Abstract
Background
There are limited data on the real-world effectiveness of direct-acting antiviral (DAA) treatment in patients coinfected with hepatitis C virus (HCV) and HIV—a population with complex challenges including ongoing substance use, cirrhosis, and other comorbidities. We assessed how patient characteristics and the appropriateness of HCV regimen selection according to guidelines affect treatment outcomes in coinfected patients.
Methods
We included all patients who initiated DAA treatment between November 2013 and July 2017 in the Canadian Co-Infection Cohort. Sustained virologic response (SVR) was defined as an undetectable HCV RNA measured between 10 and 18 weeks post-treatment. We defined treatment failure as virologic failure, relapse, or death without achieving SVR. Bayesian logistic regression was used to estimate the posterior odds ratios (ORs) associated with patient demographic, clinical, and treatment-related risk factors for treatment failure.
Results
Two hundred ninety-five patients initiated DAAs; 31% were treatment-experienced, 29% cirrhotic, and 80% HCV genotype 1. Overall, 92% achieved SVR (263 of 286, 9 unknown), with the highest rates in females (97%) and lowest in cirrhotics (88%) and high-frequency injection drug users (89%). Many patients (38%) were prescribed regimens that were outside current clinical guidelines. This did not appreciably increase the risk of treatment failure—particularly in patients with genotype 1 (prior odds ratio [OR], 1.5; 95% credible interval [CrI], 0.38–6.0; posterior OR, 1.0; 95% CrI, 0.40–2.5).
Conclusions
DAAs were more effective than anticipated in a diverse, real-world coinfected cohort, despite the use of off-label, less efficacious regimens. High-frequency injection drug use and cirrhosis were associated with an increased risk of failure.
Keywords: direct-acting antivirals, hepatitis C virus, HIV coinfection, treatment failure, treatment guidelines
Direct-acting antivirals (DAAs) are safe and highly effective treatments that can cure more than 90% of individuals chronically infected with hepatitis C virus (HCV) [1, 2]. Ambitious World Health Organization (WHO) targets have been set for HCV elimination; however, treatment must be scaled up in populations that traditionally have been difficult to engage in care [3–6]. Due to the high prevalence of HCV [7] and subsequent increased morbidity and mortality from liver disease [8] and community transmission risks, HIV-infected individuals are a priority population for HCV elimination. In clinical trials of DAAs that included HCV/HIV-coinfected individuals, treatment failures occurred infrequently [1]. Although traditionally difficult-to-treat patients are typically excluded from such trials [9], recent real-world clinical studies have shown similarly low rates of treatment failure [10–13]. Despite the advances in treatment, however, challenges remain that place coinfected persons at increased risk of DAA treatment failure. These include advanced and decompensated cirrhosis, ongoing substance use [14], important drug–drug interactions with antiretrovirals and other concomitant medications [15], and persistent HIV-related immunosuppression [16]. To date, studies have lacked more detailed information on risk behaviors, substance use, and the clinical rationale for treatment selection.
Although uncommon, treatment failures are costly. Even in the context of lower medication prices, the cost of retreatment is substantial. Failure to cure also carries the potential ongoing risk of new infections and progressive liver disease. Thus, optimizing success with a first DAA regimen should be the goal. We sought to determine how patient characteristics and the appropriateness of HCV treatment selection according to HCV treatment guidelines impact treatment outcomes for HCV/HIV-coinfected patients. Using statistical methods appropriate for small samples, we assessed risk factors for DAA treatment failure in a large, representative cohort of Canadian coinfected patients with detailed information on current risk behaviors, substance use, clinical parameters, and prescribing decisions. Our aim was to help clinicians identify people who require specific therapies, added support, or additional monitoring to minimize treatment failure in this priority population.
METHODS
Study Population
The Canadian Co-infection Cohort (CCC) includes more than 1800 patients recruited from HIV clinic populations at 18 centers across 6 Canadian provinces [17]. We retrospectively identified all coinfected patients who initiated all-oral, interferon-free DAAs between November 1, 2013, and June 30, 2017. Treatment-related information, including start and stop dates, HCV genotype, and viral load measurements, was obtained using detailed standardized data forms. All of the participants provided informed consent. Our study was approved by the community advisory committee of the Canadian Institutes of Health Research–Canadian HIV Trials Network, the McGill University Health Centre Research Ethics Board, and by all institutional ethics boards of participating centers.
Outcome
We defined sustained virologic response (SVR) as an undetectable HCV RNA measured between 10 and 18 weeks post-treatment. We then classified each failure to achieve SVR as either (1) an undetectable HCV RNA during treatment (ie, virologic failure), with or without early treatment discontinuation; (2) a detectable HCV RNA measurement after an undetectable measurement at any time up until 12 weeks after the end of treatment (ie, relapse); or (3) death before the assessment of SVR. HCV RNA was assessed in local laboratories using either a qualitative (COBAS Ampliprep/TaqMan HCV Test, v2.0, Roche Molecular Systems) or quantitative assay (Abbott RealTime PCR, Abbott Molecular Inc.).
Risk Factors
We evaluated demographic, clinical, treatment, and adherence-related risk factors for DAA treatment failure, identified a priori and using information readily available at treatment initiation. Cirrhosis was defined by clinical diagnosis and included both compensated and decompensated cirrhosis (ie, presence of ascites, varices, spontaneous bacterial peritonitis, portal hypertension, or encephalopathy). Chronic comorbidities that may impact treatment outcomes were [18]: hepatitis B virus (HBV) coinfection (surface antigen positivity), chronic renal failure (estimated glomerular filtration rate ≤60 mL/min/1.73 m2), or diabetes (clinical diagnosis or 2 consecutive measurements of fasting glucose ≥7 mmol/L or nonfasting glucose ≥11.1 mmol/L). Off-label or low-efficacy regimens were defined as any prescribed DAA medications that were outside the current clinical guidelines of the American Association for the Study of Liver Disease (AASLD) and Infectious Disease Society of America (IDSA), updated in September 2017 [19]. Problematic substance use was defined as recent high-frequency injection drug use (IDU) or hazardous alcohol consumption. High-frequency IDU was assessed by self-report of injecting crack cocaine or methamphetamines in the last year, as these drugs are associated with multiple daily injections and a high degree of risk-taking behavior [20]. Hazardous alcohol consumption was assessed by self-report using Alcohol Use Disorders Identification Test (AUDIT-C) score cutoffs (ie, ≥3 for females, ≥4 for males) [21]. Detectable HIV RNA was defined as ≥50 copies/mL.
