (EF) following ACS showed no significant difference between the two groups.29 In contrast, 1 study showed no change in TST from 3 days to 2 weeks post-ACS.40
Figure 3. Development of polysomnography sleep outcomes following acute coronary syndrome.
(A) Total sleep time (B) sleep efficiency, (C) wake after sleep onset, (D) sleep latency, (E) slow wave sleep, (F) REM sleep, and (G) sleep architecture after ACS. ACS = acute coronary syndrome, REM = rapid eye movement, S1 = stage N1 sleep, S2 = stage N2 sleep, S3 = stage N3 sleep, S4 = stage N4 sleep, SWS = slow wave sleep.
Two studies included healthy controls32,34; these showed a significant reduction of SE and SWS, and increased stage N1 sleep in patients with myocardial infarction (MI) compared to healthy controls. Four studies compared patients with and without SDB.30,33,35,36 Two of these studies30,33 showed significantly reduced REM sleep in the SDB population; however, the remaining two studies found no difference between the groups.
Two studies measured actigraphy,42,43 one of which42 showed that nighttime sleep periods were more frequent and of short duration in the CCU compared to the regular ward. However, the remaining sleep outcomes were similar between the CCU and ward.
The PSQI was measured in 4 studies.44–47 Immediately following a MI, the mean PSQI was 8.1,47 after 1 month the mean PSQI was reduced to 5.2,46 and at 2 months the mean PSQI was 6.13.44 The PSQI was significantly lowered (worsened) when the MI happened during sleep.45 Three studies used the ISI to measure sleep,31,48,49 and after the onset of a MI the mean ranged from 9.6 to 11.7.
Sleep in Relation to Coronary Heart Disease
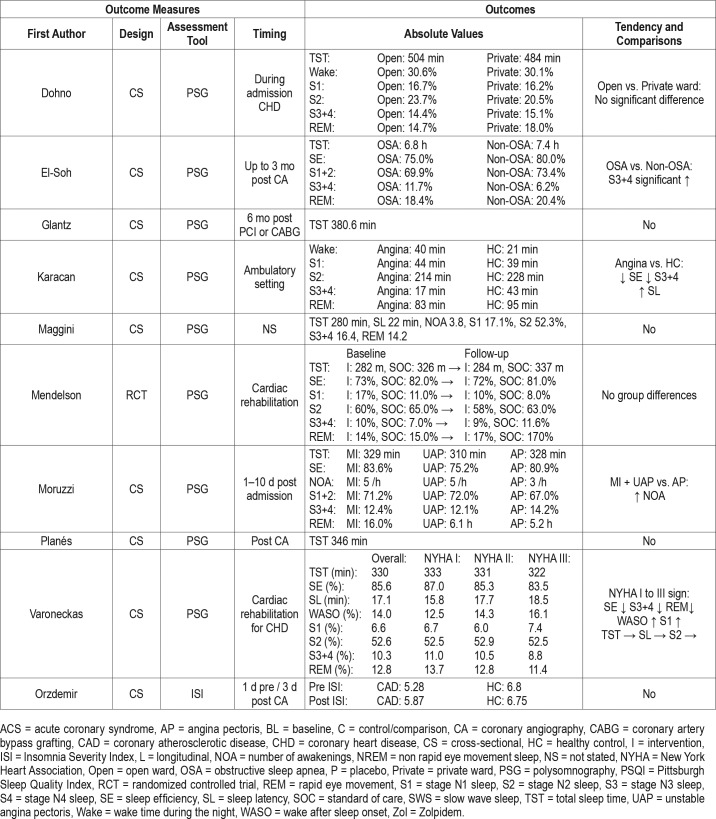
Thirteen studies used PSG to evaluate sleep in their study population (Table 3).50–62 The timing of sleep assessment was very heterogeneous, and all of the 13 studies save one59 were cross-sectional in design. Three studies measured PSG following percutaneous coronary intervention (PCI), where TST tended to increase as time passed, however, cross-study comparisons were challenging due to their heterogeneity.53,56,60 Two studies assessed sleep during cardiac rehabilitation,59,62 of which the former study was the largest in the current review (> 3,000 patients included).62 In this study, the TST was 330 minutes and the SE 85.6%. Furthermore, the population was stratified according to the New York Heart Association (NYHA) classification. With increasing NYHA class the SE, SWS, and REM sleep were reduced and WASO and stage N1 sleep were significantly increased. During cardiac rehabilitation an additional 4-week exercise program was not shown to have a significant effect on sleep.59 In contrast, Zolpidem (a hypnotic) resulted in a significantly increased TST and SWS in another randomized controlled crossover trial.54
Table 3.
Sleep outcomes in studies of coronary heart disease.
