Abstract
Background
Hepatitis C virus (HCV) cirrhosis is the leading indication for liver transplantation in the United States, although nonalcoholic steatohepatitis (NASH) is on the rise. Increasingly effective HCV antivirals are available, but their association with diagnosis-specific liver transplantation rates and early graft survival is not known.
Methods
The Scientific Registry of Transplant Recipients database records were retrospectively stratified by HCV antiviral era: interferon (2003-2010), protease inhibitors (2011-2013), and direct-acting antivirals (2014 to present). Kaplan-Meier, χ2, and multivariable Cox proportional hazards regression models evaluated the effects of antiviral era and etiology of liver disease on transplantation rates and graft survival over 3 years.
Results
Liver transplants for HCV decreased (35.3% to 23.6%), whereas those for NASH and alcoholic liver disease increased (5.8% to 16.5% and 15.6% to 24.0%) with each advancing era (all P < 0.05). Early graft survival improved with each advancing era for HCV but not for hepatitis B virus, NASH, or alcoholic liver disease (multivariable model era by diagnosis interaction P < 0.001). Era-specific multivariable models demonstrated that the risk of early graft loss for NASH was 22% lower than for HCV in the interferon era (hazard ratio, 0.78; 95% confidence interval, 0.64-0.96; P = 0.02) but risks associated with these diagnoses did not differ significantly in the protease inhibitor (P = 0.06) or direct-acting antiviral eras (P = 0.08).
Conclusions
Increasing effectiveness of HCV antivirals corresponds with decreased rates of liver transplantation for HCV and improved early graft survival. As the rates of liver transplant for NASH continue to increase, focus will be needed on the prevention and effective therapies for this disease.
Liver transplantation has saved almost 500 000 life-years in the United States since 1987, with 30% of patients undergoing liver transplantation for hepatitis C virus (HCV)-related liver disease.1 Approximately 0.7% of the United States population harbors HCV RNA and twice as many patients have HCV-specific antibodies indicating prior infection.2 Until recently, the only treatment to eradicate HCV infection consisted of interferon (IFN) plus ribavirin, which was successful in only a minority of patients and had significant treatment-limiting side effects.3,4 Recurrence of HCV is universal after liver transplantation; for patients with sufficient follow-up, nearly all of those transplanted for HCV demonstrated biopsy proven cirrhosis within 5 years.5 Additionally, early HCV cholestatic recurrence, which limits graft survival, has historically affected up to 10% of liver transplants for HCV.6,7 The recurrence of HCV additionally resulted in significantly decreased graft and patient survival compared with liver transplantation for other indications, including hepatitis B virus (HBV), alcoholic liver disease (ALD), and nonalcoholic steatohepatitis (NASH).8,9 Liver transplantation for HCV thus prolonged recipients' lives but ultimately did not cure them of liver disease.
The past 5 years have seen a field-changing shift in the treatment of HCV with the advent of protease inhibitors (PI) in 2011 and direct-acting antiviral (DAA) regimens in late 2013. There have been 12 agents approved for treating HCV since 2011, including combinations effective for treating all 6 major genotypes. These highly effective new medications allow for the nearly universal eradication of HCV in both the pretransplant and posttransplant states with much less morbidity than IFN-based therapy.10,11 The impact of modern HCV treatment options on the development of end-stage liver disease and on the field of liver transplantation is only beginning to be elucidated.12 As outcomes after transplantation for HCV have previously been worse than other major indications for transplant, it is important for the medical and liver transplant practitioners charged with stewarding this scarce resource to understand how these new therapies influence posttransplant outcomes.
After the development of effective HCV therapy, it is important to examine its effect on liver transplantation and outcomes for patients with HCV. The aim of this study is to evaluate the effects of diagnosis and antiviral treatment era on: (1) temporal trends in transplantation rates and (2) graft survival over the first 3 years after deceased donor liver transplantation in patients with HCV, HBV, NASH and ALD.