Statistical Analysis
We assessed predictors of DAA treatment failure in our coinfected population using logistic regression. All risk factors for DAA treatment failure were measured before or no later than 45 days after treatment initiation. We took a Bayesian approach to analysis to limit the bias in estimates when multivariate regression models are fit to “sparse data” [22–24]. For each model parameter, we asserted “weakly” informative prior distributions—distributions with credible intervals (CrIs) that we think would be viewed as liberally inclusive by knowledgeable clinicians [25]. Informative priors restrict each model parameter to a range of clinically plausible values, reducing the influence of outliers [26, 27]. We categorized risk factors as possibly or probably harmful, possibly or probably beneficial, or of uncertain direction. For each category, we then assigned a wide normal distribution, with a variance of 0.5, as the prior log odds ratio, to reduce the probability of extreme effect sizes [24]. We considered cirrhosis and the presence of chronic comorbidities to be possibly harmful (prior odds ratio [OR], 1.5; 95% credible interval [CrI], 0.38–6.0), whereas off-label and low-efficacy regimens and problematic substance use were probably harmful (prior OR, 2.0; 95% CrI, 0.5–8.0). We also included covariates for sex, Indigenous ethnicity, age (per 10 years), and detectable HIV RNA as a measure of patient adherence to treatment. We considered increasing age and sex to be risk factors of uncertain direction (prior OR, 1.0; 95% CrI, 0.25–4.0) and Indigenous ethnicity and detectable HIV RNA to be possibly and probably harmful, respectively. We used SAS/STAT 14.1 (Cary, NC) for our analysis, using a BAYES statement in PROC GENMOD to fit our Bayesian model. We report prior and posterior credible intervals; the difference between these intervals shows the information contributed by our data [23].
Sensitivity Analysis
First, we reclassified patients taking 8 weeks of sofosbuvir/ledipasvir as a suitable, rather than off-label, regimen for genotype 1 patients if they were treatment-naïve, noncirrhotic, and had an unknown or HCV viral load <6 million IU/mL. Current AASLD/IDSA guidelines do not support the use of short-course sofosbuvir/ledipasvir in the setting of HIV coinfection [19], but a short course of this regimen is the only option in some Canadian provinces, despite limited real-world evidence in the coinfected population [28]. Second, we restricted our analysis to HCV genotype 1 patients and replaced the existing probably harmful prior for off-label, low-efficacy regimen with a possibly harmful prior, given that we expected that this risk factor would have the greatest effect on non–genotype 1 patients. Third, we separated the effect of problematic substance use into 2 covariates—high-frequency IDU and hazardous drinking—and assigned them probably and possibly harmful priors, respectively. Lastly, we included all patients who had no available treatment outcome but were part of our study population. For those with HCV RNA measurements after starting treatment, we imputed their outcome based on post-treatment measurements, where available, or if not, on an end-of-treatment response. For patients with no measurements after starting treatment, we assumed all were failures or all were successes, to assess the effect of both extremes on our results.
RESULTS
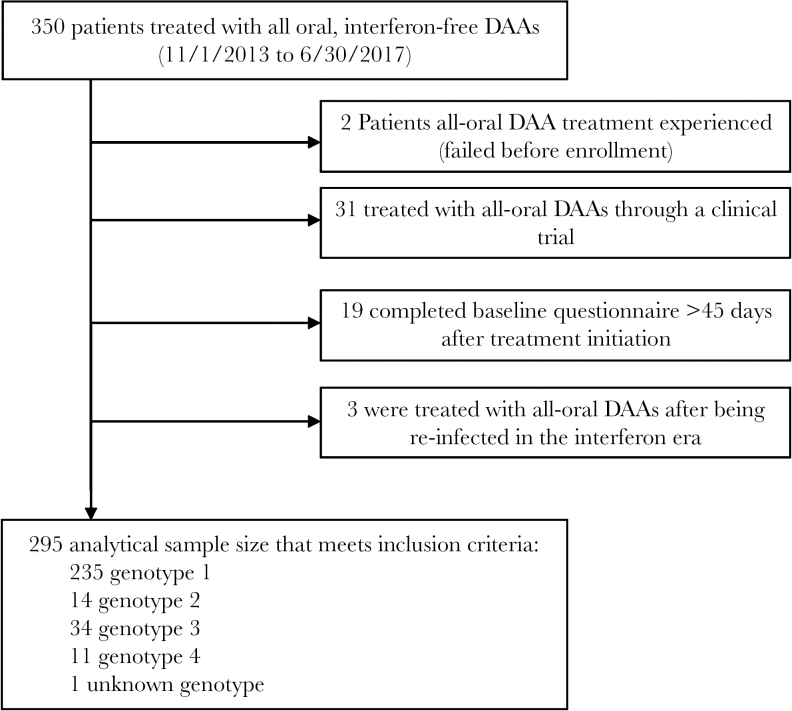
As of June 30, 2017, 350 coinfected patients initiated treatment with DAA therapy (Figure 1). We then excluded 2 patients who had been treated with DAAs before enrollment, 31 patients who were treated through a clinical trial, 19 who completed a baseline questionnaire more than 45 days after starting treatment, and 3 who were treated for a reinfection after successful interferon-based therapy. Hence our analysis was of 295 coinfected patients. Most patients were male (77%), with a median age (interquartile range [IQR]) of 52 (48–56) years; 80% had HCV genotype 1; 31% previously failed treatment with interferon; 29% were cirrhotic (Table 1). All patients were on antiretroviral therapy, with a median CD4+ cell count (IQR) of 500 (300–700) cells/μL, and 89% had suppressed HIV RNA. Recent problematic substance use was reported by 42% of patients.
