Abstract
Background
Biliary complications occur in 6% to 34% of liver transplant recipients, for which endoscopic retrograde cholangiopancreatography has become widely accepted as the first-line therapy. We evaluated long-term outcome of biliary complications in patients liver transplanted between 2004 and 2014 at Karolinska University Hospital, Stockholm.
Methods
Data were retrospectively collected, radiological images were analyzed for type of biliary complication, and graft and patient survivals were calculated.
Results
In 110 (18.5%) of 596 transplantations, there were a total of 153 cases of biliary complications: 68 (44.4%) anastomotic strictures, 43 (28.1%) nonanastomotic strictures, 24 (15.7%) bile leaks, 11 (7.2%) cases of stone- and/or sludge-related problems, and 7 (4.6%) cases of mixed biliary complications. Treatment success rates for each complication were 90%, 73%, 100%, 82% and 80%, respectively. When the endoscopic approach was unsatisfactory or failed, percutaneous transhepatic cholangiography or a combination of treatments was often successful (in 18 of 24 cases). No procedure-related mortality was observed. Procedure-related complications were reported in 7.7% of endoscopic retrograde cholangiopancreatography and 3.8% of percutaneous transhepatic cholangiography procedures. Patient survival rates, 1, 3, 5, and 10 years posttransplant in patients with biliary complications were 92.7%, 80%, 74.7%, and 54.1%, respectively, compared with 92%, 86.6%, 83.7%, and 72.8% in patients free from biliary complications (P < 0.01). Similarly, long-term graft survival was lower in the group experiencing biliary complications (P < 0.0001).
Conclusions
Endoscopic and percutaneous approaches for treating biliary complications are safe and efficient and should be considered complementing techniques. Despite a high treatment success rate of biliary complications, their occurrence still has a significant negative impact on patient and graft long-term survivals.
Biliary tract complications account for substantial morbidity and has been reported to negatively influence the graft and patient survival in patients undergoing liver transplantation (LTx).1,2 Depending on diagnostic criteria, biliary complications occur in 6% to 34% of all LTx, and most frequently consist of biliary strictures, bile leaks (BLs), and biliary stone disease.3-5 Biliary strictures are divided into groups of anastomotic (occurring at the site of liver graft bile duct anastomosis) and nonanastomotic (occurring elsewhere in the biliary tree). Anastomotic strictures (AS) appear in 3% to 14% of LTx patients, caused by improper surgical technique, fibrotic healing, and/or ischemia, and can often be successfully treated without influencing graft or patient survival.2,6 Nonanastomotic strictures (NAS) occurrence rates are similiar (5%–15%), but of a more complex nature. The pathogenesis is believed to be ischemic, immunological, or both.7
Traditionally, surgical revision and percutaneous transhepatic cholangiography (PTC) have been the primary approaches to manage biliary tract complications. However, the technique of endoscopic retrograde cholangiopancreatography (ERCP) has evolved rapidly, and has become the preferred option over the last 15 to 20 years at a majority of transplantation departments, with PTC primarily reserved for the salvage of ERCP failures.8-11
Previous studies evaluating biliary complications after LTx are often limited to a certain type of complication, and with a small study population. This study provides one of the largest study populations to date, and was conducted to determine the outcome of treatment with endoscopic and/or percutaneous transhepatic approaches in patients with biliary complications after LTx. We evaluated long-term graft and patient survival among patients developing biliary complications compared with patients who did not develop biliary complications during the same period.
MATERIALS AND METHODS
Patients
All adult patients (>18 years) undergoing LTx at the Department of Transplantation Surgery, Karolinska University Hospital Huddinge, between 2004 and 2014, were included in the study. Patients who underwent acute retransplantation or died within 2 weeks after transplantation were excluded. Follow-up was until October 1, 2015. Biliary complications were assessed by the review of radiological examinations, patient journals, and our local transplant registry. Patients were divided into groups depending on type of biliary complications: (1) AS, (2) NAS, (3) BL, (4) biliary stones and/or sludge (SS), (5) mixed complications (MC), followed by ERCP or PTC intervention at least once. Bile leak was always considered as a primary complication, regardless of simultaneous complication of any other kind. The Regional Ethics Committee in Stockholm, Sweden, approved the study (dnr: 2016/777-31/4).
Methods
All endoscopic procedures were performed with a standard therapeutic duodenoscope by experienced endoscopists. Used ERCP procedures included endoscopic sphincterotomy, balloon dilatation, and biliary stent placement. Prophylactic antibiotics were administered before the procedure. Balloon dilatation was performed using 4-, 6-, 8-, or 10-mm balloons. Single or multiple plastic stents of 7 to 10 French or metal stents of 10 mm were used to treat BLs and strictures. Patients treated with plastic stents had a planned follow-up every 3 months until stricture resolution. Patients treated with metal stents had a planned removal of the stent 12 months later. Sludge and/or stones were removed using a basket and/or a standard retrieval balloon.
A percutaneous approach (PTC) was used when deemed appropriate, in case of ERCP failure or to combine ERCP examination (rendezvous procedure). The principal technique of percutaneous biliary drainage has previously been described by Ring et al.12 Patients were treated with balloon dilatation and/or catheter placement. Balloon dilatation was performed using 4- to 7-mm balloons. Percutaneous catheters (6-14 French) were inserted with a routine exchange planned every 3 months, until stricture resolution and normalization of symptoms. Exchange of stents and/or catheters was done in advance under acute circumstances in case of fever, jaundice, cholangitis, dislocation, or leak beside the catheter.
