Abstract
Study Objectives:
This study aimed to analyze the association between habitual meal timing and sleep parameters, as well as habitual meal timing and apnea severity in individuals with obstructive sleep apnea (OSA).
Methods:
Patients in whom mild to severe OSA was diagnosed were included in the study (n = 296). Sleep parameters were analyzed by polysomnography. Dietary pattern was obtained by a food frequency questionnaire and meal timing of the participants. Individuals with OSA were categorized by meal timing (early, late, and skippers).
Results:
Dinner timing was associated with sleep latency (β = 0.130, P = .022), apnea-hypopnea index (AHI) (β = 1.284, P = .033) and poor sleep quality (β = 1.140, P = .015). Breakfast timing was associated with wake after sleep onset (WASO) (β = 3.567, P = .003), stage N1 sleep (β = 0.130, P < .001), and stage R sleep (β = −1.189, P = .001). Lunch timing also was associated with stage N1 sleep (β = 0.095, P = .025), sleep latency (β = 0.293, P = .001), and daytime sleepiness (β = 1.267, P = .009). Compared to early eaters, late eaters presented lower duration of stage R sleep and greater values of sleep latency, WASO, stage N1 sleep, and AHI, in addition to increased risk of poor sleep quality and daytime sleepiness (P < .005).
Conclusions:
Late meal timing was associated with worse sleep pattern and quality and apnea severity than early meal timing. Despite some of these results having limited clinical significance, they can lead to a better understanding about how meal timing affects OSA and sleep parameters.
Citation:
Lopes TVC, Borba ME, Lopes RVC, Fisberg RM, Paim SL, Teodoro VV, Zimberg IZ, Crispim CA. Eating late negatively affects sleep pattern and apnea severity in individuals with sleep apnea. J Clin Sleep Med. 2019;15(3):383–392.
Keywords: eating duration, meal timing, nutrition, OSA, REM sleep, slow wave sleep
BRIEF SUMMARY
Current Knowledge/Study Rationale: Several recent studies demonstrated that the timing of food intake and eating duration (interval between the first and last meal of the day), independent of energy intake, have a major role in obesity, which is a well-established leading risk factor for obstructive sleep apnea and sleep disorders.
Study Impact: Given the chronic diseases and sleep disturbance that patients with obesity and obstructive sleep apnea often have, methods to improve dietary patterns and metabolic health should be encouraged for these individuals to reduce apnea-hypopnea index and improve sleep architecture.
INTRODUCTION
Obstructive sleep apnea (OSA) is a significant public health problem that is prevalent worldwide. In association with excessive daytime somnolence rates ranged from 3% to 7% in adult men and from 2% to 5% in women.1 However, defining OSA as an apnea-hypopnea index (AHI) ≥ 5 events/h—as used by the Wisconsin Sleep Cohort—the prevalence is higher; 24% in men and 9% in women aged 30 to 60 years.2 This disturbance is characterized by recurrent episodes of partial (hypopnea) or complete (apnea) obstruction of the upper airway, intermittent hypoxia, and frequent arousal from sleep.3 Symptoms of OSA include daytime sleepiness and fatigue, and sleep-related loud snoring or gasping for air, and reduced or cessation of airflow during sleep.3
Consistent findings from studies support a strong bidirectional association between OSA and metabolic diseases such as obesity.4,5 In this sense, obesity is a well-established leading risk factor for OSA, and OSA itself may promote further weight gain.4 Although the reasons underlying this reciprocal relationship remain uncertain, evidence from observational and laboratory-based studies demonstrates a relationship between sleep and factors regulating body weight, such as physical activity and food intake.6,7
Several recent studies demonstrated that the timing of food intake8,9 and eating duration (interval between the first and last meal of the day),10 independent of energy intake, have a major role in obesity. These results indicate that avoiding late meals, mainly night meals and near bedtime, could reduce weight gain and contribute to a decrease in eating duration and an increase in fasting period, which is associated with reduced adiposity, elevated lean mass, and longer sleep duration.8–10
In addition to the increased risk of obesity, late meal timing (mainly night meals), could affect sleep pattern. Different studies have observed the effect of the nutrients from these meals on sleep,11 and apnea severity12,13; nevertheless, there is little information about the isolated association between meal times and sleep patterns of individuals with sleep apnea. The few studies in this area did not find significant associations between meal timing and sleep duration or self-reported sleep parameters such as sleepiness,7,8 but none of them investigated whether meal timing is associated with sleep architecture and sleep apnea severity of individuals with OSA.
Considering the disruptive effects of obesity on sleep and apnea severity,4 an inadequate meal timing could aggravate even more the sleep pattern and apnea severity of these individuals. Thus, we hypothesized that late eating and a longer eating duration are associated with worse sleep parameters and apnea severity compared to early eating and a shorter eating duration, respectively. The objective of this study was to assess differences in sleep parameters and AHI among individuals who eat early/late and those with longer/ shorter eating duration, as well as to analyze the association between sleep parameters and apnea severity with meal timing and eating duration.
METHODS
Study Population
This cross-sectional study was conducted in individuals with sleep apnea from August 2016 to March 2017. Patients with sleep complaints and with suspected sleep apnea were referred by their physicians for polysomnography (PSG) in a private sleep clinic where the study was conducted. During the study period, all the individuals aged 20 to 60 years were invited to take part voluntarily in the research study. Volunteers were eligible for participation if they reported no apnea treatment, such as surgery or continuous positive airway pressure therapy, no previous diagnosis of sleep disorders, and no intake of medication that could affect sleep. Using G*Power software (http://www.gpower.hhu.de/), and specifying significance level of .05 and a power of 90%, a sample size of 282 participants was established with a post hoc power of 72%. Of 409 patients who agreed to participate in the research, 296 were included in the study and 113 were excluded—95 for AHI < 5 events/h, 13 for energy consumption above 4,000 kcal, 4 for incomplete PSG, and 1 outlier (body mass index [BMI] and AHI) (Figure 1).