Two studies compared patients with stable CAD with healthy controls and showed a significantly reduced SE in stable patients with CAD.51,57 Stable angina was compared to refractory angina and showed that patients with refractory an-gina had significantly reduced TST, SE and SWS.55 Another study compared MI, UAP and AP where patients with MI had increased number of awakenings (NOA).60
Five studies measured actigraphy in patients with CHD63–67 and all save one64 had cross-sectional design. The longitudinal study tested a nurse led self-care program; however, no group differences were present at follow-up 3–4 months after baseline.64 Two studies compared patients with CAD and healthy controls, where significant differences were found in females who had reduced TST and SE.63,65
Five studies used the PSQI.54,55,67–69 One study tested a relaxation program which showed no effect on sleep.69 Overall almost 70% of a CAD population had poor sleep (PSQI > 5) and additionally patients with comorbid depression and anxiety had significantly worse sleep.68 The ISI measured before and after coronary angiography did not show any changes in sleep in relation to the procedure.70
Risk of Bias Assessment
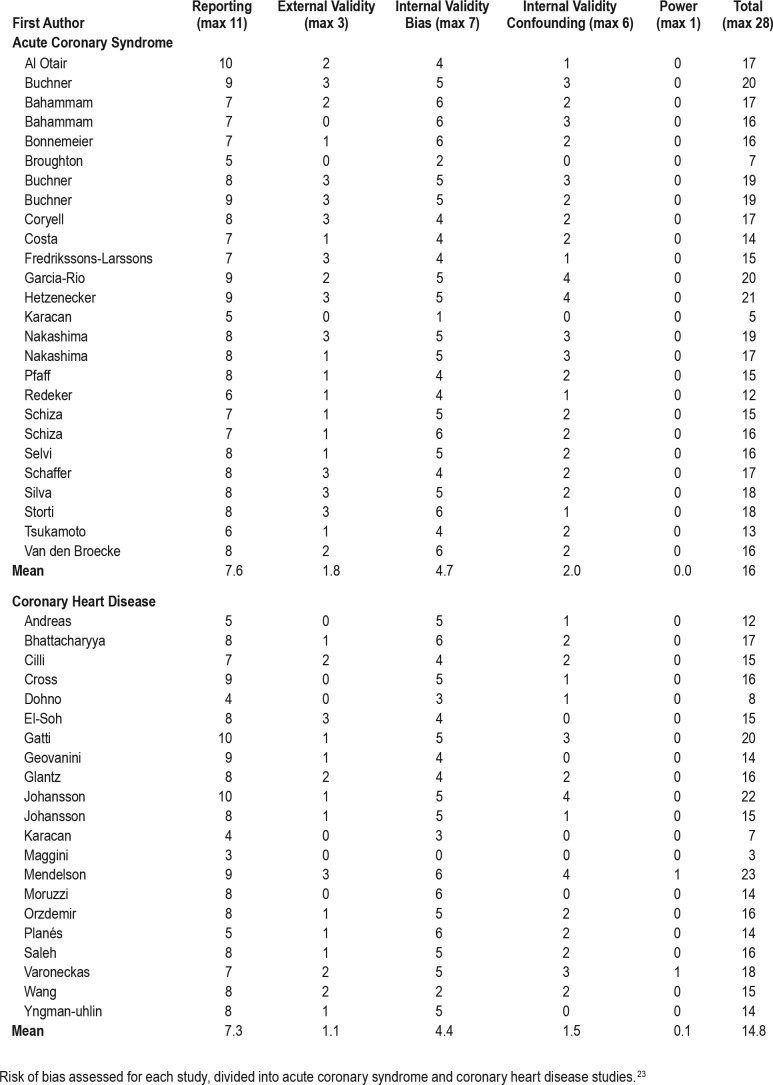
There was vast heterogeneity in study quality across studies and in sleep measurements used in relation to ACS or in a CHD population (Table 4). The mean total score was 16 and 14.8 in the ACS and CHD population, respectively.
Table 4.
Risk of bias assessment.
There was a clear tendency towards increased study quality in more recent publications (Figure 4), and all studies published before 1980 had a total score below 10. The tendency of increased study quality in recent literature seemed to be regardless of whether the study was performed in an ACS or CHD population (Figure 4).
Figure 4. Risk of bias assessment.
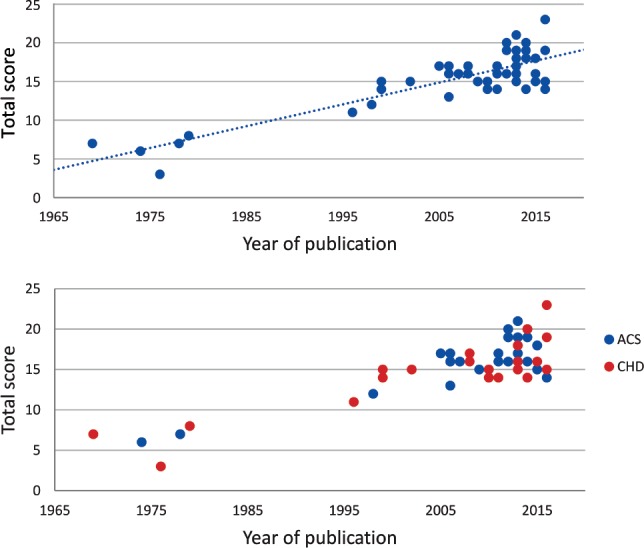
Risk of bias assessment development through time (top) and divided by ACS or CHD status (bottom). ACS = acute coronary syndrome, CHD = coronary heart disease.
DISCUSSION
The current review describes objectively measured and self-reported sleep in patients with CHD, both during an acute presentation (ACS) and a stable chronic phase. PSG was the most common sleep assessment tool followed by actigraphy and PSQI, whereas, the ISI was only sparsely used. Multiple sleep outcomes, sleep assessment points, and comparisons were applied which makes a concise reporting very challenging.