Evaluation of Treatment
Treatment success was defined as the timepoint when internal stents/PTC drainages could be removed, whereas maintaining improved cholangiographic appearance with good biliary drainage. A complication was considered recurrent when the treatment success criteria were fulfilled, and the patient later again showed abnormal cholangiographic appearance of the same or a new type, regardless the timeframe. Patients with recurrent complications, followed by successful endoscopic or transhepatic therapy, were considered to have 2 separate cases of successful treatment. Failure of treatment was defined as abnormal cholangiographic appearance at the end of treatment, or conversion from ERCP/PTC to a surgical approach.
Statistical Analysis
Data are presented as means ± standard deviation (SD) or median (interquartile range [IQR]). Student's t test or χ2 test was performed to compare data between groups. When calculating the median time to the development of a biliary complication, recurrent complications were excluded. When calculating treatment success rate, patients with ongoing treatment, and patients dying of non–treatment-related causes were excluded. Graft and patient survivals were analyzed using Kaplan-Meier methods. Mantel-Haenszel test for equality of survival curves was performed. Calculations were performed using MEDLOG release 2015-1 (Medlog Systems, Crystal Bay, NV), Graphpad Prism version 6.0c for OS X (GraphPad Software Inc., San Diego, CA), and Microsoft Excel 2013 for Windows (Microsoft Corporation, Redmond, WA). P values <0.05 were considered significant.
RESULTS
Study Population
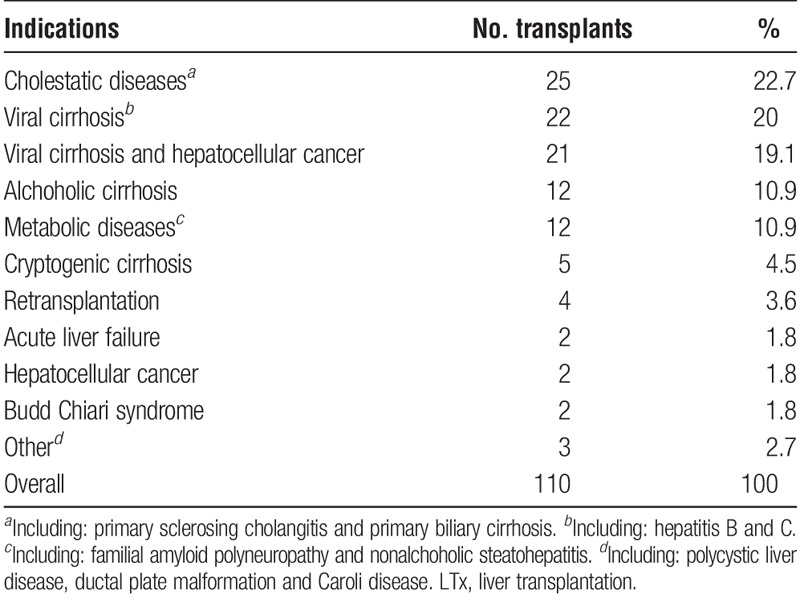
During the study period, 622 adult transplantations were performed at our clinic. Twenty-six transplantations were excluded due to either death (n = 21) or acute retransplantation (n = 5) within 2 weeks. Biliary complications were diagnosed in 110 (18.5%) of 596 LTxs, in 585 patients. One patient was included twice in the complication group after having a retransplantation because of rejection and persisting biliary complications, and again developed biliary complications in the second liver. The demographic characteristics of the patient group are shown in Table 1. There was a significant difference between patients with and without biliary complications regarding sex; males had more frequently developed biliary complications (P < 0.05). No difference was seen regarding age between the same groups. The indications for LTx in patients developing biliary complications are presented in Table 2.
TABLE 1.
Demographic data including retransplanted patients
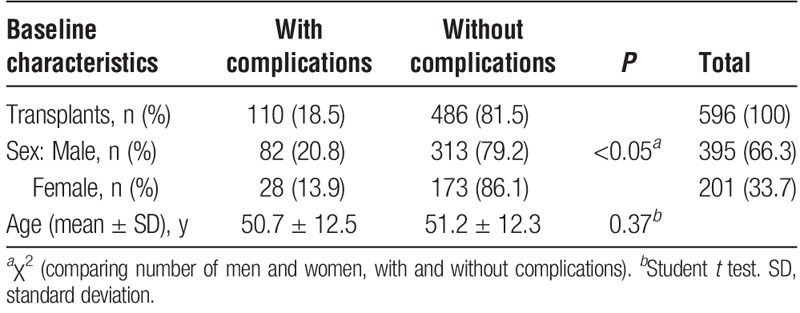
TABLE 2.