Figure 1. Study sample flow chart.
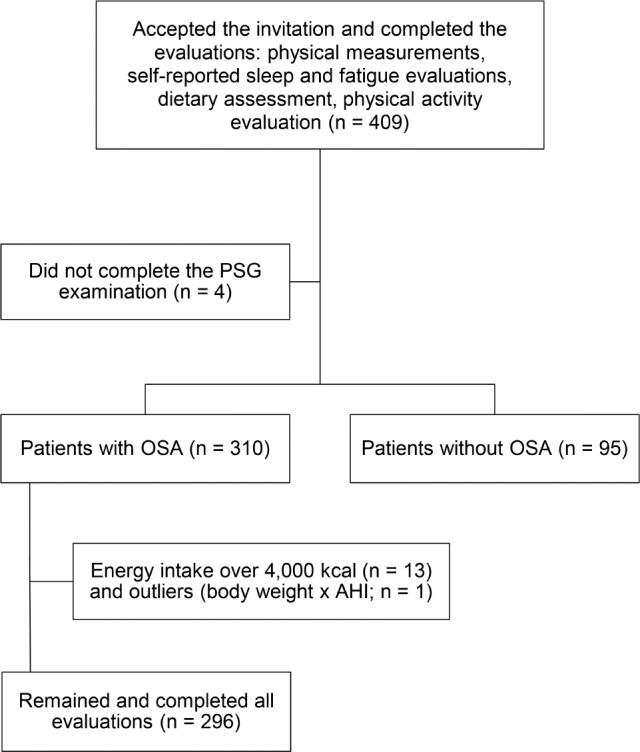
AHI = apnea-hypopnea index, OSA = obstructive sleep apnea, PSG = polysomnography.
Initially, a questionnaire was applied, which provides information about sociodemographic characteristics, personal information (age, alcohol consumption, smoking habit, and medication use), and previous diseases. Physical measurements were collected and participants also answered questionnaires about self-reported sleep and fatigue evaluations and dietary assessment, as will be described in detail later.
This study was approved by the Ethics Committee of the Federal University of Uberlandia (protocol no. 57610416.7.0000.5152) and was conducted according to international ethical standards. Informed written consent was obtained from all volunteers before starting the study.
Physical Measurements
Physical measurements were measured by trained staff using standardized methods14,15 and included: systolic blood pressure (mmHg), diastolic blood pressure (mmHg), neck circumference (NC, cm) at the thyroid cartilage level, and waist circumference (WC, cm) at the level of the umbilicus, body weight (kg), and height (m). Diastolic and systolic blood pressures were measured twice for each participant using the left arm after 10 minutes of rest and the average of two measurements was considered for analyses. The participants were weighed while barefoot, wearing light clothes, on a digital scale that measured to the nearest 0.1 kg. Height was measured using a stadiometer and the participant was positioned upright, and relaxed, with head on the Frankfurt plane. Circumferences measurements were made with a flexible and inextensible measuring tape. Participants were classified according to BMI, obtained by weight and squared height ratio.14
Sleep and Fatigue Evaluations
Sleep evaluation consisted of self-reported and objective approaches (PSG). Self-reported sleep evaluations were obtained before the PSG examination. Poor sleep quality, habitual bedtime, and sleep duration were assessed using the Pittsburgh Sleep Quality Index (PSQI) that includes 19 items and yields a score from 0 (good quality) to 21 (poor quality); a total sum greater than 5 indicates poor sleep quality.16 Daytime sleepiness was assessed using the Epworth Sleepiness Scale (ESS), a widely used and reliable predictor of daytime sleepiness.17 The ESS is an eight-item, self-administered questionnaire that is designed to provide a measure of a person's propensity to fall asleep in a variety of situations. A total score of 8 indicated excessive sleepiness.17
Fatigue was measured by the Chalder Scale, a questionnaire with 11 items that are scored on a 4-point scale from “less than usual (0)” to “much more than usual (3)”; the higher the summed score, the more fatigued the individual.18
Polysomnography
A full-night PSG was accomplished using a digital system (Alice Sleepware version 2.8.78, Respironics Inc., Murrysville, Pennsylvania, United States) at the sleep clinic during the participant's habitual sleep time. The PSG evaluations were standardized to have the same duration with an 8-hour sleep opportunity approximately. Physiological variables evaluated during PSG included: electroencephalogram (EEG) (C3/ A2, C4/A1, O2/A1, O1/A2), electrooculogram, chin and leg electromyogram, and electrocardiogram. During the PSG, airflow was monitored with nasal and oral thermocouples. Thoracic and abdominal respiratory movements were measured with inductive respiratory plethysmography, arterial oxygen saturation with pulse oximetry, while snoring was measured by tracheal microphone, and body position was measured by a sensor located on the chest. Trained technicians visually scored PSG results and the examination was carried out according to specific criteria for the definition of sleep stages.19 EEG arousals and leg movements were scored in agreement with the criteria established by The AASM Manual for Scoring Sleep and Associated Events: Rules, Terminology and Technical Specifications.19 Apneas were scored and classified according to the recommended respiratory rules for adults by the AASM Manual, and hypopneas were scored by the alternative rules.19
OSA was diagnosed in accordance with the criteria of the International Classification of Sleep Disorders.20 OSA was diagnosed in participants if they had AHI ≥ 15 or AHI 5 to 14.9 and presented at least one of these complaints: loud snoring, breathing interruptions during sleep, daytime sleepiness, and fatigue. The complaints were assessed according to Tufik et al.21: loud snoring and breathing interruptions using the second and fifth questions of the Berlin Questionnaire for sleep apnea, respectively22; daytime sleepiness using the ESS17 and the eight questions of the PSQI16; fatigue was assessed with the Chalder Fatigue Scale.18
Habitual Dietary Assessment
A semiquantitative food frequency questionnaire (FFQ) was administered in person by trained interviewers at recruitment to assess habitual dietary intake of the study participants over the past year. The FFQ was developed and validated23 to use in Brazilian studies, showing a good validity and reproducibility for estimating the food consumption of adults.23
The questionnaire is composed of 67 food items. For each food item listed, participants indicated their frequency of consumption (from 0–10 times a day, week, month, or year) and the portion consumed (small, medium, large, or extra large). Questions about recent changes in eating habits, consumption of dietary supplements, and other important foods that the instrument did not cover were also included. Eating duration was calculated as the interval between the first and last meal of the day. Participants described the times of day when meals took place and the definition of each meal was self-reported by the participant.
