Abstract
Study Objectives:
Polysomnography (PSG) is increasingly used in the assessment of infants. Newborn PSG reference values based on recent standardization are not available. This study provides reference values for PSG variables in healthy newborn infants.
Methods:
Cross-sectional study of normal term newborn infants using standardized PSG collection and American Academy of Sleep Medicine interpretation criteria.
Results:
Thirty infants born between 37 and 42 weeks gestation underwent PSG testing before 30 days of age (mean 19.6 days). The infants had a mean sleep efficiency of 71% with average proportions of transitional, NREM and REM sleep estimated at 16.1%, 43.3% and 40.6% respectively. Mean arousal index was 14.7 events/h with respiratory arousal index of 1.2 events/h. Mean apnea-hypopnea index (AHI) was 14.9 events/h. Central, obstructive, and mixed apnea indices were 5.4, 2.3, and 1.2 events/h respectively. Mean oxygen saturation in sleep was 97.9% with a nadir of 84.4%. Mean end tidal CO2 was 35.4 mmHg with an average of 6.2% of sleep time spent above end-tidal CO2 45 mmHg and 0.6% above 50 mmHg.
Conclusions:
The sleep efficiency was significantly lower and the AHI was significantly higher compared to healthy children older than 1 year. The AHI was also higher than reported in healthy infants older than 1 month. These findings suggest current severity classifications of sleep apnea may not apply to newborn infants.
Citation:
Daftarya AS, Jaloua HE, Shivelya L, Slavenb JE, Davisc SD. Polysomnography reference values in healthy newborns. J Clin Sleep Med. 2019;15(3):437–443.
Keywords: apnea, infant, polysomnography, sleep study
BRIEF SUMMARY
Current Knowledge/Study Rationale: Polysomnography (PSG) is increasingly used in the assessment of young infants, notably for airway obstruction. However, available reference data for PSG interpretation do not include newborns since procedural standardization by the AASM. Older reference values have used nonstandard methodology.
Study Impact: This study provides reference values in healthy newborns to assist with PSG interpretation and clinical decision making on major interventions at this vulnerable age.
INTRODUCTION
Infant apnea is clinically well recognized and has been a subject of considerable research over the years because of concerns for its association with sudden infant death syndrome,1 airway obstruction especially with craniofacial and chromosomal abnormalities,2–4 and decreased respiratory reserve in chronic lung disease.5 Infants spend significant amounts of each day asleep; therefore, it is intuitive that those prone to apnea would experience such events during sleep. Newborns have a higher predisposition to apnea during sleep due to various physiologic limitations including immature control of breathing, as well as higher upper airway and chest wall compliance resulting in a lower respiratory reserve.6–9 Nerve conduction delays due to neuronal immaturity have been associated with apnea in neonates.10 The Collaborative Home Infant Monitoring Evaluation study corroborated this by demonstrating a higher prevalence of apnea in infants younger than 42 weeks postconceptional age, with a maturational decline in frequency of events.1 Infant sleep apnea has been well documented to occur in the literature11; however, little is known about the long-term outcome of the condition and therefore clinicians are constantly facing dilemmas regarding when intervention is necessary.