Indications for LTx in patients with biliary complications
Transplant Operation Characteristics
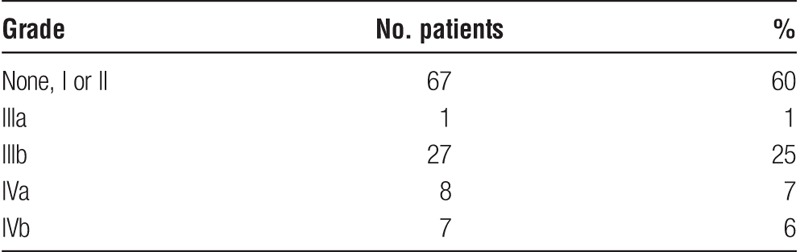
A choledochocholedochostomy (CC) was commonly made over a T-tube during the earlier years of the study. Biliary leaks occurred after T-tube placement in 9 (7.9%) of 113 transplants, compared with 15 (3.1%) of 483 transplants with other techniques (P < 0.02). Biliary complications overall occurred after T-tube placement in 22 (19.4%) of 113 transplants, compared with 88 (18.2%) of 483 transplants with other techniques (P = 0.76). Median blood loss during surgery differed for patients with or without biliary complications, 5750 mL (IQR, 2725–10 000 mL) and 3200 mL (IQR, 1425–7000 mL), respectively (P < 0.001). Detailed characteristics of the liver transplant operation for patients developing biliary complications are presented in Table 3. According to the Clavien-Dindo criteria for early postsurgical complications,13 43 (39.1%) of 110 patients that developed biliary complications had early postsurgical complications placing them in a group of IIIa or higher. This included all types of complications after transplantation. Early postsurgical complications categorized in groups II or lower were not considered relevant for this study (Table 4).
TABLE 3.
Perioperative data in liver transplanted patients with biliary complications
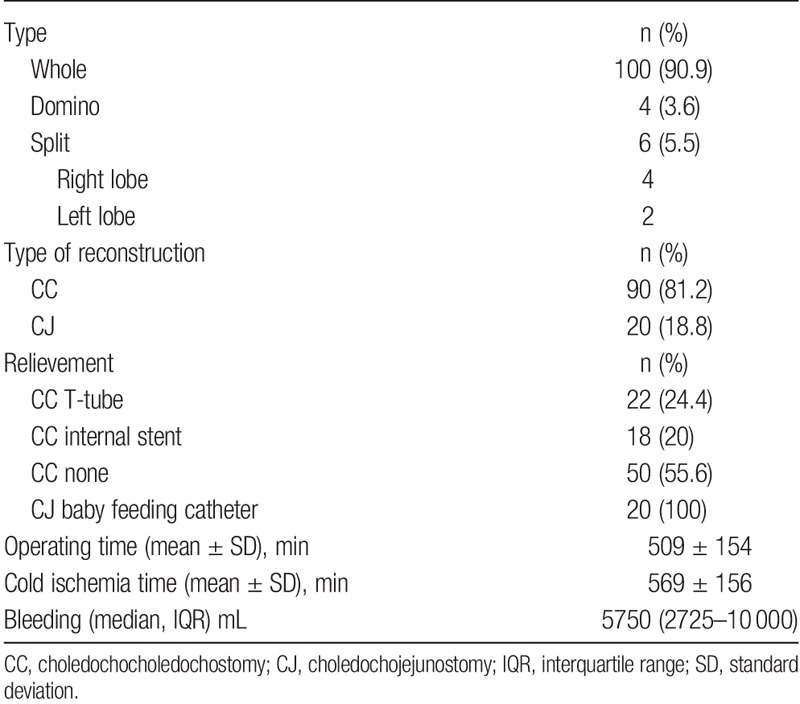
TABLE 4.
Early postsurgical complications in liver transplanted patients developing biliary complications (<30 days of operation, categorized according to the Clavien-Dindo classification)
Frequency of Biliary Complications
Primary type of biliary complication according to biliary reconstruction type is summarized in Table 5. In addition to 110 primary biliary complications, we identified concurrent, later developing, or recurrent biliary complications consisting of: 16 cases of AS, 18 cases of NAS, 4 cases of SS, and 5 cases of MC, the total number of cases of biliary complications adding up to 153. The incidence of AS and NAS, including strictures that occurred as secondary biliary complications, but excluding recurrent biliary complications, were 65 (10.9%) of 596 cases and 36 (6%) of 596 cases, respectively. The total number of transplants developing any kind of biliary stricture was 99 (16.6%) of 596 cases. The incidence of BL was 24 (4%) of 596. Median time from LTx to development of AS, NAS, BL, SS, and MC was 209 days (IQR, 56–615 days), 331 days (IQR, 123–767 days), 34 days (IQR, 25–47 days), 434 days (IQR, 182–488 days), and 105 days (IQR, 40–1190 days), respectively.
TABLE 5.