Figure 1.
Study participant flow chart. Abbreviation: DAA, direct-acting antivirals.
Table 1.
Baseline Patient Characteristics, Overall and by Hepatitis C Virus Genotypea
| HCV Genotype | |||||
|---|---|---|---|---|---|
| Overall(n = 295)b | GT1(n = 235)c | GT2(n = 14) | GT3(n = 34) | GT4(n = 11) | |
| Median age(IQR), y | 52(48–56) | 52(47–56) | 51(48–59) | 54(51–59) | 52(47–60) |
| Male sex | 227(77) | 184(78) | 10(71) | 25(74) | 7(64) |
| Indigenous ethnicityd | 38(13) | 30(13) | 3(21) | 4(12) | 1(9) |
| Detectable HIV RNA(≥50 copies/mL) | 31(11) | 27(11) | 0 | 1(3) | 2(18) |
| Median CD4 count(IQR), cells/μL | 500(300–700) | 500(330–710) | 360(260–550) | 450(230–620) | 580(330–770) |
| Nadir CD4 count ≤200 cells/μLe | 161(59) | 125(58) | 8(62) | 23(74) | 4(40) |
| HCV interferon treatment experienced | 91(31) | 72(31) | 3(21) | 10(29) | 6(55) |
| Cirrhosis | 85(29) | 65(28) | 4(29) | 13(38) | 3(27) |
| Median HCV viral load(IQR), log10 IU/mL | 6.1(5.6–6.5) | 6.1(5.7–6.4) | 6.5(5.9–6.7) | 5.8(5.1–6.4) | 5.9(5.6–6.5) |
| Any additional comorbid condition | 55(19) | 39(17) | 5(36) | 10(29) | 1(9) |
| Diabetes | 27(9) | 19(8) | 4(29) | 4(12) | 0 |
| Chronic kidney disease | 27(9) | 20(9) | 1(7) | 5(15) | 1(9) |
| Hepatitis B coinfection | 6(2) | 4(2) | 0 | 2(6) | 0 |
| Problematic substance use in last year | 124(42) | 99(42) | 5(36) | 17(50) | 2(18) |
| High frequency injection drug use | 58(20) | 47(20) | 1(7) | 10(29) | 0 |
| Hazardous alcohol consumption | 88(30) | 69(29) | 5(36) | 11(32) | 2(18) |
| Off-label or low-efficacy HCV treatmentf | 111(38) | 65(28) | 14(100) | 25(76) | 7(70) |
Abbreviations: GT, genotype; HCV, hepatitis C virus; IQR, interquartile range; IU, international units.
aAll values are No.(%), unless otherwise indicated.
bOne patient was missing data on HCV genotype.
cFor genotype 1176 patients had genotype 1a, 39 had genotype 1b, and 20 had an unspecified genotype 1.
dOverall ethnicity: Caucasian(n = 233), Indigenous(n = 38), black(n = 14), and Asian(n = 10).
eTwenty-four patients were missing data for nadir CD4 count ≤200 cells/μL: 19 for genotype 1, 1 for genotype 2, 3 for genotype 3, and 1 each for genotype 4 and the missing genotype.
fSix patients were missing data for the off-label indication: 4 for genotype 1 and 1 each for genotypes 3 and 4.
Overall, 111 patients (38%) were treated with an off-label, low-efficacy regimen (range, 28% for genotype 1 to 100% for genotype 2) (Table 2). Mutually exclusive reasons for off-label or low-efficacy indication were the use of a nonrecommended regimen, a recommended regimen with concomitant use of contraindicated antiretrovirals, or a recommended regimen without contraindication, but of a shortened duration (Table 2; Supplementary Table 1). For patients with genotype 1 (n = 231), the most common reason treatment was considered off-label or low efficacy was the use of a nonrecommended regimen (n = 36), mostly interferon-experienced patients with cirrhosis (n = 31) who did not receive ribavirin. Less frequent were the use of an acceptable regimen with a shortened duration (n = 19), mostly among patients who were treatment naïve, noncirrhotic (n = 15), and had a contraindication with HIV antiretrovirals (n = 10). For patients with other HCV genotypes (n = 57), the most common reason treatment was considered off-label or low efficacy was the use of a nonrecommended regimen (n = 43), in particular sofosbuvir and ribavirin.
Table 2.
Reasons for Off-label or Low-Efficacy Treatment in 111 Patients, by Hepatitis C Virus Genotype, According to Current HCV Treatment Guidelinesa
| Overall(n = 288) | GT1(n = 231) | GT2(n = 14) | GT3(n = 33) | GT4(n = 10) | |
|---|---|---|---|---|---|
| Regimen not recommended regardless of ART | 79 | 36b | 13 | 24 | 6 |
| Regimen not recommended only because ART contraindicated | 13 | 10 | 1 | 1 | 1 |
| Regimen recommended but prescribed duration too short | 19 | 19 | 0 | 0 | 0 |
| Total off-label, low-efficacy regimen | 111 | 65 | 14 | 25 | 7 |
Abbreviations: ART, antiretroviral therapy; GT, genotype.
aSix patients were missing data for the off-label indication: 4 for genotype 1 and 1 each for genotypes 3 and 4. One patient who had an unknown HCV genotype was excluded.
bWe assumed GT1a cirrhotic patients who used elbasvir/grazoprevir and did not have a record of baseline NS5A resistance testing to be off-label.