The Nutrition Data System for Research software (version 2014, University of Minnesota, Minneapolis, Minnesota, United States) was used to quantify the energy and nutrients consumed obtained by the FFQ. Subsequently, these data were adjusted for energy intake using the residual method.24
Physical Activity Level
Physical activity (PA) level was evaluated by the short-form International Physical Activity Questionnaire, version 6, translated into Portuguese and validated by Matsudo et al.25 Participants were considered physically active if they performed at least 150 minutes of PA per week, moderately active if they performed between 10 and 149 minutes of PA, and insufficiently active at less than 10 minutes per week.
Statistical Analysis
Initially, the normality of the data was established using Kolmogorov-Smirnov tests. Data are presented as median and interquartile range. Participants were dichotomized into early and late eaters for breakfast (median: 7:30 am), lunch (median: 12:00 pm), and dinner (median: 8:00 pm) using the median values of these meals in the population as the cutoff point.8 The results of those individuals who skipped breakfast and dinner were also used and compared with individuals who ate early and those who ate late; individuals who skipped lunch were excluded for reduced sample (three individuals). In this study, we considered as those who skipped meals the participants who reported not eating a meal (breakfast, lunch, or dinner) more than 4 days a week. Participants were also dichotomized into ≤ 12 and > 12 hours regarding eating duration (interval between the first and last meal), as proposed by Gill and Panda.10 A Kruskal-Wallis test, with Games-Howell post hoc, was used to assess median differences. Qualitative variables were analyzed using chi-square test.
In order to determine the associations between evaluated variables (meal timing, eating duration, PSG and self-reported sleep and fatigue parameters), generalized linear models (GLzMM) were used. Individual tests were done for each PSG and self-reported parameters (poor sleep quality, daytime sleepiness, and fatigue) (dependent variables) and meal timing and eating duration (independent variables), using gamma, linear, or Tweedie distributions for continuous variables. The best model was chosen based on the smaller Akaike Information Criterion resultant from the analysis. For categorical variables, ordinal and binary logistic distributions were used. To establish possible factors associated with each PSG and self-reported parameter (dependent variables), independent multivariate logistic regression models were performed using backward stepwise elimination (P ≤ .15). For collinearity diagnostic between adjustment variables, Variance Inflation Factor < 10 and tolerance > 0.1 were considered. All variables used to adjust models were in table notes. Multiple comparisons were performed using sequential Sidak post hoc test when necessary. All statistical analyses were performed using SPSS 20.0 (Chicago, Illinois, United States). For statistical significance, α error was set at 5%.
RESULTS
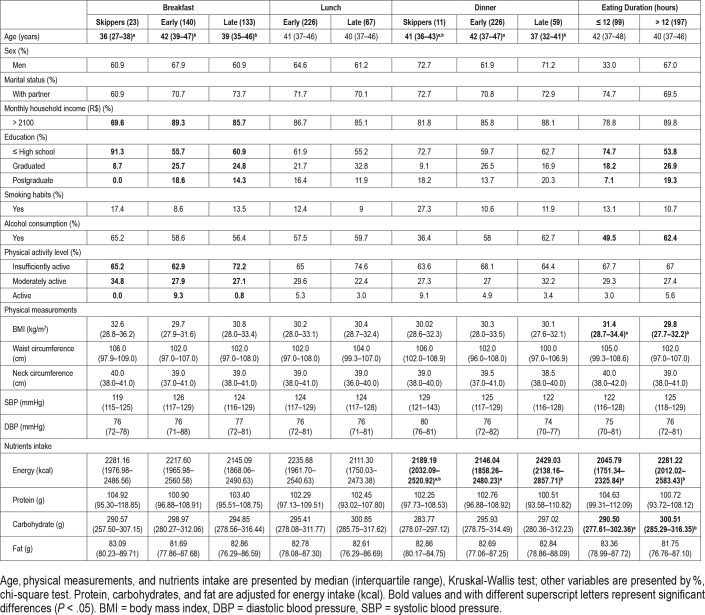
Characteristics of the population of the study based on meal timing and eating duration are shown in Table 1. Breakfast (χ = 16.773, df = 2, P < .001) and dinner (χ = 11.794, df = 2, P = .003) timing groups showed differences in age. Those who skipped breakfast were younger than those who ate early (P < .001) and late (P = .019) and those who ate dinner early were older than those who ate dinner late (P = .002).
Table 1.
Participants' characteristics according to food patterns.
Most participants had a household income at the higher level and education at the lower level. Breakfast timing was associated with household income (χ2 = 6.476, df = 2, P = .039) and education level (χ2 = 11.184, df = 4, P = .025), whereas eating duration was associated with education level (χ2 = 13.311 df = 2, P = .001) and alcohol consumption (χ2 = 4.533 df = 1, P = .033) (Table 1).