Polysomnography (PSG) is the gold standard test to evaluate for sleep apnea, and several studies have been performed over the years in healthy children with the goal of establishing normative data.12–16 Studies involving PSG during infancy have varied in testing methodology and largely excluded neonates, restricting generalizability for clinical use in the newborn age group.17 A review of infant PSG demonstrates a much higher occurrence of respiratory disturbances compared to older children.17 With the increasing use of PSG in infant sleep assessment, the American Academy of Sleep Medicine standardized this tool for infants and urged development of reference values in healthy newborns.18,19 The aim of our study was to describe the PSG findings in healthy newborn infants using recently standardized testing and interpretation criteria.20
METHODS
Healthy newborn infants were enrolled from the nurseries of regional hospitals in the Indianapolis metropolitan area. Informational posters regarding the study were posted in the postnatal wards of area hospitals as a source of awareness. A financial incentive was offered to participants. Newborns were recruited for the purposes of this study and were not part of a cohort of participants in other research studies at our institution. Individuals were identified after direct contact from families interested in participation. The maternal and infant medical records of potential participants were then screened for suitability for study participation. Any missing information was subsequently acquired by direct contact between the family and the study coordinator (LS). The study was approved by the Indiana University School of Medicine Institutional Review Board and informed consent was obtained from all parents. Newborns were defined as infants younger than 31 days for the purposes of this study. PSG tests were performed at the American Academy of Sleep Medicine (AASM) accredited sleep laboratory at Riley Hospital for Children at Indiana University Health. We included healthy newborns born at 38 to 42 weeks gestation determined based on postmenstrual age, to mothers aged 17 to 40 years at the time of delivery. Mothers who were on prenatal vita-mins and had received courses of antibiotics during pregnancy and labor were included. As neonates typically sleep for 3 to 4 hours between awakenings for feedings, we included all infants who had at least 2.5 hours of sleep time with presence of both REM and NREM sleep. Exclusion criteria included infants born to mothers with any chronic medical illness or gestational complications (hospitalization for hyperemesis gravidarum, antepartum bleeding that required hospitalization or surgical management, gestational diabetes that required more than diet control, pregnancy induced hypertension needing any intervention, antenatal steroid use or substance abuse, history of a medical or surgical illness that required hospitalization during pregnancy). Additional exclusion criteria included history of maternal smoking during or after pregnancy, any resuscitation at birth beyond airway suction and blow by oxygen for more than 5 minutes, congenital anomalies, gastroesophageal reflux requiring treatment, hypotonia, feeding difficulties, meconium stained amniotic fluid, neonatal respiratory illness—including transient tachypnea of the newborn, apnea, stridor or stertor, hyperbilirubinemia requiring phototherapy, genetic or metabolic problems and a family history of childhood apnea, congenital heart disease, metabolic disorders, inherited neurologic disorders, stillbirth, or sudden infant death syndrome. Participants were evaluated by a board certified pediatric sleep medicine physician on the day of the PSG to ensure there were no health concerns. A detailed clinical assessment to ensure newborns were healthy, with no respiratory concerns including snoring, stridor, and tachypnea, was performed by the physician.
PSG tests were attended, performed at an altitude of 732 feet above sea level, at ambient temperature of 20–23°C, and lasted 6 hours. Infants were studied in the supine position in a crib, clothed, and swaddled. The infants were allowed to feed and to use pacifiers during the study. Because neonates do not have established circadian rhythms, the studies were performed during the daytime—9:00 am to 3:00 pm. PSG tests were performed in technical accordance with standards proposed by the AASM, by a single registered polysomnography technician for consistency in collection. We collected electroencephalogram (placement of frontal [F3,F4], central [C3,C4], occipital [O1, O2] referenced to the opposite mastoid electrodes [M1,M2]), electro-oculogram, electromyogram (chin and both legs), electrocardiogram, pressure transducer and thermistor airflow, uncalibrated respiratory inductance plethysmography, oximetry, and end-tidal CO2 (ETCO2) data with video monitoring of the study for scoring support. All studies were scored by a single pediatric pulmonologist board certified in sleep medicine (AD), in accordance with the pediatric scoring rules proposed by the AASM. In addition to the routine PSG variables and indices recommended by the AASM, we calculated a respiratory arousal index for each infant, as the frequency of arousals per hour that followed respiratory events within 1 second of event termination. Fifteen studies were re-analyzed by a second board-certified pediatric sleep medicine physician (HJ) for quality control. Scorers were not blinded to the study goal. The interscorer agreement was high with a mean kappa statistic of 0.9. PSG variable data were collected using the following equipment: Natus SleepWorks PSG software (Pleasanton, California, United States), nasal airflow and side stream ETCO2 sampling using nasal sensors (Dynamedix Diagnostics, Shoreview, Minnesota, United States; Salter Labs, Arvin, California, United States), Protech 2 channel pediatric thermistor (Philips Respironics, Murrysville, Pennsylvania, United States), oximetry (Massimo Radical 7, Irvine, California, United States), and respiratory inductance plethysmography (PerfectFit, Dynamedix Diagnostics, Shoreview, Minnesota, United States), and Capnography (Capnocheck Plus, Smiths Medical PM, Inc, Waukesha, Wisconsin, United States).
