Abstract
Study Objectives:
To (1) describe outcomes from a computer decision support system (CDSS) for pediatric obstructive sleep apnea (OSA) detection in primary care; and (2) identity the prevalence of children meeting criteria for an OSA referral.
Methods:
A CDSS for OSA was implemented in two urban primary care clinics. Parents of children (age 2 to 11 years) presenting to the clinic were asked if their child snored regularly, with a positive response resulting in six additional OSA screening items. Primary care providers (PCPs) received a prompt for all snoring children, listing applicable OSA signs and symptoms and recommending further evaluation and referral for OSA.
Results:
A total of 2,535 children were screened for snoring, identifying 475 snoring children (18.7%). Among snoring children, PCPs referred 40 (15.4%) for further evaluation. The prevalence of additional OSA signs and symptoms ranged from 3.5% for underweight to 43.7% for overweight. A total of 74.7% of snoring children had at least one additional sign or symptom and thus met American Academy of Pediatrics guidelines criteria for an OSA referral.
Conclusions:
A CDSS can be used to support PCPs in identifying children at risk for OSA. Most snoring children met criteria for further evaluation. It will be important to further evaluate this referral threshold as well as the readiness of the sleep medicine field to meet this need.
Clinical Trials Registration:
Registry: ClinicalTrials.gov, Title: Evidence-based Diagnosis and Management of Pediatric Obstructive Sleep Apnea in Primary Care, Identifier: NCT02781376, URL: https://clinicaltrials.gov/ct2/show/NCT02781376
Citation:
Honaker SM, Street A, Daftary AS, Downs SM. The use of computer decision support for pediatric obstructive sleep apnea detection in primary care. J Clin Sleep Med. 2019;15(3):453–462.
Keywords: computer decision support, obstructive sleep apnea, pediatrics, primary care, sleep disorders, snoring
BRIEF SUMMARY
Current Knowledge/Study Rationale: Many children with obstructive sleep apnea (OSA) do not receive timely diagnosis and treatment. This study describes the use of a computer decision support tool for universal OSA screening in two urban pediatric primary care clinics.
Study Impact: Almost 20% of children snored and three-fourths of snoring children met American Academy of Pediatrics criteria for an OSA referral. Findings raise questions about the appropriateness and feasibility of the current referral threshold.
INTRODUCTION
An estimated 2% to 5% of children have obstructive sleep apnea (OSA), a disorder in which partial or full cessation of the airway during sleep results in fragmented sleep and hypoxia.1 Negative sequelae of untreated OSA include cardiovascular morbidity,2 neurocognitive deficits,3 worse mood and behavior,3 sleepiness,4 and reduced quality of life.3 In 2012, the American Academy of Pediatrics (AAP) published updated guidelines5 on evidence-based diagnosis and management of pediatric OSA. Two key action statements pertain specifically to screening and referral. First, clinicians should ask about snoring during routine health maintenance visits, and should perform a more focused evaluation if the child snores or presents with other signs or symptoms of OSA. Second, if a child or adolescent has regular snoring (defined as 3 or more nights per week) and any additional complaints or findings (from a defined list), the clinician should either obtain a polysomno-gram (recommendation) or refer the patient to a sleep specialist or otolaryngologist for further evaluation (option). The list of additional signs and symptoms is extensive and includes the following: labored breathing during sleep; snorts; gasps; observed apnea; enuresis; sleeping in a seated position; cyanosis; headache upon awakening; daytime sleepiness; attention deficit hyperactivity disorder (ADHD); learning problems; underweight; overweight; tonsillar hypertrophy; adenoidal facies; micrognathia; retrognathia; high-arched palate; failure to thrive; and hypertension. Although the prevalence of snoring has been estimated in multiple samples,6,7 less is known about the prevalence of children who have regular snoring and at least one additional sign or symptom of OSA. We are not aware of any studies that have reported the prevalence of children meeting AAP criteria for referral.
With regular access to most children, primary care represents an important setting for OSA screening and detection. However, despite guidelines, studies suggest very low rates of screening for snoring, evaluation, and referral/management.8–10 Even in systems where screening for snoring is automated and the primary care provider (PCP) is alerted, there may be un-warranted practice variation in PCP response to a positive screen. One study found that the prevalence of a PCP reporting concern for OSA in a snoring child ranged from 0% to 63% (variability among providers) and that OSA risk factors (ie, overweight; ADHD status) did not predict which snoring children would elicit concern for OSA.11 Barriers to effective OSA detection in primary care are multifaceted, and include minimal training in sleep medicine for both physicians12,13 and nurse practitioners,14 a large number of topics to address in a brief visit, and both provider15,16 and parent17 misinformation about OSA.
Computer decision support is one tool that could be applied to support PCPs in evidence-based OSA detection by automating screening for snoring and risk factors and communicating a child's risk within the electronic health record (EHR). Computer decision support systems have been found to improve provider adherence to guidelines in multiple areas of child health, including asthma,18 and developmental surveillance,19 but have not yet been applied to OSA.
The goals of the current research are to: (1) describe the implementation and outcomes of a novel computer decision support system (CDSS) for pediatric OSA detection; and (2) report the prevalence of children meeting criteria for referral per AAP guidelines.