MATERIALS AND METHODS
Database, Inclusion Criteria, and Data Encoding
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, waitlisted candidates, and transplant recipients in the United States, submitted by members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration, US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. After institutional review board approval, SRTR Standard Analysis Files (June 2017 release) transplant records were linked with candidate, donor and follow-up data elements.
Records were identified for adult (age ≥18 years) deceased donor whole liver transplant recipients based on SRTR-defined primary diagnoses and classified as: (1) HCV (AHN type C, Cirrhosis type C, and Alcoholic cirrhosis with HCV), (2) HBV (AHN type B ABSAg+ and cirrhosis type B HBSAg+), (3) NASH (cirrhosis fatty liver), (4) ALD (alcoholic cirrhosis), and (5) other. Antiviral era was classified based on transplant date and stratified using the approach of Flemming et al13 as: IFN (January 2003 to December 2010), PI (January 2011 to December 2013), and DAA (January 2014 to May 2017). An additional inclusion criterion was Model for End-stage Liver Disease (MELD) score at transplant 15 or greater and no prior transplant. A laboratory of MELD ≥15 was chosen as this is the threshold MELD at which the benefit of liver transplant becomes apparent, and the focus of the study was on patients with decompensated liver disease. The Liver Donor Risk Index (LDRI) was computed using previously reported methods.14
Statistical Methods
The effect of antiviral era on the proportions of persons transplanted within each diagnosis category (HCV, HBV, NASH, ALD, other) was evaluated using the χ2 test. Pairwise z-tests were used to evaluate differences between diagnosis-specific between-era proportions. Among persons transplanted for HCV, HBV, NASH and ALD, survival analysis methods tested the main and interaction effects of diagnosis and era on graft survival (event = retransplantation or death) over the first 3 years after liver transplantation. Observations were censored: (1) on the date of retransplantation or death if these events occurred within 3 years or (2) at the 3-year follow-up date if they occurred >3 years after liver transplantation. This approach equilibrated follow-up time between the antiviral therapy eras, for which total follow-up time necessarily differed.
Kaplan-Meier analyses with log-rank tests were conducted to evaluate the unadjusted effects on graft survival of era within each diagnosis and of diagnosis within each era. Cox multivariable models tested the effects of diagnosis, era, and whether the effect of diagnosis differed by era (via the diagnosis by era interaction effect) on the likelihood of graft loss over 3 years. All multivariable models adjusted for recipient age (years), laboratory MELD score at transplant and whether the recipient was HCV antibody positive, had been approved for hepatocellular carcinoma (HCC) exception points, was on life support or dialysis at transplant, or had diabetes. Additional donor-related covariates were LDRI score and whether diabetic. Covariables were chosen based on their statistical significance in prior studies and clinical experience. In addition to the comprehensive multivariable model that directly tested the era by diagnosis interaction effect, separate multivariable models evaluated the effect of diagnosis (reference = HCV) within each era. These secondary multivariable models adjusted for the recipient and donor characteristics included in the comprehensive model. Cases having missing data for 1 or more covariable were excluded from the multivariable models. All analyses were conducted using IBM SPSS statistical software (Version 24.0; Armonk, NY, IBM Corp.).