Statistical Analysis
Univariate analyses were performed for both descriptive purposes and to determine the distributions of the variables of interest. Correlation analyses and analyses of variance (ANOVAs) were performed to test for associations between the PSG variables of interest and the independent clinical and demographic variables, with correlation analyses being performed with continuous independent variables and ANOVA models being performed with categorical independent variables. If variables were skewed, Spearman nonparametric analyses were performed for continuous variables and log transformations were performed on the PSG variables when necessary for ANOVAs, to follow ANOVA assumptions. All analyses were performed using SAS v9.4 (SAS Institute, Cary, North Carolina, United States). The value of significance was P < .05 in this study.
RESULTS
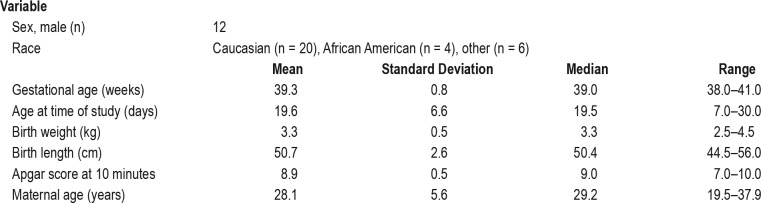
A total of 38 healthy newborns were enrolled in the study, of whom 31 underwent PSG. Seven infants did not show for the PSG within the newborn period and were excluded. One record from the 31 completed studies was excluded due to corruption of the raw data after collection; therefore, data from 30 newborns were analyzed and are reported (Figure 1). Demographic and clinical information on the participants is summarized in Table 1. Twelve males and 18 female infants participated. Twenty (66.7%) were Caucasian, four (13.3%) were African American, and six (20%) were Latino or part Latino-Caucasian. Twenty-six babies were delivered vaginally and four were delivered via cesarean section. The mean age at time of study was 19.6 days (median 19.5, range 7 to 30 days).
Figure 1. Study consort diagram.
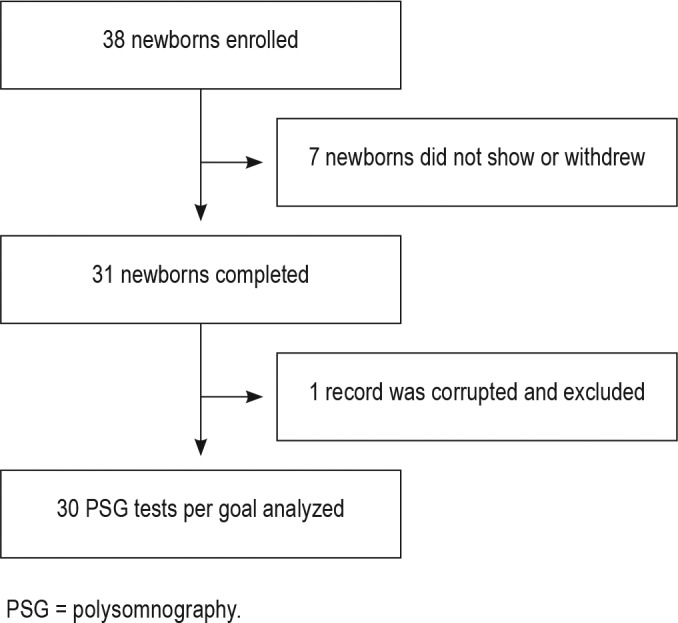
PSG = polysomnography.
Table 1.
Demographic and clinical characteristics (n = 30).