METHODS
CHICA System
Child Health Improvement through Computer Automation (CHICA), an innovative CDSS that has been operating in primary care clinics at our institution since 2004, is described elsewhere in detail.20–22 Briefly, CHICA is a rule-based CDSS that runs as a “bolt on” service to the EHR. CHICA's Arden Syntax rules encode a full array of preventive care guidelines as well as several disease management guidelines. When a child is registered for an appointment, the EHR sends a standard HL7 ADT (registration) message containing the child's record to CHICA. CHICA applies hundreds of rules to the data to select 20 tailored yes/no questions that are displayed on an electronic tablet given to the parents by the registration clerk. The questions are answered by the family in the waiting room and sent back to CHICA. Some questions can trigger additional surveys. CHICA applies additional rules to the EHR data and the data from the family to select six prioritized alerts to show the PCP. The PCP accesses CHICA from within the EHR by pressing a “CHICA” button, opening a tab within the EHR that displays six alerts. Each alert includes up to six check boxes with which the PCP can indicate his or her response to the prompt. The PCP can then insert the prose into his or her note. CHICA content areas are organized by modules, each of which targets a particular area of pediatric health with specific decision support rules.
CHICA OSA Module
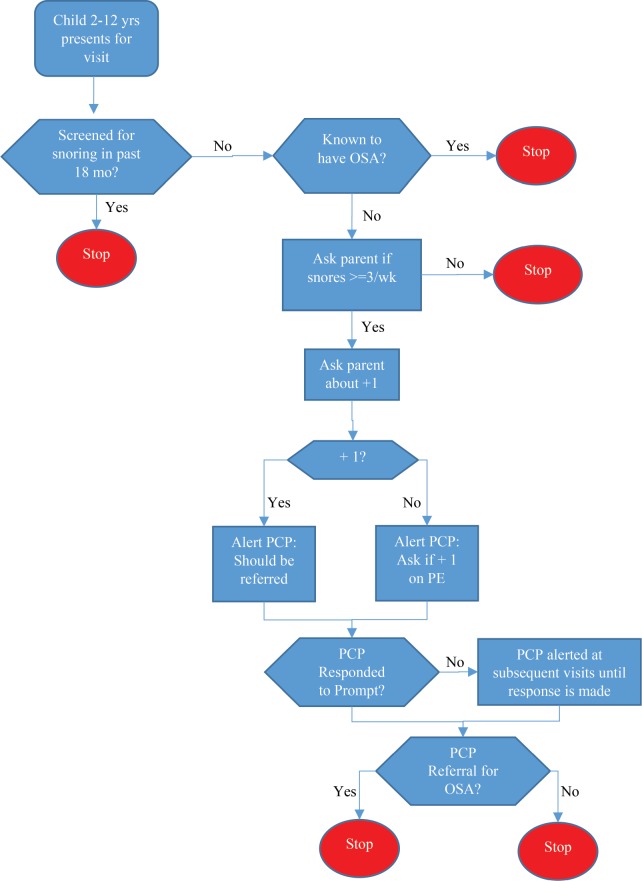
In February 2017, the CHICA OSA module (Figure 1) was incorporated into the existing CHICA system. Caregivers received the following item: “Does [CHILD NAME] consistently snore three or more nights per week?” The frequency of 3 nights per week was selected based on AAP guidelines.5 The item was presented in English or Spanish, depending on the family's preferred language. An affirmative response automatically triggered six additional items on the tablet assessing OSA symptoms, specifically labored breathing, enuresis, waking with a snort, apnea, morning headache, and daytime sleepiness. Items were from the Pediatric Sleep Questionnaire23 and were selected because they corresponded directly to symptoms and signs of OSA listed in the AAP guidelines. The enuresis item was included in the screening only for children ages 5 years and older. Additional risk factors (underweight, overweight, ADHD) were identified from existing data in the CHICA database (eg, body mass index [BMI]) from the target visit. The PCP then received one of two EHR prompts (Figure 2), listing applicable symptoms applying to that child (Figure 2A) or recommending that the PCP conduct a physical examination to identify potential signs (Figure 2B). PCPs could endorse any combination of the following responses: suspect OSA; do not suspect OSA; known to have OSA; refer for sleep study; refer to an ear nose and throat specialist (ENT); refer for a sleep medicine consult. PCPs could also print handouts for patients on OSA and/ or on the polysomnogram. Checking the polysomnography (PSG) referral box generates a prepopulated referral form for the PCP to sign.
Figure 1. CHICA OSA detection algorithm.
In CHICA OSA, children are screened for snoring every 18 months. Snoring children receive 6 additional OSA screening items, and the CHICA system assesses whether other signs (ie, overweight; ADHD) are known. The PCP then receives a prompt at that visit indicating that the child snores and listing applicable signs and patient-reported symptoms. If the child does not have any additional signs or symptoms, the PCP receives a prompt indicating that the child snores and asking the PCP to do a physical examination to assess for OSA signs such as tonsillar hypertrophy. If the PCP does not respond to a prompt, the prompt presents at the next visit. Once the PCP responds to a prompt, the detection module stops, though the child is screened again for snoring after 18 months. ADHD = attention deficit hyperactivity disorder, CHICA = Child Health Improvement through Computer Automation, OSA = obstructive sleep apnea, PCP = primary care physician, PE = physical examination
Figure 2. Sample provider alerts.