Treatment was successful for 263 of 286 patients with known treatment outcomes (SVR: 92%); 9 had an unknown outcome. Characteristics of the 23 treatment failures are shown in Table 3. The reasons for treatment failure were virologic failure (n = 11), relapse (n = 9), and death (n = 3). Among the 9 patients with an unknown outcome, 2 had an undetectable HCV RNA measurement at end of treatment, with the remainder having no information on HCV RNA after starting treatment. SVR rates were higher among genotypes 1 (93%) and 3 (94%) than genotypes 2 (85%) and 4 (80%) and were higher among females than males (97% vs 90%). Among genotype 1 patients, SVR was higher among genotype 1b (37/38, 97%) than genotype 1a (159/173, 92%). SVR rates were lower among patients with cirrhosis than those without cirrhosis (88% vs 94%) and among those reporting high-frequency IDU compared with those not (89% vs 93%). There was little difference in SVR rates between those on a recommended regimen than those on an off-label or low-efficacy regimen (93% vs 91%).
Table 3.
Characteristics of the 23 Hepatitis C Virus Treatment Failures Seen in the Canadian Co-Infection Cohort
| Patient | GT | HCV Treatment Experienced | Cirrhosis | HCV Treatment | Prescribed Duration, wk | Actual Duration, wk | Contraindicated Antiretrovirals | Off-Label, Low-Efficacy | Substance Usea | Comorbiditiesb |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1a | No | No | ELB/GRZ | 12 | 8 | None | No | IDU, alcohol | None |
| 2 | 1a | No | No | SOF/LED | 12 | 8 | None | No | IDU | None |
| 3 | 1a | No | No | SOF/LED | 12 | 11 | None | No | None | None |
| 4 | 1a | No | No | SOF/LED | 12 | 11 | None | No | IDU, alcohol | None |
| 5 | 1a | No | No | SOF/LED | 12 | 12 | None | No | None | None |
| 6 | 1a | No | No | SOF/LED | 12 | 12 | None | No | IDU | None |
| 7 | 1a | No | Yes | SOF+RBV | 12 | 5 | None | Yes | None | Kidney disease |
| 8 | 1a | No | Yes | SOF/LED | 8 | 8 | None | Yes | IDU | None |
| 9 | 1a | No | Yes | SOF/LED | 24 | 24 | None | No | None | None |
| 10 | 1a | Yes | No | SOF/LED | 12 | 12 | None | No | None | None |
| 11 | 1a | Yes | No | SOF/LED | 12 | 12 | None | No | IDU | None |
| 12 | 1a | Yes | Yes | SIM+SOF+RBV | 12 | 6 | None | Yes | None | None |
| 13 | 1a | Yes | Yes | SOF/LED | 24 | 24 | None | Yes | None | None |
| 14 | 1a | Yes | Yes | SOF/LED+RBV | 12 | 12 | None | No | Alcohol | None |
| 15 | 1b | Yes | Yes | SOF/LED | 12 | 7 | None | No | None | Diabetes |
| 16 | 1 | No | No | ELB/GRZ | 12 | 12 | Darunavir | Yes | None | Kidney disease |
| 17 | 1 | No | No | SOF/LED | 12 | 9 | None | No | None | None |
| 18 | 2 | No | No | SOF+RBV | 12 | 10 | None | Yes | None | Kidney disease |
| 19 | 2 | No | Yes | SOF+RBV | 12 | 5 | None | Yes | None | Diabetes |
| 20 | 3 | No | No | SOF+RBV | 24 | 24 | None | Yes | Alcohol | None |
| 21 | 3 | Yes | Yes | SOF+RBV | 24 | 24 | None | Yes | Alcohol | Kidney disease |
| 22 | 4 | No | No | SOF/LED | 12 | 12 | None | No | None | None |
| 23 | 4 | Yes | Yes | SOF/LED | 24 | 24 | None | Yes | None | None |
Abbreviations: ELB, elbasvir; GRZ, grazoprevir; GT, genotype; HCV, hepatitis C virus; IDU, injection drug use; LED, ledipasvir; RBV, ribavirin; SIM, simeprevir; SOF, sofosbuvir.
aHigh-frequency IDU was defined by self-report of injecting crack/cocaine or methamphetamines in the last year before treatment initiation. Hazardous alcohol use was defined by self-report of consumption in the last year before treatment initiation resulting in AUDIT-C scores ≥3 for females or ≥4 for males.
bDiabetes was defined through clinical diagnosis or 2 consecutive measurements of fasting glucose ≥7 mmol/L or nonfasting glucose ≥11.1 mmol/L before treatment initiation. Kidney disease was defined as a single estimated glomerular filtration rate measurement ≤60 mL/min/1.73 m2 before treatment initiation.
Treatment-specific SVR rates by genotype, cirrhosis status, and treatment experience are shown in Supplementary Table 2. Sofosbuvir/ledipasvir was the most frequently used regimen (n = 201), followed by sofosbuvir and ribavirin (n = 32), mainly used by genotype 2 and 3 patients. Ribavirin was a component of treatment for 29 patients (11%), of whom 21 were cirrhotic or treatment experienced. Among treatment-naïve patients, noncirrhotic genotype 1 patients with an HCV viral load <6 million IU/mL treated with sofosbuvir/ledipasvir, SVR rates were 100% (15/15) in those prescribed for 8 weeks and 95% (74/78) among those prescribed for 12 weeks.
Estimates of risk factors for treatment failure were broadly in line with our expectations, in that posterior intervals were contained within prior intervals (Table 4). However, females were less likely to fail treatment than anticipated (prior OR, 1.0; 95% CrI, 0.25–4.0; posterior OR, 0.52; 95% CrI, 0.19–1.3). Off-label, low-efficacy HCV treatment was not as detrimental as anticipated, either in the main analysis (prior OR, 2.0; 95% CrI, 0.5–8.0; posterior OR, 1.2; 95% CrI, 0.57–2.7), when sofosbuvir/ledipasvir for 8 weeks was reclassified as acceptable for certain patients (prior OR, 2.0; 95% CrI, 0.5–8.0; posterior OR, 1.4; 95% CrI, 0.62–3.0), or when the analysis was restricted to genotype 1 patients (prior OR, 1.5; 95% CrI, 0.38–6.0; posterior OR, 1.0, CrI, 0.40–2.5). Problematic substance use was not as harmful as anticipated (prior OR, 2.0; 95% CrI, 0.5–8.0; posterior OR, 1.3; 95% CrI, 0.61–2.8); however, when we separated the effects of alcohol consumption and IDU, the risk of treatment failure for those reporting high-frequency IDU (posterior OR, 2.0; 95% CrI, 0.85–4.6) was consistent with expectations.