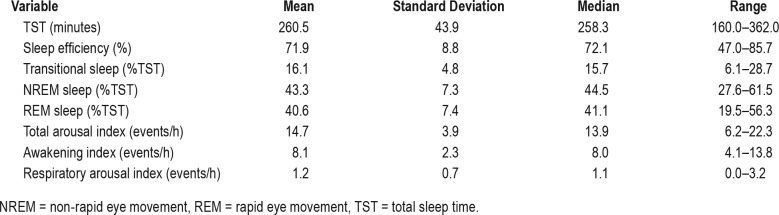
Sleep architectural variables are summarized in Table 2 and respiratory variables in Table 3. The mean duration of NREM sleep was 111.5 minutes (range 69–145); REM sleep, 105.3 minutes (33– 151.5); and transitional, 40 minutes (15–68.5).
Table 2.
Sleep architecture variables.
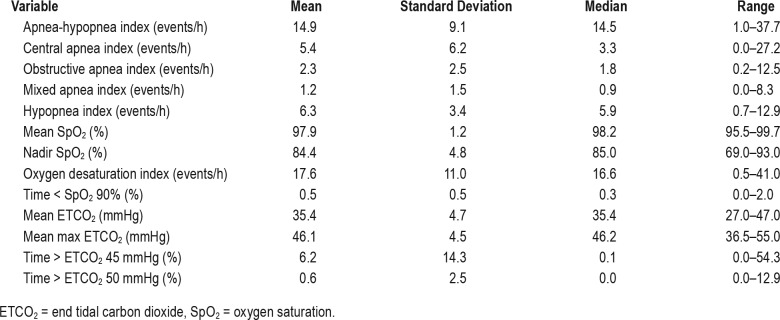
Table 3.
Respiratory variables during sleep.
The mean apnea-hypopnea index (AHI) was 14.9 events/h (median 14.5, range 1 to 37), with hypopneas being the most common respiratory events, followed by central, obstructive, and then mixed sleep apneas. There was a preponderance of respiratory events in REM sleep for a mean REM AHI of 23.6 events/h (median 20.6, range 2 to 92.6). The mean duration of central apneas was 5.7 seconds (median 5.6, range 4.3 to 8.1) with an average maximum duration of 8.3 seconds (median 8.4, range 4.3 to 13.8). The mean duration of obstructive apneas was 6 seconds (median 5.9, range 4.6 to 8.3) with an average maximum duration of 7.6 seconds (median 7.5, range 4.9 to 11.5) and the mean duration of mixed apneas was 6.6 seconds (median 6.7, range 4.3 to 9.2) with an average maximum duration of 8 seconds (median 7.5, range 4.9 to 13.5). The mean duration of hypopneas was 6.8 seconds (median 6.7, range 4.9 to 9.7) with an average maximum duration of 11.2 seconds (median 10.8, range 7.3 to 17.3 seconds). Periodic breathing was present in 12 of the newborns (40%) for a mean of 1.16% of total sleep time (median 0, range 0 to 11.3%). We did not find statistically significant correlations between the AHI or periodic breathing and the following variables: birth weight, birth length, gestational age, maternal age, Apgar score, birth order, or age at time of study.
The median oxygen saturation during sleep was 98% with nadir of 85% (range: 95.5 to 99.7% and 69 to 93% respectively). The median proportion of sleep time less than 90% oxygen saturation was 0.3% of total sleep time (range 0 to 2%). The median ETCO2 in sleep was 35.4 mmHg with a peak of 46.2 mmHg. Ten infants spent more than 1% of total sleep time above an ETCO2 of 45 mmHg, of whom three spent more than 25% of total sleep time above an ETCO2 of 45 mmHg. Two newborns spent sleep time above an ETCO2 of 50 mmHg and none had more than 5% of total sleep time above an ETCO2 50 mmHg.
DISCUSSION
To our knowledge this is the first study that provides PSG reference values for infants younger than 1 month utilizing the recently standardized AASM testing and scoring guidelines.20 Our data corroborate that newborns spend relatively equal proportions of time in NREM and REM sleep (after excluding transitional sleep).21 Respiratory events were common in our participants, with most being brief and less than 10 seconds in duration. No events exceeded 20 seconds duration in our study. The AHI was higher in our newborns than established normative data in older children12,13,22 with a higher prevalence of respiratory events in REM as compared to NREM sleep.23
Oxygen desaturations were common in our participants, but brief and self-resolving, with a cumulative sleep time spent below a saturation of 90% never exceeding 2% of total sleep time. The mean ETCO2 was lower in our participants than in older children14 and exceeded 50 mmHg in fewer than 1% of the participants.