Individuals with eating duration > 12 hours showed significantly lower BMI than ≤ 12 hours (χ = 4.171, df = 1, P = .041). Most participants were insufficiently active and physical activity level was associated with breakfast timing (χ2 = 12.998, df = 4, P = .011) (Table 1). No differences in blood pressure were observed between the groups, with only 27% of participants using antihypertensive medication (data not shown).
Dinner timing groups showed differences in energy intake (χ = 10.114, df = 2, P = .006), with individuals who ate late showing significantly more energy intake than those who ate early (P = .003). Individuals with eating duration > 12 hours had higher energy (χ = 8.118, df = 1, P = .004) and carbohydrates intake (χ = 4.127, df = 1, P = .042) than ≤ 12 hours, which had higher protein intake (χ = 4.431, df = 1, P = .035) (Table 1).
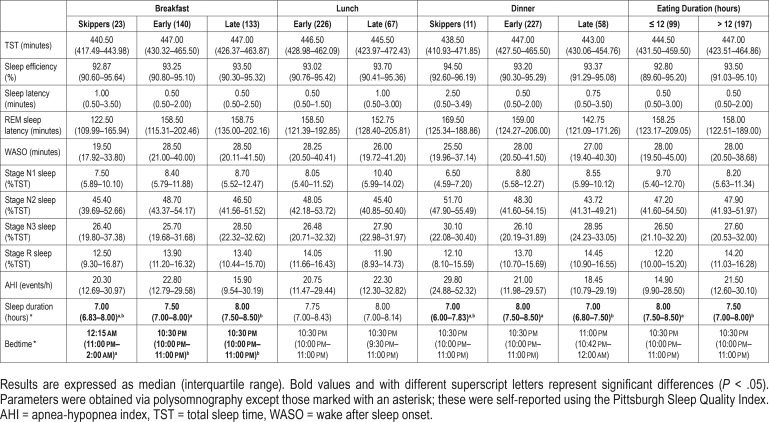
PSG and self-reported sleep parameters (sleep duration and bedtime) according to meal timing and eating duration are presented in Table 2. Self-reported sleep duration (χ = 6.346, df = 2, P = .002) and bedtime (χ = 9.657, df = 2, P < .001) were different between breakfast timing groups. The individuals who ate an early breakfast had higher self-reported sleep duration than those who ate a late breakfast (P = .002), and those who skipped breakfast slept later than those who ate early and late (P = .036 for both). Self-reported sleep duration was also different between dinner timing (χ = 10.250, df = 2, P < .001) and eating duration (χ = 4.410, df = 1, P = .036) groups. As for these data, those who ate dinner early and eating duration ≤ 12 hours presented higher self-reported sleep duration than in individuals who ate late (P < .001) and individuals with eating duration > 12 hours, respectively.
Table 2.
Objective and self-reported sleep parameters by meal timing.
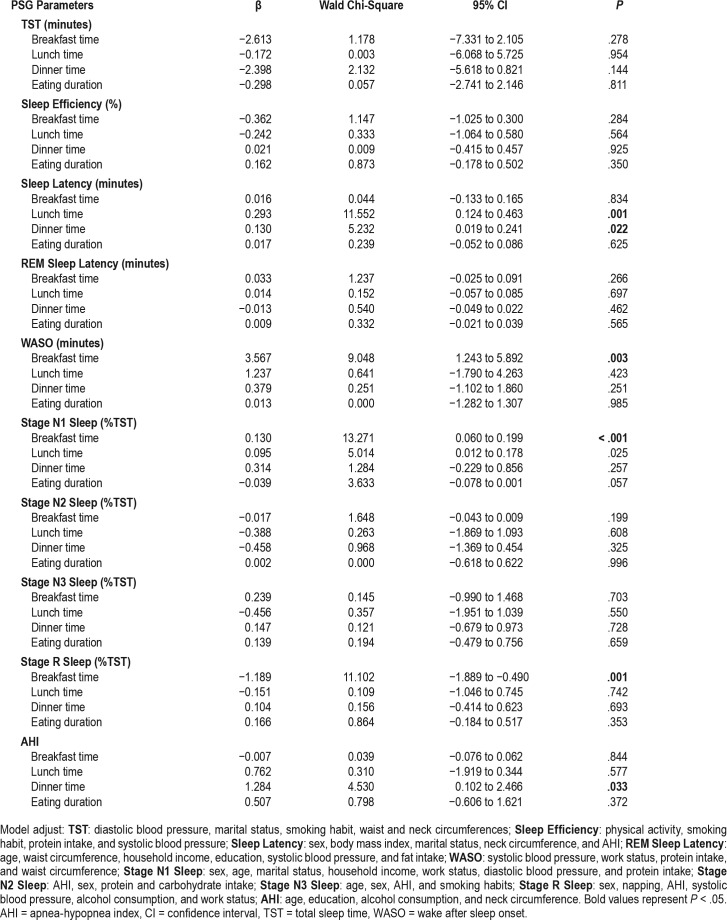
The association between meal patterns (meal timing and eating duration) and PSG parameters were analyzed and displayed in Table 3. The GLzMM showed that in patients with OSA, breakfast timing was associated with wake after sleep onset (WASO) even after being adjusted for different variables (Table 3 note) (β = 3.567, CI = 1.243–5.892, P = .003). Mul -tiple comparisons showed that individuals who ate breakfast late had higher values of WASO (mean = 39.77, standard error [SE] = 2.22) than those who eat early (mean 33.21, SE = 2.02) (P = .024) and breakfast skippers (mean = 28.61, SE = 3.76) (P = .021). Moreover, breakfast timing was also associated with stage N1 sleep (β = 0.130, confidence interval [CI] = 0.060– 0.199, P < .001) and stage R sleep (β = −1.189, CI = −1.889 to −0.490, P = .001) stages. Individuals who eat an early breakfast (mean = 8.80, SE = 0.91) presented reduced time in stage N1 sleep in comparison with those who eat late (mean = 10.78, SE = 1.01) (P = .038). For stage R sleep, no differences were found between groups in the multiple comparisons test.