Table 4.
Prior and Posterior Estimates of Risk Factors for Hepatitis C Virus Treatment Failure Among HIV-Coinfected Patients in the Canadian Co-Infection Cohort Who Initiated Treatment With Direct-Acting Antivirals
| Main Analysis | Sensitivity Analyses | ||||
|---|---|---|---|---|---|
| Reclassified Off-label | Genotype 1 | Two Substance Use Effects | |||
| Prior OR(95% CrI) | Posterior OR(95% CrI) | Posterior OR(95% CrI) | Posterior OR(95% CrI) | Posterior OR(95% CrI) | |
| Age(per 10-y increase) | 1.0(0.25–4.0) | 1.4(0.81–2.4) | 1.3(0.75–2.2) | 1.3(0.71–2.4) | 1.4(0.83–2.5) |
| Female sex | 1.0(0.25–4.0) | 0.52(0.19–1.3) | 0.49(0.19–1.2) | 0.52(0.18–1.4) | 0.51(0.19–1.3) |
| Indigenous ethnicity | 1.5(0.38–6.0) | 1.5(0.55–3.9) | 1.8(0.68–4.4) | 1.4(0.47–3.7) | 1.4(0.54–3.7) |
| Chronic comorbidities | 1.5(0.38–6.0) | 1.3(0.56–3.1) | 1.3(0.55–3.0) | 1.1(0.43–3.0) | 1.3(0.56–3.0) |
| Detectable HIV viral load | 2.0(0.5–8.0) | 1.6(0.58–3.9) | 1.5(0.56–3.7) | 1.4(0.51–3.7) | 1.5(0.58–3.8) |
| Cirrhosis | 1.5(0.38–6.0) | 1.8(0.79–3.9) | 1.6(0.72–3.6) | 1.8(0.74–4.5) | 1.8(0.80–4.1) |
| Off-label, low-efficacy regimen | 2.0(0.5–8.0) | 1.2(0.57–2.7) | 1.4(0.62–3.0) | 1.0(0.40–2.5)b | 1.2(0.57–2.8) |
| Problematic substance usea | 2.0(0.5–8.0) | 1.3(0.61–2.8) | 1.2(0.56–2.6) | 1.5(0.62–3.4) | N/A |
| Hazardous alcohol use | 1.5(0.38–6.0) | N/A | N/A | N/A | 0.94(0.40–2.1) |
| High-frequency injection drug use | 2.0(0.5–8.0) | N/A | N/A | N/A | 2.0(0.85–4.6) |
Abbreviations: CrI, credible interval; N/A, not applicable; OR, odds ratio.
aPatient self-reported high-frequency injection drug use or hazardous alcohol consumption in the last year before treatment initiation.
bReplaced the probably harmful prior OR with a possibly harmful prior OR of 1.5(95% CrI, 0.38–6.0) for this specific sensitivity analysis.
Sensitivity analyses where we imputed missing treatment outcomes under optimistic and pessimistic scenarios produced results consistent with those of the main analysis (Supplementary Table 3).
DISCUSSION
We found that all-oral DAA therapies were very effective in a real-world setting. Many of our patients were historically considered difficult to treat—active substance users (42%) and those with HCV genotype 3 (12%), interferon-based treatment experience (31%), or cirrhosis (29%). The 92% SVR rate in these patients is, however, largely comparable to that reported for coinfected patients both in clinical trials [2] and in other real-world settings [9, 11, 12, 16, 29].
Our goal was to determine how HCV treatment selection according to guidelines and baseline patient characteristics predicts treatment outcome. DAA treatments were more effective in real-world coinfected patients than we anticipated. Many patients (38%) were prescribed regimens that were outside current clinical guidelines. Contrary to our expectations, this did not greatly increase the risk of treatment failure—particularly in patients with genotype 1 (prior OR, 1.5; 95% CrI, 0.38–6.0; posterior OR, 1.0; 95% CrI, 0.40–2.5).
In genotype 1 patients, most off-label or low-efficacy regimens were those without ribavirin in cirrhotic or interferon-based treatment-experienced patients, or with a shorter duration than recommended in treatment-naïve noncirrhotics. Reclassifying 8 rather 12 weeks of sofosbuvir/ledipasvir as an acceptable regimen for genotype 1 patients if treatment-naïve, noncirrhotic, and with HCV RNA <6 million IU/mL, as is the case in HCV monoinfection, had little additional influence on treatment failure. In this particular situation, the shorter treatment may be reasonable despite current guidelines [30]. Although 10 genotype 1 patients had potential drug–drug interactions, we identified only 1 case where such interactions could have possibly contributed to treatment failure (a patient receiving elbasvir/grazoprevir and darunavir).
Note that at the time patients were treated, the treatment received might have been the best available and recommended by the guidelines of the day (eg, sofosbuvir/ribavirin for genotypes 2 and 3). However, even then it was known that the first DAA treatments for genotype 2 and 3 were not as effective as those available for genotype 1 [31]. Using current guidelines to define off-label or low-efficacy regimens allows us to assess the impact of earlier suboptimal treatment in real-world data. The low efficacy of early DAAs used for our genotype 2 and 3 patients most likely had little impact on the overall SVR rate in our study because we had relatively few patients with these genotypes. In any event, new highly effective pangenotypic regimens such as sofosbuvir/velpatasvir and glecaprevir/pibrentasvir mean that genotype is no longer a barrier to successful treatment [32].