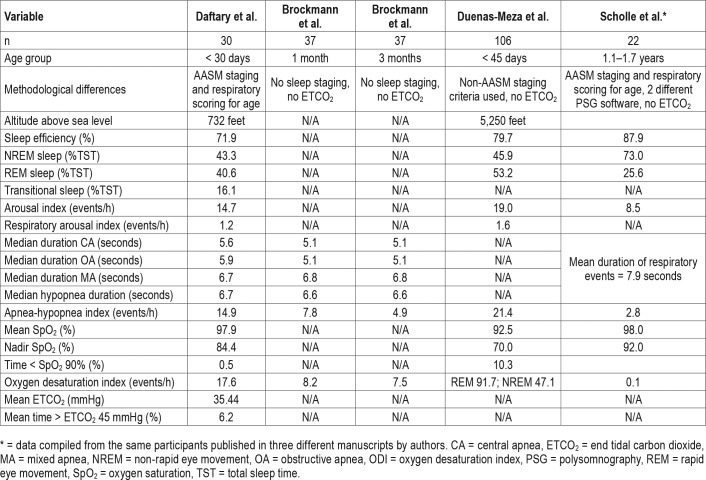
Previously published literature on respiratory and sleep parameters in infants has used varying methodology limiting current clinical generalizability.17 Although our study used the new sleep staging recommendations from the AASM, the sleep efficiency, arousal indices and relative proportions of REM and NREM sleep are similar to data from previously reported literature21,23 (Table 4). Consistent with previously reported data using PSG in infants, our study shows that the mean AHI in healthy newborns is higher than older children.17,24,25 Our study revealed that hypopneas are the most frequent respiratory events, followed by central apneas, obstructive apneas and mixed apneas, in that order. Brockmann et al. reported reference values for respiratory events in healthy infants at 1 and 3 months of age and also found central apneas to be more prevalent than obstructive apneas, which in turn were more prevalent than mixed apneas; however, their hypopnea index was much lower than in our study.24 Potential explanations may be the older age of participants, differences in sensitivity of their monitoring equipment, altitude of the study (not reported), or underestimation of hypopneas (if significant portions of nasal airflow data were disregarded due to artifact as was implied). The duration of respiratory events was similar between Brockmann et al. and our study (Table 4). Brock-mann et al. and Duenas-Meza et al.25 reported that obstructive respiratory events are normal in infants. Obstructive respiratory events have been described based on airway endoscopy in infants, and are attributed most commonly to airway dynamic collapse with trend towards spontaneous resolution with airway maturation26,27
Table 4.
Published PSG parameter values in healthy infants.
Although there is some lack of consensus, an AHI > 1 event/h is considered abnormal in the pediatric age group, which is based off normative data from older children.13,28 Using such a low threshold can potentially result in overtreatment of sleep apnea in young infants, particularly if treatment guidelines from the American Academy of Pediatrics were extrapolated to this age group.29 Our study results support consideration of a different severity classification for sleep apnea in infancy. The AASM defines pediatric hypoventilation as > 25% of total sleep time above an ETCO2 of 50 mmHg.30 The faster respiratory rates of young infants preclude plateauing of the ETCO2 wave form (Figure 2), resulting in the potential for underestimation of CO2 as compared to formal blood gas testing. It is therefore not surprising that the CO2 values we report are lower than those in normative data studies performed in older children. Our study shows that capnography can be performed in newborns and provides reference values for noninvasive CO2 estimation using this modality. Capnography data have not been described by Brockmann et al. or Duenas-Meza et al. in their infant PSG reference studies.
Figure 2. Comparison of ETCO2 tracings.