RESULTS
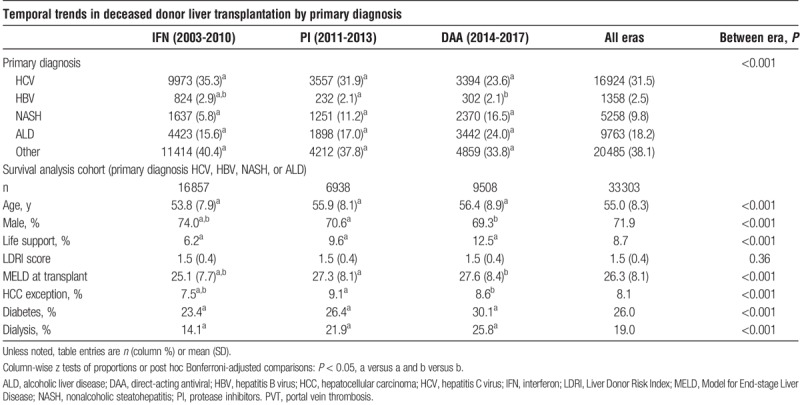
A total of 53 788 patients with decompensated end-stage liver disease as defined by a laboratory MELD score of 15 or greater were transplanted during the study period of whom 61.9% were transplanted for HCV, HBV, NASH, or ALD. The primary diagnosis for deceased donor liver transplantation changed significantly over the 15-year period (P < 0.001) (Table 1). The percentage of recipients transplanted for decompensated HCV progressively decreased from 35.3% in the IFN era to 23.6% in the DAA era (all column-wise, P < 0.05). This corresponded with a tripling of transplantation for recipients with decompensated NASH, from 5.8% in the IFN era to 16.5% in the DAA era (all column-wise, P < 0.05). There was also an era-related increase in the transplantation of patients with decompensated ALD (15.6% IFN era versus 24.0% DAA era) (all column-wise, P < 0.05), whereas the percentage of patients transplanted for decompensated HBV remained relatively stable (2.9% IFN era versus 2.1% DAA era). Era-related changes in recipient characteristics extended beyond primary diagnosis at transplantation. Recipient age at transplant and diabetes progressively increased over time (all pairwise, P < 0.05); Laboratory MELD score at transplant increased between the IFN and PI eras and remained stable thereafter. The percentage of patients receiving life support at time of transplant doubled from 6.2% in the IFN era to 12.5% in the DAA era. Correspondingly, over a quarter of patients (25.8%) required pretransplant dialysis in the DAA era compared with 14.1% in the IFN era.
TABLE 1.
Cohort characteristics
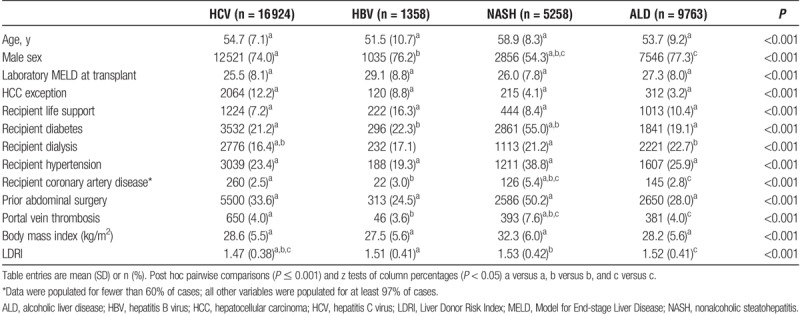
Many characteristics of liver transplant recipients varied significantly by diagnosis (column-wise, P < 0.05) (Table 2). The HCV recipients had the lowest laboratory MELD at transplant (25.5) and highest percentage of recipients with an HCC exception (12.2%). The HCV recipients also received the highest-quality organs as evidenced by the lowest LDRI score of 1.47. Conversely, NASH recipients were older at transplant and more often had comorbidities of diabetes (55%), hypertension (38.8%), and CAD (5.4%). The NASH recipients were larger (body mass index average, 32.3) and more likely to have had a prior abdominal operation (50.2%) or portal vein thrombosis (7.6%). Interestingly, nearly half of all NASH recipients were female but females comprised only about one quarter of the other recipients.
TABLE 2.
Survival analysis cohort characteristics by diagnosis
Unadjusted Kaplan Meier estimated probability of graft survival was compared between each era, stratified by (separately within) each diagnosis (Figure 1). A significant era-related improvement in graft survival was identified in HCV recipients (all log-rank, P ≤ 0.001), with 1- and 3-year point estimates increasing from 83.3% and 71.9% in the IFN era to 90.9% and 79% in the DAA era. Era-related improvement in graft survival was also noted for ALD recipients when comparing the PI or DAA era to IFN era. No differences were seen in graft survival between the IFN and DAA eras for either HBV or NASH recipients. Similarly, unadjusted Kaplan-Meier estimated probability of graft survival was compared between each of the 4 diagnoses, stratified by the 3 eras (Figure 2). In the IFN era, HCV recipients had the lowest 1- and 3-year graft survivals of any diagnosis (log-rank, P < 0.001 versus other diagnoses). However, by the DAA era, NASH recipients had lower graft survival (87.5% 1 year, 77.9% 3 years) compared with both HCV (90.0% 1 year, 79% 3 years) and ALD (90.7% 1 year, 78.9% 3 years) recipients (log-rank P ≤ 0.01).