Liver transplant patients with biliary complications according to primary type of complication and reconstruction
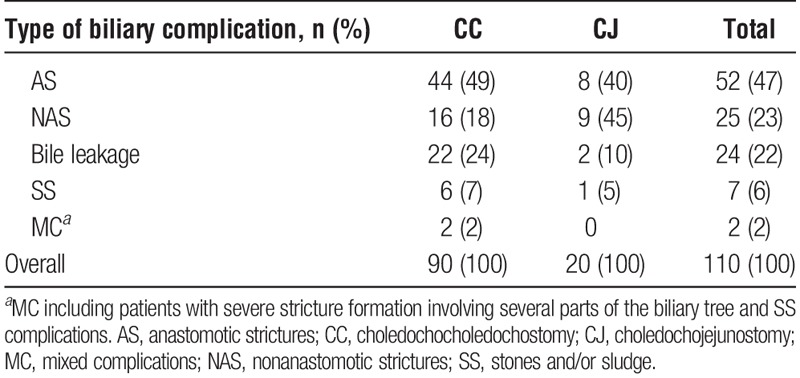
Treatment Results
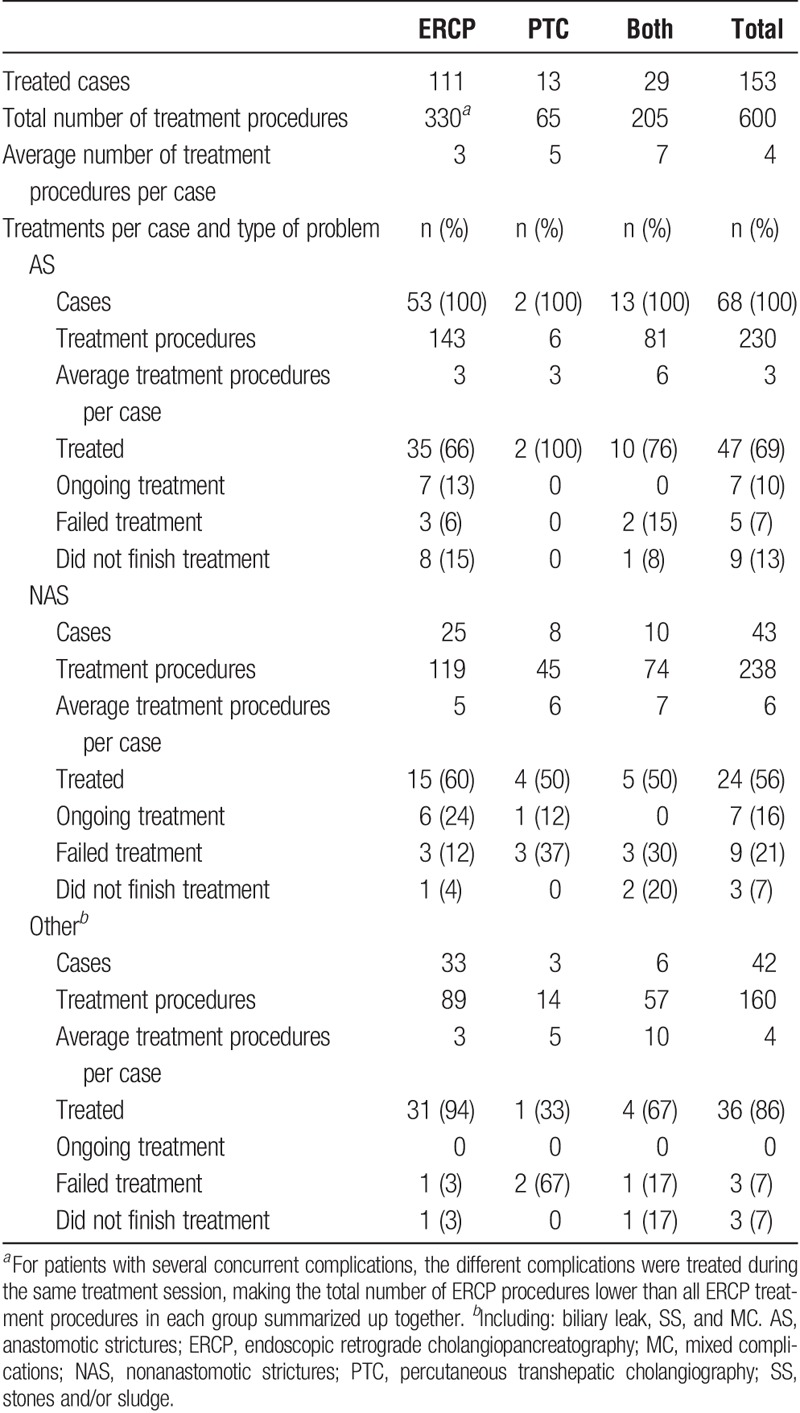
In Table 6, data are presented on treatments used and the results obtained. ERCP and/or PTC were successful in 47 (90.4%) of 52 AS cases, 24 (72.7%) of 33 NAS cases, 23 (100%) of 23 BL cases, 9 (81.8%) of 11 SS cases, and 4 (80%) of 5 MC cases, adjusting for patients who did not finish treatment and patients with ongoing treatment. In 3 patients with AS and 1 with MC where ERCP treatment was unsuccessful to reach or pass the stricture, PTC was used with success. A mixed approach of ERCP and PTC treatment was successful in 7 cases of AS, 5 cases of NAS, 1 case of BL and 2 cases of MC. Two patients with AS, 4 patients with NAS, 2 patients with SS and 1 patient with MC needed a retransplant, and 2 patients with complete anastomotic stenosis was re-anastomosed surgically. One AS patient with failed ERCP, and 1 NAS patient with failed PTC, had spontaneous resolution of symptoms. In 3 patients with NAS, ERCP and/or PTC were not able to reach the stricture, and decision was made to await further treatment. All patients who did not finish treatment died from non–treatment-related causes. The recurrence rate after successful AS and NAS treatment was 3/47 (6.4%) and 7/24 (29.2%) respectively.