(A) PCPs receive this prompt for snoring children who have one or more additional OSA symptoms. Symptoms that apply to that child are listed in the prompt. (B) Providers receive this prompt for snoring children who do not have additional identified OSA symptoms. AAP = American Academy of Pediatrics, ENT = ear, nose and throat specialist, OSA = obstructive sleep apnea, PCP = primary care physician, PSG = polysomnography.
Some signs and symptoms were not assessed using the CHICA OSA module, specifically sleeping in a seated position, cyanosis, learning problems, and hypertension, either because they were not available within our computer decision support system (hypertension; learning problems) or because there was not an item from the Pediatric Sleep Questionnaire that specifically matched that symptom (cyanosis; sleeping in a seated position). As noted previously, the PCP was prompted to assess for physical examination findings (eg, tonsillar hypertrophy) that were considered signs/symptoms for OSA per guidelines. However, physical examination findings (aside from under-weight/overweight) are not reported here as their prevalence was not available within the CHICA database.
Setting and Participants
This study was conducted between February 2016 and April 2017, and is part of a larger ongoing trial comparing intervention clinics (those utilizing CHICA OSA module) to control clinics. All children ages 2 to 11.9 years presenting for a sick- or well-child visit were eligible. The CHICA OSA module was incorporated into two clinic sites in the Eske-nazi Health System in Indianapolis, Indiana, both of which used the EPIC EHR. The Institutional Review Board of the Indiana University School of Medicine approved this study and waived informed consent, as the module was conducted as part of routine clinical practice. The primary investigator met with each PCP on at least one occasion to review the AAP guidelines as well as the CHICA OSA module content, and provided each PCP with a written summary of the guidelines and module content, as well as a copy of the guidelines manuscript.
Data Collection and Analysis
We extracted data from the CHICA system for eligible children whose caregivers were presented with the snoring screening item during the study time frame. Data were analyzed using SAS software, version 9.4 (SAS Institute, Inc., Cary, North Carolina, United States). We calculated the frequency at which caregivers endorsed snoring and additional OSA symptoms. Additional child and family demographic variables and OSA signs were extracted from the CHICA database. We further calculated rates of PCP response to the associated prompt, and the frequency of specific PCP responses.
As an exploratory analysis, we conducted Pearson chi-square tests to identify potential predictors of OSA referral. This analysis included all snoring children for whom the PCP received and responded to the prompt. The primary outcome was receipt of a referral for OSA (referral versus no referral). All referral types (ie, PSG, ENT, sleep consult) were combined into one variable, as we were not sufficiently powered to analyze these outcomes separately.
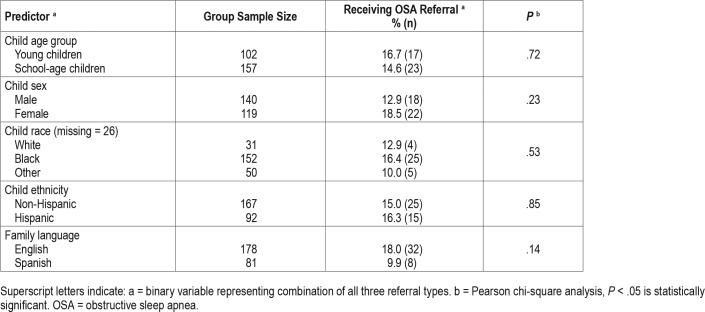
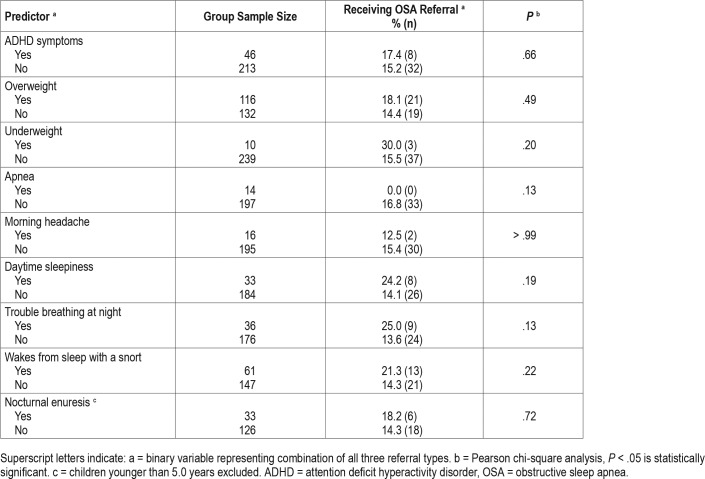
The following demographic variables were analyzed: age group (young children 2 to 5.9 years, school-aged children 6 to 11.9 years); sex (male, female); race (black, white, other); ethnicity (non-Hispanic, Hispanic); and preferred language (Spanish, English). In addition, nine OSA signs or symptoms were analyzed. Six symptoms were assessed via single parent-report items (apnea, morning headache, waking with a snort, daytime sleepiness, trouble breathing at night, enuresis) and were considered either present (parent responds “yes”) or absent (parent responded “no” or “I don't know”). Underweight and over-weight status were derived based on the child's BMI on the date of the visit (underweight = BMI < 5; overweight = BMI ≥ 85). The presence of ADHD symptoms was defined as prior initiation of ADHD evaluation documented in CHICA, and either a “yes” outcome for an ADHD diagnosis or no listed outcome (children with a “no” outcome were not included in this category). Because the outcome of the ADHD evaluation was often missing, ADHD diagnosis could not be used as a variable. Finally, we examined a provider characteristic, number of years since training (above and below the median). For this factor, we excluded three providers who practiced in the clinics but did not respond to any OSA prompts.