FIGURE 1.
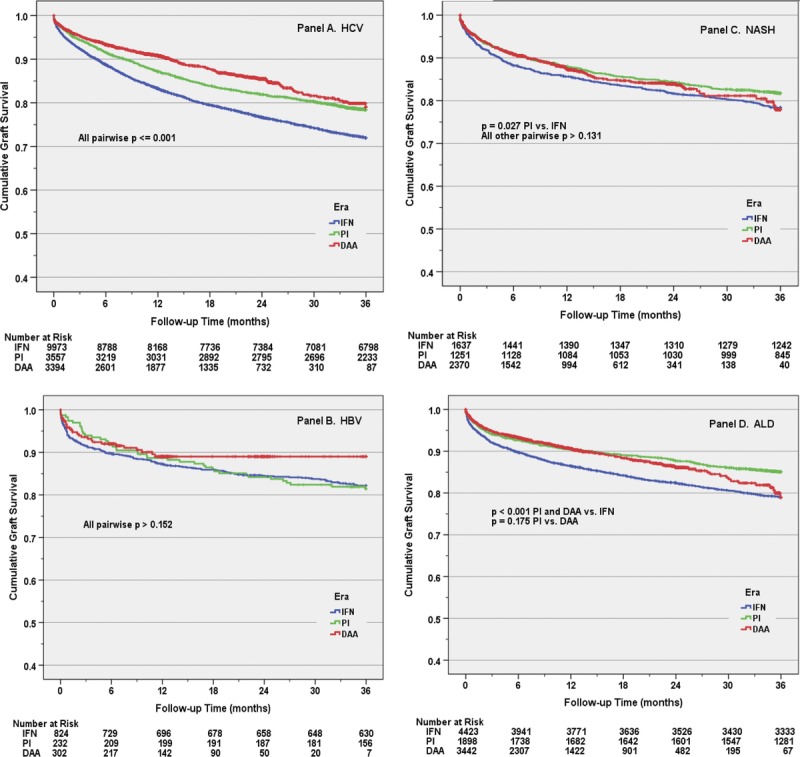
The effect of antiviral treatment era on early graft survival by diagnosis. Panel A depicts the improved graft survival among patients with hepatitis C virus (HCV) with each advancing era (all pairwise log rank P ≤ 0.001). This temporal relationship was not observed in recipients with hepatitis B virus (HBV) (panel B), nonalcoholic steatohepatitis (NASH) (panel C), or alcoholic liver disease (ALD) (panel D). DAA, direct-acting antiviral; IFN, interferon; PI, protease inhibitors.
FIGURE 2.
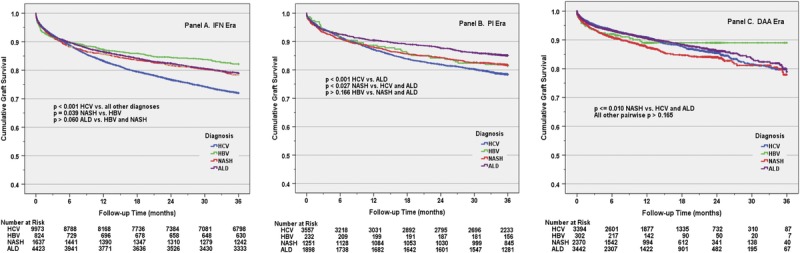
The effect of diagnosis on early graft survival by antiviral treatment era. This figure illustrates the changing relationships between the diagnosis of hepatitis C virus (HCV) and other indications, particularly nonalcoholic steatohepatitis (NASH), on early graft survival during the interferon (IFN) era (panel A), protease inhibitors (PI) era (panel B), and direct-acting antiviral (DAA) era (panel C). ALD, alcoholic liver disease; HBV, hepatitis B virus.