TABLE 6.
Treatment summary
Procedure-related Complications
There were no major procedure-related complications reported in 418 ERCP and 182 PTC procedures. After ERCP, 7 patients developed moderate, and 7 patients mild pancreatitis, according to the Cotton criteria.14 In addition, 7 cases of hemorrhage, 6 cases of cholangitis and 5 cases of sepsis were reported, adding up to a complication rate of 7.7% in the ERCP group. In the PTC group, complications were limited to 6 cases of sepsis and 1 case of cholangitis, with a total complication rate of 3.8%.
Graft and Patient Survivals
Graft survival was impaired in patients with biliary complications compared with the graft survival in patients without biliary complications (Figure 1). The 1-, 3-, 5-, and 10-year graft survival rates for patients with biliary complications were 90%, 73.8%, 67.4% and 44%, respectively. The corresponding graft survival rates for patients without complications were 91.6%, 86.1%, 82.9%, and 71.8%, respectively (P < 0.0001).
FIGURE 1.
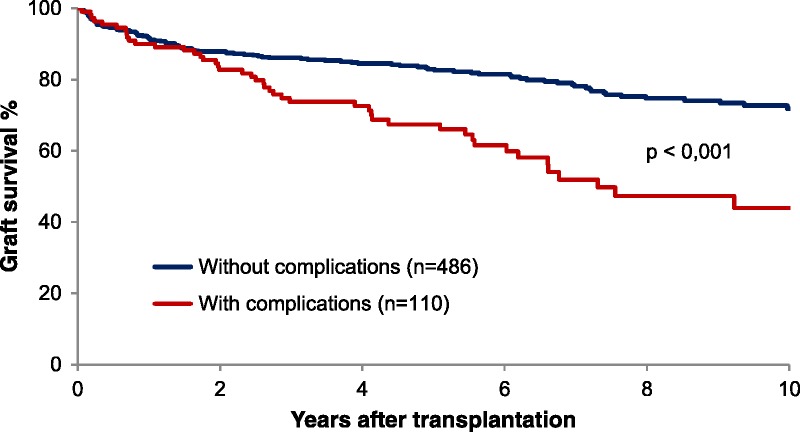
Long term graft survival in patients with or without biliary complications after LTx, including retransplantations fulfilling the study inclusion criteria of 2 weeks graft survival. LTx, liver transplantation.
Figure 2 displays patient survival rate in 109 patients developing biliary complications, and a control group of 476 patients without biliary complications. The 1-, 3-, 5-, and 10-year patient survivals for patients with biliary complications were 92.7%, 80%, 74.7%, and 54.1%, respectively. The corresponding survival rates for patients without complications were 92%, 86.6%, 83.7%, and 72.8%, respectively (P < 0.01).
FIGURE 2.
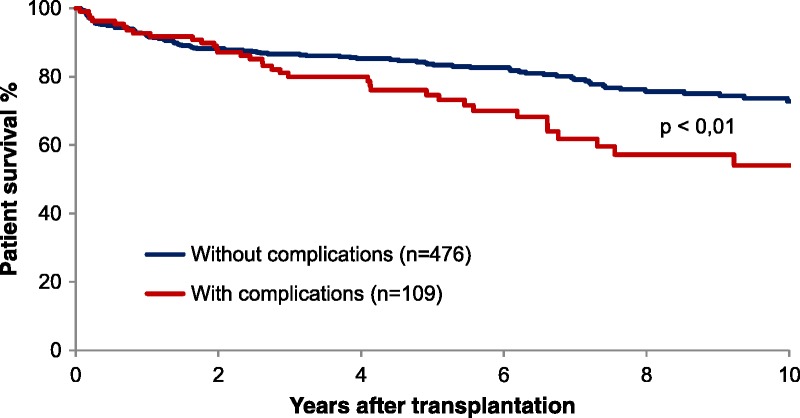
Patient survival in patients developing biliary complications or not.
DISCUSSION
In our study, we show that the overall frequency of biliary complications (18.5%) after LTx and the frequency of the most common biliary complication, ASs (44.4%), are in the ranges of previously reported complication rates of 6% to 34% and 47%, respectively.3-5,15,16 Although the range of BLs has been large historically, between 2% and 21%,8,15-20 such complications are becoming less frequent.21 Possible etiologies for developing a BL include biliary ischemia, downstream obstruction, or leak caused by T-tube removal. The use of T-tubes in CC anastomosis was considered standard procedure in the past. Advantages included the possibilities of direct measurement of bile output in the early postsurgical period, easy access in the need of radiological examination, and the ability to rapidly decompress the biliary tree if needed.9 It was also believed to decrease AS formation. Disadvantages mainly include BL upon its removal and higher overall complication rates.22 T-tubes are known to be one of the major risk factors associated with BLs,9,21 which is supported by the findings of increased rate of BLs in the group with T-tubes (P < 0.02) in our study. Few patients after the year of 2008 had a CC anastomosis with a T-tube, reflecting the shift away from its use at our department. Our low incidence of BLs (4%) may partly be due to our current restrictive use of T-tubes. However, some studies challenge the current opinion against use of T-tubes in LTx, reporting low rates of leak after removal and biliary complications overall.23,24
We know of no literature reviews concluding intraoperative blood loss to be a significant risk factor for biliary complications after LTx. However, our findings of increased intraoperative blood loss in patients developing biliary complications (P < 0.001) has been reported in a few other studies.25,26 A recent study on a large cohort of liver transplanted children did not see a correlation between intraoperative blood units transfused and the development of biliary complications,27 suggesting adults might be more sensitive to intraoperative blood loss and development of such complications.