RESULTS
Child and Family Characteristics
Participating children whose caregiver responded to a snoring item (n = 475) were 54.9% male and between the ages of 2.2 and 11.9 years with a mean age of 6.8 (SD = 2.8) years. Young children (2.2–5.9 years) comprised 41.9% of the sample whereas school-aged children (6.0–11.9 years) comprised 58.1%. Child race was reported as follows: 66.4% Black, 11.3% White, 16.5% Other/unknown, 4% multiracial, and 1.7% Asian or Pacific Islander. Of note, most of the children in the other/ unknown category identified Hispanic ethnicity (95.7%) and were most likely white Hispanic, based on the overall demographics of our local population in Indianapolis. For ethnicity, 33.3% identified as Hispanic or Latino. Spanish was the preferred language for 30.3% of families, with 93% of these children being seen by a bilingual PCP for the visit at which the screening occurred. Insurance type was as follows: 86.5% Medicaid; 7.4% Medicare, 5.1% commercial insurance, and 0.9% other.
Provider Characteristics
Providers in the two clinics (n = 16) were primarily physicians who completed pediatric residency (43.7%), but also included physicians trained in family medicine (12.5%), physicians who completed both internal medicine and pediatrics residencies (31.2%), and nurse practitioners (12.5%). A total of 31% of providers were female, with a mean of 15.1 years (range 1 to 44 years) since completion of residency training. For 11 responses, no provider was recorded in the CHICA system.
Patient Flow
Patient flow is summarized in Figure 3. Across the two clinic sites, caregivers of 2,626 eligible children were presented with a snoring item. A total of 2,535 caregivers responded, for an overall response rate of 96.5%. Of those who responded, 475 caregivers (18.7%) reported that their child snored 3 or more days per week. These 475 children were included in the analyses of the prevalence of OSA signs and symptoms. Although parents of all children identified as snoring were presented with the six additional items asking about OSA signs and symptoms, these items were not completed for some children, likely due to time (ie, additional items are presented only after the original twenty yes/no screening items are completed). The six screening items were completed for 403 children (86.7%), partially completed (ie, at least one item completed) for 6 children (1.3%), and not completed for 66 children (13.9%). All of the 475 children were included in the descriptive analysis of OSA signs and symptoms, however, as data were available on at least one risk factor (eg, BMI; ADHD status) for all children.
Figure 3. Study flow diagram.
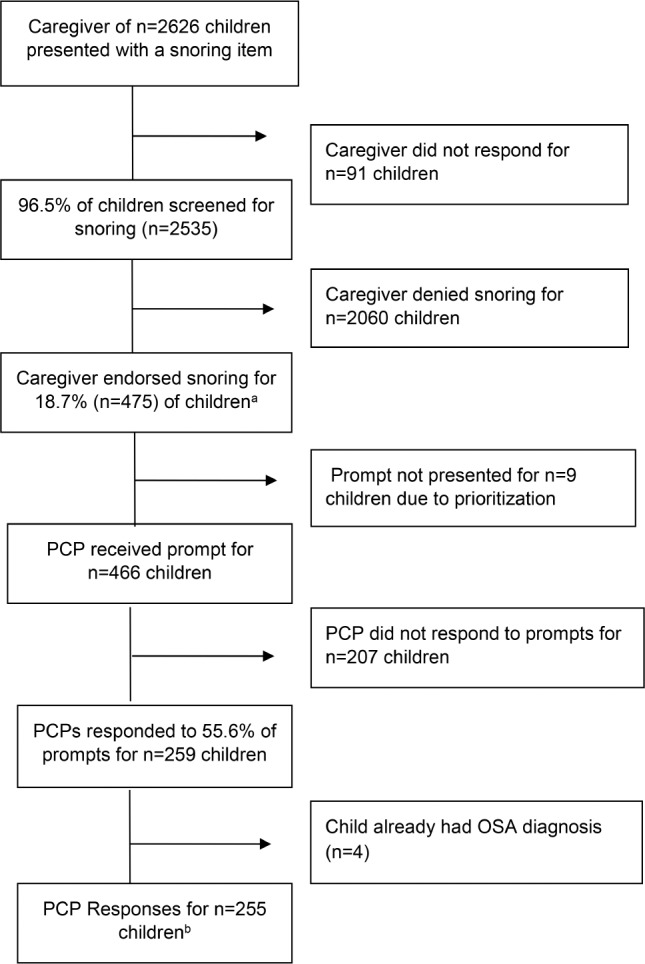
Superscript letters indicate: a = sample for analyses of prevalence of OSA signs and symptoms, b = sample for analyses of PCP response to prompt. OSA = obstructive sleep apnea, PCP = primary care provider.