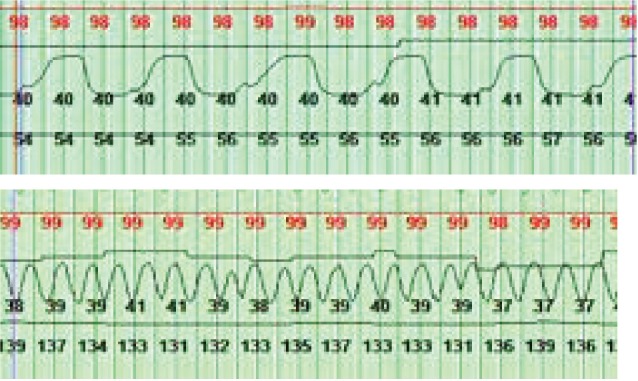
Top: ETCO2 tracing of a 16-year-old. Bottom: ETCO2 tracing of a study participant. Top tracing shows plateauing of the wave form, barely seen in the newborn ETCO2 tracing. ETCO2 = end-tidal carbon dioxide.
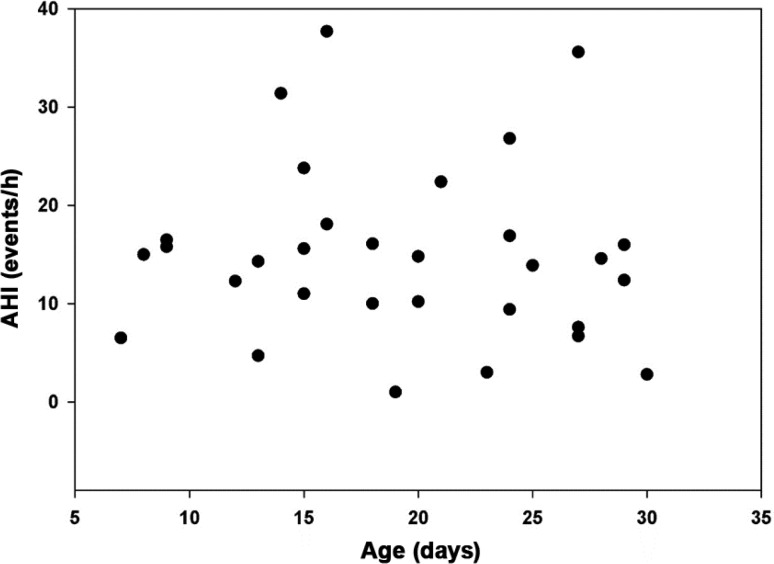
With the increasing use of PSG in treatment decisions ranging from apparent life-threatening episodes (now brief resolved unexplained events), to oxygen supplementation in neonatal chronic lung disease, to surgical interventions for neonatal airway obstructive disorders, reference values from healthy infants are important for treatment decisions.3,5,31 Although the AHI is the most commonly used index to base clinical decisions, our data show a wide range in this index in newborns. As seen in Figure 3, there is considerable intersubject variability in the AHI. We did not find significant correlations with demographic variables to explain this variance, which could be partly because of our small sample size, but may also be a reflection of the inherent immaturity of control of breathing and immature pulmonary physiology characteristic of this age group. The oxygen saturation nadir in our study is lower than in normative data studies performed at older ages. This can be explained by the respiratory physiologic limitations at this age.32 These limitations include not only the need to dynamically maintain functional residual capacity above closing volume, but also the predominance of fetal hemoglobin in the newborn with its parabolic oxygen dissociation curve enabling rapid desaturation when O2 reserves are depleted.
Figure 3. Distribution of AHI by age of newborns.
AHI = apnea-hypopnea index.
Our study has several limitations, including potential for selection bias from those interested and completing the study as well as an overrepresentation of Caucasians. Our study has a relatively small number of participants along with exhaustive exclusion criteria that may limit generalizability to all populations. The results show a high degree of variance, which may also be a consequence of the sample size. Currently recommended hypopnea scoring guidelines by the AASM render it challenging to clearly distinguish obstructive from central hypopneas due to physiologic thoracoabdominal asynchrony at this age. We did not use esophageal pressure monitoring to differentiate hypopneas as obstructive versus central, which can be helpful in this regard. Because of funding constraints, our cross-sectional study is unable to provide follow-up PSG data in the study participants to document the trajectory of respiratory events over time. Such a follow-up study would be required to provide true normative data on sleep and PSG variables through infancy.