In the literature, the incidence of biliary strictures after LTx ranges from 5% to 15%.2,15-17,28 In our study, the stricture incidence (16.6%) seems to be high. However, when subgrouping the incidence in relation to the model for end-stage liver disease (MELD), our results are in line with those described by Sundaram and colleagues,21 showing a significantly higher biliary stricture incidence in the post-MELD era (15.4% vs. 6.4%, P < 0.001). The increased use of extended criteria for donor livers, and the acceptance of organs from marginal donors, has led to an increased rate of biliary stricture formation.29 Other potential factors contributing to the increased stricture incidence are the access to more advanced diagnostic methods, the tendency of more actively treating borderline cases of biliary complications, and the growing ERCP competence available. Additionally, we have a relatively high proportion of patients transplanted for primary sclerosing cholangitis in our material. These patients are known to develop more often biliary strictures in the new liver.30
Anastomotic strictures are most of the times believed to be the result of technical issues at the anastomosis which, when combined with biliary ischemia, initiate a localized fibrotic response. Other possible contributing factors are prolonged ICU stay, sex mismatch transplants, and postoperative BLs.6,31 The optimal endoscopic treatment of ASs have been suggested to be a combination of large diameter balloon dilatation and prolonged biliary stenting over 12 months.32-34 Strictures often respond well even to shorter treatment duration, but recurrence rates have been reported to be higher.6,35,36 The recurrence rate of ASs in our study (6.4%) is lower than the reported rate in a meta-analysis (9%).37 One factor contributing to our low stricture recurrence rate might be the fact that all except 4 of the patients successfully treated with ERCP received stent treatment. A treatment approach using stents is known to lead to less recurrent strictures, as opposed to dilatation treatment only.38 The overall success rate for endoscopic and/or percutaneous treatment of anastomotic (90.4%) and nonanastomotic (72.8%) strictures is in the upper range of the results of several other authors evaluating endoscopic and/or percutaneous treatments.2,6,15,18,32-36,39-44 The lower treatment success rate (72.8%) and higher recurrence rate of NASs (29.2%) suggest that it is, generally, a more challenging complication to handle, and the optimal way approach these strictures remains uncertain. We found NASs to be the dominating primary complication type (45%) among patients with a choledochojejunostomy. Seven of the 9 patients in this group were transplanted because of primary sclerosing cholangitis. This may be 1 factor that contribute to the lower treatment success rates among patients with NASs. The high success rates in treating biliary leaks (100%) and SS (81.8%) are in line with the results of other studies.15,18,43,45
The PTC technique proved to be of important value in patients where an endoscopic approach was unsatisfactory or failed. Several patients also benefitted from rendezvous procedures combining ERCP and PTC. However, a primary PTC approach frequently requires more treatment procedures until resolution of the biliary complication. Additionally, the percutaneous drainage carry higher risk of infection and bring discomfort to the patient, as compared to ERCP stenting. We did find ERCP to have a doubled procedure-related complication rate (7.7% vs. 3.8%). However, no complication was severe. Our current regimen is that ERCP is the first line of treatment for this patient group, with complementing PTC therapy in case of ERCP failure.
The male proportion of patients developing biliary complications was significantly larger than the female proportion, comparing to the sex distribution in the group not developing biliary complications. This tendency was reported in another study, but the numbers were not significant.43 The proportion of males have previously been reported to be dominant among patients developing biliary complications.37
To our knowledge, this study is the first to show that, despite a high success rate in treating biliary complications after LTx, both graft and patient survivals were significantly impaired. This correlation has previously been reported only in retransplanted patients,46 and other studies have reported graft but not patient survival to be impaired in patients developing nonanastomotic biliary strictures.2,47 In contrast to the present study, biliary strictures overall are not known to affect graft and patient survival,2,6 and a recent study found biliary complications to not affect graft and patient survival in liver transplanted patients.43
In summary, we conclude that both endoscopic and percutaneous therapies are safe and efficient for treating biliary complications after LTx, and that surgery should be saved as a last resort. Our findings also indicate that by improvement in prevention of biliary complications one could improve patient and graft survivals after LTx.
ACKNOWLEDGMENTS
The authors thank Marie E. Larsson (research engineer, Division of Transplantation Surgery, Karolinska University Hospital) for contribution to the statistical analysis.
Footnotes
Published online 25 February, 2019.
The authors declare no funding or conflicts of interests.
J.R. retrieved and interpreted the data, and prepared the article. E.B. initiated the project, designed the study, interpreted the data, and prepared the article. U.A., performed the majority of the endoscopic procedures, designed the study, interpreted the data, and prepared the article. B.G.E. performed a considerable number of the liver transplantations, interpreted the data, and contributed to the discussion. G.N., designed the study, performed a considerable number of the liver transplantations, interpreted the data, and prepared the article.