Table 3.
Effects of meal timing and eating duration on PSG parameters.
The timing of lunch intake was associated with sleep latency (β = 0.293, CI = 0.124–0.463, P = .001) and stage N1 sleep (β = 0.095, CI = 0.012–0.178, P = .025). Regarding sleep latency, those who ate lunch early had lower latency (mean = 2.18, SE = 0.52) than those who ate lunch late (mean = 3.14, SE = 0.52) (P = .023). The same occurred for stage N1 sleep (mean early = 8.85, SE = 0.89; mean late = 11.33, SE = 1.38; P = .021) (Table 3).
Dinner timing was associated with sleep latency (β = 0.130, CI = 0.019–0.241, P = .022) and AHI (β = 1.284, CI = 0.102– 2.466, P = .033). In this case, those who ate dinner early showed lower sleep latency (mean = 2.08, SD = 0.42) than those who ate dinner late (mean = 3.30, SD = 0.59) (P = .047). For AHI, those who ate dinner early had lower AHI (mean = 21.41, SE = 1.26) than those who skipped dinner (mean = 34.38, SE = 5.08) (P = .036) and those who ate late (mean = 26.89, SE = 2.20) (P = .047) (Table 3).
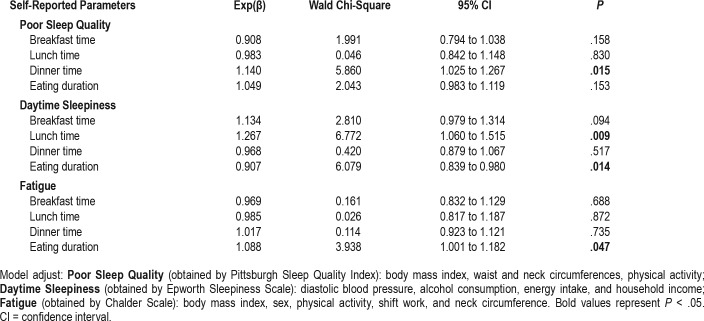
Table 4 presents the association between meal patterns (meal timing and eating duration) and self-reported sleep and fatigue parameters. The timing of dinner intake was positively associated with poor sleep quality (Exp(β) = 1.140, CI = 1.025–1.267, P = .015). Lunch timing (Exp(β) = 1.267, CI = 1.060–1.515, P = .009) and eating duration (Exp(β) = 0.907, CI = 0.839–0.980, P = .014) were positively and negatively associated, respectively, with daytime sleepiness; with respect to fatigue, only eating duration showed a positive association (Exp(β) = 1.088, CI = 1.001–1.182, P = .047). No significant associations were found for other parameters.
Table 4.
Effects of meal timing and eating duration on self-reported sleep and fatigue parameters.
DISCUSSION
Although some recent studies have shown associations between meal timing8,9 and eating duration10 with sleep duration and quality, few studies evaluated these associations through objective sleep measures and focused on sleep apnea patients. In the current study, the GLzMM analysis showed that habitual late meal intake was associated with worse sleep parameters, even after adjustments for confounders. We found that eating late was positively associated with poor sleep quality and daytime sleepiness, and individuals who ate late had longer WASO, time in stage N1 sleep, and sleep latency, and higher AHI than those who ate early. Even with some of these results being limited in terms of clinical significance, it can be suggested that the timing of meal intake itself could affect sleep and apnea severity of these individuals. Individuals with sleep apnea already have sleep disruptions characteristic of OSA, which could result in worse sleep pattern (alteration in sleep duration, efficiency, latency, sleep stages, and WASO).26 Previous studies conducted in volunteers without OSA suggested that sleep disruptions could be aggravated by the timing27 and nutrient intake11 of meals, mainly evening meals, which may result in worse sleep quality and daytime sleepiness, as shown in the current study (Table 3 and Table 4). Furthermore, those who ate dinner late showed a modest higher AHI than those who ate early (Table 3), even though no differences were found in BMI, WC, and NC between individuals who ate late and those who ate early (Table 1), which would be expected as evening meals are associated with weight gain8,9 and with OSA severity.4 These results lead us to believe that not only obesity but also the habit of eating late— in particular the evening meal—could be associated with OSA severity. Studies in humans12 and animals28 found that a high-fat meal before bedtime increased the number of apneas, suggesting that both meal timing and nutrient intake could affect AHI. Another potential explanatory mechanism would be the variation in leptin levels, which could be increased after meals, and could also induce apnea and affect AHI to enhance hyper-capnic sensitivy.26 However, this theme is controversial in the literature.29
A higher food intake preceding the sleeping period is associated with negative aspects in sleep patterns, such as impairment of the consolidation of sleep onset and an increase in the number of awakenings.11 In the current study, individuals who eat close to bedtime had a modest higher sleep latency (Table 3). Considering that sleep apnea volunteers who had late breakfast or lunch could also be the individuals who had dinner close to bedtime—as preceding meal timing and resulting satiety largely determine the size and time of the following meal30—the higher values of WASO, sleep latency, and stage N1 sleep of late breakfast and lunch eaters (Table 3) may also be explained by the habit of eating late.