CONCLUSIONS
Our study provides reference values for sleep architecture, respiratory events, and gas exchange in healthy newborns using standardized PSG methodology. The study suggests that asymptomatic newborns can have a wide range of obstructive and central respiratory events. We hope our data will provide a guide to newborn PSG interpretation, as this diagnostic modality is increasingly used for clinical management. Studies with larger numbers of healthy neonates serially monitored by PSG over several time points through infancy will be helpful in confirming the high variance of events and establishing normative data by describing the natural progression of the AHI with age and maturation during this critical period of growth and development.
DISCLOSURE STATEMENT
Work for this study was performed at the Riley Hospital for Children, Indianapolis, IN. All authors have reviewed and approved this manuscript. Financial support was provided by the Department of Pediatrics Research Fund Award, Indiana University School of Medicine. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the families that participated in this study and the staff that supported its execution. We also acknowledge the grant support by the Department of Pediatrics, Indiana University School of Medicine to enable this study.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- ETCO2
end tidal carbon dioxide
- O2
oxygen
- PSG
polysomnography
REFERENCES
- 1.Ramanathan R, Corwin MJ, Hunt CE, et al. Cardiorespiratory events recorded on home monitors: Comparison of healthy infants with those at increased risk for SIDS. JAMA. 2001;285(17):2199–2207. doi: 10.1001/jama.285.17.2199. [DOI] [PubMed] [Google Scholar]
- 2.Goffinski A, Stanley MA, Shepherd N, et al. Obstructive sleep apnea in young infants with Down syndrome evaluated in a Down syndrome specialty clinic. Am J Med Genet A. 2015;167A(2):324–330. doi: 10.1002/ajmg.a.36903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy VS. Evaluation of upper airway obstruction in infants with Pierre Robin sequence and the role of polysomnography--review of current evidence. Paediatr Respir Rev. 2016;17:80–87. doi: 10.1016/j.prrv.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Pavone M, Caldarelli V, Khirani S, et al. Sleep disordered breathing in patients with Prader-Willi syndrome: A multicenter study. Pediatr Pulmonol. 2015;50(12):1354–1359. doi: 10.1002/ppul.23177. [DOI] [PubMed] [Google Scholar]
- 5.McGrath-Morrow SA, Ryan T, McGinley BM, Okelo SO, Sterni LM, Collaco JM. Polysomnography in preterm infants and children with chronic lung disease. Pediatr Pulmonol. 2012;47(2):172–179. doi: 10.1002/ppul.21522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poets CF. Apnea of prematurity: What can observational studies tell us about pathophysiology? Sleep Med. 2010;11(7):701–707. doi: 10.1016/j.sleep.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Papastamelos C, Panitch HB, England SE, Allen JL. Developmental changes in chest wall compliance in infancy and early childhood. J Appl Physiol. 1995;78(1):179–184. doi: 10.1152/jappl.1995.78.1.179. [DOI] [PubMed] [Google Scholar]
- 8.Colin AA, Wohl ME, Mead J, Ratjen FA, Glass G, Stark AR. Transition from dynamically maintained to relaxed end-expiratory volume in human infants. J Appl Physiol. 1989;67(5):2107–2111. doi: 10.1152/jappl.1989.67.5.2107. [DOI] [PubMed] [Google Scholar]
- 9.Henschen M, Stocks J, Brookes I, Frey U. New aspects of airway mechanics in pre-term infants. The Eur Respir J. 2006;27(5):913–920. doi: 10.1183/09031936.06.00036305. [DOI] [PubMed] [Google Scholar]
- 10.Henderson-Smart DJ, Pettigrew AG, Campbell DJ. Clinical apnea and brainstem neural function in preterm infants. N Engl J Med. 1983;308(7):353–357. doi: 10.1056/NEJM198302173080702. [DOI] [PubMed] [Google Scholar]
- 11.Henderson-Smart DJ, Cohen G. Apnoea in the newborn infant. Aust Paediatr J. 1986;(22 Suppl 1):63–66. [PubMed] [Google Scholar]