REFERENCES
- 1.Duffy JP, Kao K, Ko CY, et al. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg. 2010;252:652–661. [DOI] [PubMed] [Google Scholar]
- 2.Graziadei IW, Schwaighofer H, Koch R, et al. Long-term outcome of endoscopic treatment of biliary strictures after liver transplantation. Liver Transpl. 2006;12:718–725. [DOI] [PubMed] [Google Scholar]
- 3.Gholson CF, Zibari G, McDonald JC. Endoscopic diagnosis and management of biliary complications following orthotopic liver transplantation. Dig Dis Sci. 1996;41:1045–1053. [DOI] [PubMed] [Google Scholar]
- 4.Tung BY, Kimmey MB. Biliary complications of orthotopic liver transplantation. Dig Dis. 1999;17:133–144. [DOI] [PubMed] [Google Scholar]
- 5.Akamatsu N, Sugawara Y, Hashimoto D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int. 2011;24:379–392. [DOI] [PubMed] [Google Scholar]
- 6.Verdonk RC, Buis CI, Porte RJ, et al. Anastomotic biliary strictures after liver transplantation: causes and consequences. Liver Transpl. 2006;12:726–735. [DOI] [PubMed] [Google Scholar]
- 7.Buis CI, Hoekstra H, Verdonk RC, et al. Causes and consequences of ischemic-type biliary lesions after liver transplantation. J Hepato-Biliary-Pancreat Surg. 2006;13:517–524. [DOI] [PubMed] [Google Scholar]
- 8.Pfau PR, Kochman ML, Lewis JD, et al. Endoscopic management of postoperative biliary complications in orthotopic liver transplantation. Gastrointest Endosc. 2000;52:55–63. [DOI] [PubMed] [Google Scholar]
- 9.Kochhar G, Parungao JM, Hanouneh IA, et al. Biliary complications following liver transplantation. World J Gastroenterol. 2013;19:2841–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macias-Gomez C, Dumonceau JM. Endoscopic management of biliary complications after liver transplantation: an evidence-based review. World J Gastrointest Endosc. 2015;7:606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangiavillano B, Pagano N, Baron TH, et al. Outcome of stenting in biliary and pancreatic benign and malignant diseases: a comprehensive review. World J Gastroenterol. 2015;21:9038–9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ring EJ, Oleaga JA, Freiman DB, et al. Therapeutic applications of catheter cholangiography. Radiology. 1978;128:333–338. [DOI] [PubMed] [Google Scholar]
- 13.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–393. [DOI] [PubMed] [Google Scholar]
- 15.Park JS, Kim MH, Lee SK, et al. Efficacy of endoscopic and percutaneous treatments for biliary complications after cadaveric and living donor liver transplantation. Gastrointest Endosc. 2003;57:78–85. [DOI] [PubMed] [Google Scholar]
- 16.Rerknimitr R, Sherman S, Fogel EL, et al. Biliary tract complications after orthotopic liver transplantation with choledochocholedochostomy anastomosis: endoscopic findings and results of therapy. Gastrointest Endosc. 2002;55:224–231. [DOI] [PubMed] [Google Scholar]
- 17.Greif F, Bronsther OL, Van Thiel DH, et al. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann Surg. 1994;219:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thuluvath PJ, Atassi T, Lee J. An endoscopic approach to biliary complications following orthotopic liver transplantation. Liver Int. 2003;23:156–162. [DOI] [PubMed] [Google Scholar]
- 19.Thethy S, Thomson BNj, Pleass H, et al. Management of biliary tract complications after orthotopic liver transplantation. Clin Transpl. 2004;18:647–653. [DOI] [PubMed] [Google Scholar]
- 20.O'Connor TP, Lewis WD, Jenkins RL. Biliary tract complications after liver transplantation. Arch Surg. 1995;130:312–317. [DOI] [PubMed] [Google Scholar]
- 21.Sundaram V, Jones DT, Shah NH, et al. Posttransplant biliary complications in the pre- and post-model for end-stage liver disease era. Liver Transpl. 2011;17:428–435. [DOI] [PubMed] [Google Scholar]
- 22.Scatton O, Meunier B, Cherqui D, et al. Randomized trial of choledochocholedochostomy with or without a T tube in orthotopic liver transplantation. Ann Surg. 2001;233:432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gastaca M, Matarranz A, Munoz F, et al. Biliary complications in orthotopic liver transplantation using choledochocholedochostomy with a T-tube. Transplant Proc. 2012;44:1554–1556. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Andujar R, Oron EM, Carregnato AF, et al. T-tube or no T-tube in cadaveric orthotopic liver transplantation: the eternal dilemma: results of a prospective and randomized clinical trial. Ann Surg. 2013;258:21–29. [DOI] [PubMed] [Google Scholar]
- 25.Selvakumar N, Saha BA, Naidu SC. Is duct to duct biliary anastomosis the rule in orthotopic liver transplantation? Indian J Surg. 2013;75:368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gastaca M, Matarranz A, Martinez L, et al. Risk factors for biliary complications after orthotopic liver transplantation with T-tube: a single-center cohort of 743 transplants. Transplant Proc. 2014;46:3097–3099. [DOI] [PubMed] [Google Scholar]