In the current study, those who ate a late breakfast and lunch showed longer time in stage N1 sleep in relation to those who ate early. This outcome, together with reductions in stage N2 sleep, stage R sleep, and slow wave sleep are common in individuals with obesity and apnea,26 and it represents a common sign of severely disrupted sleep31 and poor sleep quality.32 Although those who ate an early and late breakfast and lunch were above the recommended time (2% to 5% of total sleep time [TST]) in stage N1 sleep (early breakfast = 8.80, late breakfast = 10.78, early lunch = 8.85, late lunch = 11.33), our results lead us to believe that meal timing may contribute even more to the increase of stage N1 sleep in late eaters, aggravating sleep quality. Ghrelin levels could also affect the time in stage N1 sleep, because this hormone—which is higher in individuals with sleep apnea33—could be enhanced after meals and increase non-rapid eye movement sleep and slow wave sleep.34,35 This hormone may also have influenced the negative association between breakfast timing and stage R sleep found in our study (Table 3), because individuals who are late breakfast eaters could also have been the individuals who had dinner late. Thus, the increase in ghrelin34,35 and other hormones related to eating in the evening—such as cortisol and insulin36,37 could decrease stage R sleep.38,39 Nevertheless, it is unclear whether physiological changes in hormone level from intake of a meal are enough to affect sleep architecture.40
The time in stage R sleep may also be affected by nocturnal body temperature, which could be increased after meal intake39 decreasing the stage R sleep.41 Because sleep onset is triggered by the reduction in body temperature,42 thermogenic events, such as evening meals, may improve alertness at bedtime and delay sleep onset.39,43 rather than to an altered energy intake.39 In addition, Roky et al.39 found that these changes in temperature rhythm may be accompanied by alterations in the circadian pattern of melatonin secretion,44 which could also interfere with sleep onset. Thus, it is possible that late meal timing observed in this study may have caused an increase in body temperature and altered melatonin secretion resulting in worse results of individuals who are late eaters regarding self-reported sleep quality, as well as WASO, stage N1 sleep, and even sleep latency that showed a clinically minor, but significant, difference between the meal timing groups (Table 3 and Table 4). Additional studies are necessary to evaluate the relationship between late meal intake, body temperature, and sleep pattern in individuals with sleep apnea to validate this association and possible mechanisms involved.
Studies have shown that the absence or presence of food at night changes the type of nutrient that will be oxidized, which could interfere with sleep stages.45 In this way, studies with animals46 and humans40,47 have described that not eating close to bedtime could help fat oxidation and, because of this, increase slow wave sleep. In contrast, meals close to this time favor the oxidation of other nutrients such as carbohydrates.40 However, the mechanism behind this relationship is not well described.40 This information may support the results found in our study whereby individual who were late eaters showed worse sleep patterns, such as higher sleep latency (Table 3).
Several studies have reported that reductions in sleep time and quality could contribute to an increase in obesity.7,48 This relationship may be partly explained by the reduced duration of overnight fasting after short sleep duration increases the wakefulness period and, consequently, eating duration that contributes to increased caloric intake.7 Time-restricted eating (without changes in caloric intake and physical activity) may improve sleep quality and prevent weight gain, benefits that appear to be mediated by circadian rhythms.10 Gill and Panda10 found that volunteers who were overweight with an eating period of more than 14 hours decreased their body weight and improved sleep after a reduction in eating period to 10 to 11 hours per day for 16 weeks. In the current study, we found that apnea volunteers with an eating period greater than 12 hours showed higher energy and carbohydrate intake (Table 1), as showed in another study10; however, no relationship between eating duration and sleep parameters (Table 3) was found.
Usually, there is no reduction in sleep time in individuals with OSA26; however, we expected that individuals with greater eating duration, as found among individuals who are later eaters, would have a shorter sleep and worse sleep pattern than those with a lower eating duration (Table 3). One of the hypotheses for this result is that a late night snack, which was included in the eating period as the last meal, may have contributed to increasing the eating period without affecting sleep, because it usually consists of a smaller food portion and, probably, lower calorie content, not interfering in metabolic factors that could affect sleep in the way that dinner does.
In the current study, eating duration was negatively associated with daytime sleepiness. This result can explain, at least in part, the fact that individuals with higher eating duration presented with higher intake of carbohydrates (Table 1). Some study results49,50 have suggested that the amount of carbohydrates and the glycemic index may have an important influence on sleep patterns, especially on inducing sleepiness and decreasing sleep latency. However, more studies are necessary to confirm the causality between carbohydrate intake and sleepiness.
This study has some limitations. As individuals with sleep apnea volunteered for this study there is a potential for selection bias in that the dietary intakes of these participants may not be completely representative. Another point is that the nutrient results depend on the respondents' memory and could be affected by measurement error in dietary intake, a common limitation of epidemiological studies. However, the characteristics of the participants are typical of these populations and the food questionnaire used was previously validated. Because our objective was to evaluate habitual dietary intake, we assessed food intake of participants by food frequency questionnaire, which did not allow us to obtain the distribution of energy for each meal and its dietary composition for adjustment of the statistical analysis. However, we have considered macronutrient intake throughout the day for confounders. Although this study design can detect associations, a limitation of the study is its cross-sectional design, which does not allow determination of cause and effect between the meal timing and eating duration patterns and sleep parameters. Also, although a high power of test was used in the sample size calculation and our sample being higher than calculated (n = 296), we had a moderate post hoc statistical power (72%). Even so, we found significant associations between meal timing and sleep that cannot be invalidated. This statistical power could limit the feasibility to detect other significant results. Therefore, we emphasize the need for future studies with large sample sizes and higher power of the test in order to explore more associations between meal timing and sleep parameters and consequently, results with greater clinical significance. We also highlight that during PSG, the first night in the laboratory could affect the duration of some sleep stages; nevertheless, it is a common limitation in studies that use PSG and some of them reported no significant effect on the results. In addition, we also used self-reported parameters to compare to the habitual sleep pattern of these individuals, such as bedtime and sleep duration by PSQI.