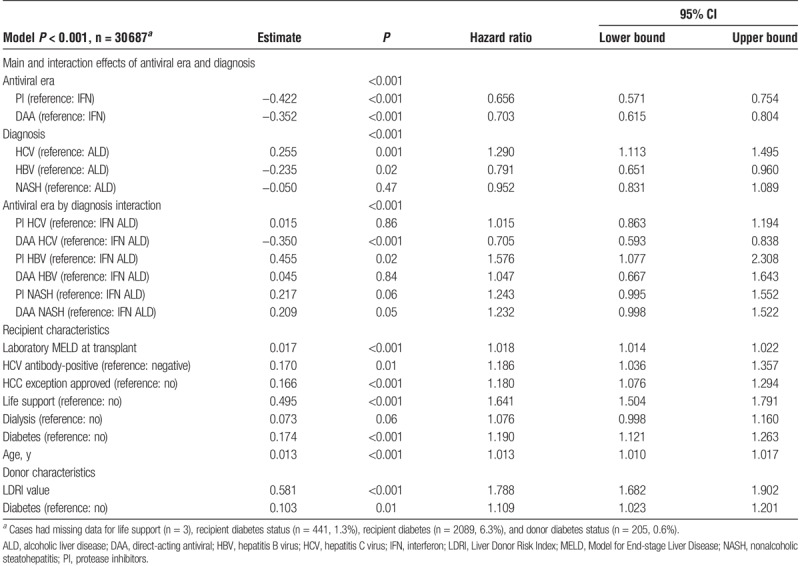
Multivariable modeling identified increasing recipient age, presence of recipient diabetes, increasing laboratory MELD score, need for life support, and HCC exception as independent predictors of graft failure over the first 3 years (Table 3). The donor characteristics of increasing LDRI score and diabetes were also associated with an increased risk of graft failure over the first 3 years. Although there were indications of both era- and diagnosis-related differences in graft survival, a statistically significant era by diagnosis interaction effect was identified (P < 0.001) precluding interpretation of these findings in isolation. Specifically, the likelihood of graft loss for a given diagnosis varied between eras as illustrated by the significantly decreased risk of graft loss for HCV recipients in the DAA era when compared with ALD recipients in the IFN era (HR 0.705; 95% CI 0.593-0.838; DAA HCV interaction contrast, Table 3). Interestingly, although not reaching statistical significance, there was a trend toward an increased risk of graft loss for NASH patients in both the PI and DAA eras when compared with the IFN era ALD reference group.
TABLE 3.
Multivariable model of the likelihood of graft failure within 3 years
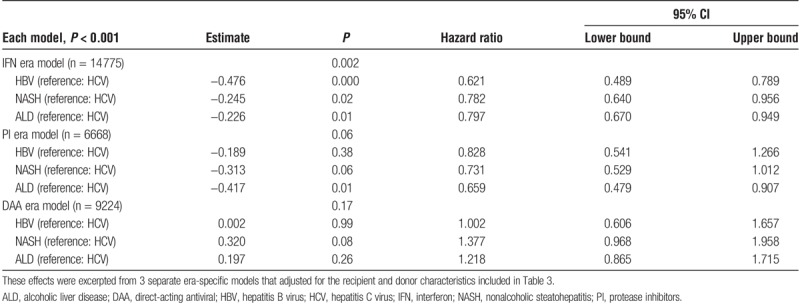
Additional era-specific multivariable modeling was performed to further evaluate the effects of diagnosis within each era in the setting of the previously-described era by diagnosis interaction effect (Table 4). These effects were excerpted from 3 separate era-specific models that adjusted for the recipient and donor characteristics included in Table 3. After adjusting for the recipient and donor characteristics, the era-specific models demonstrate that during the IFN era HBV, NASH, and ALD had a significantly decreased risk of graft failure compared with HCV. However, by the PI era only ALD had a significantly decreased risk of graft failure compared with HCV. In the most recent DAA era HCV primary diagnosis conferred no difference in risk of graft failure as compared with HBV, NASH, or ALD.