During the study timeframe, no PCP prompt was generated for nine snoring children because other health issues were prioritized for those children via the CHICA prioritization scheme.20 Sixteen different PCPs received a total of 466 automated prompts in response to a positive screen for snoring. Of these, PCPs responded to prompts for 259 children on that visit date, a 55.6% response rate. Because unanswered prompts present again at the child's next visit, the overall response rate tends to increase gradually over time.
PCP Response to the Prompt
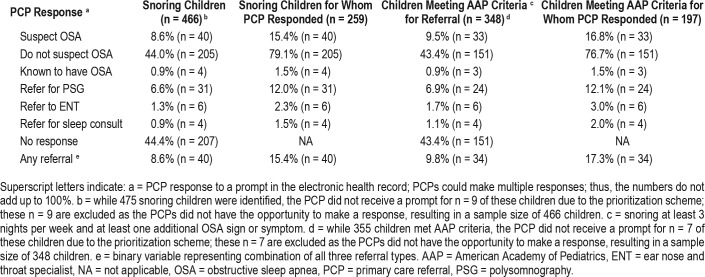
PCP responses to the prompt are reported in Table 1. For those children for whom PCPs responded to the prompt (n = 259), PCPs were most likely to endorse not suspecting OSA (n = 205; 79.1%), with PSG representing the most frequent type of referral (n = 31; 12.0%) compared to ENT (n = 6; 2.3%) or sleep medicine consult (n = 4; 1.5%). Responses patterns were similar when considering only those children known to meet AAP criteria for referral (ie, snoring and at least one additional sign/ symptom), with PCPs endorsing not suspecting OSA (n = 151; 76.7%) most often. Examining referral as a binary variable (ie, whether or not a child received any of the three referrals), when PCPs responded to the prompt they referred 15.4% (n = 40) of snoring children and 17.3% (n = 34) of children with snoring and another sign/symptom (ie, meeting AAP criteria for referral).
Table 1.
Primary care provider response to OSA prompt.
OSA Signs and Symptoms
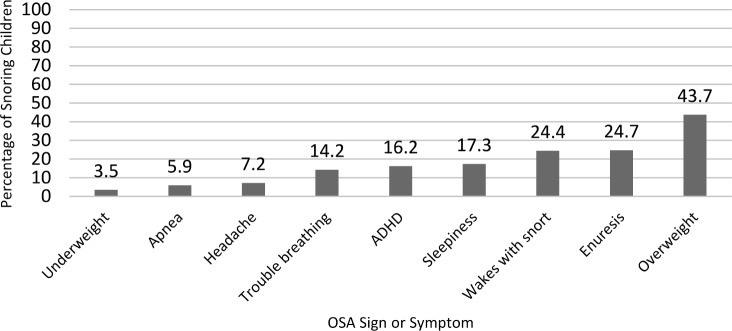
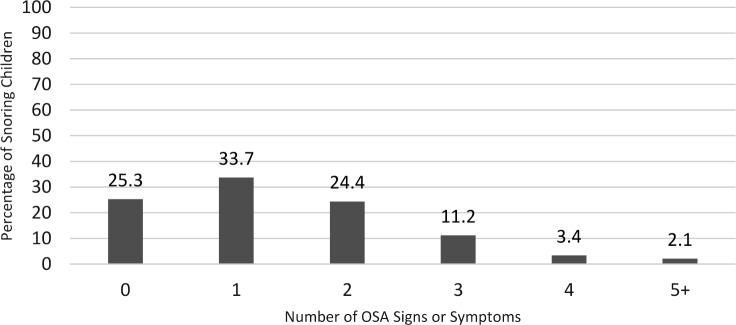
The frequency of the nine OSA signs/symptoms ranged from 3.5% (underweight) to 43.7% (overweight), and are depicted in Figure 4. The number of symptoms or signs applying to a particular child ranged from 0 to 8 (Figure 5), with 74.7% of children having at least one sign or symptom and 41.0% having at least two signs/symptoms. For patients for whom PCPs indicated concern for OSA, made a referral, or both, 85% had at least one sign or symptom. For patients for whom PCPs indicated no concern for OSA, 73.7% (n = 314) had at least one sign or symptom.
Figure 4. Frequency of OSA signs and symptoms in snoring children (n = 475).
ADHD = attention deficit hyperactivity disorder, OSA = obstructive sleep apnea.
Figure 5. Number of additional OSA signs and symptoms in snoring children (n = 475, range = 0–8).
OSA = obstructive sleep apnea.
Associations Between OSA Referral and Potential Predictive Factors
Rates of OSA referral are presented by child demographic factors (Table 2) and by OSA signs and symptoms (Table 3). None of these associations was statistically significant. Years since training was significantly associated with OSA referral (P = .03): providers who completed training more recently (within the median of 15.5 years) were significantly more likely to make an OSA referral than providers who completed training more than 15.5 years ago (24.1% versus 12.2%).
Table 2.
Predictors of OSA referral: child demographic factors.
Table 3.