Among patient-related factors, the presence of cirrhosis was an important risk factor for treatment failure. Recent studies have reported similar findings in coinfected patients [12, 16, 29]. This is of particular importance in the setting of HIV coinfection, where liver disease progression is accelerated and liver-related mortality is a leading cause of death. Furthermore, coinfected patients may have additional comorbid conditions that increase the risk of fatty liver disease and associated liver complications. Because the off-label/low-efficacy indicator could reflect an inappropriate treatment for cirrhotics, this indicator and the cirrhosis indicator are potentially correlated. Re-running the main analysis without the cirrhosis indicator led to only a slight increase in the estimated association between off-label/low-efficacy treatment and treatment failure (posterior OR, 1.4; 95% CrI, 0.67–3.0). This implies that cirrhosis is indeed an independent risk factor for treatment failure despite current strategies—longer treatment or adding ribavirin [33]. There remains little reason to delay HCV therapy in HIV-infected patients when delay increases the risk of treatment failure and untreated patients are at greater risk of end-stage liver disease, with transplantation seldom being an option [34].
The other important risk factor for treatment failure in our patients was high-frequency IDU. This potential harm only became apparent when we separated this exposure from hazardous alcohol use; illustrating the detrimental effects of specific risk behaviors may be missed if general indicators of substance use are used. The destabilizing effects of injection cocaine and methamphetamine use likely impact treatment outcomes through reduced adherence. These are also the same patients at high risk of HCV transmission [35, 36] and reinfection [26], so, although SVR rates are lower, treatment is still effective and should not be withheld. This finding stresses the need for integrative HCV care that includes addiction therapies for drugs other than opioids, adherence support, and harm reduction services to improve treatment outcomes in this priority population for HCV elimination [5, 37].
One unexpected finding was that females responded better to DAA treatment than anticipated, even after controlling for risk behaviors, such as IDU and alcohol consumption, that are more prevalent in men, behaviors that typically increase the risk of failure by reducing adherence. The implications of this finding are unclear: the same finding has been reported in other real-world settings [29, 38, 39], although without controlling for these risk behaviors. This raises the possibility that there may be biologic explanations given that HCV infection affects men and women differently [40].
Not completing treatment for the prescribed duration was likely to have been one of the most important reasons for treatment failure in our cohort. Not completing treatment is an important component of nonadherence, although patients might also take treatment for the prescribed duration but sporadically miss doses. Among our 23 treatment failures, 10 patients took treatment for less than the prescribed 12 weeks (all were treated for at least 5 weeks) (Table 3). In a preliminary analysis, before making a distinction between prescribed and actual treatment durations, these 10 patients were considered to be on an off-label, low-efficacy regimen; this risk factor was then strongly associated with failure (posterior OR, 2.4; 95% CrI, 1.1–5.3). Unfortunately, future nonadherence cannot be measured before starting treatment and does not seem easy to predict with readily available risk factors such as ethnicity, high-frequency injection drug use, and detectable HIV RNA, although all had at least some association with treatment failure.
HIV-related immunosuppression has been associated with treatment failure [16, 29]. We intentionally did not include baseline CD4+ cell count in our models, because low values may simply reflect cirrhosis [41, 42]. In response to recent studies, we subsequently added nadir CD4+ <200 cells/µL to the main model, assigning this a probably harmful prior (prior OR, 2.0; 95% CrI, 0.5–8.0) to reflect the ORs reported by Berenguer et al. [29], but we found no association with treatment failure (posterior OR, 0.84; 95% CrI, 0.38–1.9). This additional sensitivity analysis does not support the hypothesis that immune suppression influences treatment failure.
Our study has several strengths. First, our patients were recruited from a range of real-world clinical settings—community-based and tertiary care centers in urban and more rural areas of Canada. Our study has a significant proportion of traditionally understudied patient populations, so our findings can likely be generalized to other settings serving a diverse HCV/HIV-coinfected population. Second, we collected detailed substance use data not usually available in clinical cohorts and additional HCV treatment data with which to assess treatment outcome in between routine semi-annual follow-up visits. Third, we took a Bayesian approach to assessing the influence of risk factors selected a priori, so that existing clinical knowledge would constrain estimates to a clinically plausible range despite a modest sample size and an expected failure rate of less than 10%. Finally, we carried out several sensitivity analyses to examine the robustness of our results to different assumptions about missing data, alternative models, and prior expectations.
Our findings should be considered in light of several limitations. Although we used methods to minimize small sample regression bias, there remained a limited number of DAA failures, so our estimates are imprecise. With few failures and few patients of black ethnicity, we could not consider this ethnicity as a risk factor, nor, for the same reason, could we consider any effects associated with different genotypes or subtypes. We note that these were not risk factors in other studies [12, 29]. Again, with few failures, we could not explore which components of our composite off-label or low-efficacy treatment indicator were the most important. Although treatment adherence seems an important cause of treatment failure, the risk factors we considered seem unable to predict future nonadherence; hence we could not identify specific patient populations where intervention might improve treatment success beyond those injecting drugs with high frequency.
CONCLUSIONS
All-oral DAA treatment was more effective in real-world HCV/HIV-coinfected patients than anticipated, across a range of patient characteristics that have led the coinfected to be considered more difficult to treat. Clearly treatment could still be improved; patients with cirrhosis, in particular, continue to have an increased risk of treatment failure. Adherence still presents a challenge: Possible solutions are integrated HCV and addiction care and, ultimately, the development of shorter-duration treatments (ie, ≤6 weeks). But our data support the view that HCV/HIV-coinfected patients can no longer be considered a difficult-to-treat population.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank all study coordinators and nurses for their assistance with study coordination, participant recruitment, and care.
Financial support. The Canadian Co-infection Cohort is funded by the Canadian Institutes of Health Research (CIHR; FDN-143270), the CIHR-Canadian HIV Trials Network (CTN-222), and the Fonds de Recherche Québec-Santé, Réseau SIDA/Maladies Infectieuses (FRQ-S). C.R. is supported by a postdoctoral fellowship from the CIHR. M.B.K. was supported by a Chercheur National career award from the FRQ-S.