TABLE 4.
Effects of diagnosis abstracted from 3 era-specific multivariable models
DISCUSSION
The development of effective treatment for HCV infection and the progressive obesity epidemic have dramatically altered the landscape of liver transplantation in the United States. Our study examined the indication for liver transplantation over 3 eras spanning 2003 to the current time and confirms that the incidence of liver transplantation for complications of HCV-related end-stage liver disease is progressively declining while the incidence of transplantation for NASH is on the rise. Our study additionally aimed to evaluate outcomes over 3 years and found improved outcomes for patients with HCV over the eras, after accounting for era-related effects.
The effect of HCV antiviral treatment has also been examined in terms of the liver transplant waitlist. Flemming et al13 analyzed listing rates using the SRTR database from 2003 to 2015 for decompensated cirrhosis and HCV, HBV, or NASH in the DAA era compared with the IFN and PI eras and noted that waitlisting for HCV with decompensated cirrhosis declined by >30% in the DAA era. Similarly, European studies have reported delisting rates in patients with HCV since the advent of the current antiviral therapies.15 Perricone et al15 examined patients with HCV on the waitlist for liver transplant and found that 30% were delisted, with a <10% incidence of liver-related complications in the subsequent 2 years after delisting. Kwong et al16 examined the OPTN database to examine changes in waitlist mortality in listed patients with HCV. The study reported a decrease in waitlist mortality in the DAA era for patients with HCV, and the authors presumed this change to be at least in part due to the advent of DAA therapy. Correspondingly, Bowring et al17 examined the SRTR data and found an increase in the use of HCV livers (6.9% in 2010 to 16.9% in 2015). In summary, effective HCV antiviral therapy has led to a decrease in HCV liver transplants, a decrease in HCV patients being listed for liver transplant, an increase in HCV patients being removed from the liver transplant waitlist and an increase in the use of HCV donor livers.
The question that inevitably follows is whether HCV antiviral treatment has resulted in improved longer-term outcomes after liver transplantation. Cholankeril et al18 evaluated the main effects of HCV diagnosis in the UNOS database and comparably-defined transplant antiviral eras on 1-year survival after liver transplantation. They noted reduced short-term 1-year mortality and graft failure in liver transplant recipients with HCV in the DAA era compared with the pre-DAA era. Our study aimed to evaluate outcomes over 3 years and found improved outcomes for patients with HCV over the eras. Axelrod et al19 examined SRTR data from 2007 to 2016 and merged these data with national pharmaceutical claims to specifically examine the impact of posttransplant antiviral HCV treatment. The authors found that posttransplant antiviral therapy improved outcomes for HCV patients up to 3 years after liver transplant. As the use of HCV antiviral therapies become more widespread (and hopefully more accessible) the long-term effectiveness of these therapies and their effect on liver transplant outcomes will be further elucidated. Further investigations into the timing of treatment surrounding liver transplant and the treatment of HCV patients with liver disease but without cirrhosis are needed.