Predictors of OSA referral: OSA signs and symptoms.
DISCUSSION
Computer decision support is a feasible approach for automating OSA screening and detection. While a direct comparison with control clinics is pending, rates of screening and referral are high compared to previous studies examining primary care systems without a computer decision support module. With the CHICA OSA module, 96.5% of children were screened for snoring, compared to 2.2% to 24.4%8,9 in previous studies on systems without automated screening for snoring. The percentage of snoring children receiving a referral (to ENT, PSG or sleep consult) with CHICA OSA was 8.6%, compared to 0% in another system,8 though one study did find a documentation of referral in 30.2% of snoring children when snoring was assessed (in 24.4% of children).9
Nonetheless, there are areas in which the CHICA OSA module is limited. The PCP response rate was 55.6%. As noted previously, the system is designed such that unanswered prompts are presented again at subsequent visits, meaning that the response rate increases over time. Further, the PCP response rate to date for the CHICA OSA module is comparable to the PCP response rates in CHICA modules for other areas of pediatric health.21 However, the response rate suggests that many snoring children are likely not receiving a focused evaluation for OSA within these clinics. There is also considerable variability in the response rates of individual PCPS to the module, ranging from 0% to 93.7%. Also consistent with findings from the CHICA system more broadly, we have several PCPs who are regular CHICA users and others who rarely use the CHICA system. We employed multiple strategies to engage PCPs with the CHICA system, including: (1) engaging PCPs in module development; (2) regular meetings with the study investigator, CHICA team members, PCPs, and clinic staff to review the module and address concerns; and (3) the use of a technical patient liaison who visits clinics quickly to train new staff and providers and troubleshoot technical issues. Further, the CHICA system includes many of the facets that have been associated with successful utilization of clinical decision support systems, specifically integration into workflow, provision of recommendations, computer-based decision support, and support at the time and location of provider decision-making.24
When PCPs did respond to the CHICA OSA module prompts, they indicated that they did not suspect OSA for more than three-fourths of patients who met AAP criteria for referral, and referred only 17.3%. There are a number of possible reasons why a PCP may not have suspected OSA in a child with snoring and one or more additional OSA sign/symptom(s). We know from previous data within our clinics that approximately 20% parents who report snoring on the tablet then subsequently deny snoring to the PCP.11 PCPs may elect to treat the snoring and reevaluate or to engage in watchful waiting. Families may not be interested in further evaluation for OSA. It is also possible that PCPs hold some misperceptions about risk for OSA, such as the belief that tonsillar hypertrophy is highly predictive and that OSA is unlikely in its absence. PCPs may be using a more stringent threshold for suspecting OSA (eg, snoring and two or more additional symptoms, or snoring plus less frequent symptoms such as observed apnea). Long wait times for PSG may be a barrier in some health systems; however, during the study period the wait time at our pediatric sleep center was relatively short, ranging from 1 to 3 months. Although we have had informal discussions with our providers regarding their decision-making, a more systematic evaluation of PCP beliefs and perceptions regarding OSA detection is needed.
Our exploratory analysis examining associations between OSA referral and potential predictive factors should be interpreted with caution due to low statistical power. Nonetheless, descriptively our data suggest that certain OSA signs and symptoms may be associated with higher rates of OSA referral, indicating that PCPs may weigh certain symptoms more heavily than others in their decision-making. For example, 24.2% of snoring children with daytime sleepiness received a referral, compared to 14.1% of snoring children who denied daytime sleepiness. However, rates of referral were more similar for other signs or symptoms. For example, 17.4% of snoring children with ADHD symptoms received a referral, compared to 15.2% who did not. Findings also raise questions about the potential role of the family's preferred language on OSA referral. When Spanish was the preferred language, 9.9% of snoring children received a referral, compared to 18.0% when English was the preferred language. This trend is consistent with our previous study showing that PCPs were significantly less likely to have concern for OSA for children in Spanish-speaking families.11 Similarly, referral rates for Hispanic ethnicity (16.3%) versus non-Hispanic ethnicity (15.0%) suggest that language rather than ethnicity may be the driving factor. Another possible factor is insurance coverage. Some Spanish-speaking families in our system have children who are undocumented, and thus are not eligible for Medicaid insurance. PCPs may be more reluctant to refer children (or families may be more reluctant to accept a referral) when the burden of paying for the service is high.
We did find that providers who completed training more recently were more likely to make an OSA referral. However, previous findings on the role of years since training have been mixed. For example, one study of provider OSA knowledge did not find differences based on years since training,15 whereas our previous study in five clinics found that providers who had been out of training longer were actually more likely to have concern for OSA. It may be that years since training as a predictive factor is primarily dependent on the specific providers practicing in that setting.
It is important to consider both the appropriateness and feasibility of the AAP guidelines threshold for OSA referral. Almost three-fourths of snoring children had at least one OSA sign or symptom, and thus met criteria for OSA referral. Of note, this rate represents a minimum of children meeting AAP criteria in our sample, as there were some signs/symptoms that we did not assess. Because the causes of pediatric OSA are variable (eg, adiposity; retrognathia; hypotonia; tonsillar hypertrophy), it has proven difficult to predict through clinical interview and physical examination which children have OSA. In fact, the positive predictive value of a history and physical examination for OSA diagnosis was found to be 65% and 46% respectively, similar to chance.1 The limited predictability of history and examination may necessitate a low referral threshold and a high rate of resulting false positives (ie, negative polysomnograms). More research on OSA predictors is needed, particularly in a general population with universal screening (versus a population of children referred for PSG, a sample with a selection bias).