Together our findings showed that individuals with sleep apnea who have late meals and higher eating duration presented worse sleep pattern and apnea severity in addition to higher risk of fatigue and poor sleep quality than individuals who are earlier eaters and individuals with lower eating duration. Therefore, the current study supports the idea that individuals who are late eaters and individuals with higher eating duration have physiopathologic alterations that contribute to different disturbances, including sleep. Further studies in this area are required to provide causality in the relationship between meal timing and eating duration with sleep, especially regarding sleep architecture, and possible mechanisms involved. However, these results may imply that methods to improve dietary patterns and metabolic health should be encouraged for patients with obesity and OSA to reduce AHI and improve sleep architecture.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This work has been supported in part by Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG). The authors report no conflicts of interest.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- BMI
body mass index
- EEG
electroencephalogram
- ESS
Epworth Sleepiness Scale
- FFQ
food frequency questionnaire
- GLzMM
Generalized Linear Models
- ICSD
International Classification of Sleep Disorders
- NC
neck circumference
- OSA
obstructive sleep apnea
- PA
physical activity
- PSG
polysomnography
- PSQI
Pittsburgh Sleep Quality Index
- TST
total sleep time
- WASO
wake after sleep onset
- WC
waist circumference
REFERENCES
- 1.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandes JFR, Araújo LS, Kaiser SE, Sanjuliani AF, Klein MRST. The effects of moderate energy restriction on apnoea severity and CVD risk factors in obese patients with obstructive sleep apnoea. Br J Nutr. 2015;114(12):2022–2031. doi: 10.1017/S0007114515004018. [DOI] [PubMed] [Google Scholar]
- 5.Gilardini L, Lombardi C, Redaelli G, et al. Glucose tolerance and weight loss in obese women with obstructive sleep apnea. PLOS One. 2013;8(4):e61382. doi: 10.1371/journal.pone.0061382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27(4):661–666. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 7.Reid KJ, Baron KG, Zee PC. Meal timing influences daily caloric intake in healthy adults. Nutr Res. 2014;34(11):930–935. doi: 10.1016/j.nutres.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee YC, Ordovás JM, Scheer FAJL. Timing of food intake predicts weight loss effectiveness. Int J Obes. 2013;37(4):604–611. doi: 10.1038/ijo.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bandín C, Scheer FAJL, Luque AJ, et al. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: a randomized, crossover trial. Int J Obes. 2015;39(5):828–833. doi: 10.1038/ijo.2014.182. [DOI] [PubMed] [Google Scholar]
- 10.Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22(5):789–798. doi: 10.1016/j.cmet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crispim CA, Zimberg IZ, dos Reis BG, Diniz RM, Tufik S, de Mello MT. Relationship between food intake and sleep pattern in healthy individuals. J Clin Sleep Med. 2011;7(6):659–664. doi: 10.5664/jcsm.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trakada G, Steiropoulos P, Zarogoulidis P, et al. A fatty meal aggravates apnea and increases sleep in patients with obstructive sleep apnea. Sleep Breath. 2014;18(1):53–58. doi: 10.1007/s11325-013-0847-y. [DOI] [PubMed] [Google Scholar]
- 13.Cao Y, Wittert G, Taylor AW, Adams R, Shi Z. Associations between macronutrient intake and obstructive sleep apnoea as well as self-reported sleep symptoms: results from a cohort of community dwelling Australian men. Nutrients. 2016;8(4):207. doi: 10.3390/nu8040207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Obesity: Preventing and managing the global epidemic. Geneva, Switzerland: WHO; 2000. Report of a WHO consultation on obesity. WHO Technical Report Series no. 894. [PubMed] [Google Scholar]
- 15.Whitworth JA World Health Organization, International Society of Hypertension Writing Group. World Health Organization (WHO)/ International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21(11):1983–1992. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 17.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 18.Chalder T, Berflowitz G, Pawlikowaska T, et al. Development of fatigue scale. J Psychosom Res. 1993;37(2):147–153. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 19.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 20.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 21.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441–446. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 23.Selem SSC, Carvalho AM, Verly-Junior E, et al. Validade e reprodutibilidade de um questionário de frequência alimentar para adultos de São Paulo, Brasil. Rev Bras Epidemiol. 2014;17(4):852–859. doi: 10.1590/1809-4503201400040005. [DOI] [PubMed] [Google Scholar]
- 24.Willett WC, Howe GR, Kushi LW. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4):1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 25.Matsudo S, Araújo T, Matsudo V, et al. International Physical Activity Questionnaire (IPAQ): study of validity and reliability in Brazil. Brazilian J Phys Activity Health. 2001;6:5–18. [Google Scholar]