Concurrent with the decline of HCV liver transplants is the rise of NASH liver disease. Pandemic obesity is thought to be driving the increase of nonalcoholic fatty liver disease and NASH cirrhosis in the United States and globally.20 The burden of NASH on liver transplantation is anticipated to rise sharply, with decompensated cirrhosis and HCC attributed to NASH each projected to increase by well over 100% in the United States by 2030.21 The NASH liver disease is often viewed as the liver manifestation of metabolic syndrome, and there is interplay with obesity, diabetes, hyperlipidemia and hypertension. These patients have more comorbidities compared to patients without NASH as described in our study. Recipient age, diabetes, coronary artery disease, and portal vein thrombosis are all known risk factors associated with inferior patient and graft survival postliver transplant. All of these risk factors are more prevalent in NASH recipients and may account for the lower 1- and 3-year graft survivals in the modern DAA era when compared with HCV, HBV, and ALD recipients. Although recipients with NASH have “equivalent” survival when compared with non-NASH recipients after adjustment for age and diabetes, these represent artificial corrections as over 50% of patients with NASH have diabetes, and the pathophysiology of NASH is such that it takes longer to develop into cirrhosis. Additionally, the same risk factors leading to the development of NASH cirrhosis, metabolic syndrome and obesity, remain largely untreated postoperatively with as many as 90% of patients transplanted for NASH redeveloping NAFLD and up to 40% developing NASH in some cohorts.22-25 The same factors that cause NASH predispose these patients to progressive cardiovascular disease postoperatively despite the highly restrictive selection process for cardiovascular disease and obesity in candidates needing liver transplantation, resulting in high decline rates for patients with NASH cirrhosis. In fact, cardiovascular disease remains one of the leading causes of mortality after liver transplant, and this is likely to rise as the percentage of liver transplants performed for NASH cirrhosis increases.26,27 Although vast resources and time have been dedicated to the treatment of HCV with great success, there is currently no pharmacologic agent approved for treatment of NASH or NASH recurrence, which will soon overwhelm the liver transplant system.
This study is limited by the unavoidably shorter duration of follow up for the most recent DAA period. For this reason, we limited the follow-up to the first 3 years for all antiviral era cohorts. Although short-term outcomes may now be similar among groups the long term outcomes may diverge beyond 3 years. The limitations related to the use of a large national database include the lack of granularity of the data, namely the use of antiviral therapy and the timing of administration of antiviral therapy. The data can thus demonstrate era-related effects, but are unable to definitively confirm outcomes being related to the use of specific antiviral therapies.
In the current era of DAA HCV therapy, liver transplantation for HCV is on the decline with improved outcomes. This is being counterbalanced by a rapid increase in transplantation for NASH, which now has the lowest overall 1- and 3-year graft survival of the diagnoses studied. Treatment and prevention of nonalcoholic fatty liver disease prior to the development of end-stage liver disease and prevention of recurrence posttransplant must be aggressively pursued.
Footnotes
Published online 20 February, 2019.
The authors declare no conflicts of interest.
As a condition of the SRTR data use agreement for Standard Analysis Files, we note the following: The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
N.F.P. participated in the research design and writing of the article. I.D.F. participated in the research design, writing of the article, data analysis, and critical revision. L.K.M. participated in the writing of the article, interpretation of data, and critical revision. S.A.R. participated in the data analysis. R.P. participated in the interpretation of data, critical revision. S.P.A. participated in the research design, writing of article, interpretation of data, and critical revision.
REFERENCES
- 1.Rana A, Gruessner A, Agopian VG, et al. Survival benefit of solid-organ transplant in the United States. JAMA Surg. 2015;150:252–259. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017;152:1090–1099.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berenguer M, Palau A, Aguilera V, et al. Clinical benefits of antiviral therapy in patients with recurrent hepatitis C following liver transplantation. Am J Transplant. 2008;8:679–687. [DOI] [PubMed] [Google Scholar]