As the AAP guidelines represent the current evidence-based standard, it is also important to consider the readiness of the sleep medicine field to meet this need. Although referral to ENT or for a sleep medicine consult is considered an option, only referral for PSG has sufficient evidence to be graded a recommendation. Yet there are only an estimated 3,000 to 3,500 sleep centers in the United States,25 of which only a portion treat children younger than 12 years. In our sample, 14.0% of all children screened met criteria for OSA referral. Based on an estimate of the population of United States children between the ages of 2 and 11 years in 2017 (40.8 million),26 an estimated 5.7 million United States children would meet criteria for an OSA referral. Thus, there appears to be a discrepancy between the necessary and available resources to adhere to the current guidelines.
Some limitations of this study should be noted. The use of two primary care clinics from the same health system may limit generalizability of findings, both in terms of physician behavior and decision-making and the frequency of OSA signs and symptoms. Our patient population had a higher prevalence of characteristics known to increase OSA risk, specifically overweight/obese status and black race, compared to the overall United States population. This may have resulted in an overestimate of the prevalence of children meeting the referral threshold. At the same time, there were several OSA signs/symptoms that were not available in the current sample (eg, tonsillar hypertrophy; hypertension). In this sense, our reported prevalence of children with snoring and at least one additional OSA sign or symptom is likely an underestimate. Findings have fewer implications for countries outside of the United States that follow different guidelines for OSA detection. As noted previously, our understanding of PCP decision-making is limited to their selection from six prescribed checkboxes. Our analysis of which symptoms were associated with higher rates of OSA referral was essentially descriptive due to low statistical power. Further, we were not able to assess more downstream effects, such as completion of referral appointments, OSA diagnosis, OSA treatment, and symptom improvement after treatment. A final limitation is that our study was not sufficiently powered to analyze patient and/or provider predictors of OSA referral or PCP response to the prompt. Nevertheless, this study is the first that we know of that describes the frequency of children meeting AAP criteria for OSA referral in a primary care sample with universal screening, and has important implications for OSA detection.
In conclusion, the use of a computer decision support system (CHICA OSA module) facilitated universal screening for snoring, assessment of a number of additional OSA signs/symptoms, and PCP notification of OSA risk. CHICA OSA was designed to improve detection of pediatric OSA, and a randomized controlled trial evaluating this module is ongoing. We hypothesize that clinics randomized to use the CHICA OSA module will have higher rates of appropriate OSA referral (defined as referral to PSG or ENT), compared to clinics using a control module, which includes screening for snoring but not other OSA signs or symptoms. Findings from the current study, however, highlight some limitations of the use of computer decision support systems such as the CHICA OSA module for OSA detection. PCP response was moderate, and often PCPs did not suspect OSA or make a referral in children meeting referral criteria, for unknown reasons. Most snoring children met AAP criteria for referral, which raises questions about the appropriateness and feasibility of the current guidelines. Additional research will be important for exploring strategies to enhance the effectiveness of computer decision support for OSA, understanding PCP and family decision-making around OSA referral, identifying with greater specificity which children are at risk for OSA, and improving our capacity as a field to evaluate and manage pediatric OSA.
DISCLOSURE STATEMENT
Work for this study was performed at Indiana University School of Medicine. All authors have seen and approved the manuscript. Stephen Downs is the co-inventor of CHICA and the president of Digital Health Solutions, LLC, a company created to license the CHICA software. Currently, there are no patents. Sarah M. Honaker is a consultant for Google, LLC. The remaining authors report no conflicts of interest.
ACKNOWLEDGMENTS
This research was made possible by an award from the American Academy of Sleep Medicine Foundation. This publication was also made possible with support from the Indiana Clinical and Translational Sciences Institute funded by a KL training award (Grant Number KL2TR002530) from the National Institutes of Health, National Center for Advancing Translational Sciences Award. The authors thank the dedicated providers and staff at the two participating Eskenazi Health clinic sites, as well as all of the participating children and families. We further wish to acknowledge the technical expertise and efforts of the individual members of the Child Health Informatics and Research Development Lab (CHIRDL) team, which provides programming and technical support for CHICA, and the Pediatric Research Network (PReSNet) at Indiana University School of Medicine for regulatory support.