- 27.Feier FH, Seda-Neto J, da Fonseca EA, et al. Analysis of factors associated with biliary complications in children after liver transplantation. Transplantation. 2016;100:1944–1954. [DOI] [PubMed] [Google Scholar]
- 28.Colonna JO, II, Shaked A, Gomes AS, et al. Biliary strictures complicating liver transplantation. Incidence, pathogenesis, management, and outcome. Ann Surg. 1992;216:344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nemes B, Gaman G, Doros A. Biliary complications after liver transplantation. Expert Rev Gastroenterol Hepatol. 2015;9:447–466. [DOI] [PubMed] [Google Scholar]
- 30.Hildebrand T, Pannicke N, Dechene A, et al. Biliary strictures and recurrence after liver transplantation for primary sclerosing cholangitis: a retrospective multicenter analysis. Liver Transpl. 2016;22:42–52. [DOI] [PubMed] [Google Scholar]
- 31.Moser MA, Wall WJ. Management of biliary problems after liver transplantation. Liver Transpl. 2001;7:S46–S52. [DOI] [PubMed] [Google Scholar]
- 32.Tabibian JH, Asham EH, Han S, et al. Endoscopic treatment of postorthotopic liver transplantation anastomotic biliary strictures with maximal stent therapy (with video). Gastrointest Endosc. 2010;71:505–512. [DOI] [PubMed] [Google Scholar]
- 33.Tringali A, Barbaro F, Pizzicannella M, et al. Endoscopic management with multiple plastic stents of anastomotic biliary stricture following liver transplantation: long-term results. Endoscopy. 2016;48:546–551. [DOI] [PubMed] [Google Scholar]
- 34.Holt AP, Thorburn D, Mirza D, et al. A prospective study of standardized nonsurgical therapy in the management of biliary anastomotic strictures complicating liver transplantation. Transplantation. 2007;84:857–863. [DOI] [PubMed] [Google Scholar]
- 35.Zoepf T, Maldonado-Lopez EJ, Hilgard P, et al. Balloon dilatation vs. balloon dilatation plus bile duct endoprostheses for treatment of anastomotic biliary strictures after liver transplantation. Liver Transpl. 2006;12:88–94. [DOI] [PubMed] [Google Scholar]
- 36.Pasha SF, Harrison ME, Das A, et al. Endoscopic treatment of anastomotic biliary strictures after deceased donor liver transplantation: outcomes after maximal stent therapy. Gastrointest Endosc. 2007;66:44–51. [DOI] [PubMed] [Google Scholar]
- 37.Peng C, Ma C, Xu G, et al. The efficacy and safety of endoscopic balloon dilation combined with stenting in patients with biliary anastomotic strictures after orthotopic liver transplantation. Cell Biochem Biophys. 2015;72:385–397. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz DA, Petersen BT, Poterucha JJ, et al. Endoscopic therapy of anastomotic bile duct strictures occurring after liver transplantation. Gastrointest Endosc. 2000;51:169–174. [DOI] [PubMed] [Google Scholar]
- 39.Weber A, Prinz C, Gerngross C, et al. Long-term outcome of endoscopic and/or percutaneous transhepatic therapy in patients with biliary stricture after orthotopic liver transplantation. J Gastroenterol. 2009;44:1195–1202. [DOI] [PubMed] [Google Scholar]
- 40.Rizk RS, McVicar JP, Emond MJ, et al. Endoscopic management of biliary strictures in liver transplant recipients: effect on patient and graft survival. Gastrointest Endosc. 1998;47:128–135. [DOI] [PubMed] [Google Scholar]
- 41.Morelli J, Mulcahy HE, Willner IR, et al. Long-term outcomes for patients with post-liver transplant anastomotic biliary strictures treated by endoscopic stent placement. Gastrointest Endosc. 2003;58:374–379. [DOI] [PubMed] [Google Scholar]
- 42.Poley JW, Lekkerkerker MN, Metselaar HJ, et al. Clinical outcome of progressive stenting in patients with anastomotic strictures after orthotopic liver transplantation. Endoscopy. 2013;45:567–570. [DOI] [PubMed] [Google Scholar]
- 43.Mejia GA, Olarte-Parra C, Pedraza A, et al. Biliary complications after liver transplantation: incidence, risk factors and impact on patient and graft survival. Transplant Proc. 2016;48:665–668. [DOI] [PubMed] [Google Scholar]
- 44.Faleschini G, Vadala di Prampero SF, Bulajic M, et al. Predictors of endoscopic treatment outcome in the management of biliary complications after orthotopic liver transplantation. Eur J Gastroenterol Hepatol. 2015;27:150–154. [DOI] [PubMed] [Google Scholar]
- 45.Saab S, Martin P, Soliman GY, et al. Endoscopic management of biliary leaks after T-tube removal in liver transplant recipients: nasobiliary drainage versus biliary stenting. Liver Transpl. 2000;6:627–632. [DOI] [PubMed] [Google Scholar]
- 46.Enestvedt CK, Malik S, Reese PP, et al. Biliary complications adversely affect patient and graft survival after liver retransplantation. Liver Transpl. 2013;19:965–972. [DOI] [PubMed] [Google Scholar]
- 47.Guichelaar MM, Benson JT, Malinchoc M, et al. Risk factors for and clinical course of non-anastomotic biliary strictures after liver transplantation. Am J Transplant. 2003;3:885–890. [DOI] [PubMed] [Google Scholar]