- 4.Selzner N, Guindi M, Renner EL, et al. Immune-mediated complications of the graft in interferon-treated hepatitis C positive liver transplant recipients. J Hepatol. 2011;55:207–217. [DOI] [PubMed] [Google Scholar]
- 5.Firpi RJ, Clark V, Soldevila-Pico C, et al. The natural history of hepatitis C cirrhosis after liver transplantation. Liver Transpl. 2009;15:1063–1071. [DOI] [PubMed] [Google Scholar]
- 6.Berenguer M, Prieto M, Rayon JM, et al. Natural history of clinically compensated hepatitis C virus-related graft cirrhosis after liver transplantation. Hepatology. 2000;32(4 pt 1):852–858. [DOI] [PubMed] [Google Scholar]
- 7.Verna EC, Abdelmessih R, Salomao MA, et al. Cholestatic hepatitis C following liver transplantation: an outcome-based histological definition, clinical predictors, and prognosis. Liver Transpl. 2013;19:78–88. [DOI] [PubMed] [Google Scholar]
- 8.Pfitzmann R, Schwenzer J, Rayes N, et al. Long-term survival and predictors of relapse after orthotopic liver transplantation for alcoholic liver disease. Liver Transpl. 2007;13:197–205. [DOI] [PubMed] [Google Scholar]
- 9.Forman LM, Lewis JD, Berlin JA, et al. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889–896. [DOI] [PubMed] [Google Scholar]
- 10.Kwo PY, Mantry PS, Coakley E, et al. An interferon-free antiviral regimen for HCV after liver transplantation. N Engl J Med. 2014;371:2375–2382. [DOI] [PubMed] [Google Scholar]
- 11.Saab S, Rheem J, Jimenez MA, et al. Effectiveness of ledipasvir/sofosbuvir with/without ribavarin in liver transplant recipients with hepatitis C. J Clin Transl Hepatol. 2017;5:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakobsen JC, Nielsen EE, Feinberg J, et al. Direct-acting antivirals for chronic hepatitis C. Cochrane Database Syst Rev. 2017;9: CD012143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flemming JA, Kim WR, Brosgart CL, et al. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology. 2017;65:804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. [DOI] [PubMed] [Google Scholar]
- 15.Perricone G, Duvoux C, Berenguer M, et al. Delisting HCV-infected liver transplant candidates who improved after viral eradication: outcome 2 years after delisting. Liver Int. 2018;38:2170–2177. [DOI] [PubMed] [Google Scholar]
- 16.Kwong A, Kim WR, Mannalithara A, et al. Decreasing mortality and disease severity in hepatitis C patients awaiting liver transplantation in the United States. Liver Transpl. 2018;24:735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowring MG, Kucirka LM, Massie AB, et al. Changes in utilization and discard of hepatitis C-infected donor livers in the recent era. Am J Transplant. 2017;17:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cholankeril G, Li AA, March KL, et al. Improved outcomes in HCV patients following liver transplantation during the era of direct-acting antiviral agents. Clin Gastroenterol Hepatol. 2018;16:452–453. [DOI] [PubMed] [Google Scholar]
- 19.Axelrod DA, Schnitzler MA, Alhamad T, et al. The impact of direct-acting antiviral agents on liver and kidney transplant costs and outcomes. Am J Transplant. 2018;18:2437–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 21.Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhati C, Idowu MO, Sanyal AJ, et al. Long-term outcomes in patients undergoing liver transplantation for nonalcoholic Steatohepatitis-related cirrhosis. Transplantation. 2017;101:1867–1874. [DOI] [PubMed] [Google Scholar]
- 23.Yalamanchili K, Saadeh S, Klintmalm GB, et al. Nonalcoholic fatty liver disease after liver transplantation for cryptogenic cirrhosis or nonalcoholic fatty liver disease. Liver Transpl. 2010;16:431–439. [DOI] [PubMed] [Google Scholar]
- 24.Dureja P, Mellinger J, Agni R, et al. NAFLD recurrence in liver transplant recipients. Transplantation. 2011;91:684–689. [DOI] [PubMed] [Google Scholar]
- 25.Contos MJ, Cales W, Sterling RK, et al. Development of nonalcoholic fatty liver disease after orthotopic liver transplantation for cryptogenic cirrhosis. Liver Transpl. 2001;7:363–373. [DOI] [PubMed] [Google Scholar]
- 26.VanWagner LB, Lapin B, Levitsky J, et al. High early cardiovascular mortality after liver transplantation. Liver Transpl. 2014;20:1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pisano G, Fracanzani AL, Caccamo L, et al. Cardiovascular risk after orthotopic liver transplantation, a review of the literature and preliminary results of a prospective study. World J Gastroenterol. 2016;22:8869–8882. [DOI] [PMC free article] [PubMed] [Google Scholar]