ABBREVIATIONS
- AAP
American Academy of Pediatrics
- ADHD
attention deficit hyperactivity disorder
- CDSS
computer decision support systems
- CHICA
Child Health Improvement through Computer Automation
- EHR
electronic health record ENT, ear, nose, and throat specialist
- OSA
obstructive sleep apnea
- PCP
primary care provider
- PSG
polysomnography
REFERENCES
- 1.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714–e755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharjee R, Kheirandish-Gozal L, Pillar G, Gozal D. Cardiovascular complications of obstructive sleep apnea syndrome: evidence from children. Prog Cardiovasc Dis. 2009;51(5):416–433. doi: 10.1016/j.pcad.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Gozal D, Kheirandish-Gozal L. Neurocognitive and behavioral morbidity in children with sleep disorders. Curr Opin Pulm Med. 2007;13(6):505–509. doi: 10.1097/MCP.0b013e3282ef6880. [DOI] [PubMed] [Google Scholar]
- 4.Gottlieb DJ, Vezina RM, Chase C, et al. Symptoms of sleep-disordered breathing in 5-year-old children are associated with sleepiness and problem behaviors. Pediatrics. 2003;112(4):870–877. doi: 10.1542/peds.112.4.870. [DOI] [PubMed] [Google Scholar]
- 5.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576–584. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 6.Bonuck KA, Chervin RD, Cole TJ, et al. Prevalence and persistence of sleep disordered breathing symptoms in young children: a 6-year population-based cohort study. Sleep. 2011;34(7):875–884. doi: 10.5665/SLEEP.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chervin RD, Archbold KH, Panahi P, Pituch KJ. Sleep problems seldom addressed at two general pediatric clinics. Pediatrics. 2001;107(6):1375–1380. doi: 10.1542/peds.107.6.1375. [DOI] [PubMed] [Google Scholar]
- 9.Erichsen D, Godoy C, Granse F, Axelsson J, Rubin D, Gozal D. Screening for sleep disorders in pediatric primary care: are we there yet? Clin Pediatr (Phila) 2012;51(12):1125–1129. doi: 10.1177/0009922812464548. [DOI] [PubMed] [Google Scholar]
- 10.Honaker SM, Meltzer LJ. Sleep in pediatric primary care: a review of the literature. Sleep Med Rev. 2016;25:31–39. doi: 10.1016/j.smrv.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Honaker SM, Dugan T, Daftary A, et al. Unexplained practice variation in primary care providers' concern for pediatric obstructive sleep apnea. Acad Pediatr. 2018;18(4):418–424. doi: 10.1016/j.acap.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Mindell JA, Bartle A, Ahn Y, et al. Sleep education in pediatric residency programs: a cross-cultural look. BMC Res Notes. 2013;6:130. doi: 10.1186/1756-0500-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mindell JA, Bartle A, Wahab NA, et al. Sleep education in medical school curriculum: a glimpse across countries. Sleep Med. 2011;12(9):928–931. doi: 10.1016/j.sleep.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Mindell JA, Owens JA. Sleep problems in pediatric practice: clinical issues for the pediatric nurse practitioner. J Pediatr Heal Care. 2003;17(6):324–331. doi: 10.1016/s0891-5245(03)00215-3. [DOI] [PubMed] [Google Scholar]
- 15.Tamay Z, Akcay A, Kilic G, Suleyman A, Ones U, Guler N. Are physicians aware of obstructive sleep apnea in children? Sleep Med. 2006;7(7):580–584. doi: 10.1016/j.sleep.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Uong EC, Jeffe DB, Gozal D, et al. Development of a measure of knowledge and attitudes about obstructive sleep apnea in children (OSAKA-KIDS) Arch Pediatr Adolesc Med. 2005;159(2):181–186. doi: 10.1001/archpedi.159.2.181. [DOI] [PubMed] [Google Scholar]
- 17.Owens JA. The practice of pediatric sleep medicine: results of a community survey. Pediatrics. 2001;108(3):E51. doi: 10.1542/peds.108.3.e51. [DOI] [PubMed] [Google Scholar]
- 18.Carroll AE, Anand V, Dugan TM, Sheley ME, Xu SZ, Downs SM. Increased physician diagnosis of asthma with the child health improvement through computer automation decision support system. Pediatr Allergy Immunol Pulmonol. 2012;25(3) [Google Scholar]
- 19.Carroll AE, Bauer NS, Dugan TM, Anand V, Saha C, Downs SM. Use of a computerized decision aid for developmental surveillance and screening: a randomized clinical trial. JAMA Pediatr. 2014;168(9):815–821. doi: 10.1001/jamapediatrics.2014.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klann JG, Anand V, Downs SM. Patient-tailored prioritization for a pediatric care decision support system through machine learning. J Am Med Inform Assoc. 2013;20(e2):e267–e274. doi: 10.1136/amiajnl-2013-001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Downs SM, Anand V, Dugan TM, Carroll AE. You can lead a horse to water: physicians' responses to clinical reminders. AMIA Annu Symp Proc. 2010;2010:167–171. [PMC free article] [PubMed] [Google Scholar]
- 22.Anand V, Carroll AE, Downs SM. Automated primary care screening in pediatric waiting rooms. Pediatrics. 2012;129(5):e1275–e1281. doi: 10.1542/peds.2011-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1(1):21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 24.Kawamoto K. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765–770. doi: 10.1136/bmj.38398.500764.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirsch DB. There and back again: a current history of sleep medicine. Chest. 2011;139(4):939–946. doi: 10.1378/chest.10-1235. [DOI] [PubMed] [Google Scholar]
- 26.Kids Count Data Center. Child population by age group. [Accessed August 1, 2018]. https://datacenter.kidscount.org/data/tables/101-child-population-by-age-group.