- 26.Vgontzas AN, Tan TL, Bixler EO, Martin LF, Shubert D, Kales A. Sleep apnea and sleep disruption in obese patients. Arch Intern Med. 1994;154(15):1705–1711. [PubMed] [Google Scholar]
- 27.Dollander M. Etiology of adult insomnia. Encephale. 2002;28(6 Pt 1):493–502. [PubMed] [Google Scholar]
- 28.Ramadan W, Dewasmes G, Petitjean M, et al. Sleep apnea is induced by a high-fat diet and reversed and prevented by metformin in non-obese rats. Obesity (Silver Spring) 2007;15(6):1409–1418. doi: 10.1038/oby.2007.169. [DOI] [PubMed] [Google Scholar]
- 29.Imayama I, Prasad B. Role of leptin in obstructive sleep apnea. Ann Am Thorac Soc. 2017;14(11):1607–1621. doi: 10.1513/AnnalsATS.201702-181FR. [DOI] [PubMed] [Google Scholar]
- 30.Garaulet M, Gómez-Abellán P. Timing of food intake and obesity: a novel association. Physiol Behav. 2014;134:44–50. doi: 10.1016/j.physbeh.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Carskadon MA, Dement WC. Monitoring and staging human sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th ed. St. Louis, MO: Elsevier Saunders; 2011. pp. 16–26. [Google Scholar]
- 32.Redline S, Sotres-Alvarez D, Loredo J, et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med. 2014;189(3):335–344. doi: 10.1164/rccm.201309-1735OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chihara Y, Akamizu T, Azuma M, et al. Among metabolic factors, significance of fasting and postprandial increases in acyl and desacyl ghrelin and the acyl/desacyl ratio in obstructive sleep apnea before and after treatment. J Clin Sleep Med. 2015;11(8):895–905. doi: 10.5664/jcsm.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weikel JC, Wichniak A, Ising M, et al. Ghrelin promotes slow-wave sleep in humans. Am J Physiol Endocrinol Metab. 2003;284(2):E407–415. doi: 10.1152/ajpendo.00184.2002. [DOI] [PubMed] [Google Scholar]
- 35.Kluge M, Schussler P, Bleninger P, et al. Ghrelin alone or co-administered with GHRH or CRH increases non-REM sleep and decreases REM sleep in young males. Psychoneuroendocrinology. 2008;33(4):497–506. doi: 10.1016/j.psyneuen.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Hansen K, Sickelmann F, Pietrowsky R, Fehm HL, Born J. Systemic immune changes following meal intake in humans. Am J Physiol. 1997;273(2Pt2):R548–R553. doi: 10.1152/ajpregu.1997.273.2.R548. [DOI] [PubMed] [Google Scholar]
- 37.Iraki L, Bogdan A, Hakkou F, Amrani N, Abkari A, Touitou Y. Ramadan diet restriction modify the circadian time structure in humans. Study on plasma gastrin, insulin, glucose and calcium and on gastric pH. J Clin Endocrinol Metab. 1997;82(4):1261–1273. doi: 10.1210/jcem.82.4.3860. [DOI] [PubMed] [Google Scholar]
- 38.Buguet A, Cespuglio R, Radomski MW. Sleep and stress in man: an approach through exercise and exposure to extreme environments. Can J Physiol Pharmacol. 1998;76(5):553–561. doi: 10.1139/cjpp-76-5-553. [DOI] [PubMed] [Google Scholar]
- 39.Roky R, Chapotot F, Hakkou F, Benchekroun MT, Buguet A. Sleep during Ramadan intermittent fasting. J Sleep Res. 2001;10(4):319–327. doi: 10.1046/j.1365-2869.2001.00269.x. [DOI] [PubMed] [Google Scholar]
- 40.Yajima K, Seya T, Iwayama K, et al. Effects of nutrient composition of dinner on sleep architecture and energy metabolism during sleep. J Nutr Sci Vitaminol. 2014;60(2):114–121. doi: 10.3177/jnsv.60.114. [DOI] [PubMed] [Google Scholar]
- 41.Krueger MJ, Takahashi S. Thermoregulation and sleep. Closely linked but separable. Ann N Y Acad Sci. 1997;813:281–286. doi: 10.1111/j.1749-6632.1997.tb51706.x. [DOI] [PubMed] [Google Scholar]
- 42.Murphy PJ, Campbell SS. Night-time drop in body temperature: a physiological trigger for sleep onset? Sleep. 1997;20(7):505–511. doi: 10.1093/sleep/20.7.505. [DOI] [PubMed] [Google Scholar]
- 43.Smith A, Maben A, Brockman P. Effects of evening meals and caffeine on cognitive performance, mood and cardiovascular functioning. Appetite. 1994;22(1):57–65. doi: 10.1006/appe.1994.1005. [DOI] [PubMed] [Google Scholar]
- 44.Qasrawi SO, Pandi-Perumal SR, BaHammam AS. The effect of intermittent fasting during Ramadan on sleep, sleepiness, cognitive function, and circadian rhythm. Sleep Breath. 2017;21(3):577–586. doi: 10.1007/s11325-017-1473-x. [DOI] [PubMed] [Google Scholar]
- 45.Katayose Y, Tasaki M, Ogata H, Nakata Y, Tokuyama K, Satoh M. Metabolic rate and fuel utilization during sleep assessed whole-body indirect calorimetry. Metabolism. 2009;58(7):920–926. doi: 10.1016/j.metabol.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 46.Dewasmes G, Cohen-Adad F, Koubi H, Le Maho Y. Sleep changes in long-term fasting geese in relation to lipid and protein metabolism. Am J Physiol. 1984;247(4 Pt 2):663–671. doi: 10.1152/ajpregu.1984.247.4.R663. [DOI] [PubMed] [Google Scholar]
- 47.MacFadyen UM, Oswald I, Lewis SA. Starvation and human slow-wave sleep. J Appl Physiol. 1973;35(3):391–394. doi: 10.1152/jappl.1973.35.3.391. [DOI] [PubMed] [Google Scholar]
- 48.Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27(4):661–666. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 49.Driver HS, Shulman I, Baker FC, Buffenstein R. Energy content of the evening meal alters nocturnal body temperature but not sleep. Physiol Behav. 1999;68(1–2):17–23. doi: 10.1016/s0031-9384(99)00145-6. [DOI] [PubMed] [Google Scholar]
- 50.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]