Canadian Co-infection Cohort investigators (CTN222). Drs. Lisa Barrett, QEII Health Science Center for Clinical Research, Halifax, Nova Scotia; Jeff Cohen, Windsor Regional Hospital Metropolitan Campus, Ontario; Brian Conway, Vancouver Infectious Diseases Research and Care Centre, British Columbia; Curtis Cooper, the Ottawa Hospital Research Institute, Ontario; Pierre Côté, Clinique du Quartier Latin, Montréal, Quebec; Joseph Cox, McGill University Health Centre, Division of Infectious Diseases and Chronic Viral Illness Service, Montreal, Quebec; John Gill, Southern Alberta HIV Clinic, Calgary; Shariq Haider, McMaster University, Hamilton, Ontario; Mark Hull, British Columbia Centre for Excellence in HIV/AIDS, Vancouver; Marina Klein, McGill University Health Centre, Division of Infectious Diseases and Chronic Viral Illness Service, Montreal, Quebec; Julio Montaner, St. Paul’s Hospital, Vancouver, British Columbia; Erica Moodie, McGill University, Montreal, Quebec; Neora Pick, Oak Tree Clinic, Children’s and Women’s Health Centre of British Columbia, University of British Columbia, Vancouver; Anita Rachlis, Sunnybrook & Women’s College Health Sciences Centre, Toronto, Ontario; Danielle Rouleau, Centre Hospitalier de l’Université de Montréal, Quebec; Aida Sadr, Native BC Health Center, St-Paul’s Hospital, Vancouver, British Columbia; Steve Sanche, SHARE University of Saskatchewan, Saskatoon; Roger Sandre, HAVEN Program, Sudbury, Ontario; Mark Tyndall, Department of Medicine, Infectious Diseases Division, University of Ottawa, Ontario; Marie-Louise Vachon, Centre Hospitalier Universitaire de Québec; Sharon Walmsley, University Health Network, Toronto, Ontario; and Alex Wong, Regina Qu’Appelle Health Region, Regina General Hospital, Saskatchewan.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
The Canadian Co-Infection Cohort Investigators:
Lisa Barrett, Jeff Cohen, Brian Conway, Curtis Cooper, Pierre Côté, Joseph Cox, John Gill, Shariq Haider, Mark Hull, Marina Klein, Julio Montaner, Erica Moodie, Neora Pick, Anita Rachlis, Danielle Rouleau, Aida Sadr, Steve Sanche, Roger Sandre, Mark Tyndall, Marie-Louise Vachon, Sharon Walmsley, and Alex Wong
References
- 1. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, et al. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med 2017; 166:637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sikavi C, Chen PH, Lee AD, et al. Hepatitis C and human immunodeficiency virus coinfection in the era of direct-acting antiviral agents: no longer a difficult-to-treat population. Hepatology 2018; 67:847–57. [DOI] [PubMed] [Google Scholar]
- 3. Razavi H, Robbins S, Zeuzem S, et al. Hepatitis C virus prevalence and level of intervention required to achieve the WHO targets for elimination in the European Union by 2030: a modelling study. Lancet Gastroenterol Hepatol 2017; 2:325–36. [DOI] [PubMed] [Google Scholar]
- 4. Grebely J, Oser M, Taylor LE, Dore GJ. Breaking down the barriers to hepatitis C virus (HCV) treatment among individuals with HCV/HIV coinfection: action required at the system, provider, and patient levels. J Infect Dis 2013; 207(Suppl 1):S19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grebely J, Dore GJ, Morin S, et al. Elimination of HCV as a public health concern among people who inject drugs by 2030 - what will it take to get there? J Int AIDS Soc 2017; 20:22146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martin NK, Boerekamps A, Hill AM, Rijnders BJA. Is hepatitis C virus elimination possible among people living with HIV and what will it take to achieve it? J Int AIDS Soc 2018; 21(Suppl 2):e25062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016; 16:797–808. [DOI] [PubMed] [Google Scholar]
- 8. Peters L, Klein MB. Epidemiology of hepatitis C virus in HIV-infected patients. Curr Opin HIV AIDS 2015; 10:297–302. [DOI] [PubMed] [Google Scholar]
- 9. Saeed S, Strumpf EC, Walmsley SL, et al. Canadian Co-Infection Cohort Study How generalizable are the results from trials of direct antiviral agents to people coinfected with HIV/HCV in the real world? Clin Infect Dis 2016; 62:919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hawkins C, Grant J, Ammerman LR, et al. High rates of hepatitis C virus (HCV) cure using direct-acting antivirals in HIV/HCV-coinfected patients: a real-world perspective. J Antimicrob Chemother 2016; 71:2642–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Piroth L, Wittkop L, Lacombe K, et al. ANRS CO13 HEPAVIH study group Efficacy and safety of direct-acting antiviral regimens in HIV/HCV-co-infected patients - French ANRS CO13 HEPAVIH cohort. J Hepatol 2017; 67:23–31. [DOI] [PubMed] [Google Scholar]
- 12. Bhattacharya D, Belperio PS, Shahoumian TA, et al. Effectiveness of all-oral antiviral regimens in 996 human immunodeficiency virus/hepatitis C virus genotype 1-coinfected patients treated in routine practice. Clin Infect Dis 2017; 64:1711–20. [DOI] [PubMed] [Google Scholar]
- 13. Falade-Nwulia O, Sutcliffe C, Moon J, et al. High hepatitis C cure rates among black and nonblack human immunodeficiency virus-infected adults in an urban center. Hepatology 2017; 66:1402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Butt ZA, Shrestha N, Wong S, et al. BC Hepatitis Testers Cohort A syndemic approach to assess the effect of substance use and social disparities on the evolution of HIV/HCV infections in British Columbia. PLoS One 2017; 12:e0183609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wyles DL, Sulkowski MS, Dieterich D. Management of hepatitis C/HIV coinfection in the era of highly effective hepatitis C virus direct-acting antiviral therapy. Clin Infect Dis 2016; 63(Suppl 1):S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boesecke C, Ingiliz P, Berger F, et al. Liver cirrhosis as a risk factor for direct-acting antiviral therapy failure in real-life hepatitis C virus/human immunodeficiency virus coinfection. Open Forum Infect Dis 2017; 4(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klein MB, Saeed S, Yang H, et al. Cohort profile: the Canadian HIV-hepatitis C co-infection cohort study. Int J Epidemiol 2010; 39:1162–9. [DOI] [PubMed] [Google Scholar]