- 12.Montgomery-Downs HE, O'Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117(3):741–753. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 13.Marcus CL, Omlin KJ, Basinki DJ, et al. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis. 1992;146(5 Pt 1):1235–1239. doi: 10.1164/ajrccm/146.5_Pt_1.1235. [DOI] [PubMed] [Google Scholar]
- 14.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2-9 years old: additional data and review of the literature. Pediatr Pulmonol. 2005;40(1):22–30. doi: 10.1002/ppul.20236. [DOI] [PubMed] [Google Scholar]
- 15.Beck SE, Marcus CL. Pediatric polysomnography. Sleep Med Clin. 2009;4(3):393–406. doi: 10.1016/j.jsmc.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28(4):499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 17.Ng DK, Chan CH. A review of normal values of infant sleep polysomnography. Pediatr Neonatol. 2013;54(2):82–87. doi: 10.1016/j.pedneo.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Tapia IE, Marcus CL. Infant scoring: the force awakens. J Clin Sleep Med. 2016;12(3):291–292. doi: 10.5664/jcsm.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grigg-Damberger MM. The visual scoring of sleep in infants 0 to 2 months of age. J Clin Sleep Med. 2016;12(3):429–445. doi: 10.5664/jcsm.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry RB, Brooks R, Gamaldo C, et al. AASM scoring manual updates for 2017 (version 2.4) J Clin Sleep Med. 2017;13(5):665–666. doi: 10.5664/jcsm.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheldon SH, Ferber R, Kryger M, Gozal D, editors. Principles and Practice of Pediatric Sleep Medicine. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2012. [Google Scholar]
- 22.Scholle S, Wiater A, Scholle HC. Normative values of polysomnographic parameters in childhood and adolescence: cardiorespiratory parameters. Sleep Med. 2011;12(10):988–996. doi: 10.1016/j.sleep.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Kahn A, Dan B, Groswasser J, Franco P, Sottiaux M. Normal sleep architecture in infants and children. J Clin Neurophysiol. 1996;13(3):184–197. doi: 10.1097/00004691-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Brockmann PE, Poets A, Poets CF. Reference values for respiratory events in overnight polygraphy from infants aged 1 and 3months. Sleep Med. 2013;14(12):1323–1327. doi: 10.1016/j.sleep.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Duenas-Meza E, Bazurto-Zapata MA, Gozal D, Gonzalez-Garcia M, Duran-Cantolla J, Torres-Duque CA. Overnight polysomnographic characteristics and oxygen saturation of healthy infants, 1 to 18 months of age, born and residing at high altitude (2,640 meters) Chest. 2015;148(1):120–127. doi: 10.1378/chest.14-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bandyopadhyay A, Muston H, Slaven JE, Jalou HE, Engle WA, Daftary AS. Endoscopic airway findings in infants with obstructive sleep apnea. J Pulm Respir Med. 2018;8(1):448. [Google Scholar]
- 27.Goldberg S, Shatz A, Picard E, et al. Endoscopic findings in children with obstructive sleep apnea: effects of age and hypotonia. Pediatr Pulmonol. 2005;40(3):205–210. doi: 10.1002/ppul.20230. [DOI] [PubMed] [Google Scholar]
- 28.Carroll JL. Obstructive sleep-disordered breathing in children: new controversies, new directions. Clin Chest Med. 2003;24(2):261–282. doi: 10.1016/s0272-5231(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 29.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576–584. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 30.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniels H, Naulaers G, Deroost F, Devlieger H. Polysomnography and home documented monitoring of cardiorespiratory pattern. Arch Dis Child. 1999;81(5):434–436. doi: 10.1136/adc.81.5.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis RP, Mychaliska GB. Neonatal pulmonary physiology. Semin Pediatr Surg. 2013;22(4):179–184. doi: 10.1053/j.sempedsurg.2013.10.005. [DOI] [PubMed] [Google Scholar]