Immediately after ACS, patients tended to have reduced TST, SE, SWS, and REM sleep and increased WASO and SL. These changes gradually regressed towards what could be considered normal sleep outcomes over a period of 6 months after the ACS. Measurements of the aforementioned outcomes at 1 month are intermediate values between post-ACS and 6 months supporting a general normalization trend. Whether this normalization of sleep represent pre-ACS values is unknown, since no pre-event recording has been performed. In the available literature of the ACS population, SDB or changes in EF did not seem to affect the development described above. Compared to healthy controls the ACS population showed significantly different sleep patterns. Within the ACS population presence of SDB showed conflicting results in term of sleep alterations being present and not. Actigraphy measurements were few, however, showed more NOA in the CCU compared with the regular ward. Self-reported sleep outcomes did to a large extent reflect the findings of those showed by objectively measured sleep following ACS.
The patients with CHD were measured much more sporadically both as the studies predominantly were cross-sectional and information regarding time from diagnosis and severity of the disease were sparse. The study by Varoneckas et al.62 included more than 3,000 patients during their cardiac for insomnia, and one study in the current review54 showed increased TST and SWS in patients treated with zolpidem. A drawback of hypnotic treatment in general is the potential for abuse and dependence coupled with a modest effect.82 Before disease-specific trials aimed at investigating which treatment of insomnia should be undertaken, prospective cohort studies assessing depression, anxiety, and sleep—simultaneously and repeatedly—should be conducted. These studies would serve as the ideal basis of possible intervention trials.
A large part of the literature included in the current review includes PSG recordings performed in patients with CHD as an investigation of SBD. Some of these PSG recording are single-night recordings to diagnose SBD which might not reflect the everyday sleep pattern of the patient. The intense focus on SBD is in large part due to the known relationship between SDB and cardiovascular disease,10 where sleep apnea has been shown to be associated with increased risk of both MI and ischemic stroke.83 The Wisconsin sleep cohort study84 showed that with an increase of apnea-hypopnea index (AHI) the risk of incident coronary heart disease or heart failure were also increased. Treatment of SDB is primarily performed using CPAP; however, in a large epidemiological study, CPAP was not shown to reduce risk of MI.83 The prospective trials (SAVE and RICCADSA trials12,13) showed no preventive effects on cardiovascular events due to CPAP treatment. SBD is an important comorbid condition in relation to CHD; however, sleep disturbances are not solely related to the SBD in patients with CHD. Future studies investigating sleep in relation to CHD with sleep as the primary outcome should be undertaken.
Only 13 out of 47 studies applied a repeated measure design and only 9 out of 47 studies included healthy controls. As sleep inherently changes over time—especially in relation to acute illness—not applying a repeated measure design should be considered a limitation of the current literature. A further rehabilitation where absolute values of TST 330 minutes and SE 85.6% could be regarded as normative data for the CHD population. It was shown that increasing NYHA classification resulted in reduced SE, SWS and REM. Similar comparisons between healthy controls, stable, refractory, unstable angina, NSTEMI and STEMI showed disturbed sleep with increased disease severity. Taken together it seems that with increasing CHD severity (disease itself or NYHA classification) sleep is more severely disturbed.
Disturbances in the SWS and REM sleep have shown to be associated with reduced restitution and recovery.2 The acute changes in sleep in relation to an ACS will affect the recovery and convalescence after acute MI. The explicit causes of these sleep disturbances are unclear since they may be present to some extent before an ACS and partly caused by the treatment of ACS. Another very important factor is the influence of environmental factors in the hospital (eg, light exposure, noise and medical/nursing procedures) during the nighttime, which cannot be underestimated. Such environmental factors are most intense during the initial acute phase and early discharge to home setting should be encouraged, as is the case with current treatment. To what extent these changes effect the recovery is unclear on the basis of the current literature. If one were to compare the sleep changes following ACS with major non-cardiac surgery,71 a similar reduction is seen in SWS and REM sleep; however, 4–5 days after surgery a rebound of REM sleep and SWS follow. A similar investigation into the development of sleep after ACS would be interesting and even though the recent reduced length of stay after primary percutaneous coronary intervention72 make such a study difficult, measurement with ambulatory PSG would make it feasible.
ACS is in its nature an acute event which makes measurements of habitual sleep before an ACS very difficult. It is difficult to make inferences about the normalization of sleep following ACS due to no pre-event measurement. The build-up of an atherosclerotic plaque occurs over years and the sudden rupture of the plaque then constitutes the ACS. Patients with ACS are seldom free of atherosclerotic burden in their coronary arteries and therefore surely have CHD before the event, however without symptoms or other clinical manifestation.
The previous unsystematic investigation into sleep disturbances and/or insomnia in patients with CHD seems odd as insomnia is known to co-occur with depression and anxiety.11 Both depression and anxiety have gained ample research attention,11 however, a systematic sleep assessment has not been a part of the research design yet in cardiology. Insomnia is an independent risk factor of major depressive episodes,73,74 it increases the risk of relapse of depression,73 and perpetuates depression.75 Furthermore, literature within the current review reported worse self-reported sleep being associated with more prevalent depressive symptoms and antidepressant usage.46,48 The relationship between insomnia and depression is bidirectional,76 and treatment of insomnia in spite of co-morbid conditions (eg, depression) should be undertaken.77,78 Treatment of comorbid insomnia and depressive symptoms have shown a faster remission from depression.79,80 Both non-pharmacological (eg, cognitive behavioral therapy81) and pharmacological (eg, hypnotics) treatment options are possible limitation of the literature is the minority of studies including healthy controls, making quantifying differences between patients with CHD and healthy controls difficult. The predominance of literature has instead split a CHD population based on a clinical variable, eg, presence of SDB (+/-). Patients admitted to an elective coronary angiography based on suspected CHD would be a relevant case for both longitudinal repeated outcome assessment (with pre-procedure assessment) and comparison of cases with and without CHD based on procedure results. Based on such a sample it would be possible to assess the difference between patients with CHD and controls and the impact of a coronary angiography/PCI on sleep.
Inter-study comparisons are also made difficult due the heterogeneity in the patient populations (eligibility criteria in each study) and the variety found in timing the sleep assessment. With regard to the tools used, PSG is predominant, especially in the ACS setting, and there has been limited use of actigraphy in the period following ACS. Actigraphy compared to PSG is more cost-efficient, more accessible, and measurement for extended periods of time are feasible.85 This methodology could have clear advantages in measuring sleep in patients with CHD in a repeated measure setup. Applying an objective sleep assessment (eg, actigraphy) should be used in conjunction with a self-reported sleep assessment tool (eg, ISI)86 to fully describe the different dimensions of sleep. Sleep assessment in relation to stable CHD is in urgent need of longitudinal studies (repeated measure) measuring sleep in the different stages of CHD.
Strengths and Limitations
This review has the methodological strength that the review was prospectively registered on PROSPERO and stringently adhered to the PRISMA guidelines. An established case-definition of patients with CHD was applied and several electronic databases were searched as not to limit the literature. We used a standardized tool to assess the study quality which could evaluate both randomized and non-randomized trials.
The current review, however, also had some limitations. Opting not to include ongoing or unpublished literature could introduce reporting bias, which we cannot control in the current review. Including unpublished literature could potentially skew the results, as this literature would not have peer-reviewed. We chose not to contact authors for additional data. This effort may have provided additional details about the included studies but is unlikely to have brought additional studies into the review. After full-text evaluation, we made the choice not to include the studies reporting solely on patients undergoing CABG. Sleep patterns in heart surgery were recently reviewed by Liao et al.87 covering literature until 2010, although, the search strategy and databases were different than the current review. Furthermore, it is well established that undergoing a surgical procedure severely affects sleep patterns71,88 due to the surgical trauma, anaesthesia and admission. This has also been shown in relation to cardiac surgery where sleep disturbances may be present up to 6 months following cardiac surgery.89 Considering this we chose to only focus on patients with established CHD not undergoing surgery in the current manuscript. The CABG population could have served as a relevant reference population as they represent a population with severe disease (three vessel or main-stem occlusion), however, which sleep disturbances would be related to the surgical procedure and the effect of CHD would not be apparent. We chose to include only validated self-reported sleep assessment tools (Appendix 1). The excluded sleep assessment tools were primarily not properly validated, not developed to measure sleep separately, or only a sub-scale of a larger test battery.
CONCLUSIONS
Patients with CHD experience sleep disturbances (both in architecture and amount of sleep), which seem to be most aggravated in relation to an acute coronary event. The disturbances seem to normalize in the months following ACS; however, the exact trajectory of sleep disturbances is not known due to the limited usage of repeated measure design. In patients with more severe ischemic disease, sleep disturbances seem to be more prevalent. Future observational studies using repeated measure sleep assessment and thorough comorbidity assessment are warranted. Such studies should also investigate the clinical impact of disturbed sleep on mortality, recurrence, and quality of life, before potential intervention trials are tested in a disease specific setting.
DISCLOSURE STATEMENT
All authors have seen and approve the submitted manuscripts. The authors report no conflicts of interest.
ABBREVIATIONS
- ACS
acute coronary syndrome
- AHI
apnea-hypopnea index
- AP
angina pectoris
- CHD
coronary heart disease
- CPAP
continuous positive airway pressure
- CABG
coronary artery bypass grafting
- CCU
coronary care unit
- EF
ejection fraction
- ISI
Insomnia Severity Index
- ITT
Intention to treat
- IHD
ischemic heart disease
- MI
myocardial infarction
- NOA
number of awakenings
- NYHA
New York Heart Association
- PCI
percutaneous coronary intervention
- PSQI
Pittsburgh Sleep Quality Index
- PSG
polysomnography
- PRISMA
preferred reporting items for systematic reviews and meta-analysis
- REM
rapid eye movement
- RR
relative risk
- SDB
sleep-disordered breathing
- SE
sleep efficiency
- SL
sleep latency
- S1
stage N1 sleep
- S2
stage N2 sleep
- S3
stage N3 sleep
- S4
stage N4 sleep
- SWS
slow wave sleep
- TST
total sleep time
- UA
unstable angina
- WASO
wake after sleep onset
Appendix 1—Sleep evaluation tools.
Objective Sleep Assessment Tools
Polysomnography
Actigraphy
Self-Reported Sleep Assessment Tools
Valid
Pittsburgh Sleep Quality Index (PSQI)
Insomnia Severity Index (ISI)
Invalid
Uppsala Sleep Inventory (validation data never published in peer review)
Epworth Sleepiness Scale (ESS) (only evaluates sleepiness during daytime)
Verran and Snyder-Halpern Sleep Scale (VSH) (summation of VAS scales and not properly validated)
Groningen Sleep Quality Score (no validation article available or referenced)
Berlin Questionnaire (developed to evaluate SBD and not sleep)
Richard Sleep Questionnaire (not properly validated)
STOP-Bang Questionnaire (developed to evaluate SBD and not sleep)
SF-36 (not developed to measure sleep specifically and not validated)
Karolinska Sleepiness Scale (not properly validated)
REFERENCES
- 1.Akerstedt T, Nilsson PM. Sleep as restitution: an introduction. J Intern Med. 2003;254(1):6–12. doi: 10.1046/j.1365-2796.2003.01195.x. [DOI] [PubMed] [Google Scholar]
- 2.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92(3):1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coryell VT, Ziegelstein RC, Hirt K, Quain A, Marine JE, Smith MT. Clinical correlates of insomnia in patients with acute coronary syndrome. Int Heart J. 2013;54(5):258–265. doi: 10.1536/ihj.54.258. [DOI] [PubMed] [Google Scholar]
- 4.Leineweber C, Kecklund G, Janszky I, Akerstedt T, Orth-Gomér K. Poor sleep increases the prospective risk for recurrent events in middle-aged women with coronary disease. The Stockholm Female Coronary Risk Study. J Psychosom Res. 2003;54(2):121–127. doi: 10.1016/s0022-3999(02)00475-0. [DOI] [PubMed] [Google Scholar]
- 5.Clark A, Lange T, Hallqvist J, Jennum P, Rod NH. Sleep impairment and prognosis of acute myocardial infarction: a prospective cohort study. Sleep. 2014;37(5):851–858. doi: 10.5665/sleep.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Li W, Cui X, et al. Sleep duration and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Int J Cardiol. 2016;219:231–239. doi: 10.1016/j.ijcard.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 7.Strand LB, Tsai MK, Gunnell D, Janszky I, Wen CP, Chang SS. Self-reported sleep duration and coronary heart disease mortality: a large cohort study of 400,000 Taiwanese adults. Int J Cardiol. 2016;207:246–251. doi: 10.1016/j.ijcard.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 8.Arzt M, Hetzenecker A, Steiner S, Buchner S. Sleep-disordered breathing and coronary artery disease. Can J Cardiol. 2015;31(7):909–917. doi: 10.1016/j.cjca.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 9.Mezick EJ, Hall M, Matthews KA. Are sleep and depression independent or overlapping risk factors for cardiometabolic disease? Sleep Med Rev. 2011;15(1):51–63. doi: 10.1016/j.smrv.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Jimenez F, Sert Kuniyoshi FH, Gami A, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. Chest. 2008;133(3):793–804. doi: 10.1378/chest.07-0800. [DOI] [PubMed] [Google Scholar]
- 11.Morin CM, Ware JC. Sleep and psychopathology. Appl Prev Psychol. 1996;5(4):211–224. [Google Scholar]
- 12.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 13.Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194(5):613–620. doi: 10.1164/rccm.201601-0088OC. [DOI] [PubMed] [Google Scholar]
- 14.Daley M, Morin CM, LeBlanc M, Gregoire JP, Savard J. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep. 2009;32(1):55–64. [PMC free article] [PubMed] [Google Scholar]
- 15.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care. 2009;32(11):1980–1985. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32(4):491–497. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59(2):131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Booth A, Clarke M, Ghersi D, Moher D, Petticrew M, Stewart L. An international registry of systematic-review protocols. Lancet. 2011;377(9760):108–109. doi: 10.1016/S0140-6736(10)60903-8. [DOI] [PubMed] [Google Scholar]
- 20.Booth A, Clarke M, Ghersi D, Moher D, Petticrew M, Stewart L. Establishing a minimum dataset for prospective registration of systematic reviews: an international consultation. PLoS One. 2011;6(11):e27319. doi: 10.1371/journal.pone.0027319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson L, Thompson DR, Oldridge N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2016;(1):CD001800. doi: 10.1002/14651858.CD001800.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melbourne, Australia: Veritas Health Innovation; Covidence systematic review software. Available at www.covidence.org. [Google Scholar]
- 23.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchner S, Satzl A, Debl K, et al. Impact of sleep-disordered breathing on myocardial salvage and infarct size in patients with acute myocardial infarction. Eur Heart J. 2014;35(3):192–199. doi: 10.1093/eurheartj/eht450. [DOI] [PubMed] [Google Scholar]
- 25.BaHammam A, Al-Mobeireek A, Al-Nozha M, Al-Tahan A, Binsaeed A. Behaviour and time-course of sleep disordered breathing in patients with acute coronary syndromes. Int J Clin Pract. 2005;59(8):874–880. doi: 10.1111/j.1742-1241.2005.00534.x. [DOI] [PubMed] [Google Scholar]
- 26.BaHammam A. Sleep quality of patients with acute myocardial infarction outside the CCU environment: a preliminary study. Med Sci Monit. 2006;12(4):CR168–CR172. [PubMed] [Google Scholar]
- 27.Bonnemeier H, Nötges JK, Majunke B, et al. Ventricular repolarization dynamics during different sleep stages in the subacute phase of myocardial infarction. Pacing Clin Electrophysiol. 2007;(30 Suppl 1):S192–S197. doi: 10.1111/j.1540-8159.2007.00636.x. [DOI] [PubMed] [Google Scholar]
- 28.Broughton R, Baron R. Sleep patterns in the intensive care unit and on the ward after acute myocardial infarction. Electroencephalogr Clin Neurophysiol. 1978;45(3):348–360. doi: 10.1016/0013-4694(78)90187-6. [DOI] [PubMed] [Google Scholar]
- 29.Buchner S, Greimel T, Hetzenecker A, et al. Natural course of sleep-disordered breathing after acute myocardial infarction. Eur Respir J. 2012;40(5):1173–1179. doi: 10.1183/09031936.00172211. [DOI] [PubMed] [Google Scholar]
- 30.Buchner S, Eglseer M, Debl K, et al. Sleep disordered breathing and enlargement of the right heart after myocardial infarction. Eur Respir J. 2015;45(3):680–690. doi: 10.1183/09031936.00057014. [DOI] [PubMed] [Google Scholar]
- 31.Coryell VT, Ziegelstein RC, Hirt K, Quain A, Marine JE, Smith MT. Clinical correlates of insomnia in patients with acute coronary syndrome. Int Heart J. 2013;54(5):258–265. doi: 10.1536/ihj.54.258. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Rio F, Alonso-Fernández A, Armada E, et al. CPAP effect on recurrent episodes in patients with sleep apnea and myocardial infarction. Int J Cardiol. 2013;168(2):1328–1335. doi: 10.1016/j.ijcard.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Hetzenecker A, Buchner S, Greimel T, et al. Cardiac workload in patients with sleep-disordered breathing early after acute myocardial infarction. Chest. 2013;143(5):1294–1301. doi: 10.1378/chest.12-1930. [DOI] [PubMed] [Google Scholar]
- 34.Karacan I, Green JR, Salis PJ. Sleep in Post-Myocardial Infarction Patients. In: Eliot RS, editor. Contemporarary Problems in Cardiology. Futura Publishing; 2017. [Google Scholar]
- 35.Nakashima H, Henmi T, Minami K, et al. Obstructive sleep apnoea increases the incidence of morning peak of onset in acute myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2013;2(2):153–158. doi: 10.1177/2048872613478557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakashima H, Katayama T, Takagi C, et al. Obstructive sleep apnoea inhibits the recovery of left ventricular function in patients with acute myocardial infarction. Eur Heart J. 2006;27(19):2317–2322. doi: 10.1093/eurheartj/ehl219. [DOI] [PubMed] [Google Scholar]
- 37.Schiza SE, Simantirakis E, Bouloukaki I, et al. Sleep patterns in patients with acute coronary syndromes. Sleep Med. 2010;11(2):149–153. doi: 10.1016/j.sleep.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 38.Schiza SE, Simantirakis E, Bouloukaki I, et al. Sleep disordered breathing in patients with acute coronary syndromes. J Clin Sleep Med. 2012;8(1):21–26. doi: 10.5664/jcsm.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Storti LJ, Servantes DM, Borges M, et al. Validation of a novel sleep-quality questionnaire to assess sleep in the coronary care unit: a polysomnography study. Sleep Med. 2015;16(8):971–975. doi: 10.1016/j.sleep.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Tsukamoto K, Ohara A. Temporal worsening of sleep-disordered breathing in the acute phase of myocardial infarction. Circ J. 2006;70(12):1553–1556. doi: 10.1253/circj.70.1553. [DOI] [PubMed] [Google Scholar]
- 41.Van den Broecke S, Jobard O, Montalescot G, et al. Very early screening for sleep-disordered breathing in acute coronary syndrome in patients without acute heart failure. Sleep Med. 2014;15(12):1539–1546. doi: 10.1016/j.sleep.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 42.Al Otair H, Al-Shamiri M, Bahobail M, Sharif MM, BaHammam AS. Assessment of sleep patterns, energy expenditure and circadian rhythms of skin temperature in patients with acute coronary syndrome. Med Sci Monit. 2011;17(7):CR397–CR403. doi: 10.12659/MSM.881851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Redeker NS, Tamburri L, Howland CL. Prehospital correlates of sleep in patients hospitalized with cardiac disease. Res Nurs Health. 1998;21(1):27–37. doi: 10.1002/(sici)1098-240x(199802)21:1<27::aid-nur4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 44.Fredriksson-Larsson U, Alsén P, Karlson BW, Brink E. Fatigue two months after myocardial infarction and its relationships with other concurrent symptoms, sleep quality and coping strategies. J Clin Nurs. 2015;24(15–16):2192–2200. doi: 10.1111/jocn.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Selvi Y, Aydin A, Gumrukcuoglu HA, et al. Dream anxiety is an emotional trigger for acute myocardial infarction. Psychosomatics. 2011;52(6):544–549. doi: 10.1016/j.psym.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Shaffer JA, Kronish IM, Burg M, Clemow L, Edmondson D. Association of acute coronary syndrome-induced posttraumatic stress disorder symptoms with self-reported sleep. Ann Behav Med. 2013;46(3):349–357. doi: 10.1007/s12160-013-9512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andrechuk CR, Ceolim MF. Sleep quality and adverse outcomes for patients with acute myocardial infarction. J Clin Nurs. 2016;25(1–2):223–230. doi: 10.1111/jocn.13051. [DOI] [PubMed] [Google Scholar]
- 48.Da Costa D, Allman AA, Libman E, Desormeau P, Lowensteyn I, Grover S. Prevalence and determinants of insomnia after a myocardial infarction. Psychosomatics. 2017;58(2):132–140. doi: 10.1016/j.psym.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Pfaff KA, El-Masri MM, Fox-Wasylyshyn SM. Comparing the psychological stress between non-smoking patients and smoking patients who experience abrupt smoking cessation during hospitalization for acute myocardial infarction: a pilot study. Can J Cardiovasc Nurs. 2009;19(4):26–32. [PubMed] [Google Scholar]
- 50.Andreas S, Schulz R, Werner GS, Kreuzer H. Prevalence of obstructive sleep apnoea in patients with coronary artery disease. Coron Artery Dis. 1996;7(7):541–545. [PubMed] [Google Scholar]
- 51.Cilli A, Tatlicioğlu T, Köktürk O. Nocturnal oxygen desaturation in coronary artery disease. Jpn Heart J. 1999;40(1):23–29. doi: 10.1536/jhj.40.23. [DOI] [PubMed] [Google Scholar]
- 52.Dohno S, Paskewitz DA, Lynch JJ, Gimbel KS, Thomas SA. Some aspects of sleep disturbance in coronary patients. Percept Mot Skills. 1979;48(1):199–205. doi: 10.2466/pms.1979.48.1.199. [DOI] [PubMed] [Google Scholar]
- 53.El-Solh AA, Mador MJ, Sikka P, Dhillon RS, Amsterdam D, Grant BJ. Adhesion molecules in patients with coronary artery disease and moderate-to-severe obstructive sleep apnea. Chest. 2002;121(5):1541–1547. doi: 10.1378/chest.121.5.1541. [DOI] [PubMed] [Google Scholar]
- 54.Gatti RC, Burke PR, Otuyama LJ, Almeida DR, Tufik S, Poyares D. Effects of zolpidem CR on sleep and nocturnal ventilation in patients with heart failure. Sleep. 2016;39(8):1501–1505. doi: 10.5665/sleep.6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geovanini GR, Gowdak LHW, Pereira AC, et al. OSA and depression are common and independently associated with refractory angina in patients with coronary artery disease. Chest. 2014;146(1):73–80. doi: 10.1378/chest.13-2885. [DOI] [PubMed] [Google Scholar]
- 56.Glantz H, Thunström E, Herlitz J, et al. Occurrence and predictors of obstructive sleep apnea in a revascularized coronary artery disease cohort. Ann Am Thorac Soc. 2013;10(4):350–356. doi: 10.1513/AnnalsATS.201211-106OC. [DOI] [PubMed] [Google Scholar]
- 57.Karacan I, Williams RL, Taylor WJ. Sleep characteristics of patients with angina pectoris. Psychosomatics. 1969;10(5):280–284. doi: 10.1016/S0033-3182(69)71713-3. [DOI] [PubMed] [Google Scholar]
- 58.Maggini C, Guazzelli M, Castrogiovanni P, et al. Psychological and physiopathological study on coronary patients. Psychother Psychosom. 1976–1977;27(3–6):210–216. doi: 10.1159/000287021. [DOI] [PubMed] [Google Scholar]
- 59.Mendelson M, Lyons OD, Yadollahi A, Inami T, Oh P, Bradley TD. Effects of exercise training on sleep apnoea in patients with coronary artery disease: a randomised trial. Eur Respir J. 2016;48(1):142–150. doi: 10.1183/13993003.01897-2015. [DOI] [PubMed] [Google Scholar]
- 60.Moruzzi P, Sarzi-Braga S, Rossi M, Contini M. Sleep apnoea in ischaemic heart disease: differences between acute and chronic coronary syndromes. Heart. 1999;82(3):343–347. doi: 10.1136/hrt.82.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Planès C, Leroy M, Bouach Khalil N, et al. Home diagnosis of obstructive sleep apnoea in coronary patients: validity of a simplified device automated analysis. Sleep Breath. 2010;14(1):25–32. doi: 10.1007/s11325-009-0275-1. [DOI] [PubMed] [Google Scholar]
- 62.Varoneckas G, Podlipskyte A, Alonderis A, Martinkenas A. Sleep disordered breathing in coronary heart disease patients with mild and moderate heart failure. Health. 2013;5(8B):36–43. [Google Scholar]
- 63.Bhattacharyya MR, Molloy GJ, Steptoe A. Depression is associated with flatter cortisol rhythms in patients with coronary artery disease. J Psychosom Res. 2008;65(2):107–113. doi: 10.1016/j.jpsychores.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 64.Johansson A, Adamson A, Ejdebäck J, Edéll-Gustafsson U. Evaluation of an individualised programme to promote self-care in sleep-activity in patients with coronary artery disease -- a randomised intervention study. J Clin Nurs. 2014;23(19–20):2822–2834. doi: 10.1111/jocn.12546. [DOI] [PubMed] [Google Scholar]
- 65.Johansson A, Svanborg E, Edéll-Gustafsson U. Sleep-wake activity rhythm and health-related quality of life among patients with coronary artery disease and in a population-based sample--an actigraphy and questionnaire study. Int J Nurs Pract. 2013;19(4):390–401. doi: 10.1111/ijn.12080. [DOI] [PubMed] [Google Scholar]
- 66.Yngman-Uhlin P, Johansson A, Fernström A, Börjeson S, Edéll-Gustafsson U. Fragmented sleep: an unrevealed problem in peritoneal dialysis patients. Scand J Urol Nephrol. 2011;45(3):206–215. doi: 10.3109/00365599.2011.557025. [DOI] [PubMed] [Google Scholar]
- 67.Cross NJ, McCrae CS, Smith KM, Conti JB, Sears SF. Comparison of actigraphic and subjective measures of sleep in implantable cardioverter defibrillator and coronary artery disease patients. Clin Cardiol. 2010;33(12):753–759. doi: 10.1002/clc.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saleh DK, Nouhi S, Zandi H, Lankarani MM, Assari S, Pishgou B. The quality of sleep in coronary artery disease patients with and without anxiety and depressive symptoms. Indian Heart J. 2008;60(4):309–312. [PubMed] [Google Scholar]
- 69.Wang LN, Tao H, Zhao Y, Zhou YQ, Jiang XR. Optimal timing for initiation of biofeedback-assisted relaxation training in hospitalized coronary heart disease patients with sleep disturbances. J Cardiovasc Nurs. 2014;29(4):367–376. doi: 10.1097/JCN.0b013e318297c41b. [DOI] [PubMed] [Google Scholar]
- 70.Ozdemir PG, Selvi Y, Boysan M, Ozdemir M, Akdağ S, Ozturk F. Relationships between coronary angiography, mood, anxiety and insomnia. Psychiatry Res. 2015;228(3):355–362. doi: 10.1016/j.psychres.2015.05.084. [DOI] [PubMed] [Google Scholar]
- 71.Rosenberg J. Sleep disturbances after non-cardiac surgery. Sleep Med Rev. 2001;5(2):129–137. doi: 10.1053/smrv.2000.0121. [DOI] [PubMed] [Google Scholar]
- 72.Din JN, Snow TM, Rao SV, et al. Variation in practice and concordance with guideline criteria for length of stay after elective percutaneous coronary intervention. Catheter Cardiovasc Interv. 2017;90(5):715–722. doi: 10.1002/ccd.26992. [DOI] [PubMed] [Google Scholar]
- 73.Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4(2):104–113. doi: 10.1207/s15402010bsm0402_3. [DOI] [PubMed] [Google Scholar]
- 74.Dombrovski AY, Cyranowski JM, Mulsant BH, et al. Which symptoms predict recurrence of depression in women treated with maintenance interpersonal psychotherapy? Depress Anxiety. 2008;25(12):1060–1066. doi: 10.1002/da.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pigeon WR, Hegel M, Unützer J, et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31(4):481–488. doi: 10.1093/sleep/31.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kraus SS, Rabin LA. Sleep America: managing the crisis of adult chronic insomnia and associated conditions. J Affect Disord. 2012;138(3):192–212. doi: 10.1016/j.jad.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 77.Turek FW. Insomnia and depression: if it looks and walks like a duck. Sleep. 2005;28(11):1362–1363. [PubMed] [Google Scholar]
- 78.Neubauer DN. Current and new thinking in the management of comorbid insomnia. Am J Manag Care. 2009;(15 Suppl):S24–S32. [PubMed] [Google Scholar]
- 79.Sateia MJ. Update on sleep and psychiatric disorders. Chest. 2009;135(5):1370–1379. doi: 10.1378/chest.08-1834. [DOI] [PubMed] [Google Scholar]
- 80.Manber R, Chambers AS. Insomnia and depression: a multifaceted interplay. Curr Psychiatry Rep. 2009;11(6):437–442. doi: 10.1007/s11920-009-0066-1. [DOI] [PubMed] [Google Scholar]
- 81.Harvey AG, Tang NK. Cognitive behaviour therapy for primary insomnia: can we rest yet? Sleep Med Rev. 2003;7(3):237–262. doi: 10.1053/smrv.2002.0266. [DOI] [PubMed] [Google Scholar]
- 82.Buysse DJ. Insomnia. JAMA. 2013;309(7):706–716. doi: 10.1001/jama.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lamberts M, Nielsen OW, Lip GY, et al. Cardiovascular risk in patients with sleep apnoea with or without continuous positive airway pressure therapy: follow-up of 4.5 million Danish adults. J Intern Med. 2014;276(6):659–666. doi: 10.1111/joim.12302. [DOI] [PubMed] [Google Scholar]
- 84.Hla KM, Young T, Hagen EW, et al. Coronary heart disease incidence in sleep disordered breathing: the Wisconsin Sleep Cohort Study. Sleep. 2015;38(5):677–684. doi: 10.5665/sleep.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Madsen MT, Huang C, Gogenur I. Actigraphy for measurements of sleep in relation to oncological treatment of patients with cancer: a systematic review. Sleep Med Rev. 2015;20:73–83. doi: 10.1016/j.smrv.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 86.Palesh O, Peppone L, Innominato PF, et al. Prevalence, putative mechanisms, and current management of sleep problems during chemotherapy for cancer. Nat Sci Sleep. 2012;4:151–162. doi: 10.2147/NSS.S18895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liao WC, Huang CY, Huang TY, Hwang SL. A systematic review of sleep patterns and factors that disturb sleep after heart surgery. J Nurs Res. 2011;19(4):275–288. doi: 10.1097/JNR.0b013e318236cf68. [DOI] [PubMed] [Google Scholar]
- 88.Madsen MT, Rosenberg J, Gogenur I. Actigraphy for measurement of sleep and sleep-wake rhythms in relation to surgery. J Clin Sleep Med. 2013;9(4):387–394. doi: 10.5664/jcsm.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Redeker NS, Hedges C. Sleep during hospitalization and recovery after cardiac surgery. J Cardiovasc Nurs. 2002;17(1):56–68. doi: 10.1097/00005082-200210000-00006. [DOI] [PubMed] [Google Scholar]