- 18. El-Zayadi AR. Hepatitis C comorbidities affecting the course and response to therapy. World J Gastroenterol 2009; 15:4993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Infectious Disease Society of America (IDSA). HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. 2017. [Google Scholar]
- 20. Leri F, Stewart J, Tremblay A, Bruneau J. Heroin and cocaine co-use in a group of injection drug users in Montréal. J Psychiatry Neurosci 2004; 29:40–7. [PMC free article] [PubMed] [Google Scholar]
- 21. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 1998; 158:1789–95. [DOI] [PubMed] [Google Scholar]
- 22. Greenland S, Mansournia MA, Altman DG. Sparse data bias: a problem hiding in plain sight. BMJ 2016; 352:i1981. [DOI] [PubMed] [Google Scholar]
- 23. Greenland S. Bayesian perspectives for epidemiological research: I. Foundations and basic methods. Int J Epidemiol 2006; 35:765–75. [DOI] [PubMed] [Google Scholar]
- 24. Greenland S. Bayesian perspectives for epidemiological research. II. Regression analysis. Int J Epidemiol 2007; 36:195–202. [DOI] [PubMed] [Google Scholar]
- 25. Greenland S. Prior data for non-normal priors. Stat Med 2007; 26:3578–90. [DOI] [PubMed] [Google Scholar]
- 26. Young J, Rossi C, Gill J, et al. Canadian Co-infection Cohort Investigators Risk factors for hepatitis C virus reinfection after sustained virologic response in patients coinfected with HIV. Clin Infect Dis 2017; 64:1154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Young J, Weis N, Hofer H, et al. The effectiveness of daclatasvir based therapy in European patients with chronic hepatitis C and advanced liver disease. BMC Infect Dis 2017; 17:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kowdley KV, Sundaram V, Jeon CY, et al. Eight weeks of ledipasvir/sofosbuvir is effective for selected patients with genotype 1 hepatitis C virus infection. Hepatology 2017; 65:1094–103. [DOI] [PubMed] [Google Scholar]
- 29. Berenguer J, Gil-Martin Á, Jarrin I, et al. All-oral direct-acting antiviral therapy against hepatitis C virus (HCV) in human immunodeficiency virus/HCV-coinfected subjects in real-world practice: Madrid Coinfection Registry findings. Hepatology 2018; 68:32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ingiliz P, Christensen S, Kimhofer T, et al. Sofosbuvir and ledipasvir for 8 weeks for the treatment of chronic hepatitis C virus (HCV) infection in HCV-monoinfected and HIV-HCV-coinfected individuals: results from the German Hepatitis C Cohort (GECCO-01). Clin Infect Dis 2016; 63:1320–4. [DOI] [PubMed] [Google Scholar]
- 31. Sulkowski MS, Naggie S, Lalezari J, et al. PHOTON-1 Investigators Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection. JAMA 2014; 312:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pol S, Parlati L. Treatment of hepatitis C: the use of the new pangenotypic direct-acting antivirals in “special populations.” Liver Int 2018; 38(Suppl 1):28–33. [DOI] [PubMed] [Google Scholar]
- 33. Hézode C. Treatment of hepatitis C: results in real life. Liver Int 2018; 38(Suppl 1):21–7. [DOI] [PubMed] [Google Scholar]
- 34. Klein MB, Rockstroh JK, Wittkop L. Effect of coinfection with hepatitis C virus on survival of individuals with HIV-1 infection. Curr Opin HIV AIDS 2016; 11:521–6. [DOI] [PubMed] [Google Scholar]
- 35. Miller CL, Johnston C, Spittal PM, et al. Opportunities for prevention: hepatitis C prevalence and incidence in a cohort of young injection drug users. Hepatology 2002; 36:737–42. [DOI] [PubMed] [Google Scholar]
- 36. Cunningham EB, Jacka B, DeBeck K, et al. Methamphetamine injecting is associated with phylogenetic clustering of hepatitis C virus infection among street-involved youth in Vancouver, Canada. Drug Alcohol Depend 2015; 152:272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bruggmann P, Litwin AH. Models of care for the management of hepatitis C virus among people who inject drugs: one size does not fit all. Clin Infect Dis 2013; 57(Suppl 2):S56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Backus LI, Belperio PS, Shahoumian TA, et al. Real-world effectiveness and predictors of sustained virological response with all-oral therapy in 21,242 hepatitis C genotype-1 patients. Antivir Ther 2017; 22:481–93. [DOI] [PubMed] [Google Scholar]
- 39. Sarpel D, Wasserman I, Trochtenberg A, et al. Non-adherence is the most important risk factor for ledipasvir/sofosbuvir HCV treatment failure in the real world (conference abstract). In: The Liver Meeting; November 11–15, 2016; Boston. [Google Scholar]
- 40. Baden R, Rockstroh JK, Buti M. Natural history and management of hepatitis C: does sex play a role? J Infect Dis 2014; 209(Suppl 3):S81–5. [DOI] [PubMed] [Google Scholar]
- 41. Gandhi RT. Cirrhosis is associated with low CD4+ T cell counts: implications for HIV-infected patients with liver disease. Clin Infect Dis 2007; 44:438–40. [DOI] [PubMed] [Google Scholar]
- 42. Hull MW, Rollet K, Odueyungbo A, et al. Canadian Co-infection Cohort Investigators Factors associated with discordance between absolute CD4 cell count and CD4 cell percentage in patients coinfected with HIV and hepatitis C virus. Clin Infect Dis 2012; 54:1798